Abstract
Background:
We evaluated the in vivo activity of Bunium persicum (Boiss) essential oil on infected mice with acute toxoplasmosis.
Methods:
To evaluate prophylactic effects, male NMRI mice received B. persicum essential oil at the concentrations of 0.05 and 0.1 mL/kg for 14 days. After 24 h mice were infected intraperitonealy with 1×104 tachyzoites of T. gondii, RH strain. In order to investigate therapeutic effects, mice were infected and then received B. persicum oil at the concentrations of 0.05 and 0.1 ml/kg two times a day for 5 days. The time/mean time of death in all infected mice and the number of tachyzoites from infected mice were recorded.
Results:
The time/mean time of death of infected mice was 8 and 9 days after oral administration of B. persicum oil at the concentration of 0.05 and 0.1 mL/kg, respectively (P<0.05). In contrast, the time/mean time of death control group was 5 days. In addition, B. persicum significantly reduced the mean number of tachyzoites compared with control group. The time/mean time of death of infected mice was 6 and 7 days after oral administration of B. persicum essential oil at the concentration of 0.05 and 0.1 mL/kg, respectively. In contrast, the time/mean time of death control group was 5 days. B. persicum especially at the concentration of 0.1 ml/kg significantly reduced the mean number of tachyzoites compared with control group.
Conclusion:
The results showed the potential of B. persicum essential oil as a natural source for the production of new prophylactic agent for use in toxoplasmosis.
Keywords: Toxoplasma gondii, Bunium persicum Boiss, Prophylactic, Therapeutic
Introduction
Toxoplasmosis, caused by the protozoan parasite Toxoplasma gondii, is one of the most common parasitic infections of human and other warm-blooded animals. Nearly one-third of world populations have been exposed with this parasite (1). Human, typically, can be infected by three main routes of transmission: ingestion of tissue cysts in raw or undercooked infected meat, ingestion of food or water contaminated with sporulated oocysts shed in the feces of an infected cat and congenitally, vertical transmission from mother to fetus across the placenta (2). At present, the first choice midication to treat toxoplasmosis is combination of pyrimethamine and sulfadiazine. However, these medications have some drawbacks, because they may have some side effects including osteoporosis, sepsis and teratogenic effects especially in patients with severe disorders of the immune system such as AIDS patients (3, 4). These reasons indicate the urgent needs for development of new medications or combination therapy for treatment of toxoplasmosis.
Historically, natural products and their compounds have been the most productive source for treatment a wide range of diseases, such as infectious diseases (5). Bunium persicum (Boiss), called in Persian as “Zireh Kohi” belongs to Apiaceae family, which widely grows in the southeast of Iran (6). The plant seeds have been traditionally used as carminative, anti-spasmodic, increasing breast milk and antiepileptic treatment (7). Reviews have also reported antinociceptive, antioxidant, anti-inflammatory and antimicrobial effects of the B. persicum essential oil (8–10).
To date, no study has been conducted on the anti-Toxoplasma effects this plant. Therefore, the present study was aimed to evaluate the prophylactic and therapeutic efficacy of B. persicum essential oil on infected mice with acute toxoplasmosis.
Materials and Methods
Plant material
The seeds of B. persicum were collected in July 2013, from the wild plants, which grow in the Koohpayeh, Kerman, Iran. The taxonomic identification of the plant was confirmed by Dr. Mozaffarian, Department of Botany of the Research Institute of Forests and Rang-lands (TARI), Tehran, Iran. A voucher specimen (KF 1141) was deposited in the Herbarium Center of the Kerman Faculty of Pharmacy, Kerman, Iran.
Extraction/isolation of the essential oil
Air-dried plant materials (100 g) were subjected to hydro-distillation for 4 h using an all-glass Clevenger-type apparatus. The essential oil obtained was dried over anhydrous sodium sulfate and stored in darkness at 2–8 °C in airtight glass vials closed under nitrogen gas until testing. For the preparation of dilutions of the B. persicum, the essential oil was dissolved in olive oil as solvent (11).
Gas chromatography/mass spectrometry analysis
GC analysis of the B. persicum, the essential oil was carried out by a Shimadzu QP 5000 (FID) chromatograph HP-5 MS capillary column (30 m × 0.25 mm, film thickness 0.25 μm). Helium was used as carrier gas at a flow rate 1 ml/min (split ratio 1:20) with injection volume of 0.2 μL. Injector and detector temperatures were set at 220 and 290 °C, respectively. Oven temperature was kept at 50 °C for 3 min, gradually raised to 160 °C at 3 °C/min, held for 10 min and finally raised to 240 °C at 3 °C/min. GC/MS analysis was carried out using a Shimadzu QP 5050 operating at 70 eV ionization energy, equipped with an HP-5 capillary column (phenyl methyl siloxane, 30 m × 0.25 mm, 0.25 μm film thickness) with Helium as the carrier gas (split ratio 1:20). Retention indices were determined by using retention times of n-alkanes that had been injected after the oil under the same chromatographic conditions. The components were identified based on the comparison of their relative retention time and mass spectra with those of standards, Wiley 2001 library data of the GC/MS system and literature data (12).
Parasite
The virulent RH strain of T. gondii was obtained from the Parasitology Laboratory at the Department of Parasitology and Mycology, Kerman University of Medical Sciences, Kerman, Iran. Tachyzoites of this strain were collected by serial intraperitoneal passages in mice. Parasites (1×104) were inoculated in the mice, and after 72 h, tachyzoites were provided by repeated flushing of the peritoneal cavity by Phosphate Buffered Saline (PBS). Tachyzoites were then harvested and recovered with PBS and used in the experiments.
Animals
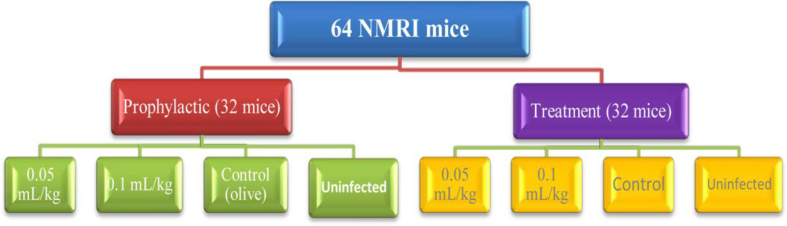
Sixty four male NMRI mice (6–8 weeks old) weighing from 20 to 25 g for establishing animal model of T. gondii were obtained from the Pasteur Institute, Tehran, Iran. Animals were housed in a colony room with a 12:12 h light/dark cycle at 21 ± 2 °C and were handled according to standard protocols for the use of laboratory animals. They were divided into eight groups; every group contained eight mice (Fig. 1).
Fig. 1: Flow chart of in vivo efficacy of the Bunium persicum (Boiss) essential oil against acute toxoplasmosis in mice.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
The protocol was approved by the Committee on the Ethics of Animal Experiments of the Kerman University of Medical Science (Permit Number: 93/110).
Establishment of acute toxoplasmosis
In this study, animal model of acute toxoplasmosis was established as described elsewhere (13). Briefly, the groups of mice were inoculated intraperitonealy with 1×104 tachyzoite of T. gondii, RH strain.
Prophylactic effects of B. persicum essential oil
To evaluate prophylactic effects of B. persicum essential oil on acute toxoplasmosis in mice, two groups of mice were received B. persicum essential oil at the concentrations of 0.05 and 0.1 mL/kg for 14 days. A group of mice was used as control that received olive oil for 14 days; while another group was contained uninfected and untreated mice. After 24 h. (fifteenth day) mice in each group were infected intraperitonealy with 1×104 tachyzoite of T. gondii, RH strain. To determine the prophylactic effects of B. persicum essential oil, the time/mean time of death in all infected mice was recorded. In addition, the number of parasites (tachyzoites) isolated from peritonea of infected mice were counted under light microscope by neubauer slide.
Therapeutic effects of B. persicum oil
In order to investigate therapeutic effects of B. persicum essential oil against acute toxoplasmosis in mice, at first, 3 groups of mice were infected intraperitonealy with 1×104 tachyzoite of T. gondii, RH strain. After 24 h, two groups of mice were received B. persicum oil at the concentrations of 0.05 and 0.1 mL/kg two times a day for 5 days. A group of mice was used as control that received olive oil for 14 days; while another group was contained uninfected and untreated mice. To assess the treatment effects of B. persicum essential oil, the time/mean time of death in all infected mice were recorded. Furthermore, the number of parasites (tachyzoites) isolated from peritonea of infected mice were counted under light microscope by neubauer slide.
Statistical analysis
Data analysis was carried out using SPSS statistical package version 17.0 (SPSS Inc., Chicago, IL, USA). Differences between test and control groups were analyzed by t-test. In addition, P<0.05 was considered statistically significant.
Results
GC/MS analysis of B. persicum oil
Table 1 indicates the results obtained by GC/MS analysis of B. persicum essential oil. Twenty-four compounds were identified in the B. persicum essential oil, which constitutes about 97.2% of this essential oil. The main components were γ-terpinene (46.1%), cuminaldehyde (15.5%), ϱ-cymene (6.7%) and limonene (5.9%).
Table 1: Essential oil composition of B. persicum seeds identified by GC-MS.
| No | Components | KI a | % Composition |
|---|---|---|---|
| 1. | α- Thujene | 926 | 0.4 |
| 2. | α- Pinene | 936 | 2.7 |
| 3. | Sabinene | 968 | 1.0 |
| 4. | β -Pinene | 976 | 2.5 |
| 5. | Myrcene | 990 | 1.8 |
| 6. | ϱ -Cymene | 1016 | 6.7 |
| 7. | α-Terpinene | 1019 | 1.3 |
| 8. | σ -Cymene | 1021 | 0.2 |
| 9. | Limonene | 1029 | 5.9 |
| 10. | γ- Terpinene | 1060 | 46.1 |
| 11. | α- Terpineolene | 1087 | 0.9 |
| 12. | ϱ-Mentha-3-ene-7-al | 1138 | 0.9 |
| 13. | Terpinene-4-ol | 1160 | 0.2 |
| 14. | α- Terpineol | 1168 | 2.2 |
| 15. | ϱ-Mentha-1,3 diene-7-al | 1176 | 0.2 |
| 16. | Cuminaldehyde | 1243 | 15.5 |
| 17. | Cuminyl alcohol | 1265 | 7.4 |
| 18. | β- Caryophyllene | 1419 | 0.2 |
| 19. | γ-Eleman | 1435 | 0.1 |
| 20. | β- Bisabolene | 1478 | 0.5 |
| 21. | β- Selinene | 1488 | 0.1 |
| 22. | Myristicin | 1491 | 0.1 |
| 23. | Germacrene B | 1558 | 0.1 |
| 24. | Dillapiol | 1631 | 0.2 |
| Total | 97.2 |
Kovats index on non-polar DB-5 ms column in reference to n-alkanes
Prophylactic effects of B. persicum essential oil
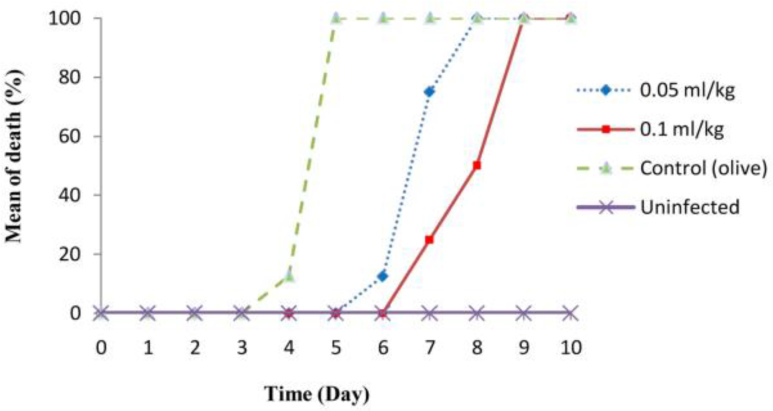
Figure 2 indicates prophylactic effects of B. persicum essential oil against acute toxoplasmosis in mice model.
Fig. 2: Prophylactic effects of Bunium persicum (Boiss) essential oil on the time/mean time of death of infected mice with acute toxoplasmosis.
The time/mean time of death of infected mice was 100%, 8 and 9 days after oral administration of B. persicum essential oil at the concentration of 0.05 and 0.1 ml/kg, respectively, while this value for control group was 5 days. In addition, mean number of tachyzoites was 192 × 104 and 64 × 104 for infected mice treated with 0.05 and 0.1 ml/kg, respectively, whereas in control group, the mean number of tachyzoites was 288×104 parasite. The obtained findings demonstrated that the difference in the time/mean time of death between of B. persicum oil at the concentration of 0.05 and 0.1 ml/kg and the control group was statistically significant (P<0.05), whereas, the difference in mean number of tachyzoites between B. persicum oil at the concentration of 0.05 ml/kg and the control group was not statistically significant.
Therapeutic effects of B. persicum oil
As shown in Fig. 3, 100% the time/mean time of death of infected mice was observed 7 and 8 days after oral administration of B. persicum essential oil at the concentration of 0.05 and 0.1 ml/kg, respectively, whereas the time/mean time of death of infected mice in control group was 100% in the fifth day. The mean number of tachyzoites was 189 × 104 and 133 × 104 for infected mice treated with 0.05 and 0.1 ml/kg, respectively, while in control group, the mean number of tachyzoites was 412×104 parasite.
Fig. 3: Therapeutic effects of Bunium persicum (Boiss) essential oil on the time/mean time of death of infected mice with acute toxoplasmosis.
Results exhibited that the difference in the time/mean time of death between of B. persicum essential oil at the concentration of 0.1 ml/kg and the control group was statistically significant (P<0.05). The difference in mean number of tachyzoites between infected mice received B. persicum essential oil and the control group was statistically significant (P<0.05).
Discussion
This study was aimed to assess in vivo prophylactic and therapeutic effects of B. persicum essential oil against acute toxoplasmosis in mice. Since last centuries, plants and their derivatives have been used as a valuable natural resource for traditional remedies (5). In recent years, development of dire effects and microbial resistance to the chemically synthesized medication has caused changes in the situation and interest in the field of ethnobotanical research (5).
Our findings showed that oral administration of B. persicum oil for 14 days especially at the concentration of 0.1 ml/kg had potent prophylactic effects against acute toxoplasmosis in mice and their survival was prolonged to 9 days, while all mice in the control group died after fifth day. Moreover, treatment of infected mice by oral administration of B. persicum essential oil for 5 days revealed remarkable therapeutic effects against acute toxoplasmosis. So that B. persicum essential oil at the concentrations of 0.05 and 0.1 ml/kg significantly reduced the time/mean time of death and the mean tachyzoites in infected mice.
So far, various pharmacological activities such as antioxidant, anti-inflammatory, and antimicrobial effects have been related to B. persicum seeds. In the GC/MS analysis of the B. persicum essential oil, the main components were found to be hydrocarbon and oxygenated monoterpenes including γ-terpinene (46.1%), cuminaldehyde (15.5%), ϱ-cymene (6.7%) and limonene (5.9%). Various studies revealed potent antibacterial, antifungal and antiparasitic activities of these compounds such as terpenic derivates, γ-terpinene, carvacrol, P-cymene, thymol, carvone, and limonene (14–18). Therefore, phytoconstituents in these plants could be responsible for their antitoxoplasmosis activity though their exact mode of action especially immunemodulatory effects is poorly understood. However, in the case of antimicrobial mechanism of some terpenoids compounds, Sikkema et al. (19) have reported that these components diffuse into pathogen and damage cell membrane structures. On the other hand, the antimicrobial activity is related to ability of terpenes to affect not only permeability but also other functions of cell membranes, these compounds might cross the cell membranes, thus penetrating into the interior of the cell and interacting with critical intracellular sites (20, 21). Concerning toxicity effects, Mandegari et al (22) have shown that the B. persicum essential oil had no mortality up to the dose of 2.5 ml/kg. According to a toxicity classification, the B. persicum showed no significant toxicity against male NMRI mice (23).
Conclusion
Findings revealed the remarkable therapeutic and prophylactic efficacy of the B. persicum essential oil against acute toxoplasmosis in mice model. Results also provided the scientific evidence that natural plants could be used in traditional medicine for the prevention and treatment of parasitic infections. However, further studies are required to evaluate exact effect of B. persicum essential oil particularly its immunomodulatory on acute toxoplasmosis.
Acknowledgments
This study was supported by Herbal and Traditional Medicine Research Center and Vice Chancellor for Research, Kerman University of Medical Sciences (project no. 93/110). The authors declare that there is no conflict of interests.
References
- 1. Hill D, Dubey J. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microb Infect. 2002; 8 (10): 634– 40. [DOI] [PubMed] [Google Scholar]
- 2. Mahmoudvand H, Saedi Dezaki E, Soleimani S, Baneshi MR, Kheirandish F, Ezatpour B, et al. Seroprevalence and risk factors of Toxoplasma gondii infection among healthy blood donors in southeast of Iran. Parasite Immunol. 2015. April 17. doi: 10.1111/pim.12198. [DOI] [PubMed] [Google Scholar]
- 3. Signorini L, Gulletta M, Coppini D, Donzelli C, Stellini R, Manca N, et al. Fatal disseminated toxoplasmosis during primary HIV infection. Curr HIV Res. 2007; 5: 273– 4 [DOI] [PubMed] [Google Scholar]
- 4. Sukthana Y. Toxoplasmosis: beyond animals to humans. Trends Parasitol. 2006; 22: 137– 42. [DOI] [PubMed] [Google Scholar]
- 5. Rocha LG, Almeida JR, Macedo RO, Barbosa-Filho JM. A review of natural products with antileishmanial activity. Phytomedicine. 2005; 12: 514– 35. [DOI] [PubMed] [Google Scholar]
- 6. Rechinger KH. Flroa Iranica. Verlagesanstalt. Graz: Akademische Druck; 1989. [Google Scholar]
- 7. Zargari A. Medicinal Plants. Tehran University, Tehran: 1996. [Google Scholar]
- 8. Hajhashemi V, Sajjadi S, Zomorodkia M. Antinociceptive and anti-inflammatory activities of Bunium persicum essential oil, hydroalcoholic and polyphenolic extracts in animal models. Pharm Biol. 2011; 49: 146– 51. [DOI] [PubMed] [Google Scholar]
- 9. Sekine T, Sugano M, Majid A, Fujii Y. Antifungal effects of volatile compounds from black zira (Bunium persicum) and other spices and herbs. J Chem Ecol. 2007; 33(11: 2123– 32. [DOI] [PubMed] [Google Scholar]
- 10. Talei G R, Mosavi Z. Chemical composition and antibacterial activity of Bunium persicum from west of Iran. Asian J Chem. 2009; 21 (6): 4749– 54. [Google Scholar]
- 11. Mahmoudvand H, Saedi Dezaki E, Kheiran-dish F, Ezatpour B, Jahanbakhsh S, Fasihi Harandi M. Scolicidal effects of black cumin seed (Nigella sativa) essential oil on hydatid cysts. Korean J Parasitol. 2014; 52 (6): 653– 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. Illinois, USA: Carol Stream, IL: Allured Publishing Corporation; 2004. [Google Scholar]
- 13. Asgari H, Keshavarz H, Shojaee S, Motazedian MH, Mohebali M, Miri R, et al. In vitro and in vivo potential of RH strain of Toxoplasma gondii (Type I) in tissue cyst forming. Iran J Parasitol. 2013; 8 (3): 367– 75. [PMC free article] [PubMed] [Google Scholar]
- 14. Giweli A, Džamić AM, Soković M, Ristić MS, Marin PD. Antimicrobial and antioxidant activities of essential oils of Satureja thymbra growing wild in Libya. Molecule. 2012; 26; 17( 5): 4836– 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Melo JO, Bitencourt TA, Fachin AL, Cruz EM, de Jesus HC, Alves PB. Antidermatophytic and antileishmanial activities of essential oils from Lippia gracilis Schauer genotypes. Acta Trop. 2013; 128 (1): 110– 15. [DOI] [PubMed] [Google Scholar]
- 16. Monzote L, García M, Pastor J, Gil L, Scull R, Maes L, et al. Essential oil from Chenopodium ambrosioides and main components: activity against Leishmania, their mitochondria and other microorganisms. Exp Parasitol. 2014; 136: 20– 6. [DOI] [PubMed] [Google Scholar]
- 17. Pearson RA, Manian A, Hall D, Harcus JL, Hewlett L. Antileishmanial activity of chlorpromazine. Antimicrob Agents Chemother. 1984; 25: 571– 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tandon JS, Srivastava V, Guru PY, Iridoids: a new class of leishmanicidal agents from Nyctanthes arbortristis. J Nat Prod. 1991; 54: 1102– 4. [DOI] [PubMed] [Google Scholar]
- 19. Sikkema J, De Bont DA, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Mol Biol Rev. 1995; 59: 201– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ismail A, Lamia H, Mohsen H, Samia S, Bassem J. Chemical composition and antifungal activity of three Anacardiaceae species grown in Tunisia. Science Int. 2013; 1: 148– 54. [Google Scholar]
- 21. Cristani M, D'Arrigo M, Mandalari G, Castelli F, Sarpietro MG, Micieli D, et al. Interaction of four monoterpenes contained in essential oils with model membranes: implications for their antibacterial activity. J Agric Food Chem. 2007; 55 (15): 6300– 8. [DOI] [PubMed] [Google Scholar]
- 22. Mandegary A, Arab-Nozari M, Ramiar H, Sharififar F. Anticonvulsant activity of the essential oil and methanolic extract of Bunium persicum (Boiss). B. Fedtsch. J Ethnopharmacol. 2012; 140: 447–51. [DOI] [PubMed] [Google Scholar]
- 23. Loomis TA. Essential of toxicology. Philadelphia: Lea and Febige, 1968. [Google Scholar]