Abstract
Rationale: Rodent studies have shown that pyruvate dehydrogenase (PDH) levels are low in sepsis. This may cause cells to shift to anaerobic metabolism, resulting in increased lactate production. Alterations in PDH during sepsis have never been studied in humans.
Objectives: The objective of this pilot study was to measure PDH activity and quantity in patients with severe sepsis.
Methods: We conducted a pilot case–control study at a single urban tertiary care center. We compared PDH activity and quantity between patients with severe sepsis admitted to the intensive care unit and healthy control subjects. PDH activity and quantity were measured in isolated peripheral blood mononuclear cells. We measured PDH activity and quantity in control subjects at baseline and in patients with sepsis at 0 (baseline), 24, 48, and 72 hours.
Measurements and Main Results: We enrolled 56 patients with sepsis and 20 control subjects with at least one blood sample being drawn from each patient. PDH activity and quantity in the sepsis group were significantly lower than the control group (P < 0.001). In multivariable linear regression adjusting for age, race, sex, and assay plate, the difference remained significant. Patients with sepsis who died had significantly lower PDH activity compared with those who survived (P = 0.03).
Conclusions: PDH activity and quantity is decreased in peripheral blood mononuclear cells of humans with severe sepsis when compared with healthy control subjects, and may be associated with mortality. Whether decreased PDH activity plays a role in lactate metabolism or whether pharmacologic modification of PDH activity may improve outcomes remains unknown.
Keywords: mitochondria, metabolism, aerobic, lactate, mortality
Elevated lactate is common in sepsis and associated with increased mortality (1–3). Although often thought to be a marker of tissue hypoperfusion, the etiology may be multifactorial. Poor perfusion plays an important role, but alterations in cellular metabolism may be significant contributors as well (4, 5).
Pyruvate dehydrogenase (PDH) is a key enzyme in aerobic metabolism. PDH converts pyruvate, the end product of glycolysis, into acetyl-coenzyme A, which then enters the Krebs cycle. Excess pyruvate, due to inadequate PDH, PDH inhibition, or other causes, can alternatively be converted to lactate via anaerobic metabolism (5). This may result in an accumulation of lactate in settings where PDH activity or levels are suppressed.
Rodent studies have demonstrated that sepsis causes a reduction in the activity and quantity of PDH, which has, in turn, been associated with increased levels of lactate (6–8). However, no study has measured PDH activity and quantity during sepsis in humans. We therefore designed this study to measure PDH levels (activity and quantity) in humans with severe sepsis and compare them with levels in healthy control subjects.
Methods
Study Design
This was a pilot, prospective, observational study of patients presenting to an urban tertiary care center. The Institutional Review Board at Beth Israel Deaconess Medical Center (Boston, MA) approved the study. Written informed consent was obtained from all participants or their legally authorized surrogate.
Participant Selection
We enrolled a convenience sample of adult (age >18 years) patients with sepsis requiring admission to an intensive care unit. The diagnosis of sepsis was based upon systemic inflammatory response syndrome criteria and presumed or proven infection. Exclusion criteria included known or suspected pregnancy or inclusion in a concurrent interventional study (NCT01070810, NCT01948063). Patients with sepsis were recruited from August 2012 to May 2014. Healthy control subjects were recruited from the emergency department or the community via flyers from October 2012 to June 2014. Inclusion criteria for control subjects included no significant past medical history or acute illness and age over 30 years to ensure similar age distribution in both groups.
Blood Processing and Data Collection
Blood was drawn from patients with sepsis at enrollment (0 hour), and 24, 48, and/or 72 hours (± 2 hours), and from all control subjects upon enrollment; 95% of patients with sepsis were enrolled within 24 hours of intensive care unit admission (median, 2.6 hours [interquartile range, 0.3–9.2 hours]). All samples were run within 20 hours of collection, as we have demonstrated previously that measurements are comparable when obtained within this time frame. PDH activity, quantity, and specific activity were measured using an assay previously described by our group (9). In brief, peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood using the density gradient separation method (Ficoll-Paque premium; GE Healthcare Bio-Science Corp., Piscataway, NJ). Functional PDH was solubilized from each sample by adding a nondenaturing detergent (lauryl maltoside) to each sample. PDH activity was preserved by adding proteinase inhibitors. PDH was then immunocaptured within each well by PDH antibodies and subjected to functional activity and quantitative microplate assays (Abcam, Inc., Cambridge, MA).
PDH activity and quantity are expressed as the initial rate of reaction, determined from the different slopes of the curves generated. They were calibrated to the total amount of protein in the sample to allow for differences in total protein content, and are expressed in optical density per minute per milligram protein (9), and PDH-specific activity was calculated (PDH activity/ln[PDH quantity]).
Demographic data (including age, race, and sex) and comorbidities were collected from all participants. Additional data collected for patients with sepsis included vital signs, laboratory values, vasopressor requirement, severity of illness scores (all collected within 4 hours of enrollment), and mortality.
Statistical Analysis
Categorical variables are presented as counts and frequencies, and continuous variables with medians and first and third quartiles given non-normality of the data. Characteristics between groups were compared with Fisher’s exact test and the Wilcoxon rank sum test. Since PDH values were all severely right-skewed, they were log-transformed before analysis. Baseline PDH values for patients with sepsis were compared with control subjects using Student’s t test. To account for potential confounding we performed multivariable linear regression with adjustments for age, sex, race, and assay plate. We used Spearman’s correlation coefficient (ρ) to assess the association between baseline PDH and clinical variables (lactate and sequential organ failure assessment [SOFA] score). Lastly, we compared PDH values between survivors and nonsurvivors in the septic group using linear repeated measures analysis with an autoregressive variance–covariance structure. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). All hypothesis tests were two sided, with a significance level (P value) of 0.05 or less. As this was an exploratory pilot trial, no adjustments were made for multiple comparisons.
Results
A total of 56 patients with sepsis were enrolled. PDH values were obtained in 38 patients at enrollment, 33 at 24 hours, 20 at 48 hours, and 14 at 72 hours. Five patients had values measured at all time points. PDH values were measured in 20 control subjects. Baseline characteristics are described in Table 1. Median ages of the septic and control groups were 70 (60–78) years and 48 (41–58) years, respectively (P < 0.001). There were 22 (39%) females in the septic group and 15 (75%) in the control group (P = 0.01).
Table 1.
Patient characteristics
| Characteristics |
Patients with Sepsis (n = 56) |
Control Subjects |
|---|---|---|
| (n = 20) | ||
| Demographics | ||
| Age, median (quartiles), yr | 70 (60–78) | 48 (41–58) |
| Sex, female, n (%) | 22 (39) | 15 (75) |
| Race, n (%) | ||
| White | 50 (89) | 13 (65) |
| African American | 1 (2) | 4 (20) |
| Asian | 2 (4) | 3 (15) |
| Other/not reported | 3 (6) | 0 (0) |
| Past medical history, n (%) | ||
| Pulmonary diseases* | 9 (16) | — |
| Cardiac disease† | 16 (28) | — |
| Cancer | 12 (21) | — |
| Diabetes | 19 (33) | — |
| Renal disease | 8 (14) | — |
| Liver disease | 9 (16) | — |
| Admission data | ||
| Lactate, median (quartiles), mmol/L‡ | 1.9 (1.1–2.6) | — |
| Lactate > 4 mmol/L, n (%)‡ | 6 (11) | — |
| APACHE II score, median (quartiles) | 14 (9–18) | — |
| SOFA score, median (quartiles) | 3 (1–6) | — |
| Vasopressor requirement, n (%) | 13 (23) | — |
| Mortality, n (%) | 12 (21) | — |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; SOFA = sequential organ failure assessment.
Includes chronic obstructive pulmonary disease and asthma.
Includes coronary artery disease and congestive heart failure.
Lactate levels were missing on seven patients.
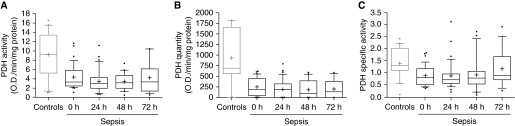
Patients with sepsis had significantly lower baseline PDH activity (3.3 [2.5–5.8] versus 9.2 [5.9–13.4]; P < 0.001), quantity (184 [5.4–307] versus 691 [573–1,642]; P = 0.01), and specific activity (0.8 [0.5–1.1] versus 1.3 [1.1–2.0]; P = 0.04) as compared with control subjects (Figure 1). The significant difference remained when adjusting for age, sex, race, and assay plate for activity (P = 0.01), but not for quantity (P = 0.20) or specific activity (P = 0.09). In the septic cohort, we found no difference over time in PDH activity (P = 0.52), quantity (P = 0.90), or specific activity (P = 0.23).
Figure 1.
Pyruvate dehydrogenase (PDH) values in patients with sepsis and control subjects. PDH activity (A), quantity (B), and specific activity (C) in control subjects and patients with sepsis at enrollment (0 hours) and 24, 48, and 72 hours thereafter. Compared with baseline values in the septic cohort, control subjects had significantly higher activity, quantity, and specific activity.
We found an inverse association between baseline lactate and PDH activity (ρ = −0.35; P = 0.05), but no significant association with quantity or specific activity. There was an inverse relationship between initial SOFA score and PDH quantity (ρ = −0.25; P = 0.03), but no significant association between SOFA score and activity or specific activity.
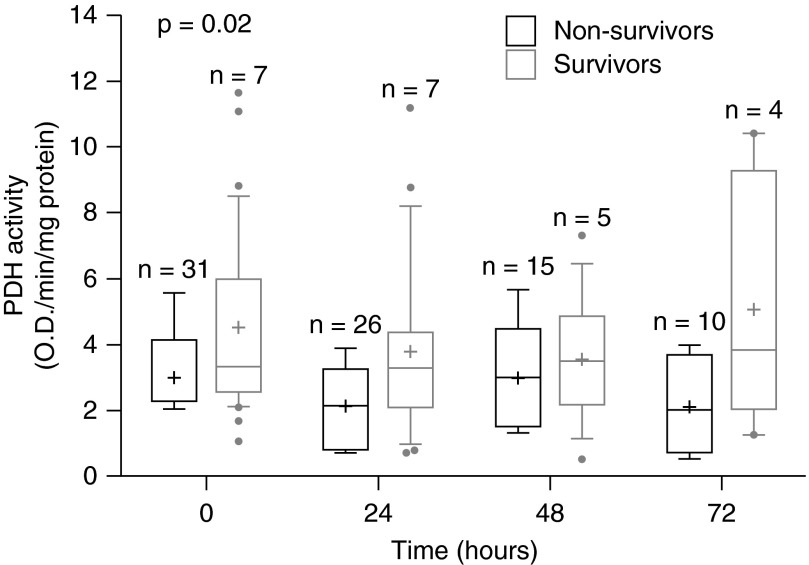
Survivors had significantly higher PDH activity as compared with nonsurvivors when analyzing all time points (61% higher [9–140%]; P = 0.02, Figure 2). This difference remained significant when adjusting for age, sex, race, and assay plate (P = 0.01). There was no significant difference in unadjusted values between survivors and nonsurvivors for quantity (207% higher [−24 to 1,145%]; P = 0.11) or specific activity (27% higher [−8 to 74%]; P = 0.14). However, the differences became significant for both quantity (P = 0.05) and specific activity (P = 0.01) after adjusting for age, sex, race, and assay plate. Of the variables chosen a priori for the multivariate analysis, only assay plate appeared to have an effect on PDH levels.
Figure 2.
Pyruvate dehydrogenase (PDH) activity in survivors and nonsurvivors. PDH activity in the septic cohort stratified by survival status. Patients who survived had significantly higher PDH activity over time as compared with those not surviving (P = 0.02).
Discussion
In this study, we found that PDH levels were significantly lower in PBMCs of patients with sepsis than in healthy control subjects, and this depression of PDH levels persisted for 72 hours. Furthermore, PBMCs in patients with sepsis who survived had significantly higher PDH levels in adjusted analysis compared with those who did not survive.
This is the first human study, to our knowledge, that confirms findings in rat models of sepsis. In a series of studies, Vary and colleagues (10) found levels of the active form of the PDH complex in rat skeletal muscle decreased by 70% in sepsis, and that this reduction persisted up to 7 days (7). Similarly, Alamdari and colleagues (8) demonstrated a 65% decrease in PDH activity 24 hours after induction of sepsis. To elucidate a possible mechanism, Alamdari and colleagues showed an increase in the inactive form of PDH preceded by marked increases in inflammatory markers (including TNF and IL-6) in sepsis. Vary and colleagues provided further support for this relationship by demonstrating that infusions of a protein that binds TNF resulted in higher active PDH levels than in control subjects with sepsis not given the TNF inhibitor (10). Looking more globally at mitochondrial dysfunction, Karamercan and colleagues (11) found that traumatic stress resulted in reduced mitochondrial oxygen consumption (a surrogate of mitochondrial function) in all tissues, including PBMCs.
Rat models of sepsis have also demonstrated that, in sepsis, lower PDH is associated with increased lactate, and that, by blocking the sepsis-associated inhibition of PDH activity, lactate levels can be lowered (10). We also found an inverse correlation between PDH activity and lactate, suggesting that PDH activity may be a clinically relevant parameter. Prior human studies have explored dichloroacetate, a medication that inhibits the conversion of active PDH to inactive PDH, in the treatment of patients with lactic acidosis. In one randomized, controlled trial, administration of dichloroacetate significantly reduced lactate levels and improved pH, but was not associated with a difference in hemodynamics or mortality (12). This study was done more than 20 years ago, however, and there have been considerable changes to critical care since. Dichloroacetate is also known to have toxicities (13), and whether other means of increasing PDH activity in the present-day septic population would be beneficial warrants exploration.
Thiamine, a cofactor necessary for PDH complex activity, may play such a role in altering PDH activity in sepsis. Thiamine deficiency is not uncommon in sepsis (14, 15), and previous case reports have found that administering thiamine to critically ill thiamine-deficient patients leads to rapid reversal of elevated lactate, as well as improvement in clinical symptoms (16–18). PDH and thiamine levels have also been found to fall with the physiologic stress of coronary artery bypass surgery in a small observational study in humans (19). Our current data demonstrating that PDH levels are low in humans in sepsis offer further support for studies into the use of thiamine as a means to reverse lactic acidosis and improve mortality in sepsis.
This was a small, exploratory, hypothesis-generating study with significant limitations, and further, larger studies are required to confirm our findings. PDH levels were measured in PBMCs as opposed to other tissues, due to the practicalities of conducting trials in humans. To our knowledge, no data on humans exist demonstrating a correlation between PDH levels in PBMCs with PDH levels in other tissues during sepsis, nor is it clear to what degree PDH levels in PBMCs would influence systemic levels of lactate. We also do not know if numbers of viable PBMCs in the septic compared with the healthy state could contribute to differences in PDH. Despite these limitations, this work demonstrates that PDH is detectable in PBMCs, and that levels are different in patients with sepsis and healthy counterparts, providing a potentially useful research tool.
Missing data is another limitation. Reasons for missing measurements included lack of laboratory personnel availability to process samples, competing studies, or patient refusal of subsequent blood draws. Due to the lack of a standardized control, our PDH assay was complicated by variability between plates. We adjusted for this both by including subjects with sepsis and control subjects on the same plate, to act as internal controls, and by including plate number in our multivariate analysis. However, there was variability even within plates; there was large variability particularly in quantity measurements (and, therefore, specific activity measurements). This level of variation in the PDH quantity and specific activity may account for the nonsignificant relationship found between these measures and control subjects and lactate and SOFA scores. Our results may also have been weakened by the overall low lactate levels in our septic population; on enrollment, only half of patients had a lactate greater than 2 mmol/ml, and only approximately 1/10 of patients had a lactate greater than 4 mmol/ml.
We were unable to adjust for comorbidities in patients with sepsis due to the number of comorbidities and the small study size, and thus are not able to establish whether differences in PDH levels are attributable to the septic state alone, or also to underlying comorbidities. In addition, PDH activity may be decreased in other inflammatory conditions or critical illness states, which was not addressed by the current study.
Conclusions
PDH levels are significantly lower in human PBMCs during sepsis when compared with healthy control subjects, and may be associated with mortality. Whether pharmacologic modification of PDH activity may improve outcomes remains unknown.
Footnotes
Supported by NHLBI grant 1K02HL107447-01A1 (M.W.D.) and American Heart Association grants 14GRNT200100002, 13CRP16930000, and 15SDG22420010 (M.W.D., K.M.B., and M.N.C.).
Author Contributions: E.N.—participation in study design, data analysis, manuscript writing; K.M.B.—oversight of study design and data analysis, manuscript writing; L.W.A.—data analysis, assistance with study design, and substantive edits of all manuscript drafts; S.M.—assistance with pyruvate dehydrogenase (PDH) assay and analysis, data collection, editing of final manuscript; J.B.—enrollment of patients, data collection, intellectual input, editing of final manuscript; M.N.C.—input into study design and planning, substantive contribution, and editing of the manuscript; X.L.—senior laboratory author (design and execution of PDH assays, data collection and analysis, editing of all drafts of manuscript); M.W.D.—senior clinical author (oversight of study conception and design, data analysis, and substantive input to all drafts of manuscript).
Originally Published in Press as DOI: 10.1513/AnnalsATS.201505-267BC on September 10, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Broder G, Weil MH. Excess lactate: an index of reversibility of shock in human patients. Science. 1964;143:1457–1459. doi: 10.1126/science.143.3613.1457. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, Weiss JW. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524–528. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33:1892–1899. doi: 10.1007/s00134-007-0680-5. [DOI] [PubMed] [Google Scholar]
- 4.Luft FC. Lactic acidosis update for critical care clinicians. J Am Soc Nephrol. 2001;12:S15–S19. [PubMed] [Google Scholar]
- 5.Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88:1127–1140. doi: 10.1016/j.mayocp.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vary TC. Increased pyruvate dehydrogenase kinase activity in response to sepsis. Am J Physiol. 1991;260:E669–E674. doi: 10.1152/ajpendo.1991.260.5.E669. [DOI] [PubMed] [Google Scholar]
- 7.Vary TC. Sepsis-induced alterations in pyruvate dehydrogenase complex activity in rat skeletal muscle: effects on plasma lactate. Shock. 1996;6:89–94. doi: 10.1097/00024382-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Alamdari N, Constantin-Teodosiu D, Murton AJ, Gardiner SM, Bennett T, Layfield R, Greenhaff PL. Temporal changes in the involvement of pyruvate dehydrogenase complex in muscle lactate accumulation during lipopolysaccharide infusion in rats. J Physiol. 2008;586:1767–1775. doi: 10.1113/jphysiol.2007.149625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Pervez H, Andersen LW, Uber A, Montissol S, Patel P, Donnino MW. Immunocapture and microplate-based activity and quantity measurement of pyruvate dehydrogenase in human peripheral blood mononuclear cells. Bioanalysis. 2015;7:583–592. doi: 10.4155/bio.14.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vary TC, Hazen SA, Maish G, Cooney RN. TNF binding protein prevents hyperlactatemia and inactivation of PDH complex in skeletal muscle during sepsis. J Surg Res. 1998;80:44–51. doi: 10.1006/jsre.1998.5324. [DOI] [PubMed] [Google Scholar]
- 11.Karamercan MA, Weiss SL, Villarroel JP, Guan Y, Werlin E, Figueredo R, Becker LB, Sims C. Can peripheral blood mononuclear cells be used as a proxy for mitochondrial dysfunction in vital organs during hemorrhagic shock and resuscitation? Shock. 2013;40:476–484. doi: 10.1097/SHK.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stacpoole PW, Wright EC, Baumgartner TG, Bersin RM, Buchalter S, Curry SH, Duncan CA, Harman EM, Henderson GN, Jenkinson S, et al. The Dichloroacetate-Lactic Acidosis Study Group. A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. N Engl J Med. 1992;327:1564–1569. doi: 10.1056/NEJM199211263272204. [DOI] [PubMed] [Google Scholar]
- 13.Chu QS, Sangha R, Spratlin J, L JV, Mackey JR, McEwan AJ, Venner P, Michelakis ED. A phase I open-labeled, single-arm, dose-escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors. Invest New Drugs. 2015;33:603–610. doi: 10.1007/s10637-015-0221-y. [DOI] [PubMed] [Google Scholar]
- 14.Donnino MW, Carney E, Cocchi MN, Barbash I, Chase M, Joyce N, Chou PP, Ngo L. Thiamine deficiency in critically ill patients with sepsis. J Crit Care. 2010;25:576–581. doi: 10.1016/j.jcrc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Manzanares W, Hardy G. Thiamine supplementation in the critically ill. Curr Opin Clin Nutr Metab Care. 2011;14:610–617. doi: 10.1097/MCO.0b013e32834b8911. [DOI] [PubMed] [Google Scholar]
- 16.Amrein K, Ribitsch W, Otto R, Worm HC, Stauber RE. Severe lactic acidosis reversed by thiamine within 24 hours. Crit Care. 2011;15:457. doi: 10.1186/cc10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giacalone M, Martinelli R, Abramo A, Rubino A, Pavoni V, Iacconi P, Giunta F, Forfori F. Rapid reversal of severe lactic acidosis after thiamine administration in critically ill adults: a report of 3 cases. Nutr Clin Pract. 2015;30:104–110. doi: 10.1177/0884533614561790. [DOI] [PubMed] [Google Scholar]
- 18.Duca J, Lum CJ, Lo AM.Elevated lactate secondary to gastrointestinal beriberi J Gen Intern Med(In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen LW, Liu X, Peng TJ, Giberson TA, Khabbaz KR, Donnino MW. Pyruvate dehydrogenase activity and quantity decreases after coronary artery bypass grafting: a prospective observational study. Shock. 2015;34:250–254. doi: 10.1097/SHK.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]