Abstract
Purpose of Review
Immune mechanisms exacerbate the severity of hypertension in humans and animal models of disease. This review summarizes recent mechanistic studies exploring the pathways whereby immunity influences salt-sensitive hypertension and renal disease.
Recent Findings
Emphasis is placed on the role of T cell subtypes, the mechanisms of T cell activation, and the identification of potential antigens or neoantigens.
Summary
Significant advancements have occurred in the search for pathways which activate the adaptive immune response. An enhanced understanding of the factors contributing to hypertension can lead to better therapies.
Keywords: Immunity, Lymphocytes, Sodium-Dependent Hypertension, Renal Disease
Introduction
Multiple reports in the past half century have documented the importance of the immune system in hypertension, vascular disease, and renal disease; these studies have been the subject of multiple review articles (1*,2,3,4,5). In the past 10–15 years, with the availability of new experimental tools, great progress has been made that has provided the fundamental links between immunity and cardiovascular disease. In particular, seminal studies by Harrison’s group used an approach with adoptive transfer of immune cells in immune deficient mice to illustrate the role of T lymphocytes in experimental hypertension (6). These studies have driven increased interest in this area of study.
A broad body of literature has demonstrated the participation of immunity in multiple models of hypertension in animals (1*), but the work in humans is less extensive. Nevertheless, data obtained from patients are consistent with observations in animal studies. It was shown in hypertensive patients that mononuclear cells infiltrate the renal interstitial spaces surrounding damaged glomeruli and tubules (7) as well as the arterioles and small arteries of the kidney (8). Subsequent studies demonstrated that hypertensive subjects have increased renal fibrosis, glomerulosclerosis, and macrophage and T-lymphocyte infiltration in the kidney when compared to normotensive individuals (9). There is therefore a clear association between the infiltration of immune cells in the kidney and hypertension and renal damage in patients; the mechanistic relationship is not well-defined, though several studies indicate that the severity of hypertension can be altered in patients receiving immunomodulatory therapy (10,11). Moreover, genetic association studies also suggest that the immune system is important in hypertension (5). Together, the histological, functional, and genetic data support the hypothesis that inappropriate immune activation plays a part in the development of hypertension and renal disease in humans.
Immunity and Salt-Sensitive Hypertension in Animals and Patients
Recent work in our laboratory has explored the role of immune mechanisms in the hypertension and renal damage that occurs in Dahl salt-sensitive (SS) rats. There are strong similarities in phenotypes between salt-sensitive hypertension and renal disease in Dahl SS rats and in humans (5). The Dahl SS strain and selected human subjects exhibit elevated arterial pressure when sodium intake is increased (5). Dahl SS rats exhibit albuminuria and renal damage that associates with sodium-sensitive hypertension; these observations are also consistent with the increased albumin excretion reported in salt-sensitive humans (5) and the renal cortical fibrosis and glomerulosclerosis that occurs in hypertensive patients (9). Finally, both Dahl SS fed high salt and hypertensive humans demonstrate an infiltration of macrophages and CD4+ and CD8+ T-cells in the kidneys (5,9). The Dahl SS and other rodent models appear to be useful to explore the role of immunity in salt-sensitive disease in the context of human disease.
Initial studies examined immunoreactive markers of infiltrating immune cells in the kidney of Dahl SS fed high salt compared to age-matched rats fed low salt. Several different approaches were used to demonstrate increased infiltration of macrophages and T-lymphocytes in the kidneys of Dahl SS rats fed a high salt diet. The infiltrating macrophages were found surrounding damaged glomeruli and in the medullary interstitial spaces near blocked renal tubules and T-lymphocytes surround damaged glomeruli and tubules as well as regions surrounding renal blood vessels (5). In contrast, normotensive Sprague Dawley rats have negligible renal damage or increased immune cell infiltration when fed a high salt diet, indicating that the infiltration of immune cells into the Dahl SS kidney is related to the disease phenotype. A subsequent gene expression analysis revealed significantly greater expression of mRNA encoding inflammatory molecules associated with T cell signaling in T cells isolated from the kidney in comparison to circulating T cells (12*). The increased expression of these and other factors indicate that infiltrating T cells in the kidney are activated and proliferating. The infiltrating immune cells may therefore be a source of proinflammatory cytokines as well as AngII, free radicals, or other factors that can influence arterial blood pressure (1,5).
To demonstrate the importance of the infiltrating immune cells in the development of salt-sensitive hypertension and renal disease, experiments were performed in which pharmacological inhibitors of the immune system were administered to Dahl SS rats during the period of high salt intake. Two mechanistically different immunosuppressive agents, administered to Dahl SS rats during the period of high salt intake, prevented the infiltration of T-cells into the kidneys of the treated animals and attenuated the development of salt-sensitive hypertension and renal damage (5). Subsequent studies utilized zinc finger nuclease (ZFN) technology to delete Recombination Activating Gene 1 (leading to a loss of mature T- and B-cells) and CD247 (leading to a loss of T-cells) in the Dahl SS genetic background. Experiments performed on these mutant rats demonstrated that elimination of the T-cells attenuated the sodium-sensitive hypertension and renal damage in the Dahl SS (12*,13). These studies demonstrated the importance of T-cells as amplifiers of salt-sensitive hypertension and renal damage. It has been interpreted that tissue damage, as a result of an initial increase in arterial pressure, triggers the inappropriate immune response in hypertension (5).
To link the Dahl SS with human disease, studies were performed to examine SH2B3, a gene linked to human hypertension and renal disease through genome wide association studies (GWAS) (14*). SH2B3 is an intracellular adaptor protein that functions as a negative regulator in many signaling pathways, including inflammatory signaling processes. A ZFN-mediated mutation of SH2B3, predicted to delete 3 amino acids in the SH2 domain, significantly attenuated the infiltration of leukocytes into the kidneys which was accompanied by a reduction of salt-sensitive hypertension and renal disease. A separate report demonstrated that mice lacking SH2B3 have an exaggerated hypertensive response to AngII (15*). Together, these studies provide further support for the immune system’s role in the pathogenesis of hypertension and end-organ damage.
Mechanism of Action of T Lymphocytes in Hypertension
As discussed above, T lymphocytes have become a focus of multiple studies interested in immune mechanisms of hypertension. Investigators have worked to delineate the T cell subtypes present in relevant tissues and elucidate the pathways leading to disease. Important roles for cytotoxic (CD8+) T cells and T helper (Th, CD4+) cells in hypertension have been established in recent studies. T helper cells are further subdivided into 4 main subtypes: Th1, Th2, Th17, and Tregs, with each subtype playing a distinct function in immune regulation. The specific contribution of these subtypes in hypertension is currently being investigated.
Recent findings have indicated deleterious roles for CD8+ T cells in hypertension. In comparison to normotensive subjects, it was shown that circulating CD8+ T cells in hypertensive subjects expressed significantly higher levels of perforin and granzyme B, two effectors of apoptosis; as well as the proinflammatory cytokines, IFNγ and TNFα (16). As described above, biopsies from patients with hypertensive renal disease reveal a significant accumulation of CD8+ and CD4+ T cells, in the kidney when compared to normotensive controls (9). As a mechanistic demonstration of the importance of different T cell subtypes, it was recently demonstrated that mice deficient in CD8+ T cells (CD8−/−) were protected from Ang II-induced hypertension and vascular dysfunction, whereas mice lacking CD4+ T cells (CD4−/−) were not (17*). Furthermore, CD8−/− mice, but not CD4−/− mice were resistant to the antinaturetic and antidiuretic effects of Ang II.
Other studies have demonstrated a role for CD4+ T cells in the modulation of blood pressure. Transfer of CD4+ T cells from pregnant rats with placental ischemia increased oxidative stress and blood pressure in normal pregnant recipients (18*). In patients with pulmonary hypertension, circulating CD4+ T cells expressed higher levels of IL-17 than controls, suggesting a polarization of T cells to the pathogenic Th17 phenotype (19*). Moreover, the ratio of Th17 cells to that of T regulatory cells (Tregs), which suppress immune responses, is increased in peripheral tissues of rats with mineralocorticoid-dependent hypertension (20*). Inhibition of IL-17 in these rats attenuated arterial hypertension as well as the renal and cardiac remodeling in the mineralocorticoid-treated rats.
T cell function may also account for sex differences in experimental hypertension. Male mice develop higher blood pressure than females when infused with Ang II; an effect abolished in mice lacking T- and B- cells. As further proof, adoptive transfer of CD3+ cells from male, but not female, wild type mice into male Rag1−/− mice recapitulated AngII-induced hypertension (21*). Of interest, in vitro stimulation of lymph node mononuclear cells in Th0 (no cytokines) or Th17 polarizing conditions with Ang II in vitro led to reduced IFNγ, TNFα, and IL-17 production in female but not male lymph node mononuclear cells. These data suggest important sex-dependent effects of T cell function in hypertension.
Activation of T Lymphocytes
The contribution of T lymphocytes in the pathogenesis of hypertension has been well documented, and a number of novel mechanisms leading to T cell activation have been proposed. The importance of the central nervous system and sympathetic tone as contributing factors in human and experimental hypertension is well-recognized (22,23). It was recently demonstrated that lesion of the anteroventral third ventricle (AV3V), a region of the circumventricular organs (CVO) in the brain, attenuated AngII-induced hypertension as well as expression of the early T cell activation marker CD69 (24). Increased dietary salt intake has been shown to significantly increase sympathetic nerve activity in Sprague-Dawley rats (25). This may arise from elevated cerebrospinal fluid [Na+] that is detected in the CVO and results in increased sympathetic drive. (26). More recently, placental growth factor (PIGF) was shown to be required for Ang II-induced hypertension and served as a neuro-immune link between the sympathetic nervous system and the splenic immune system (27**). Splenic activation of PIGF was also shown to be necessary for the costimulation of T cells which are causative players in end-organ damage in hypertension.
It is classically believed that the major histocompatibility complex on antigen-presenting cells (APCs) presents peptides to the T cell receptor (TCR). A second signal, known as costimulation then occurs, whereby CD28 on T cells interacts with the B7 ligands CD80 and CD86 on APCs. Both signals are required for the activation of T cells. Inhibition of B7-dependent costimulation prevented the interaction between T cells and APCs and attenuated AngII or deoxycorticosterone acetate (DOCA)-salt-induced models of experimental hypertension (28). Importantly, the inhibition of the B7/CD28 interaction blocked T cell activation, cytokine production, and the migration of T cells to the vascular wall. Related to this observation, our laboratory recently demonstrated that a functional TCR is necessary for the full development of salt-sensitive hypertension (12**). Genetic deletion of the CD3 zeta chain of the TCR blunted the hypertension and accompanying renal damage associated with increased salt intake in the Dahl SS. These studies demonstrate the necessary role of both the T cell receptor as well and B7/CD28 interaction in the activation of T cells that ultimately contribute to hypertension and end-organ damage.
Though the importance of T cells in hypertension is clear, recent studies have also demonstrated the importance of B cells and the production of autoantibodies in hypertension. Autoantibodies against α1- and and β1-adrenergic receptors have been shown to contribute to cardiovascular disease (29,30). Moreover, AngII type 1 receptor agonistic autoantibodies (AT1-AA) are observed in patients and in rodent models of hypertension associated with pre-eclampsia (31). It was demonstrated that depletion of B cells decreased cytokine production and blocked systemic lupus erythematosus (SLE)-induced hypertension and renal damage in mice (32*). Though adoptive transfer approaches have demonstrated that T cells are critical for the development of AngII-induced hypertension in mice (6), it is known that T cells produce cytokines which may modulate the function of other immune cells. Supporting this concept, the adoptive transfer of CD4+ T cells isolated from rats subjected to the reduced uterine perfusion pressure (RUPP) model of pre-eclampsia increased MAP in normal pregnant rats, but depletion of B cells inhibited the ability of CD4+ RUPP cells to elevate blood pressure (32). In that model, the interdependency between different immune cell types is apparent.
The mechanisms triggering the adaptive immune response in hypertension are unknown. In chronic diseases such as hypertension, activation may occur through the loss of self-tolerance, suggesting that hypertension may be an autoimmune disease. Major efforts have been taken to identify a self-antigen or neoantigen responsible for the initiation of adaptive immunity. It has been hypothesized that proteins modified via oxidation, nitrosylation, adduct formation, or posttranslational modifications create molecules that are no longer distinguished as self (2). Recent studies proposed that an autoimmune reaction to heat shock protein 70 (HSP70) could lead to salt-sensitive hypertension characterized by T cell activation and the production of regulatory T cells (33). Tolerization and overexpression studies demonstrated that HSP70 could serve as an antigen mediating the activation of T cells and development of hypertension. More recently, it has been shown that Ang II or DOCA-salt-induced reactive oxygen species formation in dendritic cells (DCs) leads to the production of isoketal adducts via lipid peroxidation. These isoketal-modified proteins activate DCs; moreover, scavenging of isoketals by 2-hydroxybenzylamine prevented the immunoreactivity normally leading to Ang II-induced hypertension (34**). Of interest, activated DCs from AngII-treated mice induced activation, proliferation, and cytokine production by T cells; and were shown to elicit hypertension in cell transfer studies. Together, these pioneering studies uncovered new pathways explaining potential mechanisms leading to autoimmunity and the subsequent development of hypertension and associated renal damage.
Sodium-Dependent Activation of T Lymphocytes
Recent advances have provided insight into the mechanisms contributing to immune activation in sodium-sensitive hypertension. It was shown that there is a dramatic induction of Th17 polarization in naïve T cells when exposed to elevated sodium concentration (35,36). The inhibition of serum- and glucocorticoid-inducible kinase-1 (SGK-1), an enzyme induced by sodium, prevented the differentiation to the Th17 phenotype, suggesting a mechanistic pathway that could be therapeutically targeted to prevent salt-induced autoimmunity. The link between salt intake, SGK-1, and Th17 polarization has recently been reviewed (37*). A longitudinal study that investigated the effects of long-term salt-intake reduction on immune status in humans demonstrated a reduction in the number of total monocytes that correlated significantly with a reduction of sodium intake. No significant differences were detected in circulating lymphocyte numbers, but IL-6 and IL-23 decreased with reduced salt intake while IL-10 increased (38*). These studies indicate that there is a potential relationship between dietary sodium intake, cytokine production, and immune status.
Conclusion
Numerous studies performed in the past 50 years have established a role for the immune system in the development of hypertension and end-organ damage. Though the mechanisms leading to activation of the immune system and the subsequent amplification of the disease process are still unresolved, studies are progressing to better understand the cell types involved in the response, the stimuli responsible for the activation of the different immune cell subtypes, and the cytokines and other factors mediating the pathogenic effects. As this field progresses, further advances should permit the development of targeted therapies to more effectively treat salt-sensitive hypertension and associated end-organ damage.
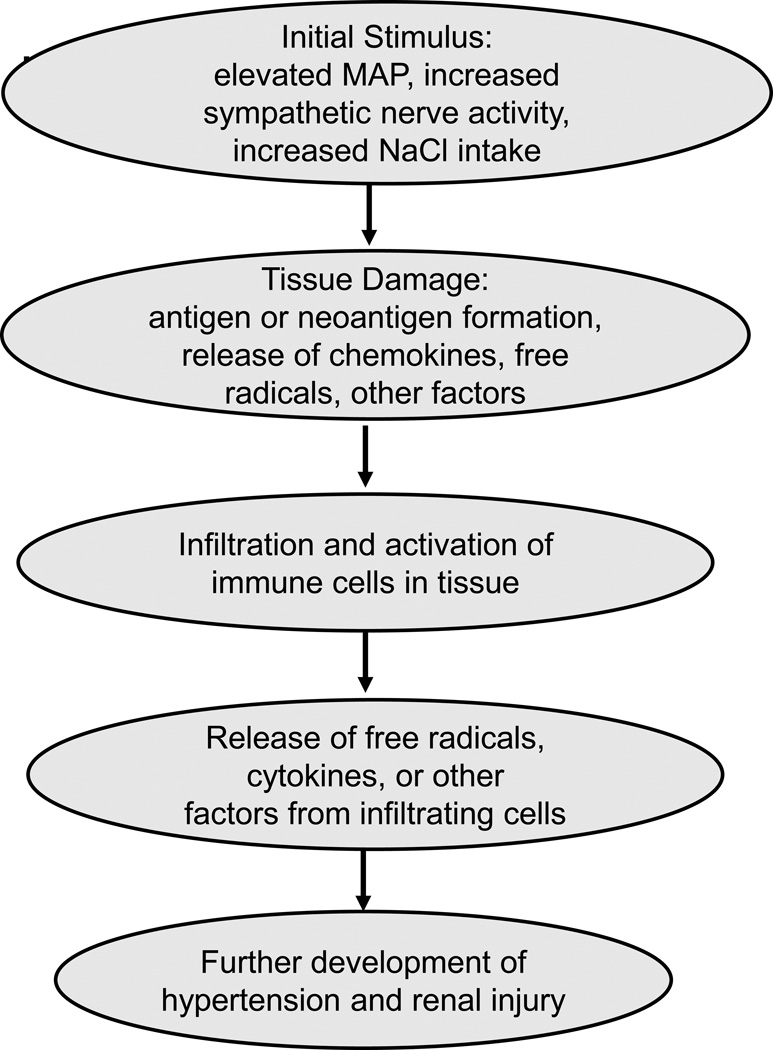
A hypothetical sequence of events, based upon the data presented in this brief review, is indicated in Figure 1. The data indicate that the inappropriate infiltration and activation of the immune system in salt-sensitive hypertension occurs in response to an initial stimulus which has been proposed to be an elevation in arterial blood pressure, increased sympathetic nerve activity, or even increased sodium intake. This stimulus results in tissue damage, antigen or neoantigen formation, and/or the release of chemokines, free radicals, or other molecules that stimulate the infiltration and activation of immune cells into the target tissue. The infiltrated immune cells may then release free radicals, cytokines, or other factors that mediate vasoconstriction, renal sodium retention, or tissue damage that promote hypertension and end-organ damage.
Figure 1.
Proposed stimuli and mechanisms of action of immune cells in salt-sensitive hypertension.
Key Points.
-
-
Recent studies indicate that activation of adaptive immune mechanisms amplifies the severity of sodium-sensitive hypertension and end-organ damage.
-
-
The activation of adaptive immunity may involve multiple factors including the sympathetic nervous system, B cells, T cells, and sodium concentration.
-
-
The mechanism of action of these immune responses involves the release of pro-inflammatory cytokines and autoantibodies.
Acknowledgments
None
Financial Support and Sponsorship
This work was partially supported by grants from the National Institutes of Health (DK96859 and HL116264) and the American Heart Association (15SFRN2391002).
Footnotes
Conflicts of Interest
None
Bibliography
- 1. Rodriguez-Iturbe B, Pons H, Quiroz Y, Lanaspa MA, Johnson RJ. Autoimmunity in the pathogenesis of hypertension. Nat Rev Nephrol. 2014;10:56–62. doi: 10.1038/nrneph.2013.248. * This review provides a comprehensive summary of immunity in hypertension.
- 2.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57(2):132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan MJ. An Update on immune system activation in the pathogenesis of hypertension. Hypertension. 2013;62:226–230. doi: 10.1161/HYPERTENSIONAHA.113.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiffrin EL. T lymphocytes: a role in hypertension? Current Opinion in Nephrology and Hypertension. 2010;19:181–186. doi: 10.1097/MNH.0b013e3283360a2e. [DOI] [PubMed] [Google Scholar]
- 5.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol. 2014;307:F499–F508. doi: 10.1152/ajprenal.00258.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sommers SC, Relman AS, Smithwick RH. Histologic studies of kidney biopsy specimens from patients with hypertension. Am J Pathol. 1958;34:685–715. [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen F. Inflammatory cellular reaction in hypertensive vascular disease in man. Acta Path Microbiol Scand Section A. 1972;80:253–256. [PubMed] [Google Scholar]
- 9.Hughson MD, Gobe GC, Hoy WE, Manning RD, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and Whites. Am J Kid Dis. 2008;52:18–28. doi: 10.1053/j.ajkd.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Herrera J, Ferrebuz A, García MacGregor E, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:218–225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 11.Seaberg EC, Muñoz A, Lu M, Detels R, Margolick JB, Riddler SA, Williams CM, Phair JP. Multicenter Cohort Study AIDS. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953–960. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 12. Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension. 2014;63(3):559–564. doi: 10.1161/HYPERTENSIONAHA.113.02191. *This study demonstrated that genetic deletion of the T cell receptor in rats attenuated salt-induced hypertension and renal damage.
- 13.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt sensitive rats attenuates hypertension and renal damage. Am J Physiol. 2013;304:R407–R414. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rudemiller NP, Lund H, Priestley JRC, Endres BT, Prokop JW, Jacob HJ, et al. Mutation of SH2B3 (LNK), a GWAS candidate for hypertension, attenuates Dahl SS hypertension via inflammatory modulation. Hypertension. 2015;65:111–1117. doi: 10.1161/HYPERTENSIONAHA.114.04736. **This study demonstrated mechanisms whereby SH2B3, a GWAS candidate gene for hypertension, can affect sodium-sensitive hypertension and end-organ damage in Dahl SS rats.
- 15. Saleh MA, McMaster WG, Wu J, Norlander AE, Samuel A, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. Journal of Clinical Investigation. 2015;125:1189–1202. doi: 10.1172/JCI76327. **This study demonstrated mechanisms whereby SH2B3, a GWAS candidate gene for hypertension, can affect experimental hypertension in AngII-infused mice.
- 16.Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62(1):126–133. doi: 10.1161/HYPERTENSIONAHA.113.00689. [DOI] [PubMed] [Google Scholar]
- 17. Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, et al. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension. 2014;64(5):1108–1115. doi: 10.1161/HYPERTENSIONAHA.114.04147. *This study illustrated that CD8+ T cells are necessary for AngII-induced hypertension.
- 18. Wallace K, Cornelius DC, Scott J, Heath J, Moseley J, Chatman K, et al. CD4+ T cells are important mediators of oxidative stress that cause hypertension in response to placental ischemia. Hypertension. 2014;64(5):1151–1158. doi: 10.1161/HYPERTENSIONAHA.114.03590. *This study demonstrated the importance of CD4+ T cells in the development of high blood pressure that occurs during pre-eclampsia.
- 19. Hautefort A, Girerd B, Montani D, Cohen Kaminsky S, Price L, et al. Th17 polarization in pulmonary arterial hypertension. Chest. 2015;147(6):1610–1620. doi: 10.1378/chest.14-1678. *This study illustrated the polarization of T cells to the Th17 phenotype in patients with pulmonary hypertension.
- 20. Amador CA, Barrientos V, Pena J, Herrada AA, Gonzalez M, Valdes S, et al. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension. 2014;63(4):797–803. doi: 10.1161/HYPERTENSIONAHA.113.02883. *This study showed the importance of Th17 cells in DOCA-salt hypertension.
- 21. Pollow DP, Uhrlaub J, Romero-Aleshire MJ, Sandberg K, Nikolich-Zugich J, Brooks HL, et al. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension. 2014;64(2):384–390. doi: 10.1161/HYPERTENSIONAHA.114.03581. *This study demonstrates that the sex differences in severity of hypertension may be due to difference in T cell function.
- 22.Guyenet PG. The sympathetic control of blood pressure. Nature Reviews Neuroscience. 2006;7(5):335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 23.Maranon RO, Lima R, Spradley FT, do Carmo JM, Zhang H, Smith A, et al. Roles for the sympathetic nervous system, renal nerves, and CNS melanocortin-4 receptor in the elevated blood pressure in hyperandrogenemic female rats. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2015;308:R708–R713. doi: 10.1152/ajpregu.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, et al. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circulation Research. 2010;107(2):263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujita M, Ando K, Kawarazaki H, Kawarasaki C, Muraoka K, Ohtsu H, et al. Sympathoexcitation by brain oxidative stress mediates arterial pressure elevation in salt-induced chronic kidney disease. Hypertension. 2012;59(1):105–112. doi: 10.1161/HYPERTENSIONAHA.111.182923. [DOI] [PubMed] [Google Scholar]
- 26.Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, et al. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. American Journal of Physiology Heart and Circulatory Physiology. 2012;302(5):H1031–H1049. doi: 10.1152/ajpheart.00899.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carnevale D, Pallante F, Fardella V, Fardella S, Iacobucci R, Federici M, et al. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity. 2014;41(5):737–752. doi: 10.1016/j.immuni.2014.11.002. **This study demonstrated that placental growth factor may link the sympathetic nervous system with the immune system in the spleen.
- 28.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, et al. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122(24):2529–2537. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahns R, Boivin V, Lohse MJ. beta(1)-Adrenergic receptor function, autoimmunity, and pathogenesis of dilated cardiomyopathy. Trends in Cardiovascular Medicine. 2006;16(1):20–24. doi: 10.1016/j.tcm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Luther HP, Homuth V, Wallukat G. Alpha 1-adrenergic receptor antibodies in patients with primary hypertension. Hypertension. 1997;29(2):678–682. doi: 10.1161/01.hyp.29.2.678. [DOI] [PubMed] [Google Scholar]
- 31.Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circulation Research. 2013;113(1):78–87. doi: 10.1161/CIRCRESAHA.113.300752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mathis KW, Wallace K, Flynn ER, Maric-Bilkan C, LaMarca B, Ryan MJ. Preventing autoimmunity protects against the development of hypertension and renal injury. Hypertension. 2014;64(4):792–800. doi: 10.1161/HYPERTENSIONAHA.114.04006. *This study demonstrated that B cell depletion attenuated systemic lupus erythematosus-induced hypertension.
- 33.Pons H, Ferrebuz A, Quiroz Y, Romero-Vasquez F, Parra G, Johnson RJ, et al. Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. American Journal of Physiology Renal Physiology. 2013;304(3):F289–F299. doi: 10.1152/ajprenal.00517.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. The Journal of Clinical Investigation. 2014;124(10):4642–4656. doi: 10.1172/JCI74084. **This article suggests that isoketals, modified in dendritic cells by reactive oxygen species, may act as a neoantigen and lead to the activation of T cells.
- 35.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Binger KJ, Linker RA, Muller DN, Kleinewietfeld M. Sodium chloride, SGK1, and Th17 activation. Pflugers Archiv : European Journal of Physiology. 2015;467(3):543–550. doi: 10.1007/s00424-014-1659-z. *This review discusses the relationship between elevated salt, serum- and glucocorticoid-inducible kinase-1 (SGK-1), and the activation of Th17 cells.
- 38. Yi B, Titze J, Rykova M, Feuerecker M, Vassilieva G, Nichiporuk I, et al. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Translational Research. 2015;166(1):103–110. doi: 10.1016/j.trsl.2014.11.007. *This longitudinal study in humans examined the effects of long-term salt-intake reduction and changes in the immune system.