Abstract
AIM: To investigate the ability of agonists of PAR-2 to stimulate release of tryptase and histamine from human colon mast cells and the potential mechanisms.
METHODS: Enzymatically dispersed cells from human colons were challenged with tc-LIGRLO, tc-OLRGIL, SLIGKV, VKGILS, trypsin, anti-IgE or calcium ionophore A23187, and the cell supernatants after challenge were collected. Tryptase release was determined with a sandwich ELISA procedure and histamine release was measured using a glass fibre-based fluorometric assay.
RESULTS: Both PAR-2 agonists tc-LIGRLO-NH2 and SLIGKV-NH2 were able to induce dose dependent release of tryptase and histamine from colon mast cells. More than 2.5 fold increase in both tryptase and histamine release was provoked by 100 μmol/mL tc-LIGRLO-NH2, in comparison with only 2.0 fold increase being stimulated by SLIGKV-NH2. The reverse peptides tc-OLRGIL-NH2 and VKGILS-NH2 at the concentrations tested had no effect on the release of these two mediators. The maximum tryptase release elicited by tc-LIGRLO-NH2 was similar to that induced by anti-IgE (10 μg/mL) or calcium ionophore (1 μg/mL), though the latter was a more potent stimulus for histamine release. Both histamine and tryptase release in response to tc-LIGRLO-NH2 were completed within 3 min. Trypsin at concentrations from 1.0 to 100 μg/mL was capable of provoking a dose dependent release of tryptase as well as histamine with a maximum of 16 ng/mL tryptase and 14 ng/mL histamine release being achieved. An approximately 80% and 70% inhibition of trypsin induced release of tryptase and histamine were observed with SBTI, respectively. Pretreatment of cells with metabolic inhibitors or pertussis toxin abolished the actions of tc-LIGRLO-NH2, SLIGKV-NH2 and trypsin.
CONCLUSION: The agonists of PAR-2 and trypsin are potent secretagogues of human colon mast cells, which are likely to contribute to the development of inflammatory disorders in human gut.
INTRODUCTION
PAR-2 is a 7-transmembrane G protein-coupled receptor, which can be activated by serine proteinase trypsin and mast cell tryptase[1], and some synthetic peptides corresponding to the N-terminal tethered ligand sequences that are unmasked by proteolytic cleavage including SLIGKV in man[2] and SLIGRL in rodent[3].
In gastrointestinal tract, PAR-2 activation has been found to be actively involved in a number of inflammatory processes including induction of granulocyte infiltration, colon edema, tissue damage in mouse[4,5], induction of gastric mucus secretion and mucosal cytoprotection[6] in rat, excitation of submucosal neurons of guinea pig small intestine[7,8] and activation of ion secretion from human colon[9]. Moreover, upregulation of expression of PAR-2 in ulcerative colitis[10] suggested a strong possibility of involvement of mast cell tryptase and trypsin in inflammatory bowel disease.
Indeed, increased numbers of mast cells were observed in chronic ulcerative colitis[11] and Crohn’s disease[12], elevated histamine levels or enhanced histamine metabolism were found in collagenous colitis, food allergy[13], Crohn’s disease[14], ulcerative colitis[14,15] and allergic enteropathy[15] and increased levels of tryptase were detected in ulcerative colitis[16], indicating that these two proinflammatory mediators are involved in the pathogenesis of certain gastrointestinal diseases.
We have reported previously that mast cell tryptase was able to activate mast cells[17,18], which presents a self-amplification mechanism of mast cell degranulation. Since the receptor of tryptase, PAR-2 was localized on human mast cells[19] and PAR-2 agonists were reported to be capable of activating rat peritoneal mast cells[20], it is likely that PAR-2 agonists and trypsin may have ability to activate human mast cells. We therefore examined the effect of trypsin and PAR-2 agonists on tryptase and histamine release from human colon mast cells in the current study.
MATERIALS AND METHODS
Reagents
The following compounds were purchased from Sigma (St. Louis, USA): collagenase (type I), hyaluronidase (type I), soy bean trypsin inhibitor (SBTI), bovine serum albumin (BSA, fraction V), penicillin and streptomycin, calcium ionophore A23187, antimycin A, human trypsin, 2-deoxy-D-glucose, pertussis toxin. Goat anti-human IgE (inactivated) was from Serotec (Kidlington, Oxford, UK). FCS and minimum essential medium (MEM) containing 25 mM N-2-hydroxylethylpiperazine-N’-2-ethane sulphonic acid (HEPES) were from Gibco (Paisley, Renfrewshire, UK). Peptides SLIGKV-NH2, VKGILS-NH2, trans-cinnamoyl-Leu-Ile-Gly-Arg-Leu-Orn-amide (tc-LIGRLO) and trans-cinnamoyl-Orn-Leu-Arg-Gly-Ile-Leu-amide (tc-OLRGIL) were > 98% purity and from Meilian Corporation (Xian, China). A polyclonal antibody and a monoclonal antibody against tryptase were donated by Dr Andrew F. Walls (University of Southampton, UK). Histamine plate was from RefLab (Copenhagen, Denmark). HEPES and all other chemicals were of analytical grade.
Dispersion of mast cells
The mast cell dispersion procedure was similar to the one described previously[21,22]. Human colon tissue was obtained from patients with carcinoma of colon at colectomy. Only macroscopically normal tissue was used for the study. After removal of fat, tissue was washed and chopped finely with scissors into fragments of 0.5-2.0 mm3, and then incubated with 1.5 mg/mL collagenase and 0.75 mg/mL hyaluronidase in MEM containing 2% fetal calf serum (1 g colon/10 mL buffer) for 70 min at 37 °C. Dispersed cells were separated from undigested tissue by filtration through nylon gauze (pore size 100 µm diameter), washed and maintained in MEM (containing 10% FCS, 200 U/mL penicillin, 200 µg/mL streptomycin) on a roller overnight at room temperature. Mast cell purity, as determined by light microscopy after stained by alcine blue, ranged from 3.5% to 5.5%.
Mast cell challenge
Dispersed cells were resuspended in HEPES buffered salt solution (HBSS, pH7.4) with 1.8 mM CaCl2 and 0.5 mM MgCl2 (complete HBSS), and 100 µL aliquots containing 4-6 × 103 mast cells were added to a 50 µL tc-LIGRLO, tc-OLRGIL, SLIGKV, VKGILS, trypsin, control secretagogue or buffer alone and incubated for 15 min at 37 °C. The reaction was terminated by the addition of 150 µL ice cold incomplete HBSS and the tubes were centrifuged immediately (500 g, 10 min, 4 °C). All experiments were performed in duplicate. For the experiments with pertussis toxin, cells were incubated with 0.1 or 1.0 µg/mL pertussis toxin for four hours at 37 °C, and then washed with HBSS before adding stimulus. Similarly, for the experiments with metabolic inhibitors, cells were incubated with 2-deoxy-D-glucose (10 mmol/L) and antimycin A (1 µmol/L) for 40 min at 37 °C before challenged with stimulus. For the measurement of total histamine concentration in certain tubes, the suspension was boiled for 6 min. Supernatants were stored at -20 °C until use.
Tryptase and histamine measurement
Tryptase concentrations were measured with a sandwich ELISA procedure with a specific polyclonal antibody against human tryptase as the capture antibody and AA5 a monoclonal antibody specific for human tryptase as the detecting antibody[23]. Histamine concentrations were determined using a glass fibre-based fluorometric assay[17].
Statistical analysis
Data are shown as mean ± SEM for the number of experiments (n) indicated, and the paired Student’s t test was applied to evaluate two independent samples. In all analyses P < 0.05 was taken as statistically significant.
RESULTS
Induction of tryptase release by agonists of PAR-2
Both PAR-2 agonists tc-LIGRLO-NH2 and SLIGKV-NH2 were able to induce dose dependent release of tryptase from colon mast cells (Figure 1). More than 2.5 fold increase in tryptase release was provoked by 100 μmol/mL tc-LIGRLO-NH2, even at a concentration as low as 1.0 μmol/mL tc-LIGRLO-NH2 was able to elicit a 2.2 fold increase in the release of tryptase. Although the potency of SLIGKV-NH2 appeared slightly weaker than that of tc-LIGRLO-NH2, it could still stimulate as much as two fold increase in the release of tryptase from colon mast cells. The reverse peptides tc-OLRGIL-NH2 and VKGILS-NH2 had no effect on the release of tryptase when being added at the concentrations from 0.1 to 300 μmol/mL (Figure 1). The quantity of tryptase released elicited by tc-LIGRLO-NH2 (100 μmol/mL) was similar to that induced by 10 μg/mL anti-IgE or 1 μg/mL calcium ionophore. Trypsin at the concentrations of 1.0-100 μg/mL was capable of provoking a dose dependent release of tryptase with a maximum of 16 ng/mL tryptase being released by 100 μg/mL trypsin (Figure 1). Approximately 80% trypsin induced release of tryptase was diminished by SBTI when trypsin and SBTI were added to cells at the same time (Figure 1).
Figure 1.
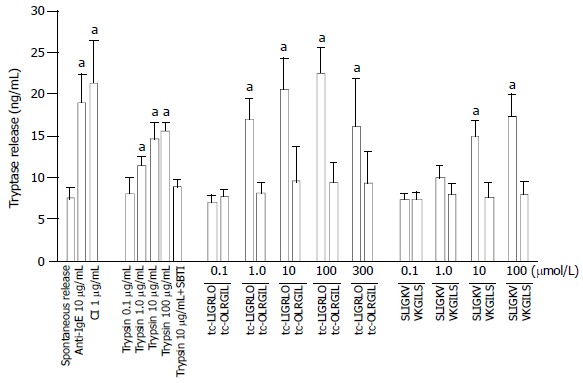
Effects of tc-LIGRLO, tc-OLRGIL, SLIGKV, VKGILS, trypsin, anti-IgE and calcium ionophore A23187 (CI) on tryptase release from colon mast cells. The values shown are mean ± SEM for four separate experiments. Stimulus or control was incubated with cells for 15 min before termination of the reactions. aP < 0.05 compared with buffer alone group (paired Student’s t test).
Induction of histamine release by agonists of PAR-2
Both PAR-2 agonists tc-LIGRLO-NH2 and SLIGKV-NH2 were also able to induce a dose dependent release of histamine from colon mast cells. More than 2.5 and 2.0 fold increases in histamine release were provoked by 100 μmol/mL tc-LIGRLO-NH2 and SLIGKV-NH2, respectively. However, histamine release elicited by tc-LIGRLO-NH2 (100 μmol/mL) was less than that induced by anti-IgE (10 μg/mL) and only half of that provoked by calcium ionophore (1 μg/mL). The reverse peptides tc-OLRGIL-NH2 and VKGILS-NH2 had no effect on the release of histamine when being added at concentrations up to 300 μmol/mL (Figure 2). Trypsin at the concentrations from 1.0 to 100 μg/mL was capable of provoking a dose dependent release of histamine with a maximum of 14 ng/mL histamine being released by 100 μg/mL trypsin. Approximately 70% trypsin induced release of histamine was reduced by SBTI when trypsin and SBTI were added at the same time to cells (Figure 2).
Figure 2.
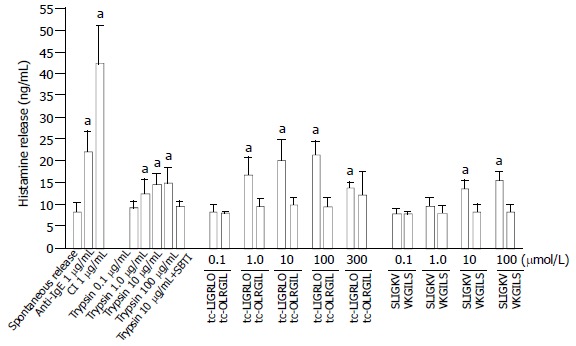
Effects of tc-LIGRLO, tc-OLRGIL, SLIGKV, VKGILS, trypsin, anti-IgE and calcium ionophore A23187 (CI) on histamine release from colon mast cells. The values shown are mean ± SEM for four separate experiments. Stimulus or control was incubated with cells for 15 min before termination of the reactions. aP < 0.05 compared with buffer alone group (paired Student’s t test).
Time course for tc-LIGRLO-NH2
The release of tryptase and histamine in response to tc-LIGRLO-NH2 was maximized within 3 min following addition of 100 μmol/mL tc-LIGRLO-NH2 to colon mast cells. The maximum release of tryptase and histamine was then maintained for at least 15 min (Figure 3). When tc-LIGRLO-NH2, anti-IgE and calcium ionophore were incubated with cells for a prolonged period of 30 min, the amount of tryptase and histamine released appeared not being increased (Table 1).
Figure 3.
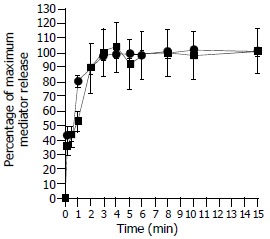
Time course for tc-LIGRLO (100 µM) induced release of tryptase (─■─) and histamine (─●─) from colon mast cells. Data shown are mean ± SEM of four separate experiments.
Table 1.
Effects of tc-LIGRLO, tc-OLRGIL, anti-IgE and cal-cium ionophore A23187 (CI) on tryptase and histamine release from colon mast cells following 30 min incubation period
| Concentration of stimulus | Tryptase (ng/mL) | Histamine (ng/mL) | |
| Buffer alone | 8.2 ± 0.6 | 9.5 ± 1.7 | |
| tc-LIGRLO | 100 µmol/mL | 22 ± 3.0a | 17 ± 1.3a |
| tc-OLRGIL | 100 µmol/mL | 8.4 ± 2.8 | 10 ± 1.9 |
| Anti-IgE | 10 µg/mL | 19 ± 4.1a | 19 ± 4.8a |
| CI | 1.0 µg/mL | 13 ± 0.6a | 34 ± 4.3a |
The values shown are mean ± SEM for four separate experiments performed in duplicate.
P < 0.05 compared with buffer alone group (paired Student’s t test).
Effects of pertussis toxin and metabolic inhibitors on tryptase and histamine release
Tryptase release induced by tc-LIGRLO-NH2, SLIGKV-NH2 and trypsin was abolished by pretreatment of colon mast cells with metabolic inhibitors or pertussis toxin (Table 2). The same treatment was also able to almost completely inhibit histamine release from mast cells (Table 3).
Table 2.
Inhibition of tc-LIGRLO, SLIGKV, trypsin, anti-IgE or calcium ionophore A23187 (CI) induced tryptase release from colon mast cells by pertussis toxin (1 µg/mL) and metabolic inhibitors
| Concentration of stimulus |
% inhibition of tryptase release |
||
| Pertussis toxin | Metabolic inhibitors | ||
| tc-LIGRLO | 100 µmol/mL | 85 ± 9.6a | 91 ± 9.3a |
| SLIGKV | 100 µmol/mL | 91 ± 4.8a | 85 ± 9.6a |
| Trypsin | 10 µg/mL | 100 ± 0a | 91 ± 3.9a |
| Anti-IgE | 10 µg/mL | 87 ± 10a | 94 ± 3.6a |
| CI | 1.0 µg/mL | 84 ± 8.8a | 95 ± 4.0a |
The values shown are mean ± SEM for four separate experiments performed in duplicate.
P < 0.05 compared with the uninhibited control group (paired Student’s t test).
Table 3.
Inhibition of tc-LIGRLO, SLIGKV, trypsin, anti-IgE or calcium ionophore A23187 (CI) induced histamine release from colon mast cells by pertussis toxin (1 µg/mL) and metabolic inhibitors
| Concentration of stimulus |
% inhibition of histamine release |
||
| Pertussis toxin | Metabolic inhibitors | ||
| tc-LIGRLO | 100 µmol/mL | 92 ± 5.4a | 98 ± 2.0a |
| SLIGKV | 100 µmol/mL | 95 ± 5.9a | 92 ± 3.8a |
| Trypsin | 10 µg/mL | 84 ± 6.8a | 88 ± 8.7a |
| Anti-IgE | 10 µg/mL | 84 ± 5.2a | 99 ± 1.1a |
| CI | 1.0 µg/mL | 97 ± 2.4a | 99 ± 0.1a |
The values shown are mean ± SEM for four separate experiments performed in duplicate.
P < 0.05 compared with the uninhibited control group (paired Student’s t test).
DISCUSSION
The finding in the current study that PAR-2 agonists were able to activate human mast cells fulfilled our hypothesis that human mast cells possess a self-amplification mechanism to IgE dependent activation through their mediator tryptase. That is to say that upon activation, mast cells release tryptase, and the released tryptase then activates their neighbouring mast cells or acts on its host mast cells through PAR-2.
The maximum 22.5 ng/mL tryptase and 21 ng/mL histamine release induced by tc-LIGRLO-NH2 were comparable to those induced by 10 mg/mL anti-IgE, indicating that the PAR-2 agonist is a potent stimulus of mast cells. SLIGKV was also able to provoke a significant tryptase and histamine release from colon mast cells, but its action appeared slightly weaker than tc-LIGRLO-NH2. This could be due to the structural difference between the two molecules. As little as 1.0 mmol/mL tc-LIGRLO-NH2 was able to stimulate a significant release of tryptase and histamine, suggesting further that this compound is a potent mast cell secretagogue, and the concentration of the stimulus is easy to be achieved under physiological conditions. It was little surprised to observe that trypsin was able to activate colon mast cells as there should be a relatively high concentration of trypsin in human intestinal tract, and some of them should be able to penetrate through the epithelial lining of intestine, particularly when paracellular permeability of colon was increased upon PAR-2 activation[4]. Nevertheless, it should be interesting to learn the physiological or pathophysiological role of mast cell activation by trypsin in gastrointestinal tract in future. The fact that reversed peptides of PAR-2 agonists had little effect on tryptase and histamine release from colon mast cells proved further the specificity of the PAR-2 agonists on mast cells. The maximum release of tryptase and histamine from colon mast cells was achieved at 3 min after adding tc-LIGRLO-NH2 to cells, suggesting that its action on mast cells was slower than that of neuropeptide substance P, which reached peak histamine release within 20 sec of incubation[24], but faster than that induced by anti-IgE and calcium ionophore, which required at least 6 min to complete[21].
The inhibition of trypsin induced tryptase and histamine release by SBTI, a mixed type of inhibitor of tryptic enzymes[25], indicating that the process was not cytotoxic and required an intact catalytic site of trypsin. Pretreatment of cells with metabolic inhibitors antimycin A which blocks the oxidative phosphorylation process of cells, and 2-deoxy-D-glucose which blocks anaerobic metabolism pathway in cells, abolished the actions of tc-LIGRLO-NH2, SLIGKV, trypsin, anti-IgE as well as calcium ionophore, indicating that the release of tryptase and histamine induced by them was a non-cytotoxic process, and was dependent on cell energy supply. Tryptase and histamine release provoked by tc-LIGRLO-NH2, SLIGKV, trypsin, anti-IgE and calcium ionophore were also inhibited by pretreatment of cells with pertussis toxin, suggesting that the degranulation process elicited by them was associated with the activation of G-protein coupled receptors[26].
Over the last decade, a number of functions of mast cell tryptase have been discovered. These include induction of microvascular leakage[27], accumulation and activation of eosinophils and neutrophils[28,29], provocation of release of IL-8 from epithelial cells[30]. Similarly, PAR-2 agonists were found to be able to provoke the inflammatory response in the rat paw[31,32] and mediate eosinophil infiltration and hyperreactivity in allergic inflammation of the airway[33]. Of particular importance is the finding that the delayed onset of inflammation was observed in protease-activated receptor-2-deficient mice[34]. These highlighted the crucial role of tryptase and its receptor PAR-2 in inflammation. The finding that PAR-2 agonists were able to activate human colon mast cells in the current study added a new concept to the tryptase and PAR-2 theory, indicating further the importance of them in the pathogenesis of inflammation. In conclusion, the agonists of PAR-2 and trypsin are potent secretagogues of human colon mast cells, which are likely to contribute to the development of inflammatory disorders in human gut.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30140023, and the Li Ka Shing Foundation, Hong Kong, China, No. C0200001
Edited by Wang XL
References
- 1.Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie JA, Schechter N, Woolkalis M, Brass LF. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 2.Hollenberg MD, Saifeddine M, al-Ani B, Kawabata A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can J Physiol Pharmacol. 1997;75:832–841. [PubMed] [Google Scholar]
- 3.Al-Ani B, Saifeddine M, Hollenberg MD. Detection of functional receptors for the proteinase-activated-receptor-2-activating polypeptide, SLIGRL-NH2, in rat vascular and gastric smooth muscle. Can J Physiol Pharmacol. 1995;73:1203–1207. doi: 10.1139/y95-172. [DOI] [PubMed] [Google Scholar]
- 4.Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, Wallace JL, Hollenberg MD, Bunnett NW, Garcia-Villar R, et al. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol. 2002;161:1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cenac N, Garcia-Villar R, Ferrier L, Larauche M, Vergnolle N, Bunnett NW, Coelho AM, Fioramonti J, Bueno L. Proteinase-activated receptor-2-induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J Immunol. 2003;170:4296–4300. doi: 10.4049/jimmunol.170.8.4296. [DOI] [PubMed] [Google Scholar]
- 6.Kawabata A, Kinoshita M, Nishikawa H, Kuroda R, Nishida M, Araki H, Arizono N, Oda Y, Kakehi K. The protease-activated receptor-2 agonist induces gastric mucus secretion and mucosal cytoprotection. J Clin Invest. 2001;107:1443–1450. doi: 10.1172/JCI10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao C, Liu S, Hu HZ, Gao N, Kim GY, Xia Y, Wood JD. Serine proteases excite myenteric neurons through protease-activated receptors in guinea pig small intestine. Gastroenterology. 2002;123:1554–1564. doi: 10.1053/gast.2002.36581. [DOI] [PubMed] [Google Scholar]
- 8.Reed DE, Barajas-Lopez C, Cottrell G, Velazquez-Rocha S, Dery O, Grady EF, Bunnett NW, Vanner SJ. Mast cell tryptase and proteinase-activated receptor 2 induce hyperexcitability of guinea-pig submucosal neurons. J Physiol. 2003;547:531–542. doi: 10.1113/jphysiol.2002.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mall M, Gonska T, Thomas J, Hirtz S, Schreiber R, Kunzelmann K. Activation of ion secretion via proteinase-activated receptor-2 in human colon. Am J Physiol Gastrointest Liver Physiol. 2002;282:G200–G210. doi: 10.1152/ajpgi.00137.2001. [DOI] [PubMed] [Google Scholar]
- 10.Kim JA, Choi SC, Yun KJ, Kim DK, Han MK, Seo GS, Yeom JJ, Kim TH, Nah YH, Lee YM. Expression of protease-activated receptor 2 in ulcerative colitis. Inflamm Bowel Dis. 2003;9:224–229. doi: 10.1097/00054725-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Stoyanova II, Gulubova MV. Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta Histochem. 2002;104:185–192. doi: 10.1078/0065-1281-00641. [DOI] [PubMed] [Google Scholar]
- 12.Furusu H, Murase K, Nishida Y, Isomoto H, Takeshima F, Mizuta Y, Hewlett BR, Riddell RH, Kohno S. Accumulation of mast cells and macrophages in focal active gastritis of patients with Crohn's disease. Hepatogastroenterology. 2002;49:639–643. [PubMed] [Google Scholar]
- 13.Schwab D, Hahn EG, Raithel M. Enhanced histamine metabolism: a comparative analysis of collagenous colitis and food allergy with respect to the role of diet and NSAID use. Inflamm Res. 2003;52:142–147. doi: 10.1007/s000110300064. [DOI] [PubMed] [Google Scholar]
- 14.Winterkamp S, Weidenhiller M, Otte P, Stolper J, Schwab D, Hahn EG, Raithel M. Urinary excretion of N-methylhistamine as a marker of disease activity in inflammatory bowel disease. Am J Gastroenterol. 2002;97:3071–3077. doi: 10.1111/j.1572-0241.2002.07028.x. [DOI] [PubMed] [Google Scholar]
- 15.Raithel M, Matek M, Baenkler HW, Jorde W, Hahn EG. Mucosal histamine content and histamine secretion in Crohn's disease, ulcerative colitis and allergic enteropathy. Int Arch Allergy Immunol. 1995;108:127–133. doi: 10.1159/000237129. [DOI] [PubMed] [Google Scholar]
- 16.Raithel M, Winterkamp S, Pacurar A, Ulrich P, Hochberger J, Hahn EG. Release of mast cell tryptase from human colorectal mucosa in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:174–179. doi: 10.1080/003655201750065933. [DOI] [PubMed] [Google Scholar]
- 17.He S, Gaça MD, Walls AF. A role for tryptase in the activation of human mast cells: modulation of histamine release by tryptase and inhibitors of tryptase. J Pharmacol Exp Ther. 1998;286:289–297. [PubMed] [Google Scholar]
- 18.He S, Gaça MD, Walls AF. The activation of synovial mast cells: modulation of histamine release by tryptase and chymase and their inhibitors. Eur J Pharmacol. 2001;412:223–229. doi: 10.1016/s0014-2999(01)00734-8. [DOI] [PubMed] [Google Scholar]
- 19.D'Andrea MR, Rogahn CJ, Andrade-Gordon P. Localization of protease-activated receptors-1 and -2 in human mast cells: indications for an amplified mast cell degranulation cascade. Biotech Histochem. 2000;75:85–90. doi: 10.3109/10520290009064152. [DOI] [PubMed] [Google Scholar]
- 20.Stenton GR, Nohara O, Déry RE, Vliagoftis H, Gilchrist M, Johri A, Wallace JL, Hollenberg MD, Moqbel R, Befus AD. Proteinase-activated receptor (PAR)-1 and -2 agonists induce mediator release from mast cells by pathways distinct from PAR-1 and PAR-2. J Pharmacol Exp Ther. 2002;302:466–474. doi: 10.1124/jpet.302.2.466. [DOI] [PubMed] [Google Scholar]
- 21.He SH, Xie H, He YS. Induction of tryptase and histamine release from human colon mast cells by IgE dependent or independent mechanisms. World J Gastroenterol. 2004;10:319–322. doi: 10.3748/wjg.v10.i3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He SH, Xie H. Modulation of histamine release from human colon mast cells by protease inhibitors. World J Gastroenterol. 2004;10:337–341. doi: 10.3748/wjg.v10.i3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckley MG, Walters C, Wong WM, Cawley MI, Ren S, Schwartz LB, Walls AF. Mast cell activation in arthritis: detection of alpha- and beta-tryptase, histamine and eosinophil cationic protein in synovial fluid. Clin Sci (Lond) 1997;93:363–370. doi: 10.1042/cs0930363. [DOI] [PubMed] [Google Scholar]
- 24.Lowman MA, Benyon RC, Church MK. Characterization of neuropeptide-induced histamine release from human dispersed skin mast cells. Br J Pharmacol. 1988;95:121–130. doi: 10.1111/j.1476-5381.1988.tb16555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He S, Gaça MD, McEuen AR, Walls AF. Inhibitors of chymase as mast cell-stabilizing agents: contribution of chymase in the activation of human mast cells. J Pharmacol Exp Ther. 1999;291:517–523. [PubMed] [Google Scholar]
- 26.Piliponsky AM, Gleich GJ, Nagler A, Bar I, Levi-Schaffer F. Non-IgE-dependent activation of human lung- and cord blood-derived mast cells is induced by eosinophil major basic protein and modulated by the membrane form of stem cell factor. Blood. 2003;101:1898–1904. doi: 10.1182/blood-2002-05-1488. [DOI] [PubMed] [Google Scholar]
- 27.He S, Walls AF. Human mast cell tryptase: a stimulus of microvascular leakage and mast cell activation. Eur J Pharmacol. 1997;328:89–97. doi: 10.1016/s0014-2999(97)83033-6. [DOI] [PubMed] [Google Scholar]
- 28.He S, Peng Q, Walls AF. Potent induction of a neutrophil and eosinophil-rich infiltrate in vivo by human mast cell tryptase: selective enhancement of eosinophil recruitment by histamine. J Immunol. 1997;159:6216–6225. [PubMed] [Google Scholar]
- 29.Temkin V, Kantor B, Weg V, Hartman ML, Levi-Schaffer F. Tryptase activates the mitogen-activated protein kinase/activator protein-1 pathway in human peripheral blood eosinophils, causing cytokine production and release. J Immunol. 2002;169:2662–2669. doi: 10.4049/jimmunol.169.5.2662. [DOI] [PubMed] [Google Scholar]
- 30.Cairns JA, Walls AF. Mast cell tryptase is a mitogen for epithelial cells. Stimulation of IL-8 production and intercellular adhesion molecule-1 expression. J Immunol. 1996;156:275–283. [PubMed] [Google Scholar]
- 31.Vergnolle N, Hollenberg MD, Sharkey KA, Wallace JL. Characterization of the inflammatory response to proteinase-activated receptor-2 (PAR2)-activating peptides in the rat paw. Br J Pharmacol. 1999;127:1083–1090. doi: 10.1038/sj.bjp.0702634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vergnolle N. Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol. 1999;163:5064–5069. [PubMed] [Google Scholar]
- 33.Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, Gater PR, Geppetti P, Bertrand C, Stevens ME. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 34.Lindner JR, Kahn ML, Coughlin SR, Sambrano GR, Schauble E, Bernstein D, Foy D, Hafezi-Moghadam A, Ley K. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. J Immunol. 2000;165:6504–6510. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]


