Abstract
Although clotrimazole was first used against fungal infections, a body of research was later developed indicating that this drug has anticancer properties as well. The mechanism of action is based on the inhibition of mitochondrial-bound glycolytic enzymes and calmodulin, which starves cancer cells of energy. Clotrimazole and its derivatives have been shown to decrease rates of cancer cell proliferation, induce G1 phase arrest, and promote pro-apoptotic factors, which lead to cell death.
Keywords: Clotrimazole, Calmodulin, Human melanoma, Hexokinase
Introduction
Clotrimazole, first synthesized by Karl Hienz Buchel (Bayer) in the late 1960s, was originally developed as an antifungal agent [1]. The first in a family of azole derivatives, it was patented by Bayer in 1972 and introduced to German markets in 1973 under the brand name Canesten. This drug's unremarkable side effects and uncomplicated metabolic profile allowed it to enjoy worldwide acceptance for the treatment of mycotic outbreaks such as vaginal yeast infections and athlete's foot. A plethora of popular products (Desenex, Lotrimin, and Lamisil) followed and, because of its utility, clotrimazole was placed on the World Health Organization's list of essential medicines. Clotrimazole is a crystalline powder with a molecular formula of C22H17ClN2 and an average molecular weight of 344.8 g/mol. It is a lipophilic molecule with a solubility of 0.49 mg/L in water. Figure 1 shows the 3-D representation of the clotrimazole molecule.
Figure 1.
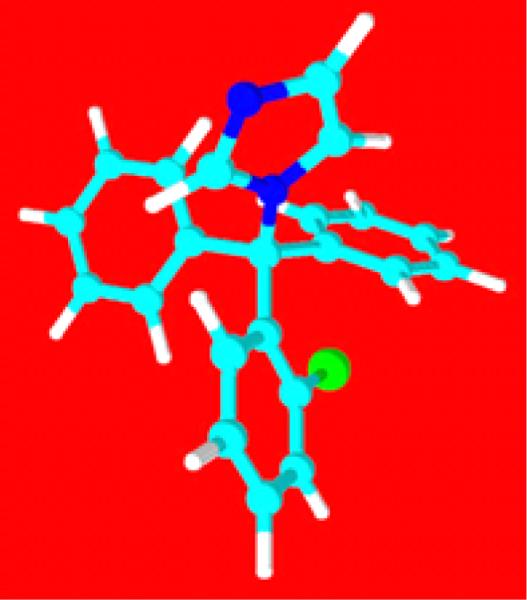
Structure of Clotrimazole
In addition to its use as an antifungal, there is promising research on using clotrimazole against other diseases (Table 1) such as sickle cell anemia, malaria, beriberi, tineapedis, Chagas disease and cancer [2-7]. The mechanism of action of clotrimazole against fungi and the trypanosomes of Chagas disease is the inhibition of ergosterol synthesis which slows microbial growth. Clotrimazole also inhibits the Ca2+-activated potassium (IK) channel and causes heme-induced membrane damage, which are responsible for its action against sickle cell disease and malaria, respectively.
Table 1.
Clotrimazole as a drug of interest against several diseases.
| Disease | Mechanism of action |
|---|---|
| Sickle cell disease [2] | Inhibition of calcium-dependent potassium channel |
| Malaria [4] | Heme-induced membrane damage |
| Chagas disease [5], Candida albicans [7], Tineapedis (Athlete's foot) [6] | Inhibition of ergosterol synthesis |
| Cancer [13] | Inhibition of calcium-dependent potassium channel, glycolytic enzymes, and G1-phase arrest |
In 1995, clotrimazole was shown to have an inhibitory effect on cancer cell proliferation and tumor growth when tested against human melanoma and glioblastoma cells in vivo [8,9]. Additional studies revealed clotrimazole to be a potent calmodulin inhibitor and that cells exposed to clotrimazole were found to have depleted calcium stores which were unable to be replenished by normal cell mechanisms [10,11]. Anti-cancer effects can also be traced to its targeting of the glycolytic enzymes hexokinase, phosphofructokinase, and aldolase [12-14]. The inhibition of glycolysis was shown against breast cancer, MCF-7, MCF10A, MDA-MB-231, HeLa, B16-F10, and Lewis lung carcinoma cell lines [13,15-19]. Further research showed the cytotoxicity of derivative compounds when clotrimazole was used as a ligand in metal coordination complexes [20,21]. In each case, clotrimazole was a potent inhibitor of glycolysis, leading to cell-cycle arrest and apoptosis.
Mechanism of Action Against Cancer
Hexokinase (HK) is the enzyme that phosphorylates glucose in the first and the rate-limiting steps of glycolysis. HK has four isoforms HK1, HK2, HK3, and HK4 (or glucokinase, GK). HK plays a crucial role in tumor growth and is the major enzyme expressed in various malignant tumors [22]. HK is bound to trans-membrane voltage-dependent anion channels (VDACs), which are located within the mitochondrial outer membrane [23]. HK will normally block the cytochrome c release from the mitochondrial outer membrane and protect the cells from apoptosis. Clotrimazole disrupts HK binding to mitochondria thus precipitating cell death [14]. Due to the fundamental role of HK in glycolytic flux in many cancers, this enzyme became an attractive target for cancer therapy. Chiara et al. has reported that clotrimazole specifically disrupts the HK-VDAC interaction in HeLa cells that causes the opening of the permeability transition pore complex, followed by release of apoptogenic proteins that cause indirect cell-death [17]. Palchaudhuri et al. treated B16-F10 cells with clotrimazole to understand whether clotrimazole induces detachment of HK from the mitochondrial outer membrane and exhibits antiproliferative activity by inhibiting glycolysis and reducing the cellular ATP level [24]. This treatment showed a 71% reduction in mitochondrial-bound HK-consistent with previous observations and reveals the mechanism of inducing cell death. Other research showed that clotrimazole also inhibited the signaling kinase protein Akt, which enhances the effect of HK dissociation [25]. Clotrimazole most likely causes dissociation of HK, cytochrome c release, and apoptosis through an allosteric interaction [26].
However, HK is not the only cellular target affected by clotrimazole; it also affects the two other important glycolytic enzymes: phosphofructokinase (PFK) and aldolase (ALD) [14]. Treating B16-F10 cells with clotrimazole was found to cause detachment of these enzymes from the cytoskeleton [12,27]. Penso et al. treated Lewis lung carcinoma and colon adenocarcinoma (CT-26) cells with clotrimazole and found a dose-dependent detachment of the PFK and ALD from the cytoskeleton [18]. Since PFK is found to be overexpressed in aggressive tumors that have an increased glycolytic flux when compared with normal tissues, this enzyme is a promising target for antineoplastic agents [28]. In another study, clotrimazole inhibited breast cancer cell proliferation through apoptosis triggered by the displacement of key glycolytic enzymes [27]. Goncalves demonstrated that when clotrimazole was tested against tumoral and nontumoral intestinal epithelial (Caco–2) cell lines, clotrimazole inhibits oxidative phosphorylation and glycolysis. In addition to decreasing ATP production which slows cell growth and proliferation, detachment of PFK can cause significant changes in cytoskeleton conformation and cell shape, which eventually lead to apoptosis [19].
Another important feature of clotrimazole is its agonistic action on calmodulin. Interference with Ca2+ metabolism and blockage of the Ca2+ - activated potassium (IK) channel by clotrimazole has been shown to reduce cancer cell proliferation [29-31]. Clotrimazole induces a release of Ca2+stores and slows their replenishment, thus inhibiting translation and slowing tumor growth [32]. Furthermore, this disruption of ion transport is severe and induces apoptosis as demonstrated by Ito against acute lymphoblastic leukemia cells [9]. When clotrimazole was tested for 86Rb efflux inhibitions, it was found that clotrimazole blocks the IK channel with an average IC50 value of 0. 92 μM [24]. These results are remarkable in that the anticancer effects were observed without an accompanying cytotoxicity [8].
Since the discovery of the popular drug cisplatin, platinum complexes have become the most widely used metal-based drugs in anticancer research [33]. The nitrogen atom present in the imidazole ring of clotrimazole facilitates coordination with transition metal ions such as Pt, as well as Ru, Pd, Cu, Co, Zn and Ni. Ravera et al. synthesized Pt(II) complexes containing bis(clotrimazole) ligands which were shown to effectively inhibit the growth of MCF-7, SKBR-3, HT-29, and B16/BL6 cell lines [21]. In another study, a series of Ru-clotrimazole compounds were synthesized which were shown to be active against multiple cancer cell lines [34]. Finally, Soledad Betanzos-Lara et al. synthesized sixteen novel CuII, CoII, ZnII, and NiII coordination complexes of clotrimazole and observed cytotoxic activity against HeLa (cervix-uterine), PC3 (prostate), and HCT-15 (colon) cell lines [20]. The SAR studies in this research showed that CuII ligated clotrimazole complexes have superior IC50values compared with other metals. It is of interest that these metal complexes are inducing apoptosis superior to that of either the parent clotrimazole or cisplatin.
The triphenylmethyl pharmacophore present in clotrimazole is the crucial element in its anti-cancer activity. Small molecules containing the triphenylmethyl motif has demonstrated potent anticancer properties [24,35-38]. All of these triphenylmethyl analogues induce cell cycle arrest in melanoma and other cancer cell lines by inhibiting cellular growth in G1- or M-phases [39]. Like S-trityl-L-cysteine, clotrimazole has no effect on tubulin polymerization which reduces the side effects observed compared with other classes of chemotherapeutic drugs [35]. Al-Qawasmeh et al. produced a series of 27 clotrimazole analogs without the imidazole moiety. This structure activity relationship study identified the large majority of these analogs as having similar G0-G1 phase arrest and antiproliferative effects as the parent molecule containing imidazole [39].
One limitation to clotrimazole's success is its poor water solubility that reduces bioavailability. Many attempts have been taken to improve clotrimazole's solubility through the use of microcapsules, thermo sensitive gels, liposomes, nanospheres or through a suspension with hydroxypropylmethyl cellulose [40-43]. Recently, inclusion of the poorly water-soluble clotrimazole with beta-cyclodextrins showed an enhanced solubility and a greater bioavailability in rats [44]. The future development of clotrimazole derivatives should center on retaining antiproliferative properties while increasing bioavailability-using information from structure relationship studies, biochemical, and cell-based assays.
Conclusions
Clotrimizole and its analogs have been shown to be clinically useful antitumor drugs, reducing the size and growth of neoplasms by interfering with plasma membrane ion transport, inducing G1-phase cell cycle arrest, and inhibiting the metabolic enzymes HK, PFK, and ALD. A more complete understanding of the mechanism of action of clotrimazole in cancer cell death will accelerate research into beneficial applications of this drug against cancer.
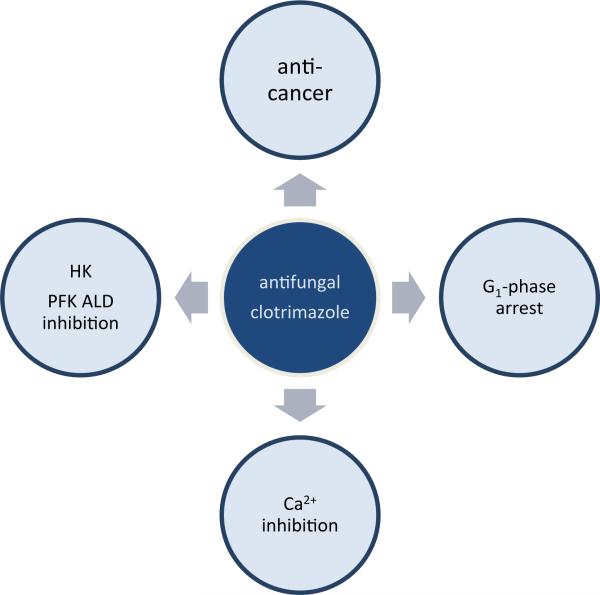
Figure 2.
Applications of clotrimazole and various anticancer mechanisms
Acknowledgements
We would like to thank the Department of Natural Sciences at Western New Mexico University, New Mexico IDeA Networks of Biomedical Research Excellence (NM-INBRE) and NIH 2P20GM103451-14.
References
- 1.Buchel KH, Draber W, Regel E, Plempel M. [Synthesis and properties of clotrimazole and other antimycotic 1-triphenylmethylimidazoles]. Arzneimittelforschung. 1972;22:1260–1272. [PubMed] [Google Scholar]
- 2.McNaughton-Smith GA Burns JF, Stocker JW, Rigdon GC, Creech C, et al. Novel inhibitors of the Gardos channel for the treatment of sickle cell disease. J Med Chem. 2008;51:976–982. doi: 10.1021/jm070663s. [DOI] [PubMed] [Google Scholar]
- 3.Stocker JW, De Franceschi L, McNaughton-Smith GA, Corrocher R, Beuzard Y, et al. ICA-17043, a novel Gardos channel blocker, prevents sickled red blood cell dehydration in vitro and in vivo in SAD mice. Blood. 2003;101:2412–2418. doi: 10.1182/blood-2002-05-1433. [DOI] [PubMed] [Google Scholar]
- 4.Huy NT, Takano R, Hara S, Kamei K. Enhancement of heme-induced membrane damage by the anti-malarial clotrimazole: the role of colloid-osmotic forces. Biol Pharm Bull. 2004;27:361–365. doi: 10.1248/bpb.27.361. [DOI] [PubMed] [Google Scholar]
- 5.Buckner FS, Urbina JA. Recent Developments in Sterol 14-demethylase Inhibitors for Chagas Disease. Int J Parasitol Drugs Drug Resist. 2012;2:236–242. doi: 10.1016/j.ijpddr.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg HL, Shwayder TA, Bieszk N, Fivenson DP. Clotrimazole/ betamethasone diproprionate: a review of costs and complications in the treatment of common cutaneous fungal infections. Pediatr Dermatol. 2002;19:78–81. doi: 10.1046/j.1525-1470.2002.00027.x. [DOI] [PubMed] [Google Scholar]
- 7.Czerninski R, Pikovsky A, Gati I, Friedman M, Steinberg D. Comparison of the efficacy of a novel sustained release clotrimazole varnish and clotrimazole troches for the treatment of oral candidiasis. Clin Oral Investig. 2014 doi: 10.1007/s00784-014-1259-5. [DOI] [PubMed] [Google Scholar]
- 8.Benzaquen LR, Brugnara C, Byers HR, Gatton-Celli S, Halperin JA. Clotrimazole inhibits cell proliferation in vitro and in vivo. Nat Med. 1995;1:534–540. doi: 10.1038/nm0695-534. [DOI] [PubMed] [Google Scholar]
- 9.Khalid MH, Tokunaga Y, Caputy AJ, Walters E. Inhibition of tumor growth and prolonged survival of rats with intracranial gliomas following administration of clotrimazole. J Neurosurg. 2005;103:79–86. doi: 10.3171/jns.2005.103.1.0079. [DOI] [PubMed] [Google Scholar]
- 10.Hegemann L, Toso SM, Lahijani KI, Webster GF, Uitto J. Direct interaction of antifungal azole-derivatives with calmodulin: a possible mechanism for their therapeutic activity. J Invest Dermatol. 1993;100:343–346. doi: 10.1111/1523-1747.ep12470043. [DOI] [PubMed] [Google Scholar]
- 11.Montero M, Alvarez J, Garcia-Sancho J. Agonist-induced Ca2+ influx in human neutrophils is secondary to the emptying of intracellular calcium stores. Biochem J. 1991;277:73–79. doi: 10.1042/bj2770073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass-Marmor L, Beitner R. Detachment of glycolytic enzymes from cytoskeleton of melanoma cells induced by calmodulin antagonists. Eur J Pharmacol. 1997;328:241–248. doi: 10.1016/s0014-2999(97)83051-8. [DOI] [PubMed] [Google Scholar]
- 13.Furtado CM, Marcondes MC, Sola-Penna M, de Souza ML, Zancan P. Clotrimazole preferentially inhibits human breast cancer cell proliferation, viability and glycolysis. PLoS One. 2012;7:e30462. doi: 10.1371/journal.pone.0030462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penso J, Beitner R. Detachment of glycolytic enzymes from cytoskeleton of Lewis lung carcinoma and colon adenocarcinoma cells induced by clotrimazole and its correlation to cell viability and morphology. Mol Genet Metab. 2002;76:181–188. doi: 10.1016/s1096-7192(02)00046-x. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Li Y, Raisch KP. Clotrimazole induces a late G1 cell cycle arrest and sensitizes glioblastoma cells to radiation in vitro. Anticancer Drugs. 2010;21:841–849. doi: 10.1097/CAD.0b013e32833e8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meira DD, Marinho-Carvalho MM, Teixeira CA, Veiga VF, Da Poian AT, et al. Clotrimazole decreases human breast cancer cells viability through alterations in cytoskeleton-associated glycolytic enzymes. Mol Genet Metab. 2005;84:354–362. doi: 10.1016/j.ymgme.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Chiara F, Castellaro D, Marin O. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS One. 2008;3:1852. doi: 10.1371/journal.pone.0001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penso J, Beitner R. Clotrimazole and bifonazole detach hexokinase from mitochondria of melanoma cells. Eur J Pharmacol. 1998;342:113–117. doi: 10.1016/s0014-2999(97)01507-0. [DOI] [PubMed] [Google Scholar]
- 19.Penso J, Beitner R. Clotrimazole decreases glycolysis and the viability of lung carcinoma and colon adenocarcinoma cells. Eur J Pharmacol. 2002;451:227–235. doi: 10.1016/s0014-2999(02)02103-9. [DOI] [PubMed] [Google Scholar]
- 20.Betanzos-Lara S, Gomez-Ruiz C, Barron-Sosa LR, Gracia-Mora I, Flores-Alamo M, et al. Cytotoxic copper(II), cobalt(II), zinc(II), and nickel(II) coordination compounds of clotrimazole. J Inorg Biochem. 2012;114:82–93. doi: 10.1016/j.jinorgbio.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Ravera M, Gabano E, Sardi M, Ermondi G, Caron G, et al. Synthesis, characterization, structure, molecular modeling studies and biological activity of sterically crowded Pt(II) complexes containing bis(imidazole) ligands. J Inorg Biochem. 2011;105:400–409. doi: 10.1016/j.jinorgbio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastomamultiforme. J Exp Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palchaudhuri R, Nesterenko V, Hergenrother PJ. The complex role of the triphenylmethyl motif in anticancer compounds. J Am Chem Soc. 2008;130:10274–10281. doi: 10.1021/ja8020999. [DOI] [PubMed] [Google Scholar]
- 25.Neary CL, Pastorino JG. Akt inhibition promotes hexokinase 2 redistribution and glucose uptake in cancer cells. J Cell Physiol. 2013;228:1943–1948. doi: 10.1002/jcp.24361. [DOI] [PubMed] [Google Scholar]
- 26.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 27.Glass-Marmor L, Morgenstern H, Beitner R. Calmodulin antagonists decrease glucose 6-bisphosphate, fructose 6-bisphosphate, ATP and viability of melanoma cells. Eur J Pharmacol. 1996;313:265–271. doi: 10.1016/0014-2999(96)00526-2. [DOI] [PubMed] [Google Scholar]
- 28.Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. ExpMol Pathol. 2009;86:174–179. doi: 10.1016/j.yexmp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Brugnara C, Armsby CC, Sakamoto M, Rifai N, Alper SL, et al. Oral administration of clotrimazole and blockade of human erythrocyte Ca(++)-activated K+ channel: the imidazole ring is not required for inhibitory activity. J Pharmacol Exp Ther. 1995;273:266–272. [PubMed] [Google Scholar]
- 30.Wulff H, Kolski-Andreaco A, Sankaranarayanan A, Sabatier JM, Shakkottai V. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr Med Chem. 2007;14:1437–1457. doi: 10.2174/092986707780831186. [DOI] [PubMed] [Google Scholar]
- 31.Nilius B, Wohlrab W. Potassium channels and regulation of proliferation of human melanoma cells. J Physiol. 1992;445:537–548. doi: 10.1113/jphysiol.1992.sp018938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aktas BH, Qiao Y, Ozdelen E, Schubert R, Sevinc S, et al. Small-Molecule targeting of translation initiation for cancer therapy. Oncotarget. 2013;4:1606–1617. doi: 10.18632/oncotarget.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frezza M, Hindo S, Chen D, Davenport A, Schmitt S, et al. Novel metals and metal complexes as platforms for cancer therapy. Curr Pharm Des. 2010;16:1813–1825. doi: 10.2174/138161210791209009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robles-Escajeda E, Martinez A, Varela-Ramirez A, Sanchez-Delgado RA, Aguilera RJ. Analysis of the cytotoxic effects of ruthenium-ketoconazole and ruthenium-clotrimazole complexes on cancer cells. Cell Biol Toxicol. 2013;29:431–443. doi: 10.1007/s10565-013-9264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez D, Ramesh C, Henson LH, Wilmeth L, Bryant BK, et al. Synthesis and characterization of tritylthioethanamine derivatives with potent KSP inhibitory activity. Bioorg Med Chem. 2011;19:5446–5453. doi: 10.1016/j.bmc.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hapuarachchige S, Montano G, Ramesh C, Rodriguez D, Henson LH, et al. Design and Synthesis of a New Class of Membrane-Permeable Triazaborolopyridinium Fluorescent Probes. J Am Chem Soc. 2011;133:6780–6790. doi: 10.1021/ja2005175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abualhasan MN, Good JA, Wittayanarakul K, Anthony NG, Berretta G, et al. Doing the methylene shuffle--further insights into the inhibition of mitotic kinesin Eg5 with S-trityl L-cysteine. Eur J Med Chem. 2012;54:483–498. doi: 10.1016/j.ejmech.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Xu W. Progress on kinesin spindle protein inhibitors as anti-cancer agents. Anticancer Agents Med Chem. 2008;8:698–704. [PubMed] [Google Scholar]
- 39.Al-Qawasmeh RA, Lee Y, Cao MY, Gu X, Vassilakos A, et al. Triaryl methane derivatives as antiproliferative agents. Bioorg Med Chem Lett. 2004;14:347–350. doi: 10.1016/j.bmcl.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Abdel-Moety EM, Khattab FI, Kelani KM, AbouAl-Alamein AM. Chromatographic determination of clotrimazole, ketoconazole and fluconazole in pharmaceutical formulations. Farmaco. 2002;57:931–938. doi: 10.1016/s0014-827x(02)01270-3. [DOI] [PubMed] [Google Scholar]
- 41.Chang JY, Oh YK, Kong HS, Kim EJ, Jang DD, et al. Prolonged antifungal effects of clotrimazole-containing mucoadhesivethermosensitive gels on vaginitis. J Control Release. 2002;82:39–50. doi: 10.1016/s0168-3659(02)00086-x. [DOI] [PubMed] [Google Scholar]
- 42.Ning M, Guo Y, Pan H, Chen X, Gu Z. Preparation, in vitro and in vivo evaluation of liposomal/niosomal gel delivery systems for clotrimazole. Drug Dev Ind Pharm. 2005;31:375–383. doi: 10.1081/ddc-54315. [DOI] [PubMed] [Google Scholar]
- 43.Yong CS, Li DX, Prabagar B, Park BC, Yi SJ, et al. The effect of beta-cyclodextrincomplexation on the bioavailability and hepatotoxicity of clotrimazole. Pharmazie. 2007;62:756–759. [PubMed] [Google Scholar]
- 44.Prabagar B, Yoo BK, Woo JS, Kim JA, Rhee JD, et al. Enhanced bioavailability of poorly water-soluble clotrimazole by inclusion with beta-cyclodextrin. Arch Pharm Res. 2007;30:249–254. doi: 10.1007/BF02977701. [DOI] [PubMed] [Google Scholar]