INTRODUCTION
The protozoa, as typically delineated in public health, are a nonmonophyletic conglomerate of unicellular eukaryotic organisms that are characterized by having animallike affinities. Most protozoa that infect the human enteric tract are characterized by having an environmentally stable stage such as a cyst or oocyst. Cysts and oocysts confer protection from environmental factors, allowing these parasites to infect other susceptible hosts through either the water or food-borne routes.
There are several parasitic protozoa that can cause enteric infections in humans, the focus here being Cryptosporidium spp, Cyclospora cayetanensis, Giardia duodenalis (syn. Giardia lamblia, Giardia intestinalis), and Cystoisospora belli (previously Isospora belli). Infections are usually characterized by gastrointestinal clinical manifestations that may include diarrhea, vomiting, abdominal cramps, and general malaise.1,2 Three of these protozoa, Cryptosporidium, Cyclospora, and Cystoisospora, were previously classified as coccidian parasites because of their intracellular location (these parasite infect enterocytes) and a complex life cycle that includes asexual (meronts) and sexual (microgametocytes and macrogametocytes) reproductive stages.3 Giardia is a flagellate, does not invade epithelial cells, and reproduces only asexually by binary fission.
The classification of eukaryotic parasites is in frequent revision because of modern systematics that incorporate bioinformatic data and cladistics classification into the traditional morphometric-based taxonomy. As of 2012, the formerly known coccidians are classified in the subgroup Apicomplexa, with Cyclospora and Cystoisospora being classified as Eimeriorinas (the sporozoites, which are the infective stages, are always enclosed in sporocysts within an oocyst) and Cryptosporidium grouped alone as a single clade (oocysts without sporocysts, containing 4 naked sporozoites).4
These parasites produce resistant stages (cysts in Giardia,5 oocysts in the coccidia), which are released into the environment. The excretion intensity of these parasites can vary significantly, from very high to low, and can be sporadic.6 Therefore, the diagnostic success of a single stool sample can be suboptimal.7 It is currently recommended to test 3 samples,8 ideally collected every other day, over a period of at least 1 week.9
Samples have to be properly preserved to assure success of the assays to be conducted. The most widely used method relies on a 2-vial collection system with sodium acetate-acetic acid-formalin (SAF); 10% buffered formalin; polyvinyl alcohol (PVA) containing fixatives such as mercury, zinc, or copper (Zn PVA, Cu PVA); or Schaudinn fluid. However, there is a trend to minimize the use of formalin (because of toxicity) and mercury (environmental impact)10; however, those alternatives may not always have high parasite recovery rates and not are always compatible with immunoassays.7
See Appendix 1 for laboratory procedures for the microscopic detection of coccidian parasites (Cryptosporidium spp, C cayetanensis, and C belli).
CRYPTOSPORIDIUM SPP
The genus Cryptosporidium was first described in 1910 by Tyzzer,11 who in 1912 also described Cryptosporidium parvum in the small intestine of mice.12 For several decades, human cryptosporidiosis was considered a benign self-resolving infection that was caused by C parvum.13 This parasite was considered to have the potential to infect a broad range of mammalian species. With the advent of the human immunodeficiency virus (HIV)/AIDS epidemic, cryptosporidiosis became an important infection, where immunocompromised patients developed nonretractable life-threatening diarrhea.14,15 Cryptosporidiosis was classified as opportunistic infection and also as an AIDS-defining illness.16 Given its public health importance, numerous studies were conducted to better understand its pathogenesis, transmission routes, therapeutic approaches, and disease-prevention strategies. The renewed interest led to important discoveries that highlighted the importance of the immune system, more specifically CD4 cells, in the clearance of infections.17,18 It was also confirmed that its primary route of transmission was waterborne and that people with immune deficiencies other than HIV were also at risk of severe disease.19,20
Data from studies using DNA-based methods started to provide evidence that not all isolates of Cryptosporidium previously identified as C parvum had the same DNA signatures.21 Further studies showed that the parasites previously described as C parvum may actually encompass several species that were morphologically identical; however, DNA data showed distinct genetic signatures and epidemiologic and biological data showed that several isolates had defined host specificities.22
Differences in DNA patterns between human and animal isolates were reported using whole DNA extracts21 and further substantiated using polymerase chain reaction (PCR)-amplified regions of the 18s small subunit ribosomal RNA gene.22–24 The systematic use of DNA-based methods, in conjunction with biological and epidemiologic studies, have led to a major revision of the genus Cryptosporidium.25–28
At present, there are 26 different species of Cryptosporidium.29 The species most frequently detected in humans are Cryptosporidium hominis, an anthroponotic parasite (infecting only humans), and C parvum, a zoonotic species. It should be noted that C parvum is most frequently detected in weaned calves but not in heifers or older cattle.30 These 2 species of Cryptosporidium have different epidemiologic distributions. Both parasites are frequent in European countries, whereas C parvum has been the species more frequently reported in the Middle East. In the United States, other industrialized nations, and other developing countries, C hominis is the parasite most frequently detected in people. Species less frequently reported are Cryptosporidium ubiquitum (previously described as cervine genotype) mainly in industrialized nations, Cryptosporidium canis and Cryptosporidium felis in nonindustrialized countries, and Cryptosporidium cuniculus, primarily in the United Kingdom. Other species with limited number of cases reported are Cryptosporidium meleagridis, Cryptosporidium viatorum, Cryptosporidium suis, Cryptosporidium muris, Cryptosporidium fayeri, Cryptosporidium andersoni, Cryptosporidium bovis, Cryptosporidium tyzzeri, Cryptosporidium erinacei, Cryptosporidium scrofarum, and Cryptosporidium xiaoi.31
Life Cycle and Biology
The latest classification of eukaryotic organisms places Cryptosporidium in its own clade, which is outside the Coccidia proper.4 Cryptosporidium spp are morphologically characterized by the presence of an attachment feeder organelle, location in the host’s cells (intracellular, but extracytoplasmic), presence of 2 functional types of oocysts (thin and thick walled), presence of a gamontlike extracellular stage, and lack of sporocysts, microphyles, and polar granules.32–35
Only 1 host is required for Cryptosporidium to complete its life cycle. Fully sporulated, thick-walled oocysts are shed in the feces. Unlike other intestinal coccidia, oocysts of Cryptosporidium spp are immediately infective when shed from the host. Infection occurs after the ingestion of oocysts in fecal-contaminated food, water, and fomites. After ingestion by a suitable host, oocysts excyst in the small intestine, releasing sporozoites. The sporozoites parasitize the epithelial cells of the intestinal tract and produce a parasitophorous vacuole located between the host cell’s cytoplasm and cell membrane. This unique intracellular but extracytoplasmatic location allows the parasite to derive nutrients via a feeder organelle.
Within the parasitophorous vacuole, sporozoites undergo asexual cycles of merogony. Mature meronts rupture from the infected host cell and take 1 of 2 pathways. Type I meronts give rise to merozoites that perpetuate the asexual cycle in the surrounding host cells. Type II meronts give rise to merozoites that initiate the sexual cycle by producing microgametes (males) or macrogametes (females). Fertilization of macrogametes by microgametes results in the formation of zygotes. Zygotes differentiate into 4 sporozoites and develop a cyst wall, becoming oocysts. Thin-walled oocysts rupture in the lumen of the intestine and perpetuate autoinfection, whereas thick-walled oocysts are shed in stool where they are immediately infectious to a susceptible host.36,37
Epidemiology
Cryptosporidiosis is ubiquitous and is reported worldwide. It is primarily transmitted through the waterborne route38; however, food-borne39–41 or direct-contact transmission can also occur.42–44 Overall, the frequency of cryptosporidiosis has shown distinct patterns between areas with high endemicity, mainly associated with other enteropathogens, and areas with low endemicity, mainly from industrialized countries, where infections are usually in low levels. There are some patterns for seasonality. For example, in European countries and New Zealand, C hominis was more frequently detected in fall, whereas C parvum was more frequently reported in spring.45
In areas of high endemicity, first cryptosporidial infections usually occur in young children, mainly by age 2 years. Most first infections are symptomatic, primarily associated with diarrhea. Detectable infections decrease with age, and cases of cryptosporidiosis are highly infrequent in children. Meanwhile, the adults living in the same settings usually do not have detectable cryptosporidiosis. Some seasonal trends have been reported.1
In industrialized nations, infections have been reported in people of all ages. Cryptosporidiosis in the United States is a reportable disease. The number of cases is consistently greater during the summer months, when outbreaks associated with the use of recreational waters are reported, and more frequently among children aged 1 to 9 years, followed by young adults (aged about 25–39 years).46–48 Foodborne outbreaks have been associated with the consumption of raw or undercooked foods49 or unpasteurized drinks.50
Clinical Manifestations
Immunocompetent people and children usually present short-term, self-limited watery diarrhea that may be accompanied with nausea and vomiting, which can lead to dehydration.1,51 People with immune deficiencies, especially with low CD4+ counts (<140/mm3)17 may present chronic and debilitating disease, which can lead to life-threatening syndromes.52
Treatment
Multiple drugs or immunotherapeutic compounds have been developed or tested for the treatment of human cryptosporidiosis. Very few of these compounds have shown therapeutic efficacy. Rehydration through the oral route is the most widely used intervention. Thus far, only nitazoxanide has been approved by the US Food and Drug Administration (FDA) for the treatment of Cryptosporidium infections in people.53 This product has shown to shorten the duration of diarrhea and parasite excretion, although it is not highly efficacious in the treatment of cryptosporidiosis in people with immune deficiencies.54 In people with HIV infections and low CD4 counts, the preferred treatment and preventative measure would be the administration of highly active antiretroviral therapies (HAART). Relapses are frequent when patients discontinue HAART.
Diagnosis
Microscopy
Cryptosporidium spp are not readily detected by routine O&P testing such as formalin-ethyl acetate (FEA) concentration or trichrome staining.9 Visualization of oocysts require special staining, such as Kinyoun MAF, Zieh-Neelsen acid-fast, or safranin (Fig. 1). Cryptosporidium may also be detected by the aurimine O stain used for mycobacteria. Direct fluorescent antibody (DFA) microscopy kits are also helpful for detection and specimen screening. Unlike several other coccidians, Cryptosporidium spp do not autofluoresce under ultraviolet (UV) light.7,9,55
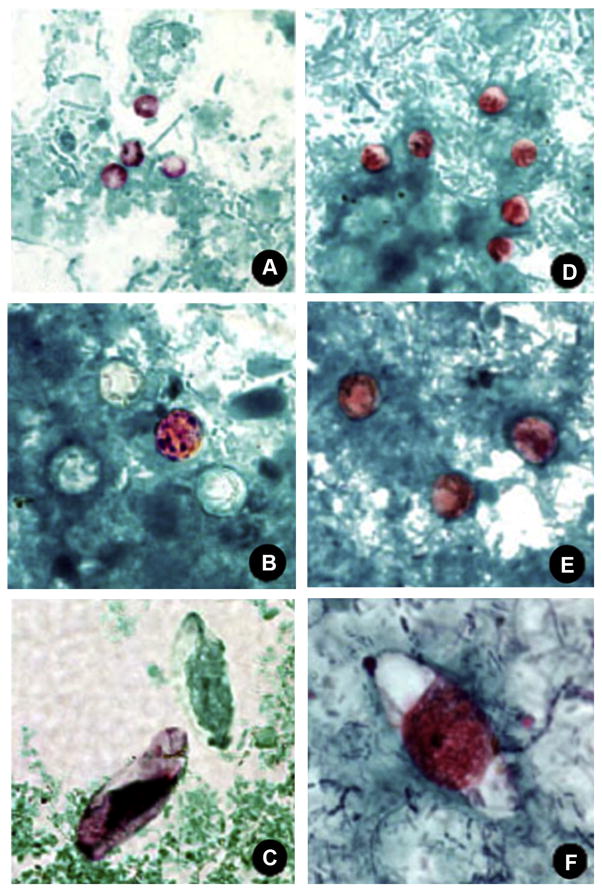
Fig. 1.
Comparison of (A, D) Cryptosporidium spp, (B, E) Cyclospora cayetanensis, and (C, F) Cystoisospora belli stained with modified acid-fast and safranin stains, original magnification × 1000. (Public domain images, courtesy of DPDx, Centers for Disease Control and Prevention, Atlanta, USA).
Antigen detection and rapid/point-of-care diagnosis
These assays are based on the detection of parasite antigens that can be detected in stool specimens. They have the advantage of simplicity and not requiring specialized trained personnel, and several products are already approved for clinical diagnosis (Table 1).56 Disadvantages are that the species identification or quantification of parasite loads is not possible.
Table 1.
Commercial diagnostic assays for the detection of protozoa, either approved by the FDA or registered for selling in the European Union, having the CE seal of European Conformity or Conformité Européenne
| Product Name | Manufacturer, Country | Sensitivity (%) | Specificity (%) | Approval |
|---|---|---|---|---|
| 1.A. Cryptosporidium | ||||
| A. Immunofluorescence assays | ||||
| Crypto-Cel IF Test | Celllabs, AUS | 100 | 100 | FDA/CE |
| B. Enzyme immunoassays | ||||
| Cryptosporidium II TEST(direct Ag/spectrophotometric/visual) | Alere/Techlab, Inc USA | 97 | 100 | FDA |
| Prospect Cryptosporidium Rapid Assay | Thermo Fisher Scientific/REMEL, USA | 100 | No data | FDA |
| RIDASCREEN Cryptosporidiuma | R-Biopharm, Germany | 100 | 97.3 | CE |
| PARA-TEC Cryptosporidium | Medical Chemical Corporation, USA | 100 | 100 | CE |
| C. Immunochromatographic/point-of-care assays | ||||
| UNI-GOLD Cryptosporidium | Trinity Biotech, Ireland/USA | 100 | 100 | FDA/CE |
| XPECT Cryptosporidium LATERAL FLOW ASSAY, MODEL 2451020 | Thermo Scientific REMEL, USA | 96.4 | 98.3 | FDA |
| Cryptosporidium Fecal ELISA Test | Cortez Diagnostics, USA | 100 | 100 | FDAa/CE |
| Crypto-Strip C-1005 (CRYPTO UNI-STRIP, CRYPTO-CIT)a | Coris BioConcept, Belgium | 95.7 | 100 | CE |
| RIDA Quick Cryptosporidium (dipstick or cassette)a | R-Biopharm, Germany | 93.8 | 100 | CE |
| CRYPTO (card and blister formats)a | CerTest Biotec, Spain | 99 | 99 | CE |
| Stick Cryptoa | Operon, Spain | 79.3 | 99.5 | CE |
| D. PCR-based assays | ||||
| KHCRYP (real-time PCR) | CEERAM S.A.S., France | No data | No data | CE |
| 1.B. Giardia duodenalis | ||||
| A. Immunofluorescent assays | ||||
| Giardia-Cel IF Test | Cellabs, Australia | 90.3 | 100 | FDA/CE |
| B. Enzyme immunoassays (antigen detection) | ||||
| Giardia lamblia ANTIGEN DETECTION MICROWELL ELISA | Ivd Research, Inc USA | 100 | 100 | FDA |
| TechLab Giardia II TEST | Techlab Inc/Alere USA | 100 | 100 | FDA |
| Giardia ELISA kit | Cortez Diagnostics, USA | 100 | 100 | FDAa/CE |
| ProSpecT Giardia Microplate Assay (direct Ag/spectrophotometric/visual) | Thermo Fisher Scientific/REMEL, USA | 97 | No data | FDA |
| ProSpecT Giardia EZ Microplate Assay | Thermo Fisher Scientific/REMEL, USA | 97 | No data | FDA |
| RIDASCREEN Giardiaa | R-Biopharm, Germany | 100 | 99.60 | CE |
| PARA-TEC Giardia lamblia | Medical Chemical Corporation, USA | 85 | 97.9 | CE |
| C. Immunochromatographic/point-of-care diagnostics | ||||
| Uni-Gold Giardia | Trinity Biotech, Ireland | 100 | 100 | CE |
| Stick Giardiaa | Operon, Spain | 93.8 | 98.9 | CE |
| Giardia lamblia (Giardia)a | CerTest Biotec, Spain | 97 | 99 | CE |
| Giardia (dipstick and cassete) | Coris BioConcept, Belgium | 96.3 | 97.8 | CE |
| D. Molecular assays | ||||
| Giardia Test Kit KHGIAR | CEERAM S.A.S., France | No data | No data | CE |
| 1.C. Dual detection: Cryptosporidium spp (C) and Giardia duodenalis(G) | ||||
| A. Immunofluorescent microscopy assays | ||||
| Crypto/Giardia-Cel IF Test | Cellabs, Australia | 100 | 100 | FDA/CE |
| MERIFLUOR Cryptosporidium/Giardia | Meridian Biosciences, USA | 100 | 100 | FDA |
| PARA-TECT Cryptosporidium/Giardia DFA 75Test Kit | Medical Chemical Corporation, USA | 100 | 100 | CE |
| IVD Crypto/Giardia DFA | IVD Research, Inc, USA | 100 | 100 | FDA |
| B. Enzyme immunoassays (antigen detection) | ||||
| Giardia/Cryptosporidium QUIK CHEK test | Techlab Inc/Alere, USA | C-100, G-98.9 | C-99.8, G-100 | FDA |
| Giardia/Cryptosporidium CHEK | Techlab Inc/Alere, USA | 97.6 | 100 | FDA |
| Crypto/Giardia Ag Combo ELISA kit | Cortez Diagnostics, USA | 99 | 100 | CE/FDAa |
| ProSpecT Giardia/Crypto (spectrophotometric) | Thermo Fisher Scientific, USA | 100 | 95 | FDA |
| C. Immunochromatographic/point-of-care assays | ||||
| XPECT Giardia/Cryptosporidium | Thermo Scientific REMEL, USA | C-96.4, G-95.8 | C-98.5, G-98.5 | FDA |
| Biosite Triage Parasite Panel | Alere/Biosite Incorporated, USA | C-91.4, G-95.1 | C-98.2, G-88.4 | FDA |
| ColorPAC Giardia/Cryptosporidium Rapid Assay | Becton Dickinson, USA | C-97.3, G-100 | C-100, G-100 | FDA |
| Crypto/Giardia Duo-Stripa | Coris BioConcept, Belgium | C-95.7, G-96.3 | C-100, G-97.8 | CE |
| ImmunoCard STAT! Cryptosporidium/Giardia Rapid Assay | Meridian Bioscience, USA | C-97.3, G-100 | C-100, G-100 | CE |
| RIDA Quick Cryptosporidium/Giardia Combi (dipstick or cassette)a | R-Biopharm, Germany | C-93.8, G-100 | C-100, G-95.2 | CE |
| RIDAQuickb Cryptosporidium/Giardia/Entamoeba Combi (dipstick or cassette)a | R-Biopharm, Germany | C-83, G-91.9 | C-93.3, G-99.5 | CE |
| CRYPTO-GIARDIA (card and blister)a | CerTest Biotec, Spain | C-99, G-97 | C-99, G-99 | CE |
| CRYPTO-GIARDIA-ENTAMOEBAa,b | CerTest Biotec, Spain | C-99, G-97 | C-99, G-99 | CE |
| Stick Crypto-Giardiaa | Operon, Spain | C-79.3, G-93.8 | C-99.5, G-98.9 | CE |
| 1.D. Molecular assays for detection of multiple enteric pathogens | ||||
| Film Array Instrumentc | Biofire Diagnostics, USA | FDA/CE | ||
| xTAG Gastrointestinal Panel (GPP)d | Luminex, USA | C-100, G-100 Cyclospora-100 |
C-99.6, G-99.5 Cyclospora-100 |
FDA/CE |
Not available in the United States.
Also for Entamoeba spp.
Multiplex assay including bacterial and viral pathogens.
Multiplex assay including the protozoa Cyclospora cayetanensis, plus bacterial and viral pathogens.
Data from Catalog of cleared and approved medical device information from FDA. Database of approved in-vitro diagnostic devices. 2014. Accessed October 20, 2014.
Molecular diagnosis
DNA-based methods are primarily used for species identification and molecular typing. PCR and PCR-related methods have been developed for the detection and identification of species within Cryptosporidium spp. Several protocols for PCR detection of Cryptosporidium DNA have been made available; however, none of these methods has received approval from FDA or for use of the European Conformity (CE) logo. These protocols allow the identification of Cryptosporidium that are of public health importance. Methods using PCR amplification followed by restriction fragment length polymorphism have been developed for the detection and differentiation of Cryptosporidium at the species level. Most of these techniques are based on the amplification of the small-subunit (SSU) rRNA gene.57 There are other PCR-based protocols that have been designed for the detection and differentiation of C parvum and C hominis, the 2 most frequent species affecting humans. However, these protocols cannot detect or differentiate other Cryptosporidium spp or genotypes.45
Subtype analyses, which are based on PCR amplification followed by DNA sequence analysis, is a powerful tool for outbreak investigations. Several subtyping tools have also been developed to characterize the diversity within C parvum or C hominis.45 This method is based on the sequence polymorphism of the GP-60 locus (also known as gp15/45/60, gp40/15), which has shown to be a robust tool in outbreak investigations.44
CYCLOSPORA CAYETANENSIS
This coccidian parasite was described in 1993.2 It is recognized as an important cause of food-borne outbreaks, both in the United States as well as in other industrialized nations. The name C cayetanensis was first proposed in 1992; however, previous studies reported organisms that later have been considered to be similar to Cyclospora.58 However, earlier reports hypothesized that those new organisms could belong to a new species of Isospora.58
In 1993, the description of oocysts, which after sporulation had 2 clearly defined sporocysts, each with 2 sporozoites, led to the organism’s classification as Cyclospora and to the species name C cayetanensis. The genus Cyclospora is currently placed among the Eimeriorina.4,59 Molecular data suggest that Cyclospora may actually belong nestled within the genus Eimeria.60,61 At present, C cayetanensis is the only species known to infect humans. Three other species are known to infect nonhuman primates, all parasites of monkeys in Africa.62
Biology
Cyclospora cayetanensis (Cyclospora) is an anthroponotic parasite. Attempts to infect other animal species have proved unsuccessful63; thus, there is no animal model to better understand its biology. The live stages of Cyclospora were described from jejunal biopsies.64 Infections start when a susceptible person ingests sporulated oocysts, a stage that is environmentally resistant. Oocysts are broken in the upper gastrointestinal tract because of partial digestion with gastric juices and digestive enzymes, leading to the release of the 2 internal cysts, called sporocysts, each with 2 infectious stages called sporozoites. On their release in the small intestine, the sporozoites infect epithelial cells where they transform into merozoites and replicate asexually, also infecting other enterocytes. After asexual replications, the merozoites differentiate into sexual stages called microgametocytes and macrogametocytes. New oocysts are formed as result of sexual reproduction between the microgametocytes and macrogametocytes. Unsporulated (and therefore noninfectious) oocysts are eventually released into the environment. Sporulation occurs in the environment. The precise factors that cause sporulation are not known, but it is estimated that it occurs in about 2 weeks.2,65
Epidemiology
Cyclospora infections have been reported in several areas of the world. It is endemic mainly in nonindustrialized nations, whereas sporadic reports associated with outbreaks have been frequently reported in industrialized countries.66 In endemic settings, cyclosporiasis is more frequent in children between the ages of 2 and 5 years, showing a marked seasonal pattern.67 For example, in Nepal, there were higher rates of infection during the summer and rainy seasons,68 whereas in Peru, most cases were detected between December and May, which are warmer months but without rain.67
In industrialized nations, cyclosporiasis has been more frequently reported in the summer months and most outbreaks have been traced back to imported fresh products. In the 1990s, most reported outbreaks in the United States were linked to imported berries. This trend has changed, and during the past 14 years, most outbreaks have been linked to leafy greens consumed raw, such as basil and herbs.
Clinical Manifestations and Treatment
Gastrointestinal manifestations associated with cyclosporiasis include diarrhea, fatigue, and abdominal cramps, which are most likely reported between 1 and 2 weeks after infection. The duration of the patent period and the severity of symptoms are different between people living in endemic and nonendemic areas. Infections in endemic settings occur mainly in children older than 2 years and are almost never detected after 10 years of age. In these cases, infections may resolve spontaneously, and as high as 50% of infected people may not show any clinical symptoms. It seems that immunity plays a significant role.
In industrialized nations, where the parasite is not endemic, most people are likely to be naive and infections are almost always symptomatic, lasting 1 to 2 weeks or more. The main symptom is diarrhea, which persists if untreated.69 Other symptoms are general malaise, lack of energy, loss of appetite, mild fever, nausea, flatulence, and abdominal cramps.67,70
In the case of people with deficits in their immune system, there are anecdotal reports of infections with a longer duration, although cyclosporiasis is not considered an opportunistic infection in HIV-infected people.71 Treatment is highly effective and is based on the administration of sulfamethoxazole trimethoprim (TMP/SMX) orally, twice daily for 7 to 10 days.72 However, no specific treatment is recommended for people who are allergic to sulfa drugs.55
Diagnostic Testing
Microscopy
As with other coccidia, C cayetanensis is not easily detected with traditional O&P procedures such as FEA concentration and trichrome stain.55 Oocysts can be better visualized in FEA concentrates if viewed under differential interference contrast or phase contrast microscopy.2,65 Oocysts can be more easily detected with permanent staining methods such as safranin or MAF staining. Oocysts stain red with both stains; however, characteristic nonuniform staining is observed with MAF and unstained oocysts are usually white and are referred to as ghost forms (see Fig. 1). In the case of safranin, oocysts stain more uniformly.55
Another characteristic of C cayetanensis is the autofluorescence of the oocyst wall under UV light, using excitation filters of 330 to 365 nm and less intense autofluorescense with filters of 450 to 490 nm.2,9,65 Autofluorescence plus morphometric characteristics are helpful when detecting Cyclospora (Fig. 2). There are currently no DFA or molecular procedures that are approved by the FDA for routine clinical diagnosis of cyclosporiasis.
Fig. 2.
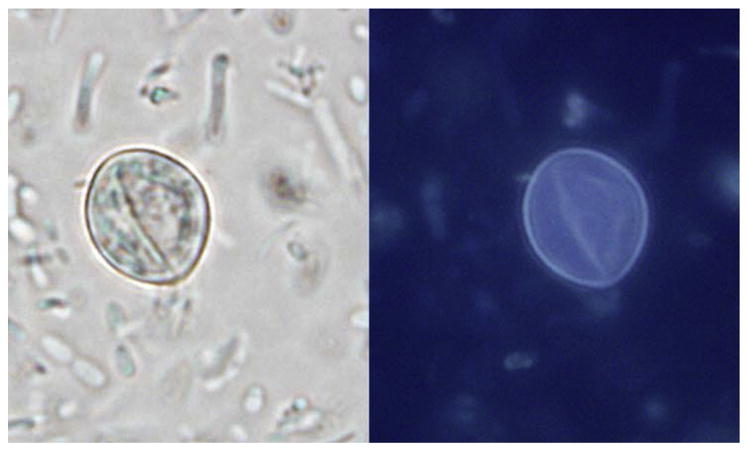
Cyclospora cayetanensis oocyst viewed under normal light (left) and ultraviolet light (right) in an unstained wet mount. (Original magnification × 1000). (Courtesy of DPDx, Centers for Disease Control and Prevention, Atlanta, USA).
CYSTOISOSPORA BELLI (PREVIOUSLY ISOSPORA BELLI)
Biology and Taxonomy
Cystoisospora belli is placed among the Eimeriorina along with Cyclospora.4 For several decades, C belli was placed in the genus Isospora until morphologic and molecular data were used to support its proper classification in the genus Cystoisospora.73,74 This genus now includes all previously classified Isospora spp that infect mammals, whereas Isospora contains only parasites that infect passeriform birds.74
Life Cycle and Biology
Cystoisospora belli is known to infect only humans and requires only the one host for completion of its life cycle, although paratenic hosts may be involved.75 Typically, partially sporulated oocysts containing 1 (rarely 2) sporoblast are shed in feces. In the environment, the sporoblast divides into 2 sporoblasts, each of which secretes a cell wall to become sporocysts. After sporulation, each sporocyst contains 4 sporozoites. Humans become infected after ingestion of fully sporulated oocysts, through contaminated food, water, and fomites. On ingestion, the sporocysts excyst in the small intestine and release the sporozoites, which invade the host epithelial cells. The sporozoites undergo asexual replication called schizogony. Mature schizonts rupture, releasing merozoites that parasitize surrounding epithelial cells, perpetuating asexual multiplication. Eventually, multinucleate meronts are formed and sexual stages develop. Macrogametocytes are fertilized by microgametocytes resulting in formation of the oocyst, which can persist in the environment for several weeks or months.55,75,76
Epidemiology
Cystoisospora belli has a worldwide distribution; however, several studies from AIDS patients with diarrhea have shown a higher prevalence of cystoisosporiasis among people from tropical or subtropical areas.77–79
Clinical Manifestations and Treatment
In immunocompetent people, C belli has been associated with diarrhea, usually lasting 6 to 10 days, and infections self-resolving in 2 to 3 weeks, although intermittent shedding may continue for an additional 2 to −3 weeks. Severe symptoms were reported among people with immunocompromised systems, which can lead to life-threatening chronic profuse diarrhea.52,80 Treatment is based on TMP/SMX.81
Microscopic Diagnosis
Because of their large size, oocysts of C belli are usually detected during route O&P examinations. However, because oocysts tend to be shed in small numbers, repeated stool examinations and concentration procedures are recommended. Coccidian-specific stains, such as MAF and safranin, are preferred if permanent stains are used for diagnosis (see Fig. 1). As with C cayetanensis, screening of wet mounts can be enhanced by using UV microscopy, with both oocyst and sporoblast/sporocyst walls capable of autofluorescence.7,9,75 There are currently no DFA or molecular procedures approved for routine clinical diagnosis of cystoisosporiasis.
GIARDIA DUODENALIS
Biology
The protozoan flagellate parasite G duodenalis is a common cause of human diarrheal disease worldwide.82 It is transmitted through the fecal-oral route, frequently through ingestion of contaminated water and food.6,76,82 This parasite has a direct life cycle, and the cysts passed in the feces are immediately infectious. These cysts can remain infectious for long periods in moist and cool environments.
Epidemiology
Giardia has a worldwide distribution.83 It affects people of all ages and has an important impact on public health. In the United States, it is more frequently reported in children aged 1 to 9 years.84 This parasite has been associated with major outbreaks and can also be present in domestic animals, such as household pets and farm animals. Giardiasis is highly underreported, with data from the United States showing that the number of annually reported cases remained steady for several years at around 20,000, whereas the estimated number of cases was 2 million.85
Through molecular genotyping methods, G duodenalis has been classified in distinct assemblages or genotypes. Assemblages A and B are the most frequently reported in humans, either in industrialized or nonindustrialized nations. These assemblages have also been reported in cattle, dogs, and cats from different countries around the world. However, other assemblages of Giardia have been reported almost exclusively in animal species: assemblages C and D in domestic and wild canids, assemblage F in cats, and assemblage E in ruminants. Therefore, G duodenalis is considered a multispecies complex, where assemblages A and B are considered to have broad host specificity and zoonotic potential.86–89
Clinical Manifestations
Approximately 40% of Giardia infections may be symptomatic, depending on the population. Symptoms may include diarrhea, cramps, bloating, nausea, and vomiting6 and may be prolonged. Infections are normally self-limiting, but chronic diarrhea may occur in children90 and a low proportion of immunocompromised people.91 There is a report that prolonged giardiasis from early childhood has been associated with poor cognitive function later in life.92
Treatment of infections is recommended only when clinical manifestations affect the well-being of the infected person. Drug of choice is metronidazole, although search for alternative therapies is ongoing.93
Diagnostic Testing
Microscopy
Because cysts are shed sporadically during an infection, detection of Giardia may require several stool samples to be examined.7,9 Giardia may be detected by microscopy, immunologic, or molecular methods. Giardia cysts and trophozoites can be readily detected via traditional methods such as trichrome staining and FEA concentrations (Fig. 3), although microscopy of whole organisms requires trained technicians as well as time to prepare and examine smears. The formalin-ether (Ritchie) concentration method is another common method used for the concentration of stool samples.55
Fig. 3.
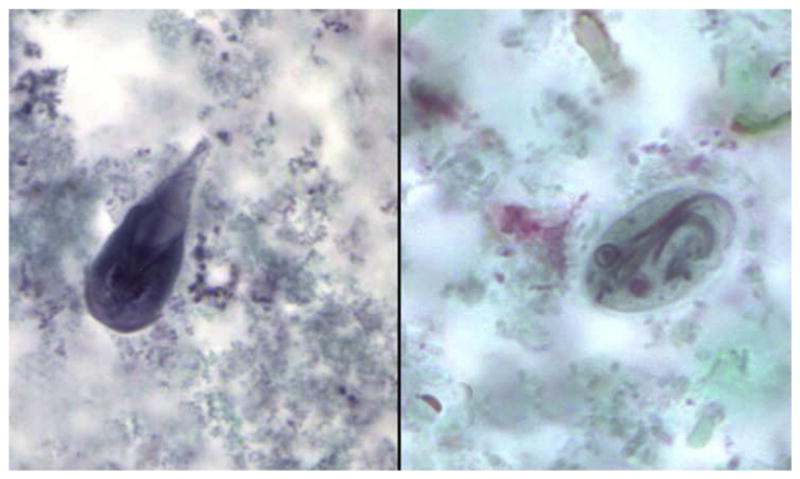
Trophozoite (left) and cyst (right) of Giardia duodenalis (trichrome, original magnification × 1000). (Courtesy of DPDx, Centers for Disease Control and Prevention, Atlanta, USA.)
Antigen detection and rapid/point-of-care diagnosis
These assays are standardized and can generate results in a short time. Tests more widely used are antigen detection immunoassays such as DFA tests that detect whole organisms and enzyme immunoassays that detect antigens in stool, which can be completed in 1 to 2 hours. They have been reported to be highly sensitive and specific (see Table 1B).94,95
PCR assays for Giardia have become more common; however, PCR amplification and sequence analysis are more frequently used for genotype/assemblage classification and are not routinely used for diagnosis. The SSU rRNA fragment, commonly amplified by PCR for other enteric parasites, is rich in GC content and thus requires special PCR conditions. Therefore, PCR-based methods have been designed to amplify other informative loci, such as the triose phosphate isomerase, glutamate dehydrogenase, and β-giardin, which are used for the taxonomic or epidemiologic classification into assemblages.88,89
SUMMARY ON DETECTION OF PARASITES
Microscopy continues to be the primary method for detection of the parasites covered in this review (Table 2), highlighting the importance of specimen processing and staining (see Table 2) and morphometric characteristics (Table 3). Rapid diagnostic assays (lateral flow/immunochromatographic cards) are also available for cryptosporidiosis or giardiasis, including approved devices for their simultaneous detection (see Table 1C). At present, there are molecular-based assays for the simultaneous detection of multiple enteric pathogens, which include virus, bacteria, and parasites. Two of these assays have been cleared by FDA and have been reported to have high sensitivity and specificity, including the detection of coinfections (see Table 1D).96,97
Table 2.
Microscopy procedures for detection of Cryptosporidium spp, Cyclospora cayetanensis, and Cystoisospora belli
| Procedure | Parasite | Advantages | Disadvantages |
|---|---|---|---|
| Wet mount (FEA concentrates) | Cryptosporidium spp | Concentration allows for better yield FEA concentrate can be used for DFA (see later in the table) |
Oocysts may be confused for yeast and other nonparasitic elements Formalin waste |
| Cyclospora cayetanensis | Concentration allows for better yield May be enhanced with differential interference contrast (DIC) or phase microscopy |
Oocysts may be confused with nonparasitic elements Formalin waste |
|
| Cystoisospora belli | Concentration allows for better yield May be enhanced with DIC or phase microscopy |
Oocysts shed sporadically so multiple collections should be tested Formalin waste |
|
|
| |||
| Trichrome stain | Cryptosporidium spp | Not recommended | Oocysts do not stain well with trichrome; likely to be confused for yeast/fungal elements Specimens in PVA usually not concentrated |
| Cyclospora cayetanensis | Not recommended | Oocysts do not stain well with trichrome Specimens in PVA usually not concentrated |
|
| Cystoisospora belli | Not recommended | Not readily detected by trichrome Specimens in PVA usually not concentrated |
|
|
| |||
| Modified Kinyoun acid-fast (MAF) stain | Cryptosporidium spp | Uniform staining of oocysts; sporozoites often visible | Oocysts may be confused with yeast and other fungal elements that often stain red to purple with MAF |
| Cyclospora cayetanensis | Oocysts can stain pink to red | Variability in staining; often many oocysts do not stain (ghost forms) and may be overlooked by inexperienced microscopists | |
| Cystoisospora belli | Sporoblasts stain red with MAF | Often there is shrinkage of oocyst wall, distorting form of oocysts | |
|
| |||
| Hot safranin stain | Cryptosporidium spp | Uniform staining of oocysts | Requires heat; messy procedure |
| Cyclospora cayetanensis | More uniform staining of oocysts | Requires heat; messy procedure | |
| Cystoisospora belli | Uniform staining of sporoblasts Oocyst wall less likely to collapse than with MAF |
Requires heat; messy procedure | |
|
| |||
| Ultraviolet microscopy | Cryptosporidium spp | Not available | Oocysts do not autofluoresce |
| Cyclospora cayetanensis | Oocyst walls autofluoresce Allows for more rapid screening |
Requires special filter with wavelength of 450–490 nm (not routine in most laboratories) | |
| Cystoisospora belli | Oocyst and sporoblast walls autofluoresce Allows for more rapid screening |
Requires special filter with wavelength of 450–490 nm (not routine in most laboratories) | |
|
| |||
| DFA | Cryptosporidium spp | Allows for rapid screening of Cryptosporidium spp Some allow for simultaneous screening of other organisms (eg, Giardia) |
Requires microscopy with fluorescence capabilities Could be expensive if not used routinely |
| Cyclospora cayetanensis | None available | None available | |
| Cystoisospora belli | None available | None available | |
Table 3.
Comparative morphology of Giardia duodenalis, Cryptosporidium spp, Cyclospora cayetanensis, and Cystoisospora belli
| Organism | Size | Other Morphologic Features | Preferred Morphologic Diagnostic Test |
|---|---|---|---|
| Giardia duodenalis | Trophozoites, 10–20 μm long Cysts 8.0–10 μm long |
Trophozoites: pyriform shape; sucking disk; 2 nuclei; 2 median bodies; 8 flagella (4 lateral, 2 ventral, 2 posterior) Cysts: ovoid shape; 2–4 nuclei; fibrils and median bodies; no flagella |
Trophozoites: direct wet mount; trichrome stain Cysts: FEA concentration wet mount; trichrome stain |
| Cryptosporidium spp | Oocysts: 4.0–6.0 μm | Oocysts: spherical shape; sporulated in feces (4 sporozoites present) | Modified acid-fast stain; Safranin stain |
| Cyclospora cayetanensis | Oocysts: 8.0–10 μm | Oocysts: spherical; unsporulated in fresh feces; refractile globules present | Modified acid-fast stain; Safranin stain; UV microscopy |
| Cystoisospora belli | Oocysts: 20–33 μm long | Oocysts: oval to ellipsoidal shape; unsporulated in fresh feces; double-layered hyaline cyst wall; single sporoblast usually present | Modified acid-fast stain; safranin stain; UV microscopy |
KEY POINTS.
Sample collection and preservation are important steps, and examination of 3 specimens from different days increases the accuracy of diagnosis.
Giardia can be detected by light microscopy during ova-and-parasite (O&P) examination, antigen detection methods (laboratory and rapid diagnostic devices), or fluorescent microscopy.
Cryptosporidium and Cyclospora are not easily detectable by O&P examination, and parasite-specific tests must be requested, such as modified acid-fast (MAF) microscopy. For Cryptosporidium, there are antigen detection assays (laboratory or rapid diagnostic devices) or antibody-based fluorescent microscopy. Properly equipped fluorescent microscopes can be used in research laboratories for confirmation of Cyclospora, as this parasite autofluoresces with the appropriate excitation wavelength.
Cystoisospora can be detected by light microscopy, and confirmation is accomplished through morphometric characteristics of samples that have been stained with safranin or acid-fast stain and also by autofluorescence.
APPENDIX 1: LABORATORY PROCEDURES FOR THE MICROSCOPIC DETECTION OF COCCIDIAN PARASITES: CRYPTOSPORIDIUM SPP, C CAYETANENSIS, AND C BELLI
| Modified Kinyoun Acid-Fast Stain |
| Specimen requirements: unfixed stool or stool preserved in 10% formalin (including FEA concentrates), SAF, or EcoFix. Procedure
Procedural notes
|
|
Hot Safranin Stain
|
| Specimen requirements: unfixed stool or stool preserved in 10% formalin (including FEA concentrates), SAF, or EcoFix. Procedure
Procedural notes
|
Footnotes
Disclosure: The authors have nothing to disclose.
References
- 1.Bern C, Hernandez B, Lopez MB, et al. The contrasting epidemiology of Cyclospora and Cryptosporidium among outpatients in Guatemala. Am J Trop Med Hyg. 2000;63(5–6):231–5. [PubMed] [Google Scholar]
- 2.Ortega YR, Sterling CR, Gilman RH, et al. Cyclospora species–a new protozoan pathogen of humans. N Engl J Med. 1993;328(18):1308–12. doi: 10.1056/NEJM199305063281804. [DOI] [PubMed] [Google Scholar]
- 3.Duszynsky DW, Upton SJ, Couch L. The coccidia of the world. [Accessed December 8, 2014];Review of coccidia in humans and animals. 2014 Available at: http://biology.unm.edu/biology/coccidia/home.html.
- 4.Adl SM, Simpson AG, Lane CE, et al. The revised classification of eukaryotes. J Eukaryot Microbiol. 2012;59(5):429–93. doi: 10.1111/j.1550-7408.2012.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe MS. Giardiasis. Clin Microbiol Rev. 1992;5(1):93–100. doi: 10.1128/cmr.5.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortega YR, Adam RD. Giardia: overview and update. Clin Infect Dis. 1997;25(3):545–9. doi: 10.1086/513745. quiz: 550. [DOI] [PubMed] [Google Scholar]
- 7.McHardy IH, Wu M, Shimizu-Cohen R, et al. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol. 2014;52(3):712–20. doi: 10.1128/JCM.02877-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiatt RA, Markell EK, Ng E. How many stool examinations are necessary to detect pathogenic intestinal protozoa? Am J Trop Med Hyg. 1995;53(1):36–9. [PubMed] [Google Scholar]
- 9.Garcia L, editor. Diagnostic medical parasitology. 5. Washington, DC: ASM Press; 2007. [Google Scholar]
- 10.Pietrzak-Johnston SM, Bishop H, Wahlquist S, et al. Evaluation of commercially available preservatives for laboratory detection of helminths and protozoa in human fecal specimens. J Clin Microbiol. 2000;38(5):1959–64. doi: 10.1128/jcm.38.5.1959-1964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyzzer EE. An extracellular coccidium, Cryptosporidium muris (Gen. Et Sp. Nov.), of the gastric glands of the common mouse. J Med Res. 1910;23(3):487–510.3. [PMC free article] [PubMed] [Google Scholar]
- 12.Tyzzer EE. Cryptosporidium parvum (sp. nov.), a coccidium found in the small intestine of the common mouse. Arch Protistenkunde. 1912;26:394–412. [Google Scholar]
- 13.Current WL, Reese NC, Ernst JV, et al. Human cryptosporidiosis in immunocompetent and immunodeficient persons. Studies of an outbreak and experimental transmission. N Engl J Med. 1983;308(21):1252–7. doi: 10.1056/NEJM198305263082102. [DOI] [PubMed] [Google Scholar]
- 14.Malebranche R, Arnoux E, Guerin JM, et al. Acquired immunodeficiency syndrome with severe gastrointestinal manifestations in Haiti. Lancet. 1983;2(8355):873–8. doi: 10.1016/s0140-6736(83)90868-1. [DOI] [PubMed] [Google Scholar]
- 15.Jonas C, Deprez C, De Maubeuge J, et al. Cryptosporidium in patient with acquired immunodeficiency syndrome. Lancet. 1983;2(8356):964. doi: 10.1016/s0140-6736(83)90473-7. [DOI] [PubMed] [Google Scholar]
- 16.Colford JM, Jr, Tager IB, Hirozawa AM, et al. Cryptosporidiosis among patients infected with human immunodeficiency virus. Factors related to symptomatic infection and survival. Am J Epidemiol. 1996;144(9):807–16. doi: 10.1093/oxfordjournals.aje.a009015. [DOI] [PubMed] [Google Scholar]
- 17.Flanigan T, Whalen C, Turner J, et al. Cryptosporidium infection and CD4 counts. Ann Intern Med. 1992;116(10):840–2. doi: 10.7326/0003-4819-116-10-840. [DOI] [PubMed] [Google Scholar]
- 18.Navin TR, Weber R, Vugia DJ, et al. Declining CD4+ T-lymphocyte counts are associated with increased risk of enteric parasitosis and chronic diarrhea: results of a 3-year longitudinal study. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(2):154–9. doi: 10.1097/00042560-199902010-00007. [DOI] [PubMed] [Google Scholar]
- 19.Soave R. Waterborne cryptosporidiosis–setting the stage for control of an emerging pathogen. Clin Infect Dis. 1995;21(1):63–4. doi: 10.1093/clinids/21.1.63. [DOI] [PubMed] [Google Scholar]
- 20.Navin TR, Juranek DD. Cryptosporidiosis: clinical, epidemiologic, and parasitologic review. Rev Infect Dis. 1984;6(3):313–27. doi: 10.1093/clinids/6.3.313. [DOI] [PubMed] [Google Scholar]
- 21.Ortega YR, Sheehy RR, Cama VA, et al. Restriction fragment length polymorphism analysis of Cryptosporidium parvum isolates of bovine and human origin. J protozool. 1991;38(6):40S–1S. [PubMed] [Google Scholar]
- 22.Sulaiman IM, Xiao L, Yang C, et al. Differentiating human from animal isolates of Cryptosporidium parvum. Emerg Infect Dis. 1998;4(4):681–5. doi: 10.3201/eid0404.980424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng MM, Xiao L, Freeman AR, et al. Genetic polymorphism among Cryptosporidium parvum isolates: evidence of two distinct human transmission cycles. Emerg Infect Dis. 1997;3(4):567–73. doi: 10.3201/eid0304.970423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao L, Sulaiman I, Fayer R, et al. Species and strain-specific typing of Cryptosporidium parasites in clinical and environmental samples. Mem Inst Oswaldo Cruz. 1998;93(5):687–91. doi: 10.1590/s0074-02761998000500022. [DOI] [PubMed] [Google Scholar]
- 25.Morgan U, Xiao L, Sulaiman I, et al. Which genotypes/species of Cryptosporidium are humans susceptible to? J Eukaryot Microbiol. 1999;46(5):42S–3S. [PubMed] [Google Scholar]
- 26.Morgan UM, Xiao L, Fayer R, et al. Variation in Cryptosporidium: towards a taxonomic revision of the genus. Int J Parasitol. 1999;29(11):1733–51. doi: 10.1016/s0020-7519(99)00109-5. [DOI] [PubMed] [Google Scholar]
- 27.Morgan UM, Xiao L, Fayer R, et al. Epidemiology and strain variation of Cryptosporidium parvum. Contrib Microbiol. 2000;6:116–39. doi: 10.1159/000060369. [DOI] [PubMed] [Google Scholar]
- 28.Xiao L, Morgan UM, Fayer R, et al. Cryptosporidium systematics and implications for public health. Parasitol Today. 2000;16(7):287–92. doi: 10.1016/s0169-4758(00)01699-9. [DOI] [PubMed] [Google Scholar]
- 29.Ryan U, Fayer R, Xiao L. Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology. 2014;141(13):1667–85. doi: 10.1017/S0031182014001085. [DOI] [PubMed] [Google Scholar]
- 30.Santin M, Trout JM, Xiao L, et al. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet Parasitol. 2004;122(2):103–17. doi: 10.1016/j.vetpar.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Valenzuela O, Gonzalez-Diaz M, Garibay-Escobar A, et al. Molecular characterization of Cryptosporidium spp. in children from Mexico. PLoS One. 2014;9(4):e96128. doi: 10.1371/journal.pone.0096128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzipori S, Widmer G. The biology of Cryptosporidium. Contrib Microbiol. 2000;6:1–32. doi: 10.1159/000060370. [DOI] [PubMed] [Google Scholar]
- 33.Rosales MJ, Cordon GP, Moreno MS, et al. Extracellular like-gregarine stages of Cryptosporidium parvum. Acta Trop. 2005;95(1):74–8. doi: 10.1016/j.actatropica.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Petry F. Structural analysis of Cryptosporidium parvum. Microsc Microanal. 2004;10(5):586–601. doi: 10.1017/S1431927604040929. [DOI] [PubMed] [Google Scholar]
- 35.Hijjawi NS, Meloni BP, Ryan UM, et al. Successful in vitro cultivation of Cryptosporidium andersoni: evidence for the existence of novel extracellular stages in the life cycle and implications for the classification of Cryptosporidium. Int J Parasitol. 2002;32(14):1719–26. doi: 10.1016/s0020-7519(02)00199-6. [DOI] [PubMed] [Google Scholar]
- 36.Fayer R. General biology. In: Fayer R, Xiao L, editors. Cryptosporidium and cryptosporidiosis. Boca Raton (FL): CRC press; 2008. pp. 1–42. [Google Scholar]
- 37.Caccio S, Putignani L. Epidemiology of human cryptosporidiosis. In: Caccio S, Widmer G, editors. Cryptosporidium parasite and disease. Wien, Austria: Springer; 2014. pp. 43–78. [Google Scholar]
- 38.Assessing the public health threat associated with waterborne cryptosporidiosis: report of a workshop. MMWR Recomm Rep. 1995;44(RR-6):1–19. [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. Foodborne outbreak of cryptosporidiosis–Spokane, Washington, 1997. MMWR Morb Mortal Wkly Rep. 1998;47(27):565–7. [PubMed] [Google Scholar]
- 40.Rose JB, Slifko TR. Giardia, Cryptosporidium, and Cyclospora and their impact on foods: a review. J Food Prot. 1999;62(9):1059–70. doi: 10.4315/0362-028x-62.9.1059. [DOI] [PubMed] [Google Scholar]
- 41.Ortega YR, Roxas CR, Gilman RH, et al. Isolation of Cryptosporidium parvum and Cyclospora cayetanensis from vegetables collected in markets of an endemic region in Peru. Am J Trop Med Hyg. 1997;57(6):683–6. doi: 10.4269/ajtmh.1997.57.683. [DOI] [PubMed] [Google Scholar]
- 42.Koch KL, Phillips DJ, Aber RC, et al. Cryptosporidiosis in hospital personnel. Evidence for person-to-person transmission. Ann Intern Med. 1985;102(5):593–6. doi: 10.7326/0003-4819-102-5-593. [DOI] [PubMed] [Google Scholar]
- 43.Navin TR. Cryptosporidiosis in humans: review of recent epidemiologic studies. Eur J Epidemiol. 1985;1(2):77–83. doi: 10.1007/BF00141796. [DOI] [PubMed] [Google Scholar]
- 44.Xiao L, Ryan U. Molecular epidemiology. In: Fayer R, Xiao L, editors. Cryptosporidium and cryptosporidiosis. Boca Raton (FL): CRC Press, and IWA Publishing; 2008. pp. 119–71. [Google Scholar]
- 45.Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol. 2010;124(1):80–9. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 46.Yoder JS, Beach MJ, et al. Centers for Disease Control and Prevention. Cryptosporidiosis surveillance–United States, 2003–2005. MMWR Surveill Summ. 2007;56(7):1–10. [PubMed] [Google Scholar]
- 47.Yoder JS, Harral C, Beach MJ, et al. Cryptosporidiosis surveillance - United States, 2006–2008. MMWR Surveill Summ. 2010;59(6):1–14. [PubMed] [Google Scholar]
- 48.Yoder JS, Wallace RM, Collier SA, et al. Cryptosporidiosis surveillance–United States, 2009–2010. MMWR Surveill Summ. 2012;61(5):1–12. [PubMed] [Google Scholar]
- 49.Ortega Y, Cama V. Foodborne transmission. In: Fayer R, Xiao L, editors. Cryptosporidium and cryptosporidiosis. Boca Raton (FL): CRC Press and IWA Publishing; 2008. pp. 289–304. [Google Scholar]
- 50.Blackburn BG, Mazurek JM, Hlavsa M, et al. Cryptosporidiosis associated with ozonated apple cider. Emerg Infect Dis. 2006;12(4):684–6. doi: 10.3201/eid1204.050796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cama VA, Bern C, Roberts J, et al. Cryptosporidium species and subtypes and clinical manifestations in children, Peru. Emerg Infect Dis. 2008;14(10):1567–74. doi: 10.3201/eid1410.071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soave R. Cryptosporidiosis and isosporiasis in patients with AIDS. Infect Dis Clin North Am. 1988;2(2):485–93. [PubMed] [Google Scholar]
- 53.New drug for parasitic infections in children. FDA Consum. 2003;37(3):4. [PubMed] [Google Scholar]
- 54.White CA., Jr Nitazoxanide: a new broad spectrum antiparasitic agent. Expert Rev Anti Infect Ther. 2004;2(1):43–9. doi: 10.1586/14787210.2.1.43. [DOI] [PubMed] [Google Scholar]
- 55.DPDx. DPDx [Web page] [Accessed December 20, 2014];Parasite diagnosis. 2013 Available at: http://www.cdc.gov/dpdx/cryptosporidiosis/dx.html.
- 56.Catalog of cleared and approved medical device information from FDA. [Accessed October 20, 2014];Database of approved in-vitro diagnostic devices. 2014 Available at: http://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm.
- 57.Xiao L, Lal AA, Jiang J. Detection and differentiation of Cryptosporidium oocysts in water by PCR-RFLP. Methods Mol Biol. 2004;268:163–76. doi: 10.1385/1-59259-766-1:163. [DOI] [PubMed] [Google Scholar]
- 58.Ashford RW. Occurrence of an undescribed coccidian in man in Papua New Guinea. Ann Trop Med Parasitol. 1979;73(5):497–500. doi: 10.1080/00034983.1979.11687291. [DOI] [PubMed] [Google Scholar]
- 59.Relman DA, Schmidt TM, Gajadhar A, et al. Molecular phylogenetic analysis of Cyclospora, the human intestinal pathogen, suggests that it is closely related to Eimeria species. J Infect Dis. 1996;173(2):440–5. doi: 10.1093/infdis/173.2.440. [DOI] [PubMed] [Google Scholar]
- 60.Pieniazek NJ, Herwaldt BL. Reevaluating the molecular taxonomy: is human-associated Cyclospora a mammalian Eimeria species? Emerg Infect Dis. 1997;3(3):381–3. doi: 10.3201/eid0303.970319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Franzen C, Muller A, Bialek R, et al. Taxonomic position of the human intestinal protozoan parasite Isospora belli as based on ribosomal RNA sequences. Parasitol Res. 2000;86(8):669–76. doi: 10.1007/pl00008550. [DOI] [PubMed] [Google Scholar]
- 62.Eberhard ML, Njenga MN, DaSilva AJ, et al. A survey for Cyclospora spp. in Kenyan primates, with some notes on its biology. J Parasitol. 2001;87(6):1394–7. doi: 10.1645/0022-3395(2001)087[1394:ASFCSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 63.Eberhard ML, Ortega YR, Hanes DE, et al. Attempts to establish experimental Cyclospora cayetanensis infection in laboratory animals. J Parasitol. 2000;86(3):577–82. doi: 10.1645/0022-3395(2000)086[0577:ATEECC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 64.Ortega YR, Nagle R, Gilman RH, et al. Pathologic and clinical findings in patients with cyclosporiasis and a description of intracellular parasite life-cycle stages. J Infect Dis. 1997;176(6):1584–9. doi: 10.1086/514158. [DOI] [PubMed] [Google Scholar]
- 65.Ortega YR, Gilman RH, Sterling CR. A new coccidian parasite (Apicomplexa: Eimeriidae) from humans. J Parasitol. 1994;80(4):625–9. [PubMed] [Google Scholar]
- 66.Ortega YR, Sterling CR, Gilman RH. Cyclospora cayetanensis. Adv Parasitol. 1998;40:399–418. doi: 10.1016/s0065-308x(08)60128-1. [DOI] [PubMed] [Google Scholar]
- 67.Bern C, Ortega Y, Checkley W, et al. Epidemiologic differences between cyclosporiasis and cryptosporidiosis in Peruvian children. Emerg Infect Dis. 2002;8(6):581–5. doi: 10.3201/eid0806.01-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sherchand JB, Cross JH. Emerging pathogen Cyclospora cayetanensis infection in Nepal. Southeast Asian J Trop Med Public Health. 2001;32(Suppl 2):143–50. [PubMed] [Google Scholar]
- 69.Huang P, Weber JT, Sosin DM, et al. The first reported outbreak of diarrheal illness associated with Cyclospora in the United States. Ann Intern Med. 1995;123(6):409–14. doi: 10.7326/0003-4819-123-6-199509150-00002. [DOI] [PubMed] [Google Scholar]
- 70.Torres-Slimming PA, Mundaca CC, Moran M, et al. Outbreak of cyclosporiasis at a naval base in Lima, Peru. Am J Trop Med Hyg. 2006;75(3):546–8. [PubMed] [Google Scholar]
- 71.Pape JW, Verdier RI, Boncy M, et al. Cyclospora infection in adults infected with HIV. Clinical manifestations, treatment, and prophylaxis. Ann Intern Med. 1994;121(9):654–7. doi: 10.7326/0003-4819-121-9-199411010-00004. [DOI] [PubMed] [Google Scholar]
- 72.Hoge CW, Shlim DR, Ghimire M, et al. Placebo-controlled trial of co-trimoxazole for Cyclospora infections among travellers and foreign residents in Nepal. Lancet. 1995;345(8951):691–3. doi: 10.1016/s0140-6736(95)90868-4. [DOI] [PubMed] [Google Scholar]
- 73.Frenkel JK. Besnoitia wallacei of cats and rodents: with a reclassification of other cyst-forming isosporoid coccidia. J Parasitol. 1977;63(4):611–28. [PubMed] [Google Scholar]
- 74.Barta JR, Schrenzel MD, Carreno R, et al. The genus Atoxoplasma (Garnham 1950) as a junior objective synonym of the genus Isospora (Schneider 1881) species infecting birds and resurrection of Cystoisospora (Frenkel 1977) as the correct genus for Isospora species infecting mammals. J Parasitol. 2005;91(3):726–7. doi: 10.1645/GE-3341.1. [DOI] [PubMed] [Google Scholar]
- 75.Lindsay DS, Dubey JP, Blagburn BL. Biology of Isospora spp. from humans, nonhuman primates, and domestic animals. Clin Microbiol Rev. 1997;10(1):19–34. doi: 10.1128/cmr.10.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marshall MM, Naumovitz D, Ortega Y, et al. Waterborne protozoan pathogens. Clin Microbiol Rev. 1997;10(1):67–85. doi: 10.1128/cmr.10.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Certad G, Arenas-Pinto A, Pocaterra L, et al. Isosporiasis in Venezuelan adults infected with human immunodeficiency virus: clinical characterization. Am J Trop Med Hyg. 2003;69(2):217–22. [PubMed] [Google Scholar]
- 78.Cranendonk RJ, Kodde CJ, Chipeta D, et al. Cryptosporidium parvum and Isospora belli infections among patients with and without diarrhoea. East Afr Med J. 2003;80(8):398–401. [PubMed] [Google Scholar]
- 79.Sorvillo FJ, Lieb LE, Seidel J, et al. Epidemiology of isosporiasis among persons with acquired immunodeficiency syndrome in Los Angeles County. Am J Trop Med Hyg. 1995;53(6):656–9. doi: 10.4269/ajtmh.1995.53.656. [DOI] [PubMed] [Google Scholar]
- 80.Modigliani R, Bories C, Le Charpentier Y, et al. Diarrhoea and malabsorption in acquired immune deficiency syndrome: a study of four cases with special emphasis on opportunistic protozoan infestations. Gut. 1985;26(2):179–87. doi: 10.1136/gut.26.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pape JW, Johnson WD., Jr Isospora belli infections. Prog Clin Parasitol. 1991;2:119–27. [PubMed] [Google Scholar]
- 82.Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14(3):447–75. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guerrant RL, Hughes JM, Lima NL, et al. Diarrhea in developed and developing countries: magnitude, special settings, and etiologies. Rev Infect Dis. 1990;12(Suppl 1):S41–50. doi: 10.1093/clinids/12.Supplement_1.S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoder JS, Gargano JW, Wallace RM, et al. Giardiasis surveillance–United States, 2009–2010. MMWR Surveill Summ. 2012;61(5):13–23. [PubMed] [Google Scholar]
- 85.Yoder JS, Harral C, Beach MJ, et al. Giardiasis surveillance - United States, 2006–2008. MMWR Surveill Summ. 2010;59(6):15–25. [PubMed] [Google Scholar]
- 86.Thompson RC, Monis P. Giardia–from genome to proteome. Adv Parasitol. 2012;78:57–95. doi: 10.1016/B978-0-12-394303-3.00003-7. [DOI] [PubMed] [Google Scholar]
- 87.Ryan U, Caccio SM. Zoonotic potential of Giardia. Int J Parasitol. 2013;43(12–13):943–56. doi: 10.1016/j.ijpara.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 88.Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev. 2011;24(1):110–40. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Monis PT, Caccio SM, Thompson RC. Variation in Giardia: towards a taxonomic revision of the genus. Trends Parasitol. 2009;25(2):93–100. doi: 10.1016/j.pt.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 90.Nundy S, Gilman RH, Xiao L, et al. Wealth and its associations with enteric parasitic infections in a low-income community in Peru: use of principal component analysis. Am J Trop Med Hyg. 2011;84(1):38–42. doi: 10.4269/ajtmh.2011.10-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cotte L, Rabodonirina M, Piens MA, et al. Prevalence of intestinal protozoans in French patients infected with HIV. J Acquir Immune Defic Syndr. 1993;6(9):1024–9. [PubMed] [Google Scholar]
- 92.Berkman DS, Lescano AG, Gilman RH, et al. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359(9306):564–71. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- 93.Kulakova L, Galkin A, Chen CZ, et al. Discovery of novel antigiardiasis drug candidates. Antimicrob Agents Chemother. 2014;58(12):7303–11. doi: 10.1128/AAC.03834-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcia LS, Shimizu RY. Evaluation of nine immunoassay kits (enzyme immunoassay and direct fluorescence) for detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimens. J Clin Microbiol. 1997;35(6):1526–9. doi: 10.1128/jcm.35.6.1526-1529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heyworth MF. Diagnostic testing for Giardia infections. Trans R Soc Trop Med Hyg. 2014;108(3):123–5. doi: 10.1093/trstmh/tru005. [DOI] [PubMed] [Google Scholar]
- 96.Taniuchi M, Verweij JJ, Sethabutr O, et al. Multiplex polymerase chain reaction method to detect Cyclospora, Cystoisospora, and Microsporidia in stool samples. Diagn Microbiol Infect Dis. 2011;71(4):386–90. doi: 10.1016/j.diagmicrobio.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khare R, Espy MJ, Cebelinski E, et al. Comparative evaluation of two commercial multiplex panels for detection of gastrointestinal pathogens by use of clinical stool specimens. J Clin Microbiol. 2014;52(10):3667–73. doi: 10.1128/JCM.01637-14. [DOI] [PMC free article] [PubMed] [Google Scholar]