Abstract
Objectives
Inadequate vitamin D (D) status is common in the very elderly yet data on dose responses to D are sparse. Our objective was to determine the dose response relationship of 25-OH D to supplemental vitamin D3 in elderly nursing home residents.
Design
Randomized double-blind investigation
Setting
Nursing Home
Participants
Eighty-one women (n=51) and men (n=30), mean age of 87.4 ±8 years enrolled and 72 completed the study.
Intervention
Sixteen weeks of oral D3 at 800, 2000, or4000 IU/day or 50,000 IU/week
Measurements
Main outcome was 25(OH) D concentrations (tandem mass spectrometry) after 16 weeks. Free 25(OH) D and iPTH were also analyzed. Safety monitoring of calcium, eGFR, adherence and clinical status was performed.
Results
25(OH)D concentrations increased with dose (p<.0001) and were higher with 50000 IU/wk (p<.0001) than other doses, higher with 4000 IU/day than 800 or 2000 IU/day but 800 IU and 2000 IU/day did not differ. One subject on 800 IU/day had concentrations <20 ng/mL. All on ≥2000 IU/day had concentrations >20 ng/mL. Free 25(OH) D concentrations rose with total D. Both total and free 25(OH)D were related to calcium concentrations ;only free 25(OH)D was related to iPTH.
Conclusion
25(OH) D increased linearly with 800-4000 IU/day and 50000 IU/week D3 without a ceiling effect. Data suggest some elderly will require over 800 IU/day D3 to ensure adequate vitamin D status. Changes in 25(OH) D with D3 were related to starting concentrations (greatest with the lowest concentrations and unchanged with 800 and 2000 IU/day if 20-40 ng/mL). Relationships between measures of D effect and free 25(OH)D suggest its potential in defining optimal 25(OH) D concentrations.
Keywords: vitamin D, elderly, 25(OH) vitamin D, iPTH, vitamin D dosing
INTRODUCTION
There is recognition of the role of vitamin D in health and disease and the negative impact of inadequate vitamin D status. 1-24 Institute of Medicine recommendations for daily vitamin D intake of 800 IU units are the same for all adults over the age of 70 years but acknowledge the paucity of data from very old people in whom physiologic, disease-related, and environmental exposure differences from younger people could affect vitamin D pharmacokinetics and pharmacodynamics. 15,25-27
Our goal was to perform a double-blind randomized trial in the very elderly to determine steady-state circulating concentrations of total 25 (OH) vitamin D in response to vitamin D3 doses of 800 IU/day, 2000 IU/day, 4000 IU/day (tolerable upper intake concentration) and 50,000 IU/week used for treatment of vitamin D deficiency. We also determined steady-state relationships between total and free 25(OH)D with calcium and intact parathyroid hormone concentrations as biomarkers of vitamin D effects.
METHODS
Overall Design
A 16 week double-blind study of long-term stay nursing home residents over age 65 years randomized to D3 doses of 800, 2000, 4000 IU/day, or 50,000 IU/week.
Participants
Participants were elderly (>65 yr) clinically stable long-term stay nursing home residents (Jewish Home, San Francisco). There were no changes in medications/diagnoses within the month prior to enrollment, or hospitalizations within 6 months of enrollment. Subjects had no hypercalcemia, history of hypercalcemia, uncontrolled thyroid or parathyroid disorders, severe renal failure (estimated GFR28 <30 ml/min/M2), active malignancies (excepting non-melanoma skin cancers), intestinal bypass surgery or small bowel resection, granulomatous diseases, contraindications or allergy to D, osteoporosis or a history of fractures and receiving over 800 IU/day D, or treatment for severe D deficiency or an investigational agent in the prior six months. They received no D supplements (D naïve) or had stable D doses for over two months prior to entry. All were able to give informed consent or had an agent able to provide consent to the project approved by the UCSF Committee on Human Research.
Intervention
Sixteen week supplementation with 800, 2000, 4000 IU/day or 50,000 IU/week D3 was given orally. Randomization was in blocks of four stratified by sex. Nurses administered one capsule daily (identically appearing D or placebo in the 50,000 IU/week group) with the meal estimated to have the highest fat intake (breakfast in 90%). Status was monitored every two weeks by interviews, and medical and nursing record reviews. Chemistry panels were analyzed at baseline, mid-study and study end. 25(OH)D and iPTH were measured at baseline and study end.
Vitamin D formulations
Capsules containing 800, 2000, and 4000 IU D3 were custom produced by Bio-Tech Pharmacal, Inc, Fayetteville, Arkansas, (www.Bio-Tech-Pharm.com) to be identical in appearance to their commercially produced 4000 and 50,000 IU D3 capsules. Analyses of capsule content were performed pre-study and at six month intervals (by Tai C. Chen, Ph.D., CTSI, Boston University, Boston, MA).
Vitamin D measurements
Total serum 25(OH) Vitamin D3 + D2 (including C3 epimer) concentrations were determined by liquid chromatography tandem mass spectrometry at Mayo Clinical Laboratories, Rochester, Minnesota, a participant in NIH Office of Dietary Supplements funded National Institute of Standards and Technology (NIST) quality assurance program for analysis of D metabolites in human serum. The assay has ~10% CV at concentrations ≥10 ng/mL.
Free 25(OH) D was directly measured by immunoassay (Future Diagnostics B.V., Wijchen, The Netherlands, http://www.future-diagnostics.nl/) as previously described. 29,30 The limit of detection of the assay was 1.9 pg/mL. In the range of concentrations measured, coefficent of variation (c.v.) was ≤ 7%.
Intact Parathyroid Hormone (iPTH) was measured at San Francisco General Hospital Clinical laboratories, San Francisco, CA using the Siemens ADVIA Centaur® assay, a two-site sandwich immunoassay using direct chemiluminometric technology.
Adherence was calculated from capsules remaining on blister packs retrieved at 2 week intervals.
Statistical Design and Data Analysis
Demographic, clinical characteristics, and baseline characteristics of groups are presented as mean ± S. D. and compared using ANOVA. Comparisons of concentrations in D3 dosing groups at study end were made by ANOVA. Adherence of 80% was pre-specified for inclusion in analyses. Results presented were Bonferonni-corrected for a mid-point safety analysis. Distribution of dropout and side effects were tested by Chi square. Relationships between total and free 25(OH) D, albumin-adjusted calcium concentrations, and iPTH were tested by linear regression. In residents without a history of supplemental vitamin D, tests for time and dose effects were by repeated measures ANOVA. Based on conservative estimates of variability and dose responses, a sample size of 24 per group was the pre-study target to detect a dose response in the form of any difference between two dose groups with an omnibus one-way ANOVA, with α=0.05 and power 0.8. Mid-study estimates based on trial data and corrected for multiple comparisons estimated 18 per group would have power 0.88-0.94 to detect a dose effect and to detect differences between groups except for 800 compared to 2000 IU/day.
ClinicalTrials.gov
The trial was registered with Clinical Trials.gov as NCT01554241.
Data and Safety Monitoring
An NIA-appointed Data Safety and Monitoring Board monitored the study before, during, and at the termination of the study.
RESULTS
Participants
Three hundred sixty three long-term stay residents were screened of whom 277 met exclusion criteria or declined to participate. Informed consent was obtained in 86 with 4 ineligible on baseline laboratory test results and one with consent withdrawn. The study was initiated in 81 residents. Subject characteristics at baseline are presented in Table 1. Mean Charlson comorbidity index was 7 ± 3 and activities of daily living score was 2 ± 2 (on average, requiring assistance in 4 of 6 activities of daily living), 25 were pre-frail, 38 were frail, one was not frail using Fried criteria31, with frailty assessment unable to be performed in 17. Resident activity was tracked. Ninety percent of participants did not leave the indoor units of the facility. For the few that did leave the indoor units, they were fully dressed for the San Francisco climate without sun exposure to their skin. At baseline sixteen residents had not previously received vitamin D supplements. The mean dose in the sixty-five residents receiving prior supplementation was 1391 ± 904 IU/day (range 200-4400 IU/day).
Table 1.
Participant Characteristics at Study Entry
| Enrolled | Completed | 800 IU/day | 2000 IU/day | 4000 IU/day | 50,000 IU/week | |
|---|---|---|---|---|---|---|
| N | 81 | 72 | 20 | 19 | 20 | 13 |
| Age (y) (range) | 87.4 ±8 (65-105) | 87.4 ±8 (65-105) | 84.9 ±8.7 (66-98) | 85.9 ±8.5 (66-101) | 89.5 ±6.6 (75-103) | 90.1 ±6.6 (79-105) |
| Men/Women | 30/51 | 23/49 | 6/14 | 7/12 | 7/13 | 3/10 |
| Race (white/Asian) | 80/1 | 72 | 19/1 | 19 | 20 | 13 |
| Weight (kg) | 69.7 ±16 | 70.2 ±16.2 | 71.1 ±18.3 | 71.1 ±13.2 | 69 ±17.3 | 69.6 ±16.4 |
| Height (cm) | 159 ±9 | 159 ±9 | 160 ±11 | 161 ±8 | 159 ±10 | 157 ±10 |
| BMI (kg/m2) | 27.4 ±5.7 | 27.5 ±5.7 | 27.5 ±6.8 | 27.6 ±5.3 | 27 ±5.4 | 28.2 ±5.7 |
| Creatinine (mg/dL) | 0.96 ± 0.34 | 0.97 ± 0.33 | 1 ± 0.3 | 0.8 ± 0.32 | 1 ± 0.4 | 1.1 ± 0.3 |
| eGFR (ml/min/1.73m2)* | 70 ± 25 | 68 ± 25 | 68 ± 28 | 81 ± 23 | 66 ± 22 | 55 ± 17 |
| Calcium (mg/dL) (Alb Adj) | 9.4 ±0.3 | 9.4 ±0.3 | 9.5 ±0.4 | 9.3 ±0.3 | 9.3 ±0.4 | 9.4 ±0.4 |
| Phosphorus (mg/dL) | 3.7 ±0.6 | 3.7 ±0.5 | 3.8 ±0.6 | 3.7 ±0.4 | 3.7 ±0.5 | 3.5 ±0.6 |
| Albumin (g/dL) | 3.6 ±0.4 | 3.6 ±0.4 | 3.6 ±0.4 | 3.7 ±0.4 | 3.6 ±0.4 | 3.7 ±0.4 |
| Total 25 (OH) D (ng/mL) | 29 ±9 | 30 ±10 | 29 ±9 | 29 ±10 | 29 ±10 | 32 ±6 |
| Free 25 (OH) D (pg/mL) | 7.9 ±2.1 | 8 ±2.2 | 7.8 ±2.6 | 8.1 ±2.1 | 8.1 ±2.3 | 7.7 ±1.5 |
Date are mean ± SD.
calculated by MDRD equation.
Seventy-two participants (89 per cent) completed the study. Five died during the study (pneumonia in two, stroke in two, and sepsis in one), two were withdrawn during hospitalization (n=2) and two met pre-determined stopping criteria of change in renal status or a renal stone. No effect of vitamin D dose assignment on dropout rates was detected ( dropouts were 3 of 20 assigned 800 IU/d, 1 of 20 assigned 2000 IU/d, 4 of 20 assigned 4000/d and 1 of 14 assigned 50,000 IU/wk) nor were differences detected in clinical characteristics between those completing the study and those who did not. An interim safety analysis found 25(OH)D concentrations over 50 ng/mL in 8 of 13 and over 80 ng/mL in one subject randomized to 50,000 IU/wk at study completion. The DSMB recommended no further randomization to this dose resulting in fewer subjects in the 50,000 IU/wk group.
Samples were obtained at a mean of 71 ±56 hours after dosing and were unrelated to time after dose. Hypercalcemia did not occur nor were corrected calcium concentrations changed by >5% (maximum changes were 0.5 and 0.6 mg/dL in two subjects).
Study Capsule Content
No significant changes in capsule content were detected during the study (April, 2013 until September, 2014). Mean content of capsules at study initiation and end were : 858 ± 29 IU and 861±45 for 800 IU capsules, 2,467 ± 69 and 2482 ± 73 IU for 2000 IU capsules, 4839 ± 202 and 4807 ± 108 IU for 4000 IU capsules, and 68,354 ± 2,296 IU and 57,542 ± 356 IU for the commercially available 50,000 IU capsules.
Adherence
Mean adherence was 96 ±7 per cent and did not differ by dose assignment. Only one subject (2000 IU/d group) had adherence below 80 per cent.
25(OH) D Concentrations
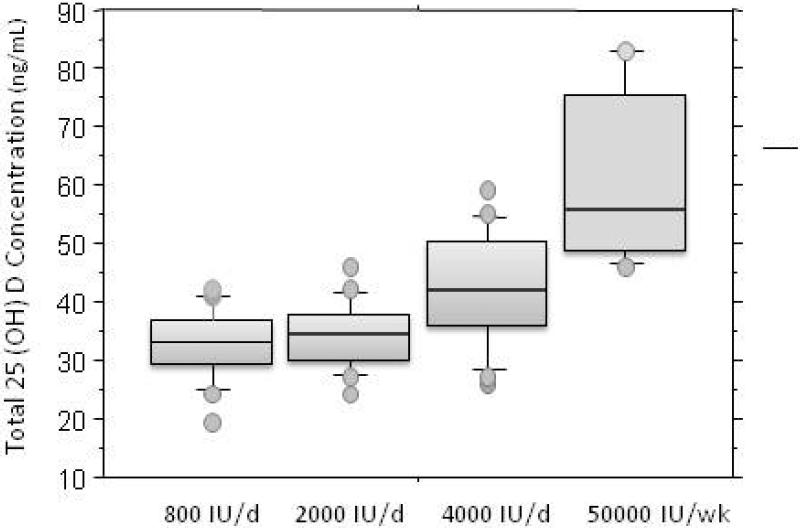
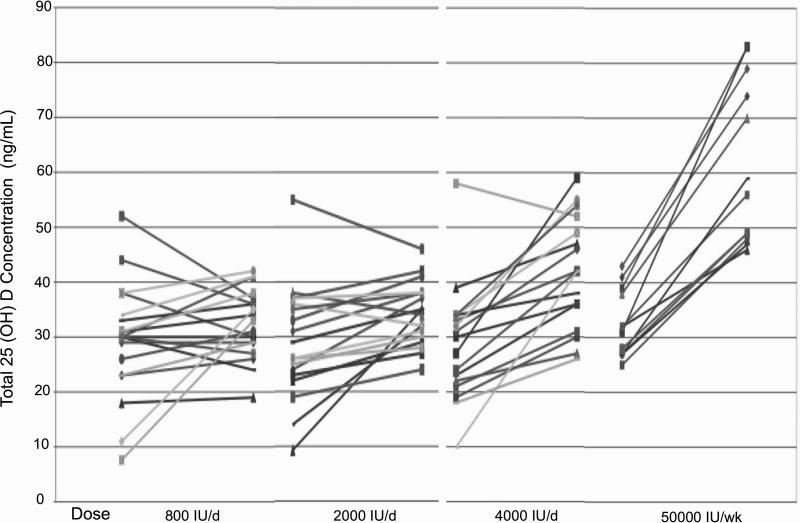
Total 25(OH)D concentrations at study end were related to dose (p<.0001). Mean data are presented in Table 2 and Figure 1 and individual responses in Figure 2. Concentrations with 50,000 IU/week were higher than all other doses, and 4000 IU day produced concentrations greater than 800 or 2000 IU/day. No difference was detected for results with 800 IU/day and 2000 IU/day (p=0.5). However, the only person failing to reach a 25(OH) D of 20 ng/mL was assigned 800 IU/day. 25(OH) D concentrations at start influenced responses (p<.001). Greatest changes in 25(OH)D were seen in those with the lowest concentrations at study start and those with concentrations of 20-40 ng/mL at study start did not have changes in 25(OH) with doses of 800 or 2000 IU/day (Figure 2 and 3). Decreases in 25(OH) D concentrations were seen in participants that had concentrations greater than 40 ng/mL at baseline and were randomized to 800 or 2000 IU/day. This can be explained on the basis of the higher vitamin D dosage that they had received prior to the study (mean of 2267 IU/day, range 1600-2800).
Table 2.
Total and Directly Measured Free 25 (OH) Vitamin D Concentrations by Vitamin D3 Dosing Assignment
| D3 Dosing Assignment (n completed of randomized) | Total 25 (OH) D (ng/mL) (range) | Between group Differences | Free 25 (OH) D (pg/mL) (range) | Between group Differences |
|---|---|---|---|---|
| 800 IU/d (20 of 23) | 33 ±6* (19-42) | vs. 2000/d, ns (p=.56) vs. 4000/d, p=.0004 vs. 50000/wk, p<.0001 |
8.7 ± 2.1 (5.7-13.6) | vs. 2000/d, ns (p=.48) vs. 4000/d, p<.002 vs. 50000/wk, p<.0001 |
| 2000 IU/d (19 of 20) | 34 ±6 (24-46) | vs. 4000/d, p<.003 vs. 50000/wk, p<.0001 |
9.5 ±1.7 (7.3-13.2) | Vs 4000/d, p<.02 vs. 50000/wk, p<.0001 |
| 4000 IU/(20 of 24) | 43 ±10 (26-59) | vs. 50000/wk, p<.0001 | 12.2 ±4.3 (7.3-23) | vs. 50000/wk, p<.0003 |
| 50,000 IU/wk (13 of 14) | 61 ±14 (46-83) | (see above) | 16.8 ± 4.3 (11.6-25.4) | (see above) |
| Dose effect | p<.0001 | P<.0001 |
Data are mean ± SD.
Statistical results are for effect of assignment (ANOVA for dose effect with Bonferroni correction for midpoint testing)
Figure 1.
25 (OH) vitamin D concentrations are presented by dosing assignment of 800, 2000, 4000 IU/day or 50000 IU/week of oral D3. The box plot shows the 10th, 25th, median, 75th and 90th percentile values. Circles represent individual values above the 90th and below the 10th percentile.
Figure 2.
Individual 25(OH)D concentration data by dosing group assignment are presented at study entry and study end. The main study outcome was concentration at study end, but individual dose response data demonstrate the greater changes for individuals with baseline 25(OH)D concentrations below 20 ng/mL, and the minimal changes in concentrations for those with baseline concentrations between 20-40 ng/mL assigned to 800 or 2000 IU/day D3, and decreases in those with concentrations over 40 ng/ml (p<.001 for effect of baseline concentration).
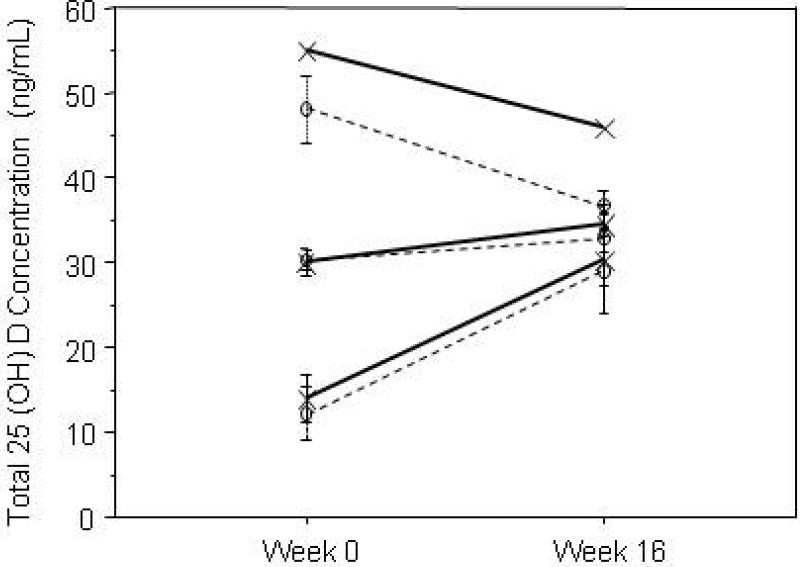
Figure 3.
Responses of 25(OH)D to oral D3 supplementation with 800 and 2000 IU/day are shown grouped by study entry 25(OH) D concentration (<20 ng/mL, 21-40 ng/mL, and >40 ng/mL; open circles and dashed lines represent data with 800 IU/day and the symbol x and solid lines represent data with 2000 IU/day). Data are mean ±S.E. (except n=1 for 2000 IU/day with baseline 25(OH)D > 40 ng/mL). The greatest changes are seen in the group with baseline concentrations of under 20 ng/mL, with no change in those with baseline concentrations of 20-40 ng/mL (p<.0001). Concentrations decreased with dosing of 800 or 2000 IU/day in those with baseline concentrations over 40 ng/mL.
Sixteen participants received D with food after crushing (FirstCrush™ electric pill crusher, Rochester, NY). A post hoc analysis found similar D concentrations in these subjects to those receiving intact capsules (30 ± 4 ng/mL for 800 IU/day (n=5) vs 34 ± 7), 31 ±4 for 2000 IU/day (n=3) vs 35 ±6, 43 ± 12 for 4000 IU/day (n=6) vs. 43±9 and 63± 10 for the two receiving 50,000 IU/wk vs. 61 ±16) although power was insufficient to eliminate a type II error (p=.64; power =.1). 25(OH) D2 concentrations were detected in ten participants at baseline (15-50% of total 25(OH)D) and in four at study end (10-25% of total). C3-epi-25(OH) D3 was detected in one participant at baseline (8% of total of 47 ng/mL) and in 2 different participants at study end (10% of 44 and 15% of 59 ng/mL total 25(OH) D).
Free 25(OH) D Concentrations
Concentrations of free 25(OH)D correlated with total 25(OH) D (r2=0.62, y=1.08 +.25*X, p<.0001) and were related to dose (see Table 2). No differences were detected between 800 IU/day compared to 2000 IU/day, but the lowest free 25(OH)D concentrations were seen with 800 IU/day. The participant with total 25(OH)D below 20 ng/mL had a free 25(OH) D concentration of 6.3 pg/mL and two other participants assigned to 800 IU/day had the only lower concentrations of 5.5 and 5.7 pg/mL.
Calcium and iPTH relationships with 25(OH)D
Calcium concentrations at study end did not differ between groups (9.4 ± 0.3 mg/dL for 800 IU/d, 9.3 ±0.3 for 2000 IU/d, 9.4± 0.4 for 4000 IU/d and 9.5 ±0.3 for 50,000IU/wk; p=0.4). Both total and free 25(OH)D were positively correlated with calcium concentrations but explained a small amount of the variation (r2=0.08, y=9.109 +.007*X, p=.02; and r2=0.07, y = 9.154 + .022 * X, p=.02, respectively). Study end iPTH concentrations did not differ between dosing groups (56 ±29 pg/mL for 800 IU/d, 43 ± 22 for 2000 IU/d, 45 ±32 for 4000 IU/d and 48 ±23 for 50,000 IU/wk; p=0.5). Free but not total 25(OH) D was inversely related to iPTH (r2= .08; y = 67.9 - 1.75 * X, p<=.02; r2= .03; y = 61.8 - .34 * X; p=.15, respectively).
Adverse effects
Hypercalcemia occurred in one subject assigned to 4000 IU D/day that resolved with discontinuation of supplemental calcium. One participant assigned to 50,000/week was withdrawn due to a renal stone on study day 4 – unblinding showed only placebo on days 1-4 (i.e. that subject had not yet received the weekly dose of 50,000 IU D). One participant assigned to 800 IU/d was withdrawn due to a decrease in eGFR to the pre-specified exclusion criteria of severe renal disease (<30 ml/min/1.73m2) but was a minimal change from 30 to 28 ml/min/1.73m2 at week 8. eGFR returned to baseline with study discontinuation and did not change with clinical rechallenge of 800 IU/day .
DISCUSSION
There are limited data on concentration responses to vitamin D in the very elderly, yet there is consensus that they are highly likely to require vitamin D supplementation because of absent or limited UVB exposure, reduced conversion of D precursor in the skin with UVB exposure, and low dietary intake of vitamin D. 27,32,33 Daily intake of 800 IU is the current recommendation of the IOM for people aged 70 or above based on estimates that this dose will produce or exceed a threshold of 25(OH)D of 20 ng/mL in 97.5% of people. 15 In this randomized double-blind study of elderly nursing home residents mean circulating 25(OH)D concentrations after 16 weeks of 800 IU daily were 33 ng/mL. The data support the IOM premise that most older people will achieve concentrations over 20 ng/mL with 800 IU/day of a formulation with documented content based on studies of somewhat younger populations. 15 Lack of detection of differences between the group receiving 800 IU and 2000 IU per day also supports the IOM recommendations. However, 1 of 20 residents did not reach a threshold of 20 ng/mL with 800 IU/day suggesting that some nursing home residents need higher doses to assure 25 (OH)D concentrations over 20 ng/mL. With 2000 IU/day, all participants had total 25(OH)D concentrations over 20 ng/mL and none had concentrations over 50 ng/mL, a concentration at which hypercalciuria may occur. These results are similar to the only prior study of U.S. nursing home residents receiving D3 (n=15) that reported a mean concentration of 32 ng/mL after six weeks of 2000 IU/day. 34 With 4000 IU/day D3, mean 25(OH)D concentrations were 43 ng/mL or 20% higher than with 800 or 2000 IU/day. Slightly over half reached concentrations over 40 ng/mL and one quarter had 25(OH)D concentrations exceeding 50 ng/mL. These 25(OH)D data with doses from 800-4000 IU/day are similar to those reported in double-blind studies of post-menopausal white and African American women randomized to 800-4800 IU/day D3 for a year. 35,36 The data also demonstrate the complex relationship between baseline 25(OH) D status and responses to supplemental D. With doses of 800, 2000, and 4000 IU/day, increases in 25(OH)D were greatest in those with the lowest baseline concentrations. Conversely, less of a change to no change was seen in those with adequate and/or higher baseline concentrations. This supports the viewpoint that supplementation will have the greatest effects in people with the lowest vitamin D status and that D status and not just dosing is an important consideration when evaluating responses both clinically and for research. 37
Concentrations with 50000 IU/wk D3 or about 7143 IU day equivalent were 42% higher than with 4000 IU/day while mean concentrations increased only 21% on average between the 2000 and 4000 IU daily doses. No plateau in the D3 dose vs. 25(OH)D response was seen. This finding differs from quadratic equation model-based predictions of a plateau in 25-OH D at about 46 ng/mL with doses over 3200 IU/day D3, although doses over 4800 IU/day were not part of the data for the model. 35 The lack of a plateau in the D3 dose vs. 25(OH)D response with doses from 1000-10,000 IU daily has previously been demonstrated in young men who achieved 25(OH)D concentrations of about 30, 60, and 84 ng/mL after single oral doses of 1000, 5000, and 10000 IU D3 per day. 38 These data demonstrate similar responses to supplemental oral D3 across the adult agespan and between races.
The results with dosing of 50,000 IU/wk were somewhat unexpected. Retrospective studies of responses to 50,000 IU/wk D2 for clinical vitamin D deficiency report mean 25(OH)D concentrations of about 33 ng/mL after at least 60 days 39 or three to 12 months. 40 In the current study, mean 25(OH)D concentrations were 61 ng/mL with concentrations over 40 ng/ml in all receiving 50,000 IU D3 weekly and exceeded 50 ng/mL in over half. Potential explanations for the differences include greater adherence in this prospective study compared to retrospective studies and that D3 and D2 differ in pharmacokinetics and pharmacodynamics.41-45 Most, but not all, of the literature suggests that lower total 25(OH)D concentrations are reached with D2 compared to D3, 41,42,46,47 including a single-blind direct comparison with 50,000 IU/week in healthy middle-aged people. 48 This may be clinically important as 50,000 IU D3 formulations are commercially available, and results with 50000 IU D3 weekly may differ from dosing with the prescription dose of 50,000 IU D2.
A novel aspect of this study is the direct measurement of free 25(OH)D. The “free hormone” hypothesis postulates that protein-bound ligands cannot freely cross the cell membrane to interact with cytoplasmic or nuclear-binding proteins while unbound “free” small lipophilic ligands can cross cell membranes and access cytoplasmic or nuclear bound proteins to exert effects. Circulating concentrations of 25(OH)D are 3 log scales higher than those of the active hormone 1,25(OH)2 D. A number of tissues express CYP27B1 and so are able to convert the circulating 25(OH)D to the active 1,25(OH)2D within the target cell. Thus, circulating free 25(OH)D could represent the driving “free” hormone of the vitamin D system. Free 25(OH) D concentrations were positively correlated with D3 dose and serum calcium and inversely correlated with iPTH. No normal range for free 25(OH)D has been established but we have reported free 25(OH) D concentrations ranging from 1-8 pg/mL in healthy people and 3.5-15 pg/mL in cirrhotics. 49 In this study, the lowest free 25(OH)D concentrations were seen in the 800 IU/day group giving further support to a potential need for doses over 800 IU/day in some elderly, although all free 25(OH)D concentrations were within the range we have seen in healthy people. Conversely, free 25(OH)D concentrations in some participants receiving 4000 IU/day and in all receiving 50,000 IU D3/wk exceeded concentrations seen in healthy subjects, stable patients, and even cirrhotics. The implications are uncertain but warrant further investigation.
Potential Limitations. The 16 week duration was based on prior investigations showing a three to four month duration of D3 dosing to reach steady-state in adults of younger to middle age.38,41,47,50 We found small increases in 25(OH)D from 8 to 16 weeks with 4000 IU/day and 50000/wk in the twelve participants who were D supplement naïve at study entry (p=.035 with Bonferroni Dunn criteria for significance of p <.0167; n=5). There may have been a small underestimation of peak steady-state concentrations for these doses in D naïve people. Sample sizes were relatively small (about 20 per group), but the same or larger than other D dose ranging studies. 35,36,41,46,47 Our results were obtained with vitamin D3 capsules that met USP standards of having at least 100% of labeled content and were within 100-140% of labeled content. Commercially available and compounded formulations can vary greatly in content and results may not be the same with formulations that do not have the same content. We studied nursing home residents but note that due to lack of financial assistance for assisted living, board and care, or retirement facilities in California, many participants were of similar age and health status to older people who have limited sunshine exposure living in the community in other states. Finally, concentration responses to vitamin D3 doses have been reported to be the same in blacks as whites 36 but we studied only whites and cannot address potential racial differences.
In summary, we provide the first data from randomized double-blind investigations of 25 (OH) D responses to 800-4000 IU D3 daily and 50,000 D3 weekly in very elderly people without sunshine exposure. Important clinical implications of this study include the potential need for doses over 800 IU/day oral D3 to assure adequate vitamin D status in some elderly and suggest that more than eight weeks of dosing is required to achieve steady-state conditions. The lack of changes in 25(OH)D with D3 supplementation with 800 and 2000 IU/day in people with normal basal concentrations questions the rationale for supplementation with these doses in such individuals. Importantly, 25(OH)D concentrations with D3 dosing from 800 IU/day to 50,000 IU did not plateau and 25(OH)D concentrations with 4000 IU/d and 50,000 IU/wk were higher than expected and possibly in the hypercalciuric range. The 25(OH)D concentrations achieved with 50000 IU D3 weekly, especially, do not support the routine use of this dose of D3 for supplementation and suggest responses should be monitored to avoid potential adverse effects during its use. The data also suggest the potential of free 25(OH) D in further defining optimal 25(OH) D concentrations.
Supplementary Material
Ancillary Figure S1: Study recruitment and completion schematic.
ACKNOWLEDGMENTS
This study was funded in part by NIH grants R21 AG 041660, and RO1 AR050023 and by the Office of Dietary Supplements, and in part with resources of the Jewish Home of San Francisco, CA, and Future Diagnostics, B.V., Wijchen, The Netherlands.
Footnotes
Author contributions: Janice Schwartz: study conception, design, data collection, data analysis, manuscript preparation.
Lynn Kane: study data collection, data entry and preliminary analysis, manuscript preparation
Dan Bikle: study conception and design, data analysis, and manuscript preparation
Sponsors: No sponsors were involved in trial design, data analysis or manuscript preparation. The NIA established the Data Safety and Monitoring Board and a representative participated in meetings.
Conflict of Interest: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Bikle D. Vitamin D and the skin. J Bone Miner Metab. 2010;28:117–130. doi: 10.1007/s00774-009-0153-8. [DOI] [PubMed] [Google Scholar]
- 2.Bikle D. Vitamin D and immune function: understanding common pathways. Curr Osteoporos Rep. 2009;7:58–63. doi: 10.1007/s11914-009-0011-6. [DOI] [PubMed] [Google Scholar]
- 3.Bikle D. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010;21:375–384. doi: 10.1016/j.tem.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams J, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick M. Vitamin D Deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 7.Holick M. Vitamin D: The other steroid hormone for muscle function and strength. Menopause. 2009;16:1077–1078. doi: 10.1097/gme.0b013e3181bd9804. [DOI] [PubMed] [Google Scholar]
- 8.Holick M, Chen T. Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 9.Ma Y, Khalifa B, Yee Y, et al. Identification and characterization of noncalcemic, tissue-selective, nonsecosteroidal vitamin D receptor modulators. J Clin Invest. 2006;116:892–904. doi: 10.1172/JCI25901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maalouf N. The noncalciotropic actions of vitamin D: recent clinical developments. Hypertension. 2008;17:408–415. doi: 10.1097/MNH.0b013e3283040c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovalenko P, Zhang Z, Yu J, et al. Dietary vitamin D and vitamin D receptor level modulate epithelial cell proliferation and apoptosis in the prostate. Cancer Prev Res (Phila) 2011;4:1617–1625. doi: 10.1158/1940-6207.CAPR-11-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleet J, Schoch R. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit Rev Clin Lab Sci. 2010;47:181–195. doi: 10.3109/10408363.2010.536429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holick M. Optimal vitamin D status for the prevention and treatment of osteoporosis. Drugs Aging. 2007;24:1017–1029. doi: 10.2165/00002512-200724120-00005. [DOI] [PubMed] [Google Scholar]
- 14.Hsia J, Heiss G, Ren H, et al. for the Women's Health Initiative (WHI) Investigators Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 15.IOM . Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: 2010. [Google Scholar]
- 16.Kampman E, Slattery M, Caan B, et al. Calcium, vitamin D, sunshine exposure, dairy products and colon cancer risk (United States). Cancer Causes Control. 2000;11:459–466. doi: 10.1023/a:1008914108739. [DOI] [PubMed] [Google Scholar]
- 17.Mak R. 1,25-dihydroxyvitamin D3 corrects insulin and lipid abnormalities in uremia. Kidney Int. 1998;53:1353–1357. doi: 10.1046/j.1523-1755.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 18.Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States. Data from the Third National Health and Nutrition Examination Survey Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 19.Melamed M, Michos E, Post W, et al. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michos E, Blumenthal R. Vitamin D supplementation and cardiovascular disease risk. Circulation. 2007;115:827–828. doi: 10.1161/CIRCULATIONAHA.106.686238. [DOI] [PubMed] [Google Scholar]
- 21.Sanders K, Stuart A, Williamson E, et al. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 22.Wang T, Pencina M, Booth S, et al. Vitamin D Deficiency and Risk of Cardiovascular Disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson K, Abrolat M, Malone L, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1775–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 24.Skinner H, Litzelman K, Schwartz G. Recent clinical trials of vitamin D3 supplementation and serum calcium levels in humans: Implications for vitamin D-based chemoprevention. Curr Opin Investig Drugs. 2010;6:678–687. [PubMed] [Google Scholar]
- 25.Schwartz J. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther. 2007;82:87–96. doi: 10.1038/sj.clpt.6100226. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz J, Abernethy D. Aging and medications: Past, present, future. Clin Pharmacol Ther. 2009;85:3–10. [Google Scholar]
- 27.MacLaughlin J, Holick M. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey A, Coresh J, Greene T, et al. Chronic kidney disease epidemiology collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Int Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz J, Lai J, Lizaola G, et al. Variability in free 25(OH)D levels in clinical populations. J Steroid Biochem Mol Biol. 2013;144(Pt A):156–158. doi: 10.1016/j.jsbmb.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz J, Lai J, Lizaola B, et al. A comparison of measured and calculated free 25 (OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014;99:1631–1637. doi: 10.1210/jc.2013-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fried L, Tangen C, Walston J, et al. Frailty in Older Adults: Evidence for a phenotype. J Gerontol Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 32.Godar DE, Pope SJ, Grant WB, et al. Solar UV doses of adult Americans and vitamin D(3) production. Dermatoendocrinol. 2011;3:243–250. doi: 10.4161/derm.3.4.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallace TC, Reider C, Fulgoni VL. Calcium and vitamin D disparities are related to gender, age, race, household income level, and weight classification but not vegetarian status in the United States: Analysis of the NHANES 2001-2008 data set. J Am Coll Nutr. 2013;32:321–330. doi: 10.1080/07315724.2013.839905. [DOI] [PubMed] [Google Scholar]
- 34.Himmelstein S, Clemens T, Rubin A, et al. Vitamin D supplementation in elderly nursing home residents increases 25(OH)D but not 1,25(OH)2D. Am J Clin Nutr. 1990;52:701–706. doi: 10.1093/ajcn/52.4.701. [DOI] [PubMed] [Google Scholar]
- 35.Gallagher J, Sai A, Templin T, et al. Dose Response to vitamin D supplementation in postmenopausal women: A randomized trial. Ann Intern Med. 2012;156:425–437. doi: 10.7326/0003-4819-156-6-201203200-00005. [DOI] [PubMed] [Google Scholar]
- 36.Gallagher JC, Peacock M, Yalamanchili V, et al. Effects of vitamin D supplementation in older African American women. J Clin Endocrinol Metab. 2013;98:1137–1146. doi: 10.1210/jc.2012-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heaney R. Vitamin D--baseline status and effective dose. N Engl J Med. 2012;367:77. doi: 10.1056/NEJMe1206858. [DOI] [PubMed] [Google Scholar]
- 38.Heaney R, Davies K, Chen T, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 39.Vande Griend JP, McQueen RB, Linnebur SA, et al. Prescription ergocalciferol dosing for vitamin D repletion: A retrospective evaluation. Pharmacotherapy. 2012;32:135–141. doi: 10.1002/PHAR.1052. [DOI] [PubMed] [Google Scholar]
- 40.Kshirsagar S, Kane L, Moore K, et al. Quantitative assessment of vitamin D supplementation on 25-OH Vitamin D levels in nursing home residents. Clin Pharmacol Ther. 2012;91(S1):PIII. [Google Scholar]
- 41.Jones KS, Assar S, Harnpanich D, et al. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99:3373–3381. doi: 10.1210/jc.2014-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romagnoli E, Mascia M, Cipriani C, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93:3015–3020. doi: 10.1210/jc.2008-0350. [DOI] [PubMed] [Google Scholar]
- 43.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–5391. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 44.Houghton L, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr. 2006;84:694–697. doi: 10.1093/ajcn/84.4.694. [DOI] [PubMed] [Google Scholar]
- 45.Tsugawa N, Nakagawa K, Kawamoto Y, et al. Biological activity profiles of 1alpha,25- dihydroxyvitamin D2, D3, D4, D7, and 24-epi-1alpha, 25-dihydroxyvitamin D2. Biol Pharm Bull. 1999;22:371–377. doi: 10.1248/bpb.22.371. [DOI] [PubMed] [Google Scholar]
- 46.Harris S, Dawson-Hughes B, Perrone G. Plasma 25-hydroxyvitamin D responses of younger and older men to three weeks of supplementation with 1800 IU/day of vitamin D. J Am Coll Nutr. 1999;18:470–474. doi: 10.1080/07315724.1999.10718885. [DOI] [PubMed] [Google Scholar]
- 47.Holick M, Biancuzzo R, Chen T, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–681. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heaney RP, Recker RR, Grote J, et al. Vitamin D3 Is more potent than vitamin D2 in humans. J Clin Endocrinol Metab. 2011;96:E447–E452. doi: 10.1210/jc.2010-2230. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz J, Lai J, Lizaola B, et al. A comparison of measured and calculated free 25 (OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014;99:1631–1637. doi: 10.1210/jc.2013-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hollis VW, Wagner CL. The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98:4619–4628. doi: 10.1210/jc.2013-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ancillary Figure S1: Study recruitment and completion schematic.