Abstract
Rationale: Experimental and epidemiological evidence suggests that neutrophils are important in the host response to tuberculosis. HIV infection, which increases the risk of tuberculosis, adversely affects neutrophil function.
Objectives: To determine the impact of HIV and antiretroviral therapy on neutrophil antimycobacterial activity.
Methods: We performed a cross-sectional comparison of neutrophil functions in 20 antiretroviral-naive HIV-infected and 20 HIV-uninfected individuals using luminescence-, flow cytometry–, and ELISA-based assays. We then conducted a prospective study in the HIV-infected individuals investigating these parameters during the first 6 months of antiretroviral therapy. Surface markers of neutrophil activation were investigated in a separate cohort using flow cytometry.
Measurements and Main Results: HIV infection impaired control of Mycobacterium tuberculosis by neutrophils (mean ratio of mycobacterial luminescence in neutrophil samples vs. serum controls at 1 hour in HIV-infected participants, 0.88 ± 0.13 vs. HIV-uninfected participants, 0.76 ± 0.14; P = 0.01; at 24 hours, 0.82 ± 0.13 vs. 0.71 ± 0.13; P = 0.01). The extent of impairment correlated with log[HIV viral load]. Neutrophil cell death after 24 hours’ incubation with M. tuberculosis was higher in the HIV-infected cohort (85.3 ± 11.8% vs. 57.9 ± 22.4% necrotic cells; P < 0.0001). Neutrophils from HIV-infected participants demonstrated significantly more CD62L-negative cells (median, 23.0 vs. 8.5%; P = 0.008) and CD16-negative cells (3.2 vs. 1.3%, P = 0.03). Antiretroviral therapy restored mycobacterial restriction and pattern of neutrophil death toward levels seen in HIV-uninfected persons.
Conclusions: Neutrophils in antiretroviral-naive HIV-infected persons are hyperactivated, eliminate M. tuberculosis less effectively than in HIV-uninfected individuals, and progress rapidly to necrotic cell death. These factors are ameliorated by antiretroviral therapy.
Keywords: tuberculosis, granulocyte, necrosis
HIV killed 1.5 million people and tuberculosis killed 1.1 million in 2013, including 360,000 with both infections (1, 2). Relatively little literature exists on the roles of neutrophils in the human host response to either pathogen. In particular, despite the crucial role played by these phagocytes in tuberculosis, as established both experimentally (3) and epidemiologically (4), and despite the close interrelation of the HIV and tuberculosis pandemics (5), no studies have previously investigated how HIV impacts neutrophil responses to mycobacteria. Data from investigators using other organisms or in vitro models suggest that neutrophils are dysfunctional in HIV-infected persons: their activation, respiratory burst, phagocytosis, microbial killing capacity, and cell death have all been described to be adversely affected (6–11).
It has been demonstrated that this impact of HIV infection on neutrophils is greatest in those with the highest level of viremia (9) or lowest number of CD4+ T cells (8). We therefore recruited HIV-infected patients who were due to start antiretroviral therapy (ART; CD4 count < 350 × 103/ml, according to South African Department of Health criteria). We aimed to compare their neutrophil antimycobacterial functions to those of HIV-uninfected control subjects from the same community in a case-control study. Many neutrophil functions have been described to improve with antiretroviral treatment (8, 9, 12), and therefore we also conducted a longitudinal study tracking the responses of HIV-infected patients over the first 6 months of therapy. Finally, a separate cohort of HIV-infected and HIV-uninfected persons was recruited to assess activation status of peripheral blood neutrophils.
Some of the results of these studies have been previously reported in the form of an abstract (13).
Methods
Patients and Recruitment
HIV-infected patients were recruited from the Ubuntu Site B clinic in Khayelitsha, South Africa if they were eligible for (CD4 count < 350 × 103/ml) and agreed to commence antiretroviral therapy. Control patients were recruited from Ubuntu or the Khayelitsha Youth Centre among people who had recently tested negative for HIV.
Ethics
Studies were approved by the University of Cape Town Research Ethics Committee (HREC 545/2010), and participants provided written informed consent.
Organisms and Labeling
The plasmid construction and electroporation of organisms has been described previously (14). Organisms were defrosted and grown to mid-log phase (72 h) before use. The growth, luminescence measurement, and fluorescein isothiocyanate (FITC) labeling of the organisms has been described previously (15, 16). Further details are included in the online supplement.
Cell Depletion and Neutrophil Isolation
CD15 is a carbohydrate adhesion molecule highly expressed on granulocytes; it has previously been described that positive selection via anti-CD15 conjugated magnetic beads is the optimal method to isolate granulocytes for functional studies in terms of purity and function (17), and we therefore used this approach.
For depletion experiments, blood was drawn into heparinized tubes, divided into aliquots, and incubated with 50 μl/ml anti-CD15 or Basic (unconjugated) Miltenyi MicroBeads at 2 to 8°C for 15 minutes, then diluted 1:1 with RPMI-1640 and pipetted into preprimed Miltenyi Biotec LS columns resting in magnets (MidiMACS Separation Unit, Miltenyi Biotec) (15). Depleted blood was collected, and CD15+ cells were recovered from the column, counted, and diluted if necessary with further RPMI-1640 to reach the required final concentration.
Isolated Neutrophil Lux Assay
This assay has been previously described in detail (15). Briefly, 400 μl of 1 × 106/ml granulocytes were infected with Mycobacterium tuberculosis-lux at a multiplicity of infection approximately 1 cfu:3 neutrophils in the presence of 10% autologous serum. Serum control samples were prepared with 400 μl RPMI-1640, 50 μl serum, and 50 μl organism suspension. Sample luminescence was measured after 1 and 24 hours’ incubation at 37°C (neutrophils are expected to act rapidly and the 1-h measurement aims to assess this; the 24-h measurement confirms that any restriction of mycobacterial metabolism persists after significant neutrophil cell death and does not therefore simply represent early phagocytosis [15]). Results were calculated as the ratio of luminescence in neutrophil samples versus autologous serum controls.
Whole Blood M. tuberculosis Lux Assay
This has been described in full previously (16, 18). Briefly, approximately 5 × 105 cfu M. tuberculosis-lux at mid-log phase in 100 μl phosphate buffered saline was inoculated into triplicate samples of 900 μl cell-depleted or Basic MicroBead-treated blood already diluted 1:1 with RPMI, as described above. Samples were incubated at 37°C on a rocking platform, and mycobacterial luminescence was measured after harvesting of assay supernatants in samples at 96 hours.
Phagocytosis Assay
This has been described in detail previously (15). Briefly, 400 μl of 1 × 106/ml granulocytes were infected with FITC-labeled M. tuberculosis-lux at a multiplicity of infection approximately 1 cfu:3 neutrophils in the presence of 10% autologous serum. Samples were processed after 30 minutes’ incubation as described (15).
Surface Activation Marker Flow Cytometry
Standard fluorochrome labeling techniques were applied to whole blood, as further detailed in the online supplement. The gating strategy is described later.
Measurement of CD4 Count, HIV Viral Load, Full Blood Count, and C-Reactive Protein
These were performed by the South African National Health Laboratory Service by flow cytometry, polymerase chain reaction, and nephelometry, respectively.
Cell Death Assay
Samples were prepared as per the neutrophil lux assay and incubated at 37°C for 24 hours. Samples were processed and analyzed similarly to assays previously described (19). We undertook two analyses, one on phenotypic “granulocytes” (defined by forward and side scatter) and the other on CD66a,c,e-positive events. CD66 receptors are involved in cell adhesion, migration, and activation of signaling pathways; they are highly expressed on granulocytes from late stages of bone marrow development but not significantly on other hematological cells (20). CD66 is also up-regulated rather than shed on activation (21). We thus interpreted CD66-positive events as granulocyte related, although they were not necessarily phenotypic granulocytes according to forward and side scatter. Further details are included in online supplement, and the flow cytometry analysis is shown in Figure E3 in the online supplement.
Human Neutrophil Peptide 1-3 ELISA and Quantiferon Gold In-Tube Assays
Human neutrophil peptide (HNP) 1-3 ELISA (Hycult Biotech, Uden, the Netherlands) and Quantiferon Gold In-Tube assays (Cellestis/Qiagen, Victoria, Australia) were performed according to manufacturers’ instructions.
Statistics
Parametric data (normality assessed using Kolmogorov-Smirnov test) were analyzed using Student t tests for two groups (paired when appropriate) or one-way analysis of variance for three or more groups with post hoc correction. Nonparametric data were analyzed using Mann-Whitney U tests or Wilcoxon signed rank tests for two groups and Kruskal-Wallis testing with Dunn correction for three or more groups. Proportions were compared with Chi-square tests. Correlations were performed using Pearson methodology.
All statistical analyses were performed using SPSS v18.0 (SPSS Inc, Chicago, IL) or GraphPad Prism v4.0. All reported P values are two-sided; P less than 0.05 was inferred as significant.
Results
Participant Characteristics and Response to Antiretroviral Therapy
Twenty-two HIV-infected and 20 HIV-uninfected participants were included in the case-control study (see Figure E1 in the online supplement). Two HIV-infected patients had a positive sputum culture for M. tuberculosis at study entry and were excluded from statistical analyses according to the study protocol. Baseline demographic and clinical characteristics of HIV-infected patients and HIV-uninfected control subjects are detailed in Table 1. The only difference between groups was in weight, with the HIV-uninfected participants being heavier (median weight, 80.6 kg vs. 65.4 kg, P = 0.004).
Table 1.
Participant characteristics at enrollment
| HIV-infected (n = 20) | HIV-uninfected (n = 20) | ||
|---|---|---|---|
| Age, yr | Median | 30 | 24.5 |
| Range | 23–70 | 18–47 | |
| Ethnicity | Xhosa | 19 (95) | 20 (100) |
| Zimbabwean | 1 (5) | 0 (0) | |
| Sex | Male | 4 (20) | 8 (40) |
| Female | 16 (80) | 12 (60) | |
| Weight, kg | Median | 65.4 | 80.6 |
| Range | 50.1–82.7 | 51.5–121.1 | |
| Current smoking | Yes | 1 (5) | 4 (20) |
| No | 19 (95) | 16 (80) | |
| Regular alcohol* | Yes | 3 (15) | 6 (30) |
| No | 17 (85) | 14 (70) | |
| BCG | Yes | 15 (75) | 12 (60) |
| No | 0 (0) | 5 (25) | |
| Unsure/unclear | 5 (25) | 3 (15) | |
| Comorbidity† | Yes | 9 (45) | 5 (25) |
| No | 11 (55) | 15 (75) | |
| Cotrimoxazole prophylaxis | Yes | 16 (80) | N/A |
| No | 4 (20) | ||
| Vitamin treatment | Vitamin B Co-Strong | 18 (90) | N/A |
| Vitamin C | 5 (25) | ||
| Other medication in previous 3 mo‡ | Yes | 6 (30) | 3 (15) |
| No | 14 (70) | 17 (85) | |
| CD4 count, ×106/L | Median | 209.5 | N/A |
| Range | 22–498 | ||
| Log [HIV viral load (copies/ml)] | Median | 4.90 | N/A |
| Range | 3.45–6.06 | ||
| IFN-γ release assay result | Positive | 6 (30) | 10 (50) |
| Negative | 14 (70) | 10 (50) | |
| Blood neutrophil count, ×109/L | Median | 2.50 | 2.60 |
| Range | 1.05–5.73 | 1.40–5.01 | |
| Serum C-reactive protein, mg/L | Median | 2.0 | 1.75 |
| Range | <1–8.7 | <1–44.2 | |
Definition of abbreviations: BCG = Mycobacterium bovis-Bacille Calmette-Guerin; N/A = not applicable.
Data presented as n (%) unless otherwise noted.
Defined as at least once per week.
Identified comorbidities: chronic sinusitis, asthma, hypertension, diet-controlled diabetes mellitus, oral candidiasis, previous psychotic episode, chronic mildly increased alanine transaminase of uncertain cause, recent diarrhea, anemia, depression.
Identified medications: salbutamol inhaler, amitriptyline, ferrous sulfate, folic acid, loperamide, nystatin, hydrochlorothiazide, other antihypertensives (unknown classes).
Of the 20 HIV-infected participants who commenced the longitudinal study, one was lost to follow-up after the first visit and one after the 3-month visit. The remaining 18 patients attended all visits. As shown in Figure 1A, most patients suppressed viral replication by 6 months, such that HIV was below the limit of detection (40 copies/ml) in blood. One patient had not suppressed, but the level was declining (111 copies/ml); another patient achieved suppression by 3 months but then lost control (9,983 copies/ml) due to confusion with medication. Median (interquartile range, IQR) CD4 count improved from 209.5 (125.5–295.5) × 103/ml to 322.5 (203.5–483.5) × 103/ml (P = 0.002), and median IQR CD4 percentage improved from 14.0% (9.3–18.4%) to 24.7% (15.5–30.8%) (P < 0.001; Figures 1B and 1C).
Figure 1.
The impact of HIV and antiretroviral therapy on neutrophil antimycobacterial activity. (A–C) Twenty antiretroviral-naive HIV-infected patients were recruited and followed for 6 months of therapy (n = 19 after baseline time point, n = 18 after Month 3). Blood was taken at baseline, 1 month, 3 months, and 6 months for determination of HIV viral load (A), CD4 count (B), and percentage (C). **P < 0.01, ***P < 0.001 (Wilcoxon signed rank test versus baseline). (D) A total of 4 × 105 CD15+ granulocytes in RPMI-1640 were incubated with 10% autologous serum and M. tuberculosis-lux. Control samples did not contain neutrophils. Mycobacterial luminescence was measured for 20 seconds after 1 hour of incubation at 37°C, and the ratio of relative light units (RLU) in neutrophil samples versus serum controls is presented. Lines represent means. (E) Data from the HIV-infected individuals in D are correlated to log[HIV viral load]. R = 0.55 (95% CI, 0.13–0.80), P = 0.015. The dotted line represents a ratio of 1 (no impact of neutrophils vs. serum controls). (F) Samples were processed as in D at the indicated intervals over 6 months of antiretroviral therapy in HIV-infected individuals; data from HIV-uninfected individuals are presented for comparison. Lines represent means; dotted line indicates ratio of 1 (no impact of neutrophils vs. serum controls).
Control of M. tuberculosis by Human Neutrophils Is Impaired by HIV Infection in a Viral Load–dependent Fashion and Improves with ART
To determine the influence of HIV and ART on neutrophil antimycobacterial activity, we used our recently described luminescence assay (15). The primary readouts are the ratios between samples containing cells versus serum-only controls at 1 hour and 24 hours; a lower ratio indicates greater restriction of mycobacterial luminescence by neutrophils.
Potential correlates of the 1-hour and 24-hour ratios of mycobacterial luminescence between neutrophil samples and serum controls are summarized in Table 2. HIV infection had a significant negative impact on mycobacterial control by neutrophils at both time points (mean ratio of mycobacterial luminescence in neutrophil samples vs. serum controls at 1 hour in HIV-infected patients, 0.88 ± 0.13; in HIV-uninfected patients, 0.76 ± 0.14; P = 0.01; at 24 hours, 0.82 ± 0.13 and 0.71 ± 0.13, respectively; P = 0.01). There was a strong correlation between the 1-hour and 24-hour cell:serum luminescence ratios across HIV-infected and HIV-uninfected participants (r = 0.64, P < 0.0001).
Table 2.
Potential correlates of neutrophil antimycobacterial activity
| Correlate | Mean Ratio of Luminescence in Neutrophil Samples vs. Serum Controls at 1 h | Univariate P Value | Mean Ratio of Luminescence in Neutrophil Samples vs. Serum Controls at 24 h | Univariate P Value | |
|---|---|---|---|---|---|
| Sex | Male | 0.79 | 0.52 | 0.75 | 0.53 |
| Female | 0.83 | 0.78 | |||
| BCG | Yes | 0.80 | 0.68 | 0.77 | 0.93 |
| No | 0.88 | 0.73 | |||
| Unsure/unclear | 0.81 | 0.78 | |||
| IGRA | Positive | 0.79 | 0.42 | 0.73 | 0.16 |
| Negative | 0.83 | 0.79 | |||
| Smoking | Yes | 0.75 | 0.29 | 0.69 | 0.18 |
| No | 0.83 | 0.78 | |||
| Regular alcohol | Yes | 0.89 | 0.11 | 0.79 | 0.54 |
| No | 0.79 | 0.76 | |||
| Comorbidity | Yes | 0.79 | 0.54 | 0.74 | 0.47 |
| No | 0.83 | 0.78 | |||
| HIV status | Positive | 0.88 | 0.01 | 0.82 | 0.01 |
| Negative | 0.76 | 0.71 | |||
| |
|
|
|||
| |
Pearson r (correlation with ratio of luminescence in neutrophil samples vs. serum controls at 1 h) | ||||
| Age |
0.02 | 0.90 | 0.17 | 0.31 | |
| Weight |
−0.33 | 0.04 | −0.12 | 0.94 | |
| Serum C-reactive protein | −0.28 | 0.09 | −0.08 | 0.64 | |
Definition of abbreviations: BCG = Mycobacterium bovis–Bacille Calmette-Guérin; IGRA = IFN-γ release assay.
Bold type indicates statistical significance (P < 0.05).
Figure 1D presents the 1-hour cell:serum mycobacterial luminescence ratios. These ratios also correlated positively with log[HIV viral load] (Pearson r = 0.55, P = 0.02; Figure 1E) in ART-naive participants, suggesting that the degree of neutrophil dysfunction is dependent on the level of viremia. A weaker inverse relationship was seen between CD4 count and 1-hour cell:serum ratio (Pearson r = −0.47, P = 0.04).
During their initial 6 months of ART, HIV-infected participants’ neutrophils improved their restriction of M. tuberculosis-lux bioluminescence versus serum control samples at 1 hour’s incubation, with a significant linear trend in results (P = 0.03; Figure 1F). Pairwise comparisons revealed a difference between baseline and 6-month results in HIV-infected participants: mean (±SD) ratio of luminescence in neutrophil versus serum samples at 1 hour was 0.88 ± 0.14 at baseline versus 0.78 ± 0.11 at 6 months (P = 0.02), when it became similar to HIV-uninfected donors’ results (0.76 ± 0.14, P = 0.59).
Twenty-four–hour cell:serum luminescence ratios also declined over time: there was no longer a significant difference between HIV-infected patients at 6 months and HIV-uninfected control subjects (mean ratio of luminescence in neutrophil vs. serum samples at 24 hours, 0.77 ± 0.16 and 0.72 ± 0.13, respectively; P = 0.27). Although pairwise comparison between baseline and 6-month 24-hour cell:serum ratios did not reveal a significant difference (0.82 ± 0.13 vs. 0.77 ± 0.16, respectively; P = 0.35), there was a significant reduction between the 1-month and 6-month time points (0.88 ± 0.13 vs. 0.77 ± 0.16, respectively; P = 0.006).
The Effect of CD15 Depletion on Mycobacterial Control Correlates Negatively with Viral Load in HIV-infected Participants
Having established impairment of antimycobacterial function in isolated neutrophils from HIV-infected people, we investigated the impact of granulocyte depletion from whole blood. Aliquots of blood from all participants at baseline and HIV-infected participants after 6 months of ART were depleted of CD15+ cells or were incubated with nonconjugated magnetic beads as controls. This blood was then infected with M. tuberculosis-lux and luminescence was measured at 96 hours. Figure 2A details the impact of neutrophil depletion on the 96-hour luminescence, presented as a ratio versus the nondepleted condition to control for variations in baseline mycobacterial growth. There was a significant effect of CD15 depletion on the control of mycobacterial luminescence in both groups (P < 0.001), similar to previous results (3).
Figure 2.
The impact of neutrophil depletion from blood on mycobacterial control in HIV-infected and HIV-uninfected people. (A) Blood was taken from 20 HIV-uninfected donors, 20 antiretroviral-naive HIV-infected donors, and 18 of the same HIV-infected donors after 6 months of antiretroviral therapy. A total of 450 μl of blood depleted of CD15+ cells or undepleted (in triplicate per donor per condition), diluted 1:1 with RPMI-1640, was infected with approximately 5 × 105 cfu M. tuberculosis-lux. Samples were incubated at 37°C for 96 hours before red cell lysis and measurement of mycobacterial luminescence on at least two 100-μl aliquots. Results are presented as the ratio of mycobacterial luminescence in the CD15-depleted conditions versus nondepleted controls. Solid lines represent means; the dotted line indicates a ratio of 1 (no impact of depletion). (B) Results from the CD15-depleted condition in HIV-infected donors at baseline are correlated versus log[HIV viral load]. R = −0.67 (95% CI, −0.32 to −0.86), P = 0.001. The dotted line indicates a ratio of 1 (no impact of depletion).
No statistically significant difference was observed between the groups. However, the impact of CD15 depletion on mycobacterial control correlated inversely with CD4 count (Pearson r = 0.47, P = 0.04) and there was a stronger, inverse relationship between the impact of neutrophil depletion and log[HIV viral load] (Pearson r = −0.67, P = 0.001), implying that in the presence of high viral loads neutrophils were less effective at controlling mycobacteria and there was minimal impact of removing neutrophils on 96-hour mycobacterial luminescence (Figure 2B).
The mean ratio of luminescence in CD15-depleted blood vs. undepleted controls was more similar to that in HIV-uninfected donors after 6 months’ ART in the HIV-infected donors (Figure 2A), although the difference vs. baseline was not significant.
Overall “lux ratios” (change in luminescence over 96 h) were also calculated for whole blood samples and corrected for contemporaneous M. tuberculosis growth in 7H9 broth to allow for differences in mycobacterial stock behavior. Lux ratios tended to be higher in HIV-infected patients than HIV-uninfected (0.76 ± 0.77 vs. 0.48 ± 0.44, P = 0.18) and to be lower at 6 months (0.64 ± 0.42) than at baseline in the HIV-infected cohort. However, there were large standard deviations in these data, and no comparisons reached significance.
Neutrophils from HIV-infected Patients Exhibit Accelerated Cell Death That is Reversed with ART
To further interrogate the compromise of neutrophil antimycobacterial activity in HIV infection, we also investigated cell death after 24 hours’ incubation. Results demonstrated a marked increase in the percentage of CD66a,c,e-positive events identified as necrotic in HIV-infected patients compared with uninfected control subjects (85.3 ± 11.8% vs. 57.9 ± 22.4%, P < 0.0001 for dual Annexin V and Viability Dye–positive events; Figure 3A). There was a corresponding decrease in the percentage of Viability Dye–negative apoptotic events (9.8 ± 2.2% vs. 31.7 ± 4.5%, P < 0.001) and viable events (2.1 ± 0.6% vs. 8.7 ± 1.7%, P = 0.001). Gating on “granulocytes” as defined by forward scatter and side scatter revealed that cells in HIV-infected participants, although far fewer in number than in HIV-uninfected, were relatively more likely to be viable or necrotic and less likely to remain in apoptosis (for HIV-infected vs. uninfected: viable events, 33.6 ± 4.1% vs. 20.1 ± 3.6%; P = 0.02; apoptotic events, 23.0 ± 3.7% vs. 63.6 ± 5.6%; P < 0.0001; Annexin V–positive necrotic events, 34.4 ± 4.2% vs. 14.3 ± 2.4%, P < 0.001; primary necrotic events, 9.0 ± 2.7% vs. 2.1 ± 0.7%, P = 0.03; Figure 3B).
Figure 3.
The impact of HIV and antiretroviral therapy on M. tuberculosis–associated neutrophil cell death. (A, B) A total of 4 × 105 CD15+ granulocytes in RPMI-1640 were incubated with 10% autologous serum and M. tuberculosis-lux. After 24 hours’ incubation at 37°C, samples were labeled with Annexin V-FITC, eFluor450 Viability Dye, and CD66a,c,e-PE for 15 minutes. Displayed are the percentages of total CD66a,c,e+ events (A) or “granulocytes” as defined by forward and side scatter (B) for HIV-infected and HIV-uninfected donors classed as viable (Annexin V negative, Viability Dye negative), apoptotic (Annexin V positive, Viability Dye negative), apoptotic and necrotic (Annexin V positive, Viability Dye positive), and primary necrotic (Annexin V negative, Viability Dye positive). (C, D) Samples were processed as in (A). The mean (SD) percentages of total CD66a,c,e+ events (C) or “granulocytes” (D) are displayed for HIV-infected donors at baseline and after 1, 3, or 6 months of antiretroviral therapy classed as viable, apoptotic, apoptotic and necrotic, and primary necrotic; data from HIV-uninfected donors are shown for comparison.
Notably, the percentage of events excluded as dead cells in the phagocytosis assay (30 min incubation; see above) due to Viability Dye positivity was also significantly higher in the HIV-infected versus -uninfected donors (4.96 ± 1.78% in HIV-infected donors vs. 3.17 ± 1.57% in HIV-uninfected donors, P = 0.01).
Neutrophil cell death was assessed in HIV-infected donors at each visit during the 6 months of ART. For all CD66a,c,e-positive events (Figure 3C) there was a decrease in necrotic events (P value for linear trend across all visits in HIV-infected participants = 0.008) and corresponding increase in apoptotic events (P value for linear trend = 0.004) over the studied period. By 6 months, results in HIV-infected participants more closely resembled those seen in HIV-uninfected participants, except that viable events remained lower (viable events, 2.6 vs. 8.7%, respectively, P = 0.002; apoptotic events, 24.5 vs. 31.7%, respectively; P = 0.28; apoptotic/necrotic events, 71.8 vs. 57.9%, respectively; P = 0.07; primary necrotic events, 1.1 vs. 1.7%, respectively; P = 0.15).
Gating on granulocytes (defined by forward and side scatter) also revealed a progressive change in the pattern of cell death (P value for linear trend in apoptotic events < 0.0001; P value for linear trend in necrotic events = 0.003). By 6 months, results in HIV-infected participants resembled those seen in HIV-uninfected participants (viable events, 26.4 vs. 20.0%, respectively; P = 0.20; apoptotic events, 53.7 vs. 63.6%, respectively; P = 0.18; apoptotic/necrotic events, 17.4 vs. 14.3%, respectively; P = 0.39; primary necrotic events, 2.6 vs. 2.1% respectively; P = 0.57; Figure 3D).
Neutrophils Are Hyperactivated in HIV-infected Patients with an Ex Vivo Surface Marker Pattern Suggesting Progression to Early Cell Death
Having established that neutrophils from HIV-infected individuals die rapidly on mycobacterial challenge, we hypothesized that this may be due to hyperactivation at baseline before pathogen encounter. To assess this possibility, separate groups of HIV-infected individuals (n = 11) and HIV-uninfected control subjects (n = 6) were recruited to study neutrophil surface markers of activation in fresh blood immediately ex vivo (participants were bled in the laboratory).
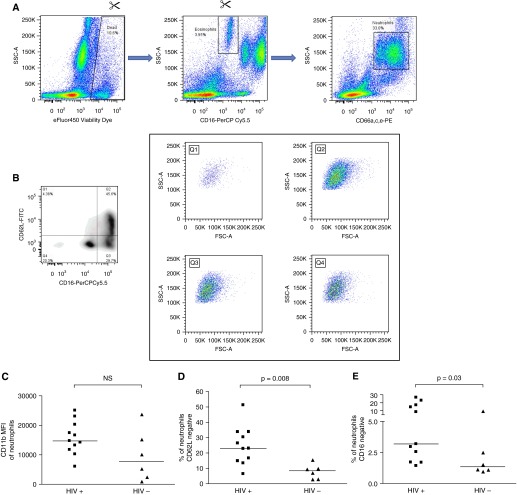
Table 3 details participant characteristics, and Figure 4 details the flow cytometry analysis strategy: note that eosinophils are excluded here because they may be gated with neutrophils on the basis of CD66 or CD11b expression but are intrinsically CD16 negative, which would confound results. Analysis demonstrated that a higher percentage of neutrophils from HIV-infected patients had shed CD62L/L-Selectin (23.0% [IQR, 14.8–33.8%] vs. 8.5% [IQR, 3.1–12.9%], P = 0.008) and CD16 (3.2% [IQR, 1.7–17.1%] vs. 1.3% [IQR, 1.1–6.2%], P = 0.03; Figure 4). There was no difference (P = 0.13) in the expression of CD11b on the neutrophils of the two groups as defined by median fluorescence intensity (MFI). The HIV-infected donors’ serum did not reflect significant evidence of active inflammation with a median (IQR) C-reactive protein of 2.6 (1.7–12.8) mg/L.
Table 3.
Participant characteristics at baseline enrollment for activation study
| HIV-infected (n = 11) | HIV-uninfected (n = 6) | ||
|---|---|---|---|
| Age, yr | Median | 33 | 37 |
| Range | 24–63 | 26–47 | |
| Ethnicity | Xhosa | 11 (100) | 6 (100) |
| Sex | Male | 3 (27.3) | 2 (33.3) |
| Female | 8 (72.7) | 4 (66.7) | |
| Current smoking | Yes | 2 (18.2) | 0 (0) |
| No | 9 (81.8) | 6 (100) | |
| Regular alcohol* | Yes | 1 (9.1) | 1 (16.7) |
| No | 10 (90.9) | 5 (83.3) | |
| BCG | Yes | 2 (18.2) | 4 (66.7) |
| No | 6 (54.5) | 0 (25) | |
| Unsure/unclear | 3 (27.3) | 2 (33.3) | |
| Comorbidity† | Yes | 6 (54.5) | 2 (33.3) |
| No | 5 (45.5) | 4 (66.7) | |
| Cotrimoxazole prophylaxis | Yes | 9 (81.8) | N/A |
| No | 2 (18.2) | ||
| Vitamin treatment | Vitamin B Co-Strong | 8 (72.7) | N/A |
| Vitamin C | 5 (45.5) | ||
| Other medication in previous 3 mo‡ | Yes | 6 (54.5) | 2 (33.3) |
| No | 5 (45.5) | 4 (66.7) | |
| CD4 count, ×106/L (n = 10) | Median | 170 | N/A |
| Range | 55–371 | ||
| Log [HIV viral load (copies/ml)] | Median | 5.14 | N/A |
| Range | 3.96–5.96 | ||
Definition of abbreviations: BCG = Mycobacterium bovis–Bacille Calmette–Guerin; N/A = not applicable.
Data presented as n (%) unless otherwise noted.
Defined as at least once per week.
Identified comorbidities: epilepsy, oral candidiasis, recent finger infection, eczema, tinea cutis, shingles, chronic cough.
Identified medications: phenytoin, nystatin, ibuprofen, paracetamol, folic acid, acyclovir, antifungal creams, steroid creams.
Figure 4.
The impact of HIV on neutrophil activation markers immediately ex vivo. (A) A total of 200 μl of blood was stained immediately after phlebotomy with fluorochromes; red cells were lysed and samples fixed before acquisition within 48 hours on a BD Fortessa flow cytometer. Singlet signals (derived from Forward Scatter [FSC] area vs. FSC height, not shown) were first gated on eFluor450 Viability Dye versus Side Scatter (SSC) to exclude dead cells. Eosinophils, defined as CD16-negative with high SSC, are excluded and then neutrophils are defined as CD66a,c,e-PE–positive events with high/intermediate SSC. (B) Neutrophils thus defined are divided into quadrants on the basis of CD62L–fluorescein isothiocyanate (FITC) and CD16-PerCP Cy5.5. The contents of each quadrant are also shown on plots of FSC versus SSC, indicating that all quadrants resemble granulocytes. (C) The median fluorescence intensity of CD11b-PE Cy7 on neutrophils is plotted for 11 HIV-infected and 6 HIV-uninfected individuals. NS = not significant. (D) The percentage of neutrophils negative for CD62L is plotted for 11 HIV-infected and 6 HIV-uninfected individuals. (E) The percentage of neutrophils negative/intermediate for CD16 is plotted for 11 HIV-infected and 6 HIV-uninfected individuals. Lines in C – E represent medians, and P values are derived from Mann-Whitney tests.
This group of HIV-infected individuals was not followed prospectively. However, we analyzed the expression of CD66a,c,e on neutrophils in the phagocytosis assay in the longitudinal study via measurement of MFI; this marker is indicative of neutrophil activation and degranulation (21). Median (IQR) MFI in HIV-infected individuals at baseline was 5,344 (3,829–7,265) versus 3,032 (2,540–4,624) after 6 months of ART and 3,241 (2,282–4,570) in HIV-uninfected donors. There was a significant difference (P < 0.05) between results from the HIV-infected group at baseline and those from the same cohort after 6 months’ ART or the HIV-uninfected participants.
Neutrophil Phagocytosis of M. tuberculosis and HNP 1-3 Concentrations in Supernatants of Neutrophil Culture Do Not Differ between HIV-infected and HIV-uninfected Participants
We also investigated other aspects of neutrophil function that might impact antimycobacterial activity: phagocytosis and HNP concentrations. Samples of neutrophils from 16 of the ARV-naive HIV-infected and 16 of the HIV-uninfected donors (cohorts described in Table 1) were incubated with FITC-labeled M. tuberculosis to assess phagocytosis (15). There was no difference between the groups at baseline, with 45.6 ± 13.5% of neutrophils in HIV-uninfected donors versus 40.7 ± 22.7% of neutrophils in HIV-infected donors internalizing mycobacteria by 30 minutes (P = 0.46). There was also no difference between the baseline and 6-month results in HIV-infected donors (P = 0.16).
Supernatants from M. tuberculosis–infected neutrophils were aspirated at 24 hours, and HNP 1-3 concentrations were measured by ELISA. There was no difference between values in HIV-infected and HIV-uninfected donors (mean 1,162 ± 726 pg/ml vs. 1,544 ± 598 pg/ml, P = 0.13) and no difference between results from baseline and after 6 months’ ART in HIV-infected participants (mean 1,162 ± 726 pg/ml vs. 1,247 ± 816 pg/ml, P = 0.75).
Discussion
This work has established that the antimycobacterial activity of human neutrophils is impaired in HIV infection and that this is associated with accelerated neutrophil cell death and restored with antiretroviral therapy.
We have previously described that neutrophils are crucial for the control of mycobacterial growth in human blood (3), and we here confirm this finding via CD15+ cell depletion experiments, which increased mycobacterial luminescence at 96 hours compared with undepleted blood. HIV infection increases risk of tuberculosis (5) and especially extrapulmonary tuberculosis (22), and our results suggest that dysfunction of neutrophils (the most common circulating white blood cell) may be associated with this. Correspondingly, we determined that neutrophils were less able to directly restrict M. tuberculosis bioluminescence in the context of HIV viremia and that this impairment correlated inversely with HIV viral load (or positively with CD4 count). Similarly, the impact of CD15 depletion on mycobacterial bioluminescence depended strongly on HIV viral load and on CD4 count: at the highest viral loads, or greatest immunological compromise, neutrophil depletion did not impair restriction of bacilli by blood. These results are comparable to those of others, including Mastroianni and colleagues, who found that neutrophil chemotaxis, oxidative burst, and fungicidal activity all correlated with CD4 count (8), and Monari and colleagues, who described that impairment of cryptococcal killing by HIV-infected patients’ neutrophils correlated with viral load (9).
Antiretroviral therapy improved the antimycobacterial capability of neutrophils over 6 months, such that restriction of M. tuberculosis-lux bioluminescence vs. serum controls in HIV-infected participants became indistinguishable from that seen in HIV-uninfected individuals. Others have similarly noted normalization of neutrophil activity in HIV-infected people after 3 months’ antiretroviral therapy (9). Our results suggest that the previously described phenomenon of improved restriction of M. tuberculosis by the blood of HIV-infected patients receiving ART (23) may be partly explained by recovery of neutrophil function.
In a large prospective multicohort study (24), the incidence of tuberculosis in HIV-infected people fell markedly from the period 0 to 3 months after commencement of antiretrovirals (13 per 100 person-years) compared with the period 3 to 5 months after commencement of antiretrovirals (6 per 100 person years). Our results suggest that restoration of neutrophil function could explain some of this protection. A more cautious interpretation restricts this hypothesis at least as far as circulating blood (as was used for our experiments), a conclusion supported by a progressive decrease in the proportion of extrapulmonary tuberculosis over the first year of ART (24).
Neither HIV infection nor antiretroviral therapy affected phagocytosis of M. tuberculosis by neutrophils or extracellular release of HNP in our experiments. The former result suggests that failure to control mycobacteria represents intracellular dysfunction. However, there is a profound impact of HIV on neutrophil cell death. Gating on all neutrophil surface marker–positive events revealed a far higher percentage of necrotic events by 24 hours in the HIV samples vs. non-HIV samples, whereas gating on phenotypic “granulocytes” suggested that cells may be rapidly progressing through apoptosis to necrosis, similar to macrophages infected with a large number of bacilli (25).
This result corresponds with previous findings in other contexts (10, 26); in particular, Pitrak and colleagues (26) discovered similarly profound differences in apoptosis rates and overall viability between HIV-infected individuals’ neutrophils and those of healthy control subjects. This probably relates to HIV-associated activation of neutrophils in vivo before they even encounter pathogens, as demonstrated by surface activation marker expression.
We found loss of CD62L (L-selectin) and CD16 on neutrophils from HIV-infected individuals. Elbim and colleagues (6) also noted lower CD62L on resting neutrophils from HIV-infected persons (and significantly higher CD11b expression in their experiments). It thus seems likely that neutrophils from HIV-infected individuals are already beyond optimum activation when they encounter bacilli; indeed, others have found that despite high baseline activation, there is poor response to further stimulation (6, 9). CD62L and CD16 are preferentially lost as neutrophils progress toward death (27). It thus follows that “exhausted” neutrophils in HIV, despite their ability to phagocytose, fail to eliminate pathogens and instead die in situ with rapid progression to necrosis.
This work has limitations. Blood is not the medium in which humans first encounter M. tuberculosis, although it contains most components of the immune system and is important for dissemination of bacilli to distant sites. HIV-infected and -uninfected participants differed in terms of weight, which itself correlated inversely with neutrophil antimycobacterial control at 1 hour (although not by 24 h). HIV-infected patients were also often receiving cotrimoxazole or vitamin supplements, whereas HIV-uninfected patients were not. However, it is reported that cotrimoxazole has no effect on neutrophil function at in vivo concentrations (28), and vitamins (up to 10 mM Vitamin C, 1 mM Vitamin B12, and 10 nM 1,25-dihydroxyvitamin D3) do not affect the ratio of luminescence in neutrophil samples versus serum in our experiments (data not shown).
Many results required analysis as ratios to control for differences in metabolic activity and intrinsic luminescence of M. tuberculosis-lux (despite using a single stock throughout). However, most of the important findings are based on intradonor comparison of conditions, and thus ratios are appropriate.
In summary, we have demonstrated that neutrophil antimycobacterial function is significantly impaired in ART-naive HIV-infected patients, whose cells experience accelerated necrotic cell death after interacting with mycobacteria. There may be important therapeutic implications from this work. Because neutrophil antimycobacterial function depends strongly on HIV viral load, the finding of a high viral load suggests commencement of ART regardless of CD4 count, further supporting a “test-and-treat” strategy for clinical outcome as well as for prevention of HIV transmission (29). Furthermore, the aberrant neutrophil response itself could potentially be targeted, with therapies such as Annexin I or evasins showing promise in other models (30, 31).
Footnotes
Supported by Wellcome Trust grants WT087754 (D.M.L.), WT104803 (R.J.W.), WT097684 (N.B.); Medical Research Council grant U1175.02.002.00014.01 (R.J.W.); Wellcome Trust Grant 077,273/Z/05 and Medical Research Council Program Grant MR/K011944/1 (B.K.); European Union grant FP7-HEALTH-2012-305578, and National Research Foundation of South Africa grant 96841.
Author Contributions: D.M.L., B.K., K.A.W., R.J.W., and A.R.M. designed experiments. D.M.L. and R.G. recruited participants. D.M.L. and N.B. performed experiments. D.M.L., K.A.W., R.J.W., and A.R.M. designed data analysis. D.M.L. drafted and all authors reviewed the manuscript. All authors approved the final version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization. Global Health Observatory (GHO) data. How many TB cases and deaths are there? [accessed 2015 Jan]. Available from: www.who.int/gho/tb/epidemic/cases_deaths/en/
- 2.World Health Organization. Global Health Observatory (GHO) Data. Number of deaths due to HIV/AIDS [accessed 2015 Jan] Available from: http://www.who.int/gho/hiv/epidemic_status/deaths/en/
- 3.Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ, Wilkinson RJ. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117:1988–1994. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe DM, Bandara AK, Packe GE, Barker RD, Wilkinson RJ, Griffiths CJ, Martineau AR. Neutrophilia independently predicts death in tuberculosis. Eur Respir J. 2013;42:1752–1757. doi: 10.1183/09031936.00140913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawn SD. AIDS in Africa: the impact of coinfections on the pathogenesis of HIV-1 infection. J Infect. 2004;48:1–12. doi: 10.1016/j.jinf.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Elbim C, Prevot MH, Bouscarat F, Franzini E, Chollet-Martin S, Hakim J, Gougerot-Pocidalo MA. Polymorphonuclear neutrophils from human immunodeficiency virus-infected patients show enhanced activation, diminished fMLP-induced L-selectin shedding, and an impaired oxidative burst after cytokine priming. Blood. 1994;84:2759–2766. [PubMed] [Google Scholar]
- 7.Coffey MJ, Phare SM, George S, Peters-Golden M, Kazanjian PH. Granulocyte colony-stimulating factor administration to HIV-infected subjects augments reduced leukotriene synthesis and anticryptococcal activity in neutrophils. J Clin Invest. 1998;102:663–670. doi: 10.1172/JCI2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mastroianni CM, Lichtner M, Mengoni F, D’Agostino C, Forcina G, d’Ettorre G, Santopadre P, Vullo V. Improvement in neutrophil and monocyte function during highly active antiretroviral treatment of HIV-1-infected patients. AIDS. 1999;13:883–890. doi: 10.1097/00002030-199905280-00003. [DOI] [PubMed] [Google Scholar]
- 9.Monari C, Casadevall A, Baldelli F, Francisci D, Pietrella D, Bistoni F, Vecchiarelli A. Normalization of anti-cryptococcal activity and interleukin-12 production after highly active antiretroviral therapy. AIDS. 2000;14:2699–2708. doi: 10.1097/00002030-200012010-00009. [DOI] [PubMed] [Google Scholar]
- 10.Salmen S, Montes H, Soyano A, Hernández D, Berrueta L. Mechanisms of neutrophil death in human immunodeficiency virus-infected patients: role of reactive oxygen species, caspases and map kinase pathways. Clin Exp Immunol. 2007;150:539–545. doi: 10.1111/j.1365-2249.2007.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis M, Gupta S, Galant S, Hakim S, VandeVen C, Toy C, Cairo MS. Impaired neutrophil function in patients with AIDS or AIDS-related complex: a comprehensive evaluation. J Infect Dis. 1988;158:1268–1276. doi: 10.1093/infdis/158.6.1268. [DOI] [PubMed] [Google Scholar]
- 12.Levine AM, Karim R, Mack W, Gravink DJ, Anastos K, Young M, Cohen M, Newman M, Augenbraun M, Gange S, et al. Neutropenia in human immunodeficiency virus infection: data from the women’s interagency HIV study. Arch Intern Med. 2006;166:405–410. doi: 10.1001/archinte.166.4.405. [DOI] [PubMed] [Google Scholar]
- 13.Lowe DM, Goliath R, Wilkinson RJ, Martineau AR. Neutrophils in HIV infection demonstrate impaired restriction of Mycobacterium tuberculosis [abstract]. Keystone Symposium Host Response in Tuberculosis (X7); Whistler, BC, Canada; March 13–18, 2013. [Google Scholar]
- 14.Snewin VA, Gares MP, Gaora PO, Hasan Z, Brown IN, Young DB. Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect Immun. 1999;67:4586–4593. doi: 10.1128/iai.67.9.4586-4593.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowe DM, Bangani N, Mehta MR, Lang DM, Rossi AG, Wilkinson KA, Wilkinson RJ, Martineau AR. A novel assay of antimycobacterial activity and phagocytosis by human neutrophils. Tuberculosis (Edinb) 2013;93:167–178. doi: 10.1016/j.tube.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kampmann B, Gaora PO, Snewin VA, Gares MP, Young DB, Levin M. Evaluation of human antimycobacterial immunity using recombinant reporter mycobacteria. J Infect Dis. 2000;182:895–901. doi: 10.1086/315766. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L, Somasundaram R, Nederhof RF, Dijkstra G, Faber KN, Peppelenbosch MP, Fuhler GM. Impact of human granulocyte and monocyte isolation procedures on functional studies. Clin Vaccine Immunol. 2012;19:1065–1074. doi: 10.1128/CVI.05715-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kampmann B, Tena GN, Mzazi S, Eley B, Young DB, Levin M. Novel human in vitro system for evaluating antimycobacterial vaccines. Infect Immun. 2004;72:6401–6407. doi: 10.1128/IAI.72.11.6401-6407.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corleis B, Korbel D, Wilson R, Bylund J, Chee R, Schaible UE. Escape of Mycobacterium tuberculosis from oxidative killing by neutrophils. Cell Microbiol. 2012;14:1109–1121. doi: 10.1111/j.1462-5822.2012.01783.x. [DOI] [PubMed] [Google Scholar]
- 20.Watt SM, Sala-Newby G, Hoang T, Gilmore DJ, Grunert F, Nagel G, Murdoch SJ, Tchilian E, Lennox ES, Waldmann H. CD66 identifies a neutrophil-specific epitope within the hematopoietic system that is expressed by members of the carcinoembryonic antigen family of adhesion molecules. Blood. 1991;78:63–74. [PubMed] [Google Scholar]
- 21.Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–446. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- 22.Naing C, Mak JW, Maung M, Wong SF, Kassim AI. Meta-analysis: the association between HIV infection and extrapulmonary tuberculosis. Lung. 2013;191:27–34. doi: 10.1007/s00408-012-9440-6. [DOI] [PubMed] [Google Scholar]
- 23.Kampmann B, Tena-Coki GN, Nicol MP, Levin M, Eley B. Reconstitution of antimycobacterial immune responses in HIV-infected children receiving HAART. AIDS. 2006;20:1011–1018. doi: 10.1097/01.aids.0000222073.45372.ce. [DOI] [PubMed] [Google Scholar]
- 24.Nicholas S, Sabapathy K, Ferreyra C, Varaine F, Pujades-Rodríguez M AIDS Working Group of Médecins Sans Frontières. Incidence of tuberculosis in HIV-infected patients before and after starting combined antiretroviral therapy in 8 sub-Saharan African HIV programs. J Acquir Immune Defic Syndr. 2011;57:311–318. doi: 10.1097/QAI.0b013e318218a713. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Hartman M, Kornfeld H. Macrophage apoptosis in tuberculosis. Yonsei Med J. 2009;50:1–11. doi: 10.3349/ymj.2009.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitrak DL, Tsai HC, Mullane KM, Sutton SH, Stevens P. Accelerated neutrophil apoptosis in the acquired immunodeficiency syndrome. J Clin Invest. 1996;98:2714–2719. doi: 10.1172/JCI119096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart SP, Ross JA, Ross K, Haslett C, Dransfield I. Molecular characterization of the surface of apoptotic neutrophils: implications for functional downregulation and recognition by phagocytes. Cell Death Differ. 2000;7:493–503. doi: 10.1038/sj.cdd.4400680. [DOI] [PubMed] [Google Scholar]
- 28.Anderson R, Grabow G, Oosthuizen R, Theron A, Van Rensburg AJ. Effects of sulfamethoxazole and trimethoprim on human neutrophil and lymphocyte functions in vitro: in vivo effects of co-trimoxazole. Antimicrob Agents Chemother. 1980;17:322–326. doi: 10.1128/aac.17.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sigaloff KC, Lange JM, Montaner J. Global response to HIV: treatment as prevention, or treatment for treatment? Clin Infect Dis. 2014;59:S7–S11. doi: 10.1093/cid/ciu267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perretti M, Dalli J. Exploiting the Annexin A1 pathway for the development of novel anti-inflammatory therapeutics. Br J Pharmacol. 2009;158:936–946. doi: 10.1111/j.1476-5381.2009.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montecucco F, Mach F, Lenglet S, Vonlaufen A, Gomes Quinderé AL, Pelli G, Burger F, Galan K, Dallegri F, Carbone F, et al. Treatment with Evasin-3 abrogates neutrophil-mediated inflammation in mouse acute pancreatitis. Eur J Clin Invest. 2014;44:940–950. doi: 10.1111/eci.12327. [DOI] [PubMed] [Google Scholar]