Abstract
Background:
Cutaneous leishmaniasis (CL) is a parasitic disease with a relatively wide distribution in different areas of the world, including Iran. The parasite is mainly diagnosed microscopically, but serological approaches might be useful for diagnosis as well. This study aimed to assess the efficacy of an immunoblotting system for serodiagnosis of cutaneous leishmaniasis in Iran.
Methods:
Sixty-one sera samples from parasitologically confirmed CL patients and 50 sera samples from healthy controls along with 50 sera sample from non-CL patients were collected. Native strain of Leishmania major was cultured in Schneider medium and soluble Leishmania antigens were prepared from amastigotes-like parasites. All of sera samples were evaluated by an immunoblotting system.
Results:
Components of 14 to 135 kDa were detectable by the sera of CL patients. From 61 sera of CL patients, 59 cases (96.7%) detected a 63 kDa subunit and 51 cases (83.6%) recognized a 32–35 kDa component. Among all subunits, the 63 kDa band showed the highest sensitivity (96.7%) and a 75 kDa band had the highest (98%) specificity.
Conclusion:
Immunoblotting has a satisfactory performance in diagnosis of CL and this test can be used, as an aid, for proper diagnosis of CL.
Keywords: Cutaneous Leishmaniasis, ELISA, IFA, Immunoblotting
Introduction
Cutaneous leishmaniasis (CL) is a serious and increasing health problem worldwide and is endemic in nearly 90 countries, including Iran (1). In Iran, the disease is mainly caused by Leishmania major and L. tropica, although Leishmania infantum has been infrequently isolated from patients with cutaneous leishmaniasis (2–5).
The laboratorial diagnosis of CL relies mainly on the direct parasitological method that searches for the Leishmania parasite in clinical samples, taken from the skin lesion of CL patients. Parasitological methods are highly specific as they demonstrate the presence of the parasite in tissues materials taken from the skin lesion, by staining the sample by Giemsa’s stain, or by cultivation of lesion materials, allowing for parasite growth. Although microscopic examination of lesion scrapings is specific, however its sensitivity is hampered by the fact that tissue distribution of parasites is not homogeneous and the density of Leishmania is low in chronically infected CL Cases (6). On the other hand, culture of lesion biopsies and aspirates, for isolating the Leishmania spp. are labor intensive, are available in reference laboratories and are positive in less than 60% of CL cases (7). Along with parasitological method, PCR-based approaches have also been widely utilized for the diagnosis of CL. Nevertheless, the persistent of parasite in the lesion after the treatment and the technical requirements indicate that molecular methods cannot be easily incorporated routinely into diagnostic protocols for CL (8–10).
Serological methods have been commonly used to diagnose visceral leishmaniasis (VL) and considered as a routine method for diagnosis of VL (11–14). Serological methods have been considered of limited importance for the diagnosis of CL. However, in recent years such methods have been evaluated, with encouraging results, for the diagnosis of CL in few studies (15–21). Among the serological tests, Western blot analysis has received much attention for the diagnosis of CL.
The current study was conducted to evaluate the effectiveness of immunoblotting, aiming at the development of better tool for proper diagnosis of CL.
Materials and Methods
Serum Samples
Sera samples were collected from sixty-one parasitologically confirmed (by microscopically examination of lesions) CL patients (41 men and 20 women) referred to university affiliated hospitals in Shiraz, southern Iran. From each patient, 5 ml of whole blood was collected. The mean age of the patients was 37 years (range 9–83 years). Most patients (23 cases) had skin lesion in their hand followed by foot (13 cases), face (8 cases), and trunk (4 cases). Few cases had skin lesion in more than one area of the body. Mean of lesion number was 3 (range 1–20) and history of the disease was from two weeks up to 20 years. Control samples (n=50) were obtained from healthy individuals who had no history of CL.
Moreover, 50 sera sample were collected from non-CL patients including patients with bacterial (n=12), parasitic (n=3) and autoimmune diseases (n=35).
Ethical approval of the study was given by the Ethics Committee of Shiraz University of Medical Sciences and consent was obtained from the participants.
Antigen preparation
Promastigote form of L. major (MRHO/IR/75/ER) were cultivated at 25°C in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum, penicillin G (100 UI/mL), and streptomycin (100 mg/ml). Moreover, amastigote-like form of L. major was cultured in Schneider medium as previously described (22). Soluble L. major antigen was prepared from the amastigote-like by washing the parasite, three times (1500×g for 10 min) with PBS. Cocktail of protease inhibitor (Sigma, P2714) was added and the sample was subjected to three rounds of freezing and thawing followed by five pulse of sonication. The lysed amastigote-like was centrifuged (1500×g for 15 min at 4 °C) and the supernatant was stored at −20 °C until use.
SDS-PAGE and immunoblotting
Soluble Leishmania antigen (SLA) of L. major amastigote-like (20 μg) was subjected to discontinuous reducing SDS-PAGE, using 5% stacking and 15% separating gels in a Bio-Rad apparatus at 25 mA/gel for 1 hour. The antigen was transferred from the gel into nitrocellulose membrane (Pall Gelman Laboratory, P/N 66485). Efficacy of transfer was checked by staining the membrane with Ponceau S stain (0.001 g/ml in 3% trichloroacetic acid). The membranes with blotted antigen were cut into strips and blocked with 5% (w/v) of skimmed milk in washing buffer (10 mM Tris, 150 mM NaCl, and 0.05%Tween 20; pH 7.4) for 2 hours. The strips were incubated with test sera (1/100 dilution in washing buffer with 1% bovine serum albumin (BSA) for 1.5 hours at room temperature. After 3 washes (each 15 min), the strips were incubated with horseradish peroxidase conjugated anti-human IgG (Sigma, A8667) at a dilution of 1/4000 (in washing buffer contained 1% BSA) for 1 h at room temperature. After 3 washes as before, bound antigens was developed using diaminobanzidine (DAB) substrate (0.1% H2O2, 0.5 mg/ml DAB (Sigma, D5637) in 50 mM Tris-HCl, pH 7.6).
Results
All the sera samples were evaluated by the immunoblotting system. Using antigens prepared from amastigotes, components of 14 to 135 kDa (14, 18, 20–29, 30–35, 38–42, 45, 48, 51–56, 62, 68, 70, 75, 95, and 135 kDa) were detectable by the sera of CL patients. From 61 sera of CL patients, 59 cases (96.7%) detected a 63 kDa subunit and 51 cases (83.6%) recognized a 32–35 kDa component (Fig. 1, 2). These components were detected by the sera of just a few of healthy subjects or non-CL patients. The highest sensitivity (96.7%) of the system was achieved with the 63-kDa subunit while the highest specificity was found with a 75-kDa subunit. The best negative predictive value (97.2%) was achieved with the 63-kDa subunit while the highest positive predictive value of the system (89.4%) was obtained with the 75-kDa subunit. Table 1 shows the performance of different subunits of soluble antigen of L. major amastigote-like in diagnosis of CL in immunoblotting system.
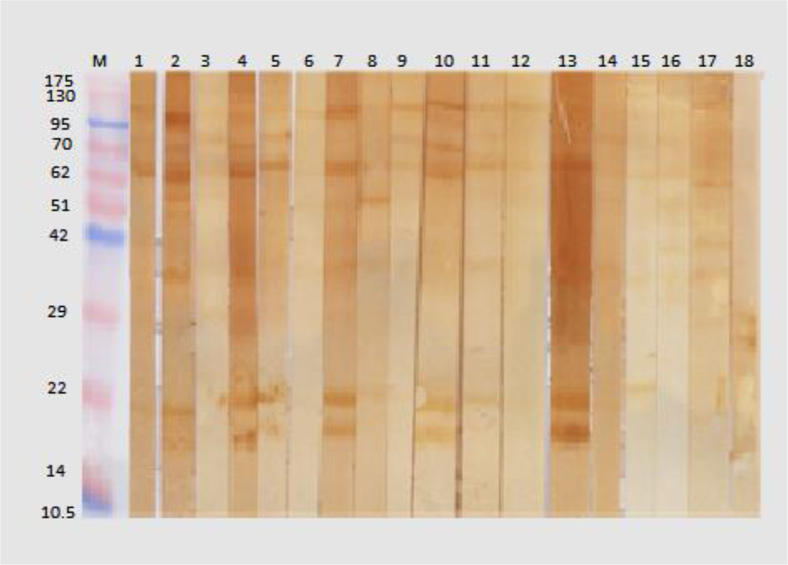
Fig. 1: Immunoblotting of sera samples from cutaneous leishmaniasis (CL) patients and controls with soluble antigen of L. major amastigotes. M, protein marker; Lanes 1–3 sera of non-CL patients; lane 4–5 sera of healthy controls; lane 6–16 sera of CL patients.
Fig. 2: Immunoblotting of sera samples from cutaneous leishmaniasis (CL) patients and controls with soluble antigen of L. major amastigotes. M, protein marker; Lanes 1–13 sera of CL patients; 14–16 sera of healthy controls; 17–18 sera of non-CL patients.
Table 1: Performance of different subunits of soluble Leishmania antigen in diagnosis of CL in immunoblotting system.
| Subunits (kDa) | Sensitivity (%) | Specificity (%) | PPV (Positive Predictive Value) (%) | NPV (Negative Predictive Value) (%) |
|---|---|---|---|---|
| 14 | 14.7 | 9.3 | 56 | 64 |
| 18 | 70.5 | 8.9 | 79.6 | 82.4 |
| 29–20 | 27.9 | 7 | 36.1 | 61.4 |
| 35–32 | 83.6 | 6.5 | 59.3 | 40.6 |
| 42–38 | 1.6 | 95 | 16.6 | 61.2 |
| 45 | 29.5 | 87 | 58 | 66.9 |
| 48 | 0 | 52.2 | 0 | 43.5 |
| 56–51 | 4.9 | 83 | 15 | 58.8 |
| 63 | 96.7 | 70 | 66.2 | 97.2 |
| 68 | 34.4 | 86 | 60 | 68 |
| 70 | 0 | 87 | 0 | 58.7 |
| 75 | 27.9 | 98 | 89.4 | 69 |
| 95 | 39.3 | 93 | 77.4 | 71.5 |
| 135 | 0 | 95 | 0 | 60.8 |
Discussion
Detection of Leishmania parasites in lesion of CL patients remains as a gold standard for diagnosis of CL (9–10). The sensitivity of direct microscopic identification of the parasite is less that 60% (7). Furthermore, the direct microscopic identification methods are much less sensitive in detecting the parasites in chronic cases, where parasitemia is low. Cultivation of tissue samples, taken from the skin lesions, might help to some extent for detecting the Leishmania parasite, but it is time-consuming and parasite cultivation cannot be easily performed in any diagnostic centers.
Serological approaches have been extensively used for the diagnosis of VL (11–14). It is mainly because robust activation of humoral immunity occurs in VL and hyper gammaglobulinemia is a feature of this form of leishmaniasis. Therefore, antibodies against different antigens of Leishmania are present in the sera of VL patients and these antibodies can be detected by serological approaches. Serological assays have a limited application in the diagnosis of CL in comparison with VL. Nevertheless, the use of the serological tools in diagnosis of CL has been evaluated in few studies with encouraging results (15–19, 23, 24). IFA and ELISA for serodiagnosis of cutaneous leishmaniasis on 61 confirmed CL patients’ revealed sensitivity of 91.6% and 83.6% and specificity of 81% and 62.7% respectively (25).
The current study evaluated the usefulness of immunoblotting in diagnosis of CL. Findings of the study demonstrated an appropriate performance for immunoblotting in the diagnosis of CL where a sensitivity of more than 95% and specificity of 70% were found for a 63-kDa subunit of L. major for diagnosis of CL. Moreover, the diagnostic performance of the 32–35 kDa and a 75 kDa antigens of amastigotes of L. major in this study were satisfactory.
The detected 63 kDa subunit, might be the well-known gp63 (Glycoproteins 63) of Leishmania surface protein which is the most important surface protein of Leishmania spp. It belongs to metalloprotease family and has major role in entrance of parasite into macrophages (26).
In Pomares et al. study, diagnostic value of Western blot was evaluated for diagnosis of CL. Serum samples from 45 patients with proven CL were all positive by immunoblotting with specific bands against 14 kDa and/or 18 kDa Leishmania antigens (27). In our study, the low molecular weight antigens of L. major had a relatively high sensitivity but their specificity was low.
Zeyrek et al. assessed the antigens of L. infantum for the diagnosis of Anthroponotic Cutaneous Leishmaniasis (ACL) caused by L. tropica and found subunits of antigens ranging from 15 to 118 kDa, recognized by patients’ sera (19). Among all bands, the 63-kDa band was found to be more sensitive (88.5%). This is consistent with our findings about the performance of the 63-kDa subunit of L. major in diagnosis of CL. The 63-kDa subunit of Leishmania amastigote antigen, which is detected by the sera of most of CL patients, might be the gp 63, a well-known glycoprotein that is present in Leishmania spp.
A protein of 60 kDa can be recognized by the sera of American cutaneous leishmaniasis (ACL) patients, and suggested that this subunit can be used for the diagnosis of ACL (28). Our results are broadly consistent with the findings of that study (28).
A few of samples from healthy controls or non-CL patients reacted with the 63 kDa in our study. Thus, a positive immunoblotting test should be interpreted in light of the patient’s clinical status.
Conclusion
Immunoblotting has a satisfactory performance in diagnosis of CL, and this test can be used for serological diagnosis of CL along with the parasitological methods.
Acknowledgments
The results described in this paper were part of MSc thesis of Marzieh Ashrafmansouri. The study was financially supported by the office of vice-chancellor for research of Shiraz University of Medical Sciences (Grant No. 3602). The authors declare that there is no conflict of interests.
References
- 1. Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012; 7 (5): e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Motazedian H, Noamanpoor B, Ardehali S. Characterization of Leishmania parasites isolated from provinces of the Islamic Republic of Iran. East Mediterr Health J. 2002; 8 (2–3): 338– 344. [PubMed] [Google Scholar]
- 3. Hajjaran H, Mohebali M, Mamishi S, Vasigheh F, Oshaghi MA, Naddaf SR, et al. Molecular identification and polymorphism determination of cutaneous and visceral leishmaniasis agents isolated from human and animal hosts in Iran. Biomed Res Int. 2013; 2013: 789326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohebali M. Visceral leishmaniasis in Iran: Review of the epidemiological and clinical features. Iran J Parasitol. 2013; 8 (3): 348– 358. [PMC free article] [PubMed] [Google Scholar]
- 5. Sarkari B, Hatam G, Ghatee M. Epidemiological features of visceral leishmaniasis in Fars province, southern Iran. Iran J Public Health. 2012; 41 (4): 94– 99. [PMC free article] [PubMed] [Google Scholar]
- 6. Souza AP, Soto M, Costa JM, Boaventura VS, de Oliveira CI, Cristal JR, et al. Towards a more precise serological diagnosis of human tegumentary leishmaniasis using Leishmania recombinant proteins. PLoS One. 2013; 8 (6): e66110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh S. New developments in diagnosis of leishmaniasis. Indian J Med Res. 2006; 123 (3): 311– 30. [PubMed] [Google Scholar]
- 8. Reithinger R, Dujardin JC. Molecular diagnosis of leishmaniasis: current status and future applications. J Clin Microbiol. 2007; 45 (1): 21– 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goto H, Lindoso JA. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti Infect Ther. 2010; 8 (4): 419– 433. [DOI] [PubMed] [Google Scholar]
- 10. Ameen M. Cutaneous leishmaniasis: advances in disease pathogenesis, diagnostics and therapeutics. Clin Exp Dermatol. 2010; 35 (7): 699– 705. [DOI] [PubMed] [Google Scholar]
- 11. Srividya G, Kulshrestha A, Singh R, Salotra P. Diagnosis of visceral leishmaniasis: developments over the last decade. Parasitol Res. 2012; 110 (3): 1065– 1078. [DOI] [PubMed] [Google Scholar]
- 12. Mikaeili F, Fakhar M, Sarkari B, Motazedian MH, Hatam G. Comparison of serological methods (ELISA, DAT and IFA) for diagnosis of visceral leishmaniasis utilizing an endemic strain. Iran J Immunol 2007; 4(2: 116– 121. [PubMed] [Google Scholar]
- 13. Sarkari B, Hatam GR, Mikaeili F, Sadeghi H, Ebrahimi S. A comparative study of antigen and antibody detection in visceral leishmaniasis using serum and urine-based ELISA. Trop Biomed. 2008; 25 (2): 96– 99. [PubMed] [Google Scholar]
- 14. Ghatei MA, Hatam GR, Hossini MH, Sarkari B. Performance of latex agglutination test (KA-tex) in diagnosis of visceral leishmaniasis in Iran. Iran J Immunol. 2009; 6 (4): 202– 207. [PubMed] [Google Scholar]
- 15. Castellano LR, Gave TC, Lira MA, Dessein H, Dessein A, Correia D, et al. Evaluation of electro-eluted antigens in the serological diagnosis of cutaneous leishmaniasis. Ann Trop Med Parasitol. 2010; 104 (4): 347– 350. [DOI] [PubMed] [Google Scholar]
- 16. Szargiki R, Castro EA, Luz E, Kowalthuk W, Machado AM, Thomaz-Soccol V. Comparison of serological and parasitological methods for cutaneous leishmaniasis diagnosis in the state of Parana, Brazil. Braz J Infect Dis. 2009; 13 (1): 47– 52. [DOI] [PubMed] [Google Scholar]
- 17. Al-Nahhas SA. Serodiagnosis of cutaneous leishmaniasis in the Syrian Arab Republic. Saudi Med J. 2009; 30 (3): 382– 386. [PubMed] [Google Scholar]
- 18. Barroso-Freitas AP, Passos SR, Mouta-Confort E, Madeira MF, Schubach AO, Santos GP, et al. Accuracy of an ELISA and indirect immunofluorescence for the laboratory diagnosis of American tegumentary leishmaniasis. Trans R Soc Trop Med Hyg. 2009; 103 (4): 383– 389. [DOI] [PubMed] [Google Scholar]
- 19. Zeyrek FY, Korkmaz M, Ozbel Y. Serodiagnosis of anthroponotic cutaneous leishmaniasis (ACL) caused by Leishmania tropica in Sanliurfa Province, Turkey, where ACL is highly endemic. Clin Vaccine Immunol. 2007; 14 (11): 1409– 1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ribeiro FC, de OS, Mouta-Confort E, Schubach TM, de Fatima MM, Marzochi MC. Use of ELISA employing Leishmania (Viannia) braziliensis and Leishmania (Leishmania) chagasi antigens for the detection of IgG and IgG1 and IgG2 subclasses in the diagnosis of American tegumentary leishmaniasis in dogs. Vet Parasitol. 2007; 148 (3–4): 200– 206. [DOI] [PubMed] [Google Scholar]
- 21. Rocha RD, Gontijo CM, Eloi-Santos SM, Teixeira-Carvalho A, Correa-Oliveira R, Ferrari TC, et al. Clinical value of anti-live Leishmania (Viannia) braziliensis immunoglobulin G subclasses, detected by flow cytometry, for diagnosing active localized cutaneous leishmaniasis. Trop Med Int Health. 2006; 11 (2): 156– 166. [DOI] [PubMed] [Google Scholar]
- 22. Habibi P, Sadjjadi SM, Owji M, Moattari A, Hatam GR, Sarkari B. Characterization of in vitro cultivated amastigote like of Leishmania major: a substitution for in vivo studies. Iran J Parasitol. 2008; 3 (1): 6– 15. [Google Scholar]
- 23. Jensen AT, Gaafar A, Ismail A, Christensen CB, Kemp M, Hassan AM, et al. Serodiagnosis of cutaneous leishmaniasis: assessment of an enzyme-linked immunosorbent assay using a peptide sequence from gene B protein. Am J Trop Med Hyg, 1996; 55(5: 490– 495. [DOI] [PubMed] [Google Scholar]
- 24. Romero LI, Paz HM, Ortega-Barria E, Bayard V, Hochberg LP, Collins KM, et al. Evaluation of serological assays based on a novel excreted antigen preparation for the diagnosis of cutaneous leishmaniasis in Panama. J Microbiol Methods. 2004; 57 (3): 391– 397. [DOI] [PubMed] [Google Scholar]
- 25. Sarkari B, Ashrafmansouri M, Hatam G, Habibi P, Abdolahi Khabisi S. Performance of an ELISA and indirect immunofluorescence assay in serological diagnosis of zoonotic cutaneous leishmaniasis in Iran. Interdiscip Perspect Infect Dis. 2014; 2014: 505134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lieke T, Nylén S, Eidsmo L, McMaster WR, Mohammadi AM, Khamesipour A, Berg L, Akuffo H. Leishmania surface protein gp63 binds directly to human natural killer cells and inhibits proliferation. Clin Exp Immunol. 2008; 153 (2): 221– 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pomares C, Despierres L, del GP, Delaunay P, Michel G, Ferrua B, et al. Western blot analysis as an aid for the diagnosis of cutaneous leishmaniasis due to Leishmania major. Trans R Soc Trop Med Hyg. 2012; 106 (7): 452– 454. [DOI] [PubMed] [Google Scholar]
- 28. Masuda A, do Nascimento SF, Guerra CS, Paranhos GS, Ferreira AW. Analysis of the specificity of human antibodies to antigens of Leishmania braziliensis braziliensis. Rev Inst Med Trop Sao Paulo. 1989; 31 (4): 228– 234. [DOI] [PubMed] [Google Scholar]