To the Editor:
Neuroendocrine hyperplasia of infancy (NEHI) is a childhood interstitial lung disease originally reported in the medical literature in 2005. Otherwise healthy former term infants exhibit insidious onset of chronic tachypnea, retractions, and hypoxemia in the first months to years of life (1). Delays in diagnosis are common as children undergo evaluation focused on more common pulmonary conditions.
Lung biopsy has been the historical gold standard for NEHI diagnosis, demonstrating near-normal histology with an increased number of neuroendocrine cells, best identified by bombesin immunostaining. However, through studies analyzing the clinical, physiologic, radiologic, and histologic features (2–5), evidence has emerged such that an American Thoracic Society clinical guideline now recommends noninvasive diagnosis of NEHI based on computed tomographic (CT) chest imaging findings in the compatible clinical context (6).
Although NEHI is clearly rare, its incidence and prevalence remain unknown. Since the original report of 15 cases from two large referral centers, the published literature tallies an additional 116 cases. However, the numbers of unique cases in these reports are undoubtedly substantially fewer, as several large referral centers have used their cohorts in multiple publications (1, 3–5). We have previously reported a single-center retrospective study in which we identified eight cases of NEHI from 1994 to 2011, encompassing an extended period of time before the establishment of our childhood interstitial lung disease program in 2012 (7). Only one NEHI case was diagnosed from 2009 to 2011. Thus, we were intrigued by the subsequent identification of five NEHI cases from within our general pediatrics service from 2012 to 2014.
Here we report the experience of two pulmonary fellows in the diagnosis of NEHI, based on these local patients. Some of the results of these studies have been previously reported in the form of an abstract (8).
Methods
After institutional review board approval (131026), we comprehensively reviewed the medical records of five children diagnosed with NEHI at Vanderbilt from 2012 to 2014. None were previously seen by pulmonary consultants at other centers.
Results
Patients were initially admitted to the general pediatric hospitalist service, with subsequent pulmonary consultation within 24 hours in all but one case. The age of presentation ranged from 1 to 7 months, and prominent findings included tachypnea, hypoxemia, and failure to thrive, but not wheezing (Table 1). Two individuals had crackles on initial exam, though all had crackles on subsequent assessments.
Table 1.
Clinical presentation
| Gestational age |
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
|---|---|---|---|---|---|
| Full term | Full term | Full term | Full term | Full term | |
| Age and reason for hospitalization* | 3.5 mo: bronchiolitis | 6 mo: tachypnea and poor weight gain | 5 mo: transient tachypnea and desaturation | 7 mo: failure to thrive; hypoxemia as incidental finding | 1 mo: tachypnea and hypoxemia |
| Key findings at pulmonary presentation | |||||
| Tachypnea | + | + | + | + | + |
| Crackles | + | − | − | − | + |
| Hypoxemia | + | + | + | + | + |
| Wheezing | − | − | − | − | − |
| Weight percentile | <Third percentile | Fourth percentile | <Third percentile | <Third percentile | 22nd percentile |
| Age at neuroendocrine hyperplasia of infancy diagnosis | 5.5 mo | 7 mo | 6 mo | 7 mo | 1.5 mo |
| Comorbidities | Oral aversion requiring g-tube; GERD | GERD | G-tube; recurrent febrile illnesses; GERD | Otitis media, trivial PDA; GERD | GERD |
| Prior respiratory hospitalization | Yes | No | Yes | No | No |
| Outcomes | |||||
| Weight percentile | |||||
| Age 12 mo | Seventh percentile | <Third percentile | <Third percentile | 14th percentile | 50th percentile† |
| Age 18 mo | Sixth percentile | 13th percentile | <Third percentile | 34th percentile | Not available |
| Age 24 mo | Not available | Fourth percentile | <Third percentile | 45th percentile | Not available |
| Supplemental O2 use | |||||
| Age 12 mo | 1 LPM continuous | 0.5–1 LPM continuous | 2 LPM continuous | 1.0–1.5 LPM continuous | 0.5–0.75 LPM continuous† |
| Age 18 mo | 1 LPM continuous | 0.5–1 LPM sleep only | 1.5 LPM continuous | 0.5–1 LPM continuous | Not available |
| Age 24 mo | Not available | Room air (25 mo) | 1 LPM continuous | 0.5–1 LPM continuous | Not available |
Definition of abbreviations: GERD = gastroesophageal reflux disease; LPM = liters per minute; PDA = patent ductus arteriosus.
Represents initial pulmonary contact.
Denotes data at 9 months of age.
The diagnosis of NEHI was made 1–2 months after the initial pulmonary consultation. Table 2 describes the diagnostic studies performed. Empiric corticosteroid therapy was not initiated before pulmonary evaluations. All infants were treated for gastroesophageal reflux based on either clinical assessment or impedance studies. Evaluation for dysphagia was performed before proceeding to chest CT scan in all cases. Mild to moderate oropharyngeal dysphagia was identified in three patients, although respiratory symptoms persisted after feeding concerns were addressed.
Table 2.
Diagnostic evaluations
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Sweat chloride | Normal | Normal | Normal | Normal | Not performed |
| Bronchoscopy | |||||
| Anatomy | Normal | Normal | Normal | Normal | Normal |
| bronchoalveolar lavage culture | No growth | No growth | No growth | Moraxella Catarrhalis | No growth |
| Lipid laden macrophages | Normal | Normal | Normal | Elevated | Normal |
| Video swallow study | Normal | Abnormal* | Abnormal* | Normal | Abnormal* |
| Impedance study | Normal | Abnormal | Abnormal | Not performed | Not performed |
| Genetic testing for surfactant disorders | Not performed | Not performed | Not performed | Negative for SFPTC, ABCA3, and NKX2.1/TTF1 | Not performed |
| Echocardiogram | Normal | Normal | Normal | Small PDA | Normal |
| Initial chest computed tomography findings | Very subtle mosaic attenuation in geographic pattern to suggest NEHI | Subtle GGO in pattern consistent with NEHI; + air-trapping | Subtle mosaic attenuation in pattern consistent with NEHI; + air-trapping | Relatively diffuse GGO, most prominent centrally and in RML and lingula | GGO, most prominent centrally and in RML and lingula; + air-trapping |
| Subsequent chest computed tomography findings (age, findings) | 18 mo: highly consistent with NEHI | Not performed | Not performed | 16 mo: more consistent with NEHI | Not performed |
Definition of abbreviations: GGO = ground glass opacity; NEHI = neuroendocrine hyperplasia of infancy; PDA = patent ductus arteriosus; RML = right middle lobe.
For patients 2 and 5, a video swallow study revealed mild oropharyngeal dysphagia with intermittent laryngeal penetration, but no aspiration. Patient 3 had moderate oropharyngeal dysphagia with one episode of trace aspiration. Of note, patient 3 was exclusively breast fed at the time of study and had difficulties taking a bottle.
Chest CT findings included a variable extent of geographic ground-glass opacities and evidence of air-trapping in some cases. Initial CT scans were highly consistent for NEHI in three cases (Figure 1) and were moderately consistent in the other two, based on either more diffuse ground glass opacity or very subtle findings. Repeat imaging approximately 1 year later in these two cases was supportive of NEHI diagnosis, and lung biopsy was not performed. None of the patients had bronchiectasis or focal parenchymal findings.
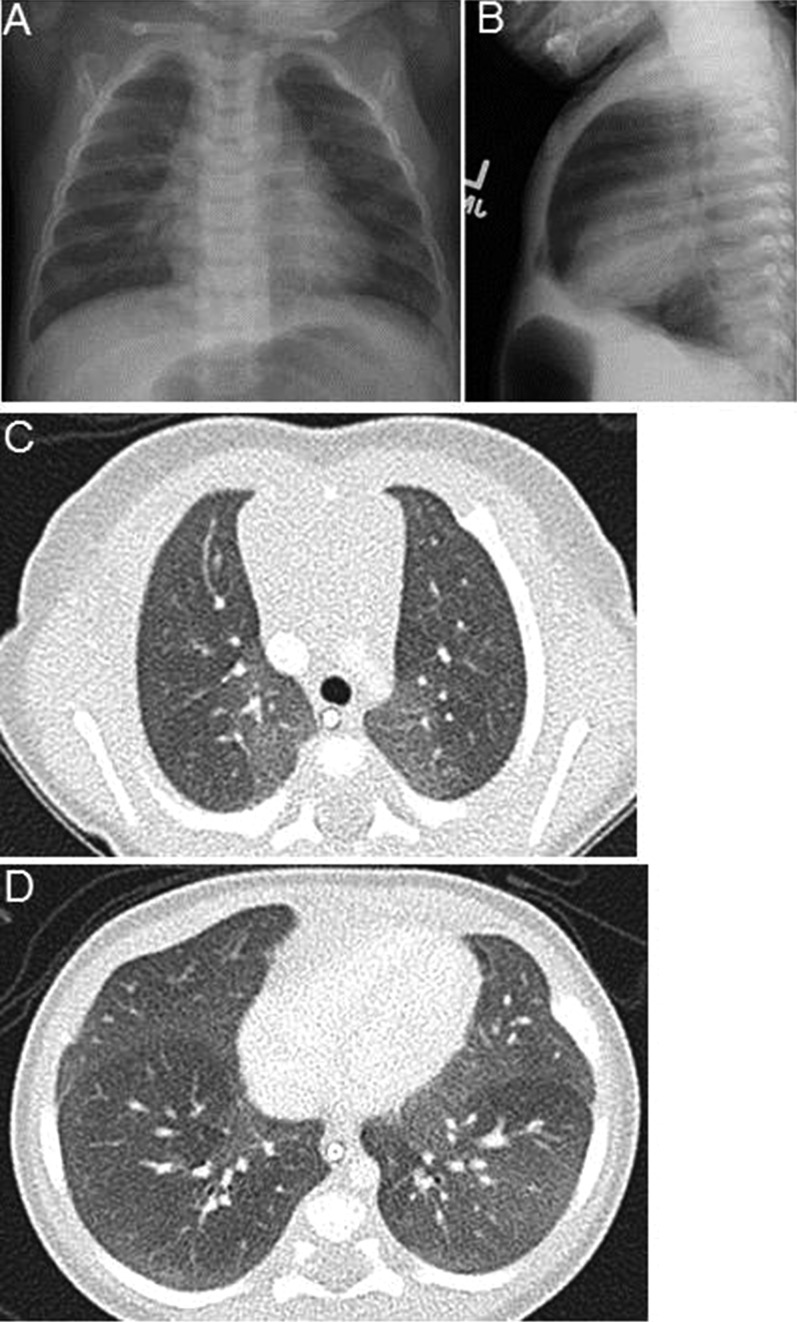
Figure 1.
Imaging findings in neuroendocrine hyperplasia of infancy cases. (A–B) Anterior-posterior and lateral chest radiographs at presentation at age 7 months for patient 4 show marked hyperinflation and mild peri-hilar opacities. (C–D) Chest computed tomography scan in patient 5 at age 1.5 months demonstrates ground glass opacities, which are most prominent centrally, and in the right middle lobe and lingula. No other significant architectural abnormalities are present.
Improvement in symptoms occurred at a variable pace. Patients 1–4 tolerated some reduction in the amount or duration of supplemental oxygen therapy by 18 months of age. Caloric supplementation was important for all individuals, with two requiring gastrostomy tube placement. Despite feeding tube placement, patient 3 continued to have difficulty with weight gain that was not clearly attributable to reflux or aspiration.
Discussion
Progress in rare diseases is often predicated on establishing sufficient numbers of well-phenotyped patients for study; awareness and timely diagnosis are challenges common to most rare diseases. During the last decade, great advances have been made in establishing diagnostic criteria for NEHI. However, there is no definitive therapy, and disease pathogenesis remains largely unknown. Pulmonary neuroendocrine cells function as oxygen sensors and chemoreceptors, serving complex roles in a variety of developmental and disease-associated contexts, with recent data suggesting genetic mechanisms may play a role in NEHI (9, 10).
Because most published reports derive from referral populations, we focused on five local cases to examine the early presentations, without the bias of prior pulmonary consultations. Several common themes emerged. First, infants displayed longstanding tachypnea, and two had previously been hospitalized for similar symptoms before pulmonary consultation. Second, failure to thrive was a key factor prompting further work-up, raising the possibility that milder cases were missed. Further, presence of gastroesophageal reflux and mild swallowing incoordination in some cases failed to account for the extent of respiratory symptoms or the nature of the CT image findings. Thus, although appropriate evaluation and clinical management of esophageal reflux or aspiration are prudent, our experience emphasizes that identification of such conditions should not exclude a diagnosis of NEHI. Prospective studies are needed to validate the sensitivity and specificity of the clinical features best used to identify NEHI.
Chest CT imaging diagnosis is increasingly the accepted diagnostic approach for NEHI. In the series by Brody and colleagues, the sensitivity of chest CT imaging for NEHI diagnosis was 78%, with specificity of 100%, compared with lung biopsy (3). Some centers have proposed infant pulmonary function tests as a diagnostic adjunct, although the specificity has not been systematically evaluated (5, 6). In our cases, NEHI diagnosis was ultimately achieved using clinical features and chest CT findings, without lung biopsy. In two cases, we had strong clinical suspicion for NEHI, but the initial chest CT images were not definitive. Our patients were managed with long-term supplemental oxygen, nutritional support as needed, and immunizations. Corticosteroids were not administered based on relative lack of scientific rationale or clinical experience to support their use in NEHI.
In summary, we speculate that NEHI is likely an under-recognized disorder. Our experience suggests that enhanced awareness led to increased case recognition in a short time frame, particularly among pediatric hospitalists and pulmonary trainees. We anticipate that more timely diagnosis will reduce the associated burden on both families and the health care system.
Footnotes
This project was supported by T32 GM07569 in clinical pharmacology (to M.G.O.),
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Deterding RR, Pye C, Fan LL, Langston C. Persistent tachypnea of infancy is associated with neuroendocrine cell hyperplasia. Pediatr Pulmonol. 2005;40:157–165. doi: 10.1002/ppul.20243. [DOI] [PubMed] [Google Scholar]
- 2.Deutsch GH, Young LR, Deterding RR, Fan LL, Dell SD, Bean JA, Brody AS, Nogee LM, Trapnell BC, Langston C, et al. Pathology Cooperative Group; ChILD Research Co-operative. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176:1120–1128. doi: 10.1164/rccm.200703-393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brody AS, Guillerman RP, Hay TC, Wagner BD, Young LR, Deutsch GH, Fan LL, Deterding RR. Neuroendocrine cell hyperplasia of infancy: diagnosis with high-resolution CT. AJR Am J Roentgenol. 2010;194:238–244. doi: 10.2214/AJR.09.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young LR, Brody AS, Inge TH, Acton JD, Bokulic RE, Langston C, Deutsch GH. Neuroendocrine cell distribution and frequency distinguish neuroendocrine cell hyperplasia of infancy from other pulmonary disorders. Chest. 2011;139:1060–1071. doi: 10.1378/chest.10-1304. [DOI] [PubMed] [Google Scholar]
- 5.Kerby GS, Wagner BD, Popler J, Hay TC, Kopecky C, Wilcox SL, Quinones RR, Giller RH, Accurso FJ, Deterding RR. Abnormal infant pulmonary function in young children with neuroendocrine cell hyperplasia of infancy. Pediatr Pulmonol. 2013;48:1008–1015. doi: 10.1002/ppul.22718. [DOI] [PubMed] [Google Scholar]
- 6.Kurland G, Deterding RR, Hagood JS, Young LR, Brody AS, Castile RG, Dell S, Fan LL, Hamvas A, Hilman BC, et al. American Thoracic Society Committee on Childhood Interstitial Lung Disease (chILD) and the chILD Research Network. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med. 2013;188:376–394. doi: 10.1164/rccm.201305-0923ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soares JJ, Deutsch GH, Moore PE, Fazili MF, Austin ED, Brown RF, Sokolow AG, Hilmes MA, Young LR. Childhood interstitial lung diseases: an 18-year retrospective analysis. Pediatrics. 2013;132:684–691. doi: 10.1542/peds.2013-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connor MG, Wurth MA, Young LR. NEHI in the house: pulmonary fellows’ experience in the diagnosis of NEHI. Am J Respir Crit Care Med. 2015;191:A4745. [Google Scholar]
- 9.Young LR, Deutsch GH, Bokulic RE, Brody AS, Nogee LM. A mutation in TTF1/NKX2.1 is associated with familial neuroendocrine cell hyperplasia of infancy. Chest. 2013;144:1199–1206. doi: 10.1378/chest.13-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popler J, Gower WA, Mogayzel PJ, Jr, Nogee LM, Langston C, Wilson AC, Hay TC, Deterding RR. Familial neuroendocrine cell hyperplasia of infancy. Pediatr Pulmonol. 2010;45:749–755. doi: 10.1002/ppul.21219. [DOI] [PubMed] [Google Scholar]