Abstract
AIM: To study the role of survivin expression induced by chemotherapy agent (doxorubicin) in the development and anti-chemotherapy of cholangiocarcinoma.
METHODS: Expression of survivin was detected by SP immunohi stochemical te chnique in 33 cases of cholangiocarcinoma, 28 cases of adjacent noncancerous bile duct, and 5 cases of benign bile duct lesions. Low concentration of doxorubicin (0.05 mg/l) was added in cultured cholangiocarcinoma cell line (QBC939). The expression of survivin was detected by RT-PCR and Western blot at 24 h and 48 h after adding doxorubicin.
RESULTS: Survivin was expressed in 24 of 33 cholangiocar-cinoma cases (72.7%). In contrast, no expression of survivin in adjacent noncancerous and benign bile duct lesions was observed (P < 0.01). No correlation was found between survivin expression and clinical features. Doxorubicin could markedly (P < 0.001) up-regulate survivin mRNA and protein expression of QBC939 cells.
CONCLUSION: Overexpression of survivin in cholangiocar-cinomas may play an important role in the development of cholangiocarcinoma, its relationship with prognosis of cholangiocarcinoma deserves further investigation. Higher expression of survivin is induced by doxorubicin in QBC939. Survivin expression may resist apoptosis induced by chemotherapy agents.
INTRODUCTION
Survivin, a member of the inhibitors of apoptosis protein (IAP) family, is characterized by a unique structure that discriminates it from other members of the IAP family. It contains only a single BIR repeat and lacks a carboxy terminal RING finger domain. Survivin is expressed in the G2/M phase of cell cycle in a cycle-regulated manner[1]. It directly binds to and inhibits both Caspase-3 and Caspase-7 activity, leading to arrest of apoptosis[2] .
Survivin expression is not detectable in differentiated normal adult cells of any organ[3], but it is abundantly expressed in embryonic tissues and in a wide range of cancer tissues[4] including neuroblastoma[5], colorectal[6], stomach[7] and breast[8] carcinomas. It has been demonstrated recently that survivin is also frequently expressed in malignant pancreatic ductal tumors[9] and pancreatic adenocarcinoma[10]. Furthermore, the prognostic value of survivin expression has been reported in several human cancers[11].
Inducing apoptosis is the mechanism of chemotherapy agents killing tumor cells. But tumor cells resist chemotherapy agents because not only they overexpress MDR1/P-glycoprotein (P-gp) but also resist apoptosis induced by chemotherapy agents. Studies demonstrating resistance of survivin-transfected cells to anticancer drug-induced apoptosis[2] and sensitization to chemotherapy by survivin antisense treatment[12] have shown that survivin is implicated in sensitization to chemotherapy.
But in cholangiocarcinoma, survivin distribution and its implication for apoptosis inhibition are not clear at present. This study aimed to study the role of survivin expression induced by chemotherapy agent (doxorubicin) in the development and anti-chemotherapy of cholangiocarcinoma.
MATERIALS AND METHODS
Materials
Thirty-three specimens were obtained from patients with cholangiocarcinoma at the Department of General Surgery, Tongji Hospital of Tongji Medical College during the period from 1993 to 2001. There were 21 males and 12 females, and the mean age of the patients was 55.1 years (range from 34 to 79 years). The patients did not receive chemotherapy, radiation therapy or immunotherapy before surgery. Five specimens of benign bile duct lesions were also obtained. Formalin-fixed, paraffin-embedded blocks of tissue samples were taken from pathological archives. Serial sections of 4 µm were prepared from the cut surface of the blocks at the maximum cross-section of the tissue sample. Representative sections were stained with H&E in order to confirm the histopathological diagnosis. Human extrahepatic cholangiocarcinoma cell line QBC939 was established by Professor Wang SG (Third Military Medical University, China) and offered to us as a gift[13]. The cells were maintained as monolayers in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 100 units/ml penicillin and 100 mg/ml streptomycin in a humidified atmosphere of 50 mL/L CO2 at 37 °C.
Methods
Immunohistochemical staining Immunohistochemical staining was carried out with the SP technique using the SP kit (Zhongshan Biotech Co., Beijing, China) after antigen retrieval by microwave pretreatment. Briefly, deparaffinized sections were immersed in a 0.1 M sodium citrate buffer (pH6.0) and heated three times for 5 min each at a 15 min interval in a microwave oven at 600 W. After quenched in 3 % hydrogen peroxide and blocked, the sections were incubated with rabbit survivin polyclonal antibody (Neomarkers, USA; dilution 1:200) overnight at 4 °C. Biotinylated antirabbit immunoglobulin and streptavidin conjugated to horseradish peroxidase were subsequently applied. Finally, 3’, 3’-diaminobenzidine was used for color development, and hematoxylin was used for counterstaining. As a negative control, the sections were processed in the absence of primary antibody. Tissue sections from a hepatocellular carcinoma with a known strong expression of survivin were used as a positive control. A scoring method was used to quantitate the survivin expression in various samples examined. A mean percentage of positive tumor cells was determined in at least five areas at × 400 magnification. Patients with scores of less than 5% were defined as negative, otherwise they were defined as positive.
RT-PCR Low concentration of doxorubicin (0.05 mg/l, Pharmacia & Upjohn Co. Ltd.) was added in cultured cholangiocarcinoma cell line (QBC939). Expression of survivin was detected by RT-PCR before adding doxorubicin and at 24 h and 48 h after adding doxorubicin. Total RNA was prepared from subconfluent cultures with TRIzol reagent (Gibco, USA) according to the manufacture’s instructions. The primers were designed to amplify a fragment of survivin cDNA based on the reported sequence for human survivin. To normalize the amount of input RNA, RT-PCR was performed with primers for constitutively expressed β-actin gene. The survivin primers were 5’-CCCCATAGAGAACATAAA-3’ (sense) and 5’-GGAATAAACCCTGGAAGTG-3’ (antisense), giving rise to a 273 base pair polymerase chain reaction product. The β-actin primers were 5’ -GTGCGTGACATTAAGGAG-3’(sense) and 5’-CTAAGTCATAGTCCGCCT- 3’ (antisense), giving rise to a 520 base pair polymerase chain reaction product. The first strand cDNA synthesis and the subsequent PCR were performed with RNA PCR kit (AMV) using a programmed temperature control system set for 35 cycles, each consisting of denaturation at 94 °C for 45 s, annealing at 50 °C for 45 s, and extension at 72 °C for 45 s. Ten µL reaction mixture was electrophoresed on a 1.5% agarose gel, and the PCR products were visualized by ethidium bromide staining and quantified by an ImageQuant software. Survivin mRNA expression level was determined by survivin/β-actin protein.
Western blot Low concentration of doxorubicin (0.05 mg/l) was added in cultured cholangiocarcinoma cell line (QBC939). Expression of survivin was detected by Western blot before adding doxorubicin and at 24 h and 48 h after adding doxorubicin. Total cells were lysed with cell-lysis buffer [50 mM Tris-Cl, pH8.0, 150 mM NaCl, 0.02% NaN3, 0.1% SDS, 100 µg/ml PMSF, 1 µg/ml Aprotinin, 1% NP-40]. Twenty µg of protein was separated on 10% of SDS-PAGE gels and transferred to NC membranes. After blocked with 5% non-fat milk, the membranes were incubated with rabbit survivin polyclonal antibody (1:1000 dilution) at 4 °C overnight. After washed three times the membranes were incubated with goat anti-rabbit IgG at room temperature for 1 hour. The signals were developed with the ECL kit (Amersham Pharmacia Biotechnology Inc.).
Statistical analysis
Association between survivin expression and various clinical and pathological variables was examined using χ2 test or Fisher’s exact test. The data of PCR and Western blot were expressed as mean ± SD. Student’s t-test was used for statistical analysis. P < 0.05 was considered statistically significant.
RESULTS
Expression of survivin and associated clinicopathological variables
Survivin was prominently found in 24 of 33 cholangiocarcinoma cases (72.7%) by immunohistochemistry. Positive staining for survivin was located in the cytoplasm of tumor cells (Figure 1). In contrast, expression of survivin was observed neither in adjacent noncancerous bile ducts nor in benign bile duct lesions (P < 0.01). No correlation was found between survivin expression and clinical features (Table 1).
Figure 1.
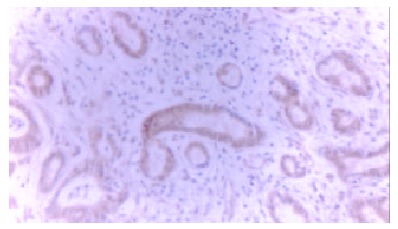
Expression of survivin in cholangiocarcinoma (× 200, SP).
Table 1.
Correlation between clinicopathological factors and survivin expression in cholangiocarcinoma
| Clinical features | n |
Survivin expression |
χ2 | P | |
| n | % | ||||
| All patients | 33 | 24 | 72.7 | ||
| Age (years) | |||||
| 60 | 18 | 13 | 72.2 | 0.0051 | 0.9431 |
| ≤ 60 | 15 | 11 | 73.5 | ||
| Sex | |||||
| Male | 21 | 15 | 71.4 | 0.0491 | 0.8246 |
| Female | 12 | 9 | 75.0 | ||
| Tumor size (cm) | |||||
| ≤ 2 | 13 | 10 | 76.9 | 0.1904 | 0.6626 |
| > 2 | 20 | 14 | 70.6 | ||
| Differentiation level | |||||
| High & Middle | 29 | 21 | 72.4 | 0.0119 | 0.9133 |
| Low | 4 | 3 | 75.0 | ||
| Metastasis | |||||
| Positive | 7 | 5 | 71.4 | 0.0076 | 0.9307 |
| Negative | 26 | 19 | 73.1 | ||
Expression level of survivin mRNA
Doxorubicin could markedly (P < 0.001) up-regulate survivin mRNA expression of QBC939 cells (Figure 2, Table 2).
Figure 2.
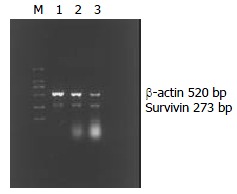
Expression of survivin in a human cholangiocarcinoma cell line. β-actin served as control. M: DL2000 marker, 1: Normal Qbc939, 2: 24 h after adding doxorubicin, 3: 48 h after adding doxorubicin.
Table 2.
Expression level of survivin mRNA
| Group | n | Survivin/β-actin | t | P |
| A | 7 | 0.4210 ± 0.0551 | bt = 45.89, dt = 12.54 | bP, dP < 0.001 |
| B | 7 | 0.8481 ± 0.0713 | bt = 26.11 | bP < 0.001 |
| C | 7 | 1.7034 ± 0.0493 |
Survivin mRNA expression level was determined by survivin/β-actin protein. Data were expressed as mean ± SD, b vs C (48 h after adding doxorubicin), d vs B (24 h after adding doxorubicin), A: Normal Qbc939.
Expression level of survivin protein
Doxorubicin could markedly (P < 0.001) up-regulate survivin protein expression of QBC939 cells (Figure 3, Table 3).
Figure 3.
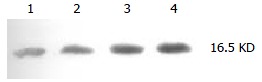
Expression of survivin in a human cholangiocarcinoma cell line. 1 and 2: Normal Qbc939, 3: 24 h after adding doxorubicin, 4: 48 h after adding doxorubicin.
Table 3.
Expression level of survivin protein
| Group | n | OD value | t | P |
| A | 6 | 204.568 ± 1.387 | bt = 17.99, dt = 11.23 | bP, dP < 0.001 |
| B | 6 | 311.105 ± 1.539 | bt = 11.02 | bP < 0.001 |
| C | 6 | 339.989 ± 1.872 |
Data were expressed as mean ± SD, b vs C (48 h after adding doxorubicin), d vs B (24 h after adding doxorubicin), A: Normal Qbc939.
DISCUSSION
Expression of survivin was detected in 72.7% of cholangiocarcinomas. In contrast, no expression of survivin in adjacent noncancerous and benign bile duct lesions was observed (P < 0.01). Using a similar polyclonal antibody, survivin expression was detected in 93% of malignant melanomas[14], 81% of basal cell carcinomas, 92% of cutaneous squamous cell carcinomas[15], 70% of hepatocellular carcinomas[16], 88% of gastric carcinomas[17], 100% of oesophageal cancers[18], 88% of pancreatic adenocarcinomas[10] and 74% of ovarian carcinomas[19]. Our study demonstrated a high expression of survivin in cholangiocarcinoma as in other human malignancies.
There was no correlation between survivin expression and any clinical or pathological characteristics of cholangiocarcinoma. A similar absence of correlation was also noted in previous observations including gastric[7,20], colorectal[6,21] and breast cancers[8]. Though many reports have shown that survivin was an independent prognostic factor for various cancers[19,22-27], such a distribution of clinicopathological features and the high prevalence of survivin expression in cholangiocarcinoma might have rendered the power of this study insufficient to demonstrate any correlation between survivin expression and any clinical or pathological characteristics. Thus, the relationship with prognosis deserves further investigation.
Substantial evidences have also shown that during chemotherapy, changes in expression levels of survivin might provide information about chemo-sensitivity or chemo-resistance of tumors[28,29]. In the present study, low concentration of chemotherapy agent doxorubicin (0.05 mg/l) could markedly (P < 0.001) up-regulate survivin mRNA and protein expression of QBC939 cells. These results suggested a direct link between survivin expression and bile duct carcinoma cell susceptibility to doxorubicin. That is, higher survivin expression may directly down-regulate chemo-sensitivity. It may be one of the mechanisms of anti-chemotherapy in cholangiocarcinoma.
In summary, a high expression of surviv in in cholangiocarcinoma may play an important role in the development of cholangiocarcinoma, the relationship with prognosis deserves further investigation. Higher expression of survivin could be induced by doxorubicin in QBC939 and might resist apoptosis induced by chemotherapy agents. These results provide several exciting therapeutic possibilities. Initial evidence in vitro and in vivo has shown that targeting survivin may provide a viable approach to kill cancer cells selectively[30,31]. Inhibition of survivin expression by molecular manipulation has been reported to improve the effectiveness of chemotherapy[12,31-33] and produce impact on radiation therapy[34-37].
Footnotes
Edited by Zhang JZ and Wang XL
Supported by National Natural Science Foundation of China, No. 30271473
References
- 1.Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 2.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- 3.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto T, Tanigawa N. The role of survivin as a new target of diagnosis and treatment in human cancer. Med Electron Microsc. 2001;34:207–212. doi: 10.1007/s007950100017. [DOI] [PubMed] [Google Scholar]
- 5.Adida C, Berrebi D, Peuchmaur M, Reyes-Mugica M, Altieri DC. Anti-apoptosis gene, survivin, and prognosis of neuroblastoma. Lancet. 1998;351:882–883. doi: 10.1016/S0140-6736(05)70294-4. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58:5071–5074. [PubMed] [Google Scholar]
- 7.Lu CD, Altieri DC, Tanigawa N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998;58:1808–1812. [PubMed] [Google Scholar]
- 8.Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6:127–134. [PubMed] [Google Scholar]
- 9.Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92:271–278. doi: 10.1002/1097-0142(20010715)92:2<271::aid-cncr1319>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer. 2002;86:886–892. doi: 10.1038/sj.bjc.6600133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altieri DC, Marchisio PC. Survivin apoptosis: an interloper between cell death and cell proliferation in cancer. Lab Invest. 1999;79:1327–1333. [PubMed] [Google Scholar]
- 12.Olie RA, Simões-Wüst AP, Baumann B, Leech SH, Fabbro D, Stahel RA, Zangemeister-Wittke U. A novel antisense oligonucleotide targeting survivin expression induces apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer Res. 2000;60:2805–2809. [PubMed] [Google Scholar]
- 13.Wang SG, Han BL, Duan HC, Chen YS, Peng ZM. Establishment of the extrahepatic cholangiocarcinoma cell line. Chin J Exp Sur. 1997;14:67–68. [Google Scholar]
- 14.Grossman D, McNiff JM, Li F, Altieri DC. Expression and targeting of the apoptosis inhibitor, survivin, in human melanoma. J Invest Dermatol. 1999;113:1076–1081. doi: 10.1046/j.1523-1747.1999.00776.x. [DOI] [PubMed] [Google Scholar]
- 15.Grossman D, McNiff JM, Li F, Altieri DC. Expression of the apoptosis inhibitor, survivin, in nonmelanoma skin cancer and gene targeting in a keratinocyte cell line. Lab Invest. 1999;79:1121–1126. [PubMed] [Google Scholar]
- 16.Ito T, Shiraki K, Sugimoto K, Yamanaka T, Fujikawa K, Ito M, Takase K, Moriyama M, Kawano H, Hayashida M, et al. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000;31:1080–1085. doi: 10.1053/he.2000.6496. [DOI] [PubMed] [Google Scholar]
- 17.Okada E, Murai Y, Matsui K, Isizawa S, Cheng C, Masuda M, Takano Y. Survivin expression in tumor cell nuclei is predictive of a favorable prognosis in gastric cancer patients. Cancer Lett. 2001;163:109–116. doi: 10.1016/s0304-3835(00)00677-7. [DOI] [PubMed] [Google Scholar]
- 18.Beardsmore DM, Verbeke CS, Sarela AI, Li AGK, Davis CL, Guillou PJ. The expression of survivin, an inhibitor of apoptosis, in esophageal cancer. Gastroenterology. 2001;120:660–668. [Google Scholar]
- 19.Cohen C, Lohmann CM, Cotsonis G, Lawson D, Santoianni R. Survivin expression in ovarian carcinoma: correlation with apoptotic markers and prognosis. Mod Pathol. 2003;16:574–583. doi: 10.1097/01.MP.0000073868.31297.B0. [DOI] [PubMed] [Google Scholar]
- 20.Zhu XD, Lin GJ, Qian LP, Chen ZQ. Expression of survivin in human gastric carcinoma and gastric carcinoma model of rats. World J Gastroenterol. 2003;9:1435–1438. doi: 10.3748/wjg.v9.i7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarela AI, Scott N, Ramsdale J, Markham AF, Guillou PJ. Immunohistochemical detection of the anti-apoptosis protein, survivin, predicts survival after curative resection of stage II colorectal carcinomas. Ann Surg Oncol. 2001;8:305–310. doi: 10.1007/s10434-001-0305-0. [DOI] [PubMed] [Google Scholar]
- 22.Ikeguchi M, Ueda T, Sakatani T, Hirooka Y, Kaibara N. Expression of survivin messenger RNA correlates with poor prognosis in patients with hepatocellular carcinoma. Diagn Mol Pathol. 2002;11:33–40. doi: 10.1097/00019606-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Ikehara M, Oshita F, Kameda Y, Ito H, Ohgane N, Suzuki R, Saito H, Yamada K, Noda K, Mitsuda A. Expression of survivin correlated with vessel invasion is a marker of poor prognosis in small adenocarcinoma of the lung. Oncol Rep. 2002;9:835–838. [PubMed] [Google Scholar]
- 24.Ikeguchi M, Kaibara N. survivin messenger RNA expression is a good prognostic biomarker for oesophageal carcinoma. Br J Cancer. 2002;87:883–887. doi: 10.1038/sj.bjc.6600546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takai N, Miyazaki T, Nishida M, Nasu K, Miyakawa I. Survivin expression correlates with clinical stage, histological grade, invasive behavior and survival rate in endometrial carcinoma. Cancer Lett. 2002;184:105–116. doi: 10.1016/s0304-3835(02)00190-8. [DOI] [PubMed] [Google Scholar]
- 26.Kappler M, Kotzsch M, Bartel F, Füssel S, Lautenschläger C, Schmidt U, Würl P, Bache M, Schmidt H, Taubert H, et al. Elevated expression level of survivin protein in soft-tissue sarcomas is a strong independent predictor of survival. Clin Cancer Res. 2003;9:1098–1104. [PubMed] [Google Scholar]
- 27.Kennedy SM, O'Driscoll L, Purcell R, Fitz-Simons N, McDermott EW, Hill AD, O'Higgins NJ, Parkinson M, Linehan R, Clynes M. Prognostic importance of survivin in breast cancer. Br J Cancer. 2003;88:1077–1083. doi: 10.1038/sj.bjc.6600776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeguchi M, Nakamura S, Kaibara N. Quantitative analysis of expression levels of bax, bcl-2, and survivin in cancer cells during cisplatin treatment. Oncol Rep. 2002;9:1121–1126. [PubMed] [Google Scholar]
- 29.Zaffaroni N, Pennati M, Colella G, Perego P, Supino R, Gatti L, Pilotti S, Zunino F, Daidone MG. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell Mol Life Sci. 2002;59:1406–1412. doi: 10.1007/s00018-002-8518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaffaroni N, Daidone MG. Survivin expression and resistance to anticancer treatments: perspectives for new therapeutic interventions. Drug Resist Updat. 2002;5:65–72. doi: 10.1016/s1368-7646(02)00049-3. [DOI] [PubMed] [Google Scholar]
- 31.Altieri DC. Blocking survivin to kill cancer cells. Methods Mol Biol. 2003;223:533–542. doi: 10.1385/1-59259-329-1:533. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto T, Manome Y, Nakamura M, Tanigawa N. Downregulation of survivin expression by induction of the ef-fector cell protease receptor-1 reduces tumor growth potential and results in an increased sensitivity to anticancer agents in human colon cancer. Eur J Cancer. 2002;38:2316–2324. doi: 10.1016/s0959-8049(02)00247-2. [DOI] [PubMed] [Google Scholar]
- 33.Chen T, Tian FZ, Cai ZH, Yin ZL, Zhao TJ. [The signal transduction pathway related to hepatocellular carcinoma apoptosis induced by survivin antisense oligonucleotide] Zhonghua Yi Xue Za Zhi. 2003;83:425–429. [PubMed] [Google Scholar]
- 34.Asanuma K, Moriai R, Yajima T, Yagihashi A, Yamada M, Kobayashi D, Watanabe N. Survivin as a radioresistance factor in pancreatic cancer. Jpn J Cancer Res. 2000;91:1204–1209. doi: 10.1111/j.1349-7006.2000.tb00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asanuma K, Kobayashi D, Furuya D, Tsuji N, Yagihashi A, Watanabe N. A role for survivin in radioresistance of pancreatic cancer cells. Jpn J Cancer Res. 2002;93:1057–1062. doi: 10.1111/j.1349-7006.2002.tb02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodel C, Haas J, Groth A, Grabenbauer GG, Sauer R, Rodel F. Spon-taneous and radiation-induced apoptosis in colorectal carcinoma cells with different intrinsic radiosensitivities: survivin as a radiore-sistance factor. Int J Radiat Oncol Biol Phys. 2003;55:1341–1347. doi: 10.1016/s0360-3016(02)04618-7. [DOI] [PubMed] [Google Scholar]
- 37.Pennati M, Binda M, Colella G, Folini M, Citti L, Villa R, Daidone MG, Zaffaroni N. Radiosensitization of human melanoma cells by ribozyme-mediated inhibition of survivin expression. J Invest Dermatol. 2003;120:648–654. doi: 10.1046/j.1523-1747.2003.12082.x. [DOI] [PubMed] [Google Scholar]


