Abstract
Background:
Naegleria spp. is a free-living amoeba of which some species including N. fowleri and N. australeinsis are highly pathogenic in human and animals. These widespread amoebae could be found in different environmental sources particularly in aquatic resources of tropical and subtropical regions. The most important source of infection is via recreational water contact. Due to the lack of thorough research regarding species of Naegleria spp. in aquatic sources, the present study was conducted.
Methods:
In the present study, 60 samples were collected from recreational water resources of Rasht city, Guilan province, north of Iran. After filtering and culturing the samples, plates were examined by microscopic method and according to the page criteria. DNA of vahlkampfiid-positive samples were then extracted using phenol-chlorophorm method. Amoebae genus was identified by targeting the ITS-region and sequencing based-approaches.
Results:
Nine (15%) samples out of a 60 total samples were positive for Naegleria spp. of which seven belonged to potentially pathogenic N. australiensis. Two other strains were belonged to non-pathogenic N. pagei.
Conclusion:
The present research was the first report of occurrence of N. australiensis and N. pagei in Rasht city, north Iran. This study reflects the occurrence of Naegleria spp. in water sources of Guilan Province, Iran.
Keywords: Naegleria, Iran, Water
Introduction
Free-living amoeba (FLA) such as Naegleria, Valhkampfia, Acanthamoeba and Vermamoeba genera are widespread protozoa that can be isolated from different natural sources particularly from water resources (1). Among these FLAs, some genera such as Naegleria fowleri, Acanthamoeba spp., and Balamuthia mandrillaris are important pathogens affecting human and animals (1–3). These genera of protozoan parasites is especially important in at risk groups such contact lens wearers and also immunecompromised patients most notably cancer patients, so numerous studies were carried out on epidemiology of these parasites in these groups (4, 5).
Naegleria genus, as well as, Vahlkampfia and Paravahlkampfia belong to Vahlkampfidae family and the mentioned genera could be pathogenic for human, but, only pathogenic Naegleria species called N. fowleri and N. australiensis are medically important (6–8). Naegleria genus consists of 47 species, of which some species (N. fowleri and N. australiensis) have been reported as pathogenic agents for human and animals (3). Primary Ameobic Meningitis (PAM) is a disease that can be caused by both species. PAM is an acute and rapidly fatal meningoencephalitis (3, 9, 10), occurs in both humans and animals (10). It is worthy to mention most of PAM cases have been reported in young adults with a history of thermal water contacts. In Iran, a single case study reported meningoencephalitis due to pathogenic N. fowleri in a six-month Iranian infant. The report suggests paying more attention on isolation and molecular characterization of Naegleria spp. in Iran (11). There is another report from Iran detecting a mixed infection of Acanthamoeba genotype T3 and Vahlkampfia in a cosmetic soft contact lens wearer (6).
Gilan Province is located in north of Iran by the Caspian Sea on the north and by the Alborz Mountains on the south, with humid subtropical climate and the heaviest rainfalls in Iran. Because of beaches and forest, the province attracts many tourists every year. Rasht is the capital city of Gilan Province and at the 2011 its population was 639951 (Wikipedia). Despite the studies suggesting Acanthamoeba occurrence in this region, the presence of Naegleria spp. in water bodies and other environmental sources has not been reported. However, in other regions of Iran, few reports exist on the presence of Naegleria spp. Two studies were conducted in hot springs of Ardebil province to explore the presence of Acanthamoeba and Vahlkampfiids (12, 13). While the prevalence of Vahlkampfidae, particularly Naegleria spp. has not been studied in Gilan Province, there are several studies about the prevalence of other FLAs, especially within Acanthamoeba genus (14, 15), which showed the predominance of pathogenic T4 in the tested samples (14).
Thus, the present study aimed to isolate Naegleria spp., from recreational water resources using both morphological and molecular-based approaches. To the best of our knowledge there were no previous research regarding Naegleria spp. in north Iran.
Materials and Methods
Sampling area, Filtration and Cultivation
Rasht is the largest Iranian city on Caspian Sea coast. It is a major trade center between Caucasia, Russia and Iran using the port of Bandar-e Anzali. Rasht is also a major tourist center with the resort of Masouleh in the adjacent mountains and the beaches of Caspian as some of the major attractions (Wikipedia). A total of 60 water samples (500–1500 ml) were collected from Rasht ponds waters (20 samples), pools (20 samples) and streams (20 samples). All of the samples were taken within summer and the temperature of the water sources at the time of sampling were 8–20 °C. The samples were transferred to protozoology laboratory, Shahid Beheshti University of Medical Sciences, Tehran, Iran within one day and stored at room temperature. Approximately 250 ml of each sample were filtered through cellulose nitrate membrane with 1.6μm pore size. After that, middle of each membrane was cut out and cultured in plates of 1.5% non-nutrient agar medium along with heat inactivated Escherishia coli (16). Then plates were incubated at room temperature up to two months.
Morphological identification and cloning
Investigation of Vahlkampfiids was started after one week and continued up to two months. Light microscope with magnification of ×100 and page’s morphological criteria were used for identification of positive plates. Positive plates were then cloned to eliminate bacteria and fungi contamination and achieving pure plates for DNA extraction. Briefly, cloning was performed by transfer of single Vahlkampfiid- amoebae to new plates according to our previous studies (4).
DNA extraction
After adding sterile normal saline, pH 7, plates were scraped to harvest the amoebae. Samples were then centrifuged three times at 500g for 5 minutes and the sediments were used for DNA extraction. DNA samples were suspended in lysis buffer and incubated at 56 °C overnight. Proteinase K was also added to samples and finally the mixture was put in boiling temperature for 30 minutes. Finally, the isolated DNA of the samples was purified using phenol-chloroform method (17).
PCR analysis and Sequencing
Internal transcribed spacer (ITS) primer was targeted for amplification of Vahlkampfiids DNA including Naegleria spp. the PCR assay primers were designed included forward 5′GAACCTGCGTAGGGATCATTT3′ and reverse primer ITS2 5′ TTTCTTTTCCTCCCCTTATTA 3′.
The PCR reaction was accomplished in a 30 μl Ampliqone (Taq DNA Polymerase Master Mix Red, Denmark) as a readymade mixture. To achieve volume of 30-μl PCR reaction, 25 μl of master mix was combined with 5 ng DNA templates and 20 pmol primers. PCR products were separated on a 1.5% agarose gel. Products were visualized by ethidium bromide stain and were imaged under UV light.
The entire PCR product was then submitted to sequencing. DNA chromatograms were tested using chromas software (version 1.45) and the sequences were then aligned. Homology analyses were done using BLAST software and each sequence was blasted against all available eukaryotic sequences in the GenBank database.
Results
Out of 60 collected water samples from park ponds, pools and streams, 9 (15%) samples were found to contain Vahlkampfiids. The trophozoite form was detected after the third days of cultivation and the cysts were observed after one week of culture. Cloning of all Vahlkamfiids was done successfully. The most contamination source was found in ponds water sources (Table 1).
Table 1: Isolated Naegleria species in water samples of Rasht city according to the source of contamination.
| Sampling sources | No. of samples | Positive samples (%) |
|---|---|---|
| Ponds | 20 | 7 (35) |
| Pools | 20 | 1 (5) |
| Streams | 20 | 1 (5) |
| Total | 60 | 9 (15) |
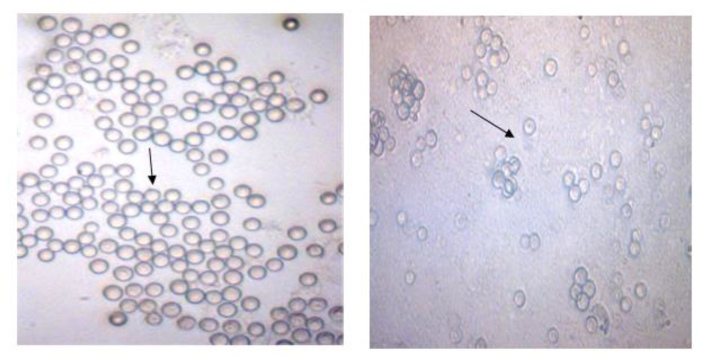
Amoebae isolated from pools only once. Vahlkampfiids were detected by their elongated shape of trophozoites, vesicular nucleus measuring between15–20 micron. The cysts were presented with smooth round wall measuring between 10–15 microns (Fig. 1, 2).
Fig. 1: Cloned Naegleria australiensis cysts in non-nutrient culture medium (note the round ectocysts).
Fig. 2: Elongated shape of Naegleria trophozoites in non-nutrient culture medium.
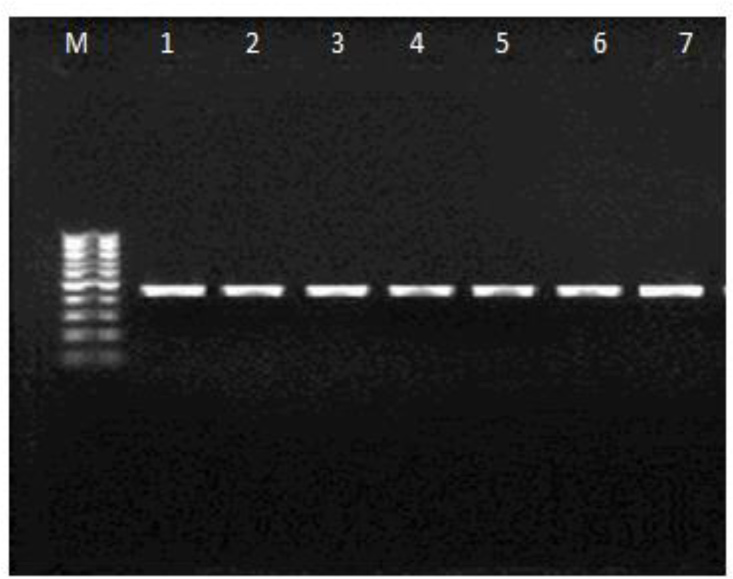
Eight of isolated amoebae showed growth on high temperatures (37–40 °C) and one strain did not growth in 40 °C. PCR analysis targeting ITS region revealed a 450 bp product in 9 isolates (15%) (Fig. 3).
Fig. 3: PCR products of some positive samples on a 1.5% agarose gel presenting 450 bp band (M= Marker, 1–7=samples).
Sequencing and homology analyses of the obtained sequences in Basic Local Alignment Search Tool (BLAST) showed that all strains belonged to the Naegleria genus. Seven strains isolated from pond 6 and stream 1 contained N. australiensis. Two other Naegleria belonged to N. pegei (Table 2).
Table 2: contaminated Sources and Naegleria species isolated from recreational area in Rasht City, Guilan province.
| Code | Source | Species |
|---|---|---|
| MN1 | Pool | N. pagei |
| MN2 | Pond | N. australiaensis |
| MN3 | Pond | N. australiaensis |
| MN4 | Pond | N. australiaensis |
| MN5 | Pond | N. australiaensis |
| MN6 | Pond | N. pagei |
| MN7 | Pond | N. australiaensis |
| MN8 | Pond | N. australiaensis |
| MN9 | Stream | N. australiaensis |
Discussion
In the present study, Naegleria spp. were detected from 15% water samples of Rasht City, Gilan Province. Based on PCR products base pairs in the examined water bodies, the most pathogenic Naegleria spp., N. fowleri, was not identified. This may justifies lack of primary amoebic meningoencephalitis (PAM) report in the region. However, other studies reported Naegleria spp. in Iran. Except one study, other studies in Iran identified non-pathogenic Naegleria and they have been isolated from environmental samples. Recently there is just one case report of PAM from Qom Province, Iran (11). In addition, there is a report of mixed infection due to Acanthamoeba genotype T3 and Vahlkampia belonging to Vahlkampfiidea family in a cosmetic soft lens wearer in Iran with poor prognosis (6).
Despite the very low prevalence of PAM in Iran, because of carrier role of Naegleria for potentially pathogenic microorganisms, like other FLAs, study regarding Naegleria occurrence in environmental sources should not be neglected (18). However, more studies are needed to investigate water sources with more sample size. In addition, vast research studies need to be carried out on to examine the etiology of meningoencephalitis.
The present study is the first research on the occurrence of Naegleria spp. in environmental sources in Rasht City. Most studies were conducted on other FLAs occurrence, particularly Acanthamoeba genus (19). Behniafar et al. reported 40% occurrence of free living amoeba including Acanthamoeba, Thecamoeba, Hartmannella, Vahlkampfiids and Vannella in surface waters of Kaleybar and Khodaafarin counties (East-Azerbaijan province)using culture and microscopic method (20). Another study was conducted to estimate the presence of Acanthamoeba in Gilan Province with 50 samples of recreational water sources. Niyyati et al. reported 30% prevalence of Acanthamoba by cultivation and morphological detection and molecular analysis. They revealed that 13-isolated Acanthamoeba belong to T4 genotype (14). In other regions of Iran, researchers have studied prevalence of Naegleria spp. in water resources. While the results of the present study indicate no occurrence of N. fowleri in water samples, two studies report the occurrence of Naegleria in hot spring of Ardebil Province. Badirzadeh et al. found 11 samples out of 28 (39.3%) hot-spring water samples to be positive for Vahlkampfiids (12). However, their research was limited to morphological identification. Solgi et al. tested 30 samples collected from hot-springs of Ardebil Province that 8 samples (26.7%) were positive for Valhkampfiid and Hartmannella (13). This discrepancy could be explained by the fact that Naegleria spp. are thermotolerant so they are possibly more prevalent in hot springs.
The findings of the present study indicate the occurrence of Vahlkampfiids in 15% of the samples. This is in disagreement with other studies reporting a higher isolation of Naegleria. Wang et al. reported Naegleria occurrence of 92.9% in environmental water of Cangchun, Northeastern China using PCR method (21). Edagawa et al. found prevalence of 68.7% of FLAs from tap-water sources in Osaka, Japan (22). This difference may be attributed to the application of various isolation methods for FLA detection. It should be mention that although N. pagei is a non-pathogenic species but it can harbor pathogenic microorganisms.
Conclusion, the present study reflects the occurrence of Naegleria spp. in water sources of Rasht City that can be potential hazard for native people and tourists, thus posting of alarming signs in recreational places could be an option for decreasing the risk. However, we recommend more research investigations focusing on N. fowleri detection and their prevalence in the environmental sources.
Acknowledgments
Dr. Maryam Niyyati was supported from National Elite Foundation for Young Associated Professors and Shahid Beheshti University of Medical Sciences. The authors declare that there is no conflict of interests.
References
- 1. Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol. 2004; 34 (9): 1001– 1027. [DOI] [PubMed] [Google Scholar]
- 2. Karanis P, Kourenti C, Smith H. Waterborne transmission of protozoan parasites: A worldwide review of outbreaks and lessons learnt. J Water Health. 2007; 5 (1): 1– 38. [DOI] [PubMed] [Google Scholar]
- 3. Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007; 50 (1): 1– 26. [DOI] [PubMed] [Google Scholar]
- 4. Niyyati M, Rahimi F, Lasejerdi Z, Rezaeian M. Potentially pathogenic free-living amoebae in contact lenses of the asymptomatic contact lens wearers. Iran J Parasitol. 2014; 9 (1): 14. [PMC free article] [PubMed] [Google Scholar]
- 5. Memari F, Niyyati M, Haghighi A, Tabaei SJS, Lasjerdi Z. Occurrence of pathogenic Acanthamoeba genotypes in nasal swabs of cancer patients in Iran. Parasitol Res. 2015; 114 (5): 1907– 1912. [DOI] [PubMed] [Google Scholar]
- 6. Niyyati M, Lorenzo-Morales J, Rezaie S, Rahimi F, Martín-Navarro CM, Mohebali M, Maghsood AH, Farnia S, Valladares B, Rezaeian M. First report of a mixed infection due to Acanthamoeba genotype t3 and Vahlkampfia in a cosmetic soft contact lens wearer in Iran. Exp Parasitol. 2010; 126 (1): 89– 90. [DOI] [PubMed] [Google Scholar]
- 7. Visvesvara GS, Sriram R, Qvarnstrom Y, Bandyopadhyay K, Da Silva AJ, Pieniazek NJ, Cabral GA. Paravahlkampfia francinae n. Sp. Masquerading as an agent of primary amoebic meningoencephalitis. J Eukaryot Microbiol. 2009; 56(4): 357–366. [DOI] [PubMed] [Google Scholar]
- 8. Marciano-Cabral F, Jamerson M, Kaneshiro E. Free-living amoebae, Legionella and Mycobacterium in tap water supplied by a municipal drinking water utility in the USA. J Water Health. 2010; 8 (1): 71– 82. [DOI] [PubMed] [Google Scholar]
- 9. Martinez AJ, Visvesvara GS. Free-living, amphizoic and opportunistic amebas. Brain Pathology. 1997; 7 (1): 583– 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Visvesvara GS, De Jonckheere JF, Sriram R, Daft B. Isolation and molecular typing of Naegleria fowleri from the brain of a cow that died of primary amebic meningoencephalitis. J CLIN MICROBIOL. 2005; 43 (8): 4203– 4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Movahedi Z, Shokrollahi MR, Aghaali M, Heydari H. Primary amoebic meningoencephalitis in an iranian infant. Case Reports In Medicine. 2012; 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Badirzadeh A, Niyyati M, Babaei Z, Amini H, Badirzadeh H, Rezaeian M. Isolation of free-living amoebae from sarein hot springs in ardebil province, iran. Iran J Parasitol. 2011; 6 (2): 1. [PMC free article] [PubMed] [Google Scholar]
- 13. Solgi R, Niyyati M, Haghighi A, Mojarad EN. Occurrence of thermotolerant Hartmannella vermiformis and Naegleria spp. In hot springs of ardebil province, northwest iran. Iran J Parasitol. 2012; 7 (2): 47. [PMC free article] [PubMed] [Google Scholar]
- 14. Niyyati M, Nazar M, Lasjerdi Z, Haghighi A, Nazemalhosseini E. Reporting of T4 genotype of Acanthamoeba isolates in recreational water sources of gilan province, northern Iran. Novel BioMed. 2015; 3 (1): 20– 24. [Google Scholar]
- 15. Mahmoudi MR, Taghipour N, Eftekhar M, Haghighi A, Karanis P. Isolation of Acanthamoeba species in surface waters of gilan province-north of iran. Parasitol Res. 2012; 110 (1): 473– 477. [DOI] [PubMed] [Google Scholar]
- 16. Rezaeian M, Niyyati M, Farnia S, Haghi AM. Isolation of Acanthamoeba spp. From different environmental sources. Iran J Parasitol. 2008; 3 (1): 44– 47. [Google Scholar]
- 17. Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. Cell. Vol. 1 New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 18. Huang S-W, Hsu B-M, Chen N-H, Huang C-C, Huang K-H, Chen J-S, Kao P-M. Isolation and identification of Legionella and their host amoebae from weak alkaline carbonate spring water using a culture method combined with pcr. Parasitol Res. 2011; 109 (5): 1233– 1241. [DOI] [PubMed] [Google Scholar]
- 19. Niyyati M, Rezaeian M. Current status of Acanthamoeba in iran: A narrative review article. Iran J Parasitol. 2015; 10 (2): 157– 163. [PMC free article] [PubMed] [Google Scholar]
- 20. Behniafar H, Niyyati M, Lasjerdi Z, Dodangeh S. High occurrence of potentially pathogenic free living amoebae in water bodies of kaleybar and khodaafarin, east azerbaijan province. Curr World Environ. 2015; 10(special issue) [Google Scholar]
- 21. Wei W, Feng W, Jiping L, Nan L, Quan L. Isolation and identification of Naegleria species from environmental water in changchun, northeastern china. Iran J Parasitol. 2014; 9 (2): 254– 259. [PMC free article] [PubMed] [Google Scholar]
- 22. Edagawa A, Kimura A, Kawabuchi-Kurata T, Kusuhara Y, Karanis P. Isolation and genotyping of potentially pathogenic Acanthamoeba and Naegleria species from tap water sources in Osaka, Japan. Parasitol Res. 2009; 105 (4): 1109– 1117. [DOI] [PubMed] [Google Scholar]