Abstract
AIM: To study the relationship between expression of platelet-derived growth factor-BB (PDGF-BB) and fibrogenesis in chronic hepatitis B.
METHODS: Hepatic tissues from 43 patients with chronic hepatitis B were embedded in paraffin. The sections were stained with HE and picric acid-sirius red to determine inflammatory activity and fibrosis stages. PDGF-BB expression was detected by immunohistochemistry and assessed semiquantatively. Levels of serum hyaluronic acid (HA), pro-collagen III (PCIII), collagen IV (IV-C) and laminin (LN) were examined by radioimmunoassay (RIA).
RESULTS: The expression level of PDGF-BB was found to be positively correlated with inflammatory activity, fibrosis stage and grade of histological findings (τ = 0.58, 0.55, 0.55, P < 0.01). The positive correlation was also observed between tissue level of PDGF-BB expression and contents of HA, PCIII, IV-C and LN in the circulation (r = 0.52, 0.32, 0.40, 0.33, P < 0.05).
CONCLUSION: PDGF-BB may play some role in the development and progression of liver fibrosis.
INTRODUCTION
In chronic liver diseases, fibrosis is one of the parameters indicating a progressive process leading to cirrhosis. Hepatic stellate cells (HSCs) are considered to play a central role in the pathogenesis of liver fibrosis[1,2]. During the process, HSCs proliferate and differentiate into myofibroblast-like cells, synthesizing various extracellular matrix (ECM) components including collagen[2,3].
Platelet-derived growth factor (PDGF) is the most potent mitogen for HSCs[4-6], which were currently indicated as the principle cells producing connective tissue in fibrotic liver[7-9]. PDGF was currently indicated as a major inflammatory growth factor playing a central role in the repair process after acute and chronic tissue injuries. Several recent studies have demonstrated a pathogenic role of PDGF in several chronic inflammatory disorders including glomerulonephritis[10,11], scleroderma[12], rheumatoid arthritis[13], idiopathic pulmonary fibrosis[14] and atherosclerosis[15,16]. The presence of inflammatory infiltrates and the excessive deposition of collagenous matrix are the prominent features of chronic hepatitis. Along these lines, PDGF may also be involved in hepatic fibrogenesis.
PDGF consists of two polypeptide chains (A and B). Three isoforms have been described, namely PDGF-AA, -AB, -BB[17-19]. Recent studies have shown that PDGF-AB and -BB isoforms are more mitogenic for HSCs than PDGF-AA[6]. Thus, the expression of the B chain is thought to be more important in hepatic fibrogenesis than that of the A chain.
In this study we used immunohistological methods to detect PDGF-BB in human liver biopsy specimens from 43 patients with chronic hepatitis B. In addition, the levels of serum hyluranic acid (HA), pro-collagen type III (PCIII), collagen type IV (IV-C) and laminin (LN) were examined by radioimmunoassay. The relationship between expression of PDGF-BB, histologic and serum parameters were evaluated.
MATERIALS AND METHODS
Liver and serum samples
Liver tissue samples with various necroinflammatory activities and at different fibrosis stages were obtained by percutaneous liver biopsies from 43 patients with HBV-related chronic hepatitis during routine diagnostic procedures. All of the patients did not receive any anti-inflammatory or anti-fibrotic treatments, such as steroids or interferon. Informed consent was obtained from the patients for the use of their specimens in the investigation. The biopsy specimens were fixed in 10% neutral formalin and embedded in paraffin. Sections of 6 μm in thickness were used for morphological and immunohistochemical examinations. Serum samples were collected and stored at -20 °C.
Histology
Paraffin sections were stained with hematoxylin and eosin (H&E). Alternatively, the Sirius red stain method was used to demonstrate fibrous tissue components. A polarization microscope (DMLB, Leica, Wetzlar, Germany) was used to distinguish type I from type III collagen fibers[20]. Grades of necroinflammation (0 - 4) and stages of fibrosis (0 - 4) were assessed according to the well-established criteria[21,22]. The grades and stages were scored as follows: 0 = 20, 1 = 21, 2 = 22, 3 = 23, and 4 = 24.
Immunohistochemistry
The deparaffinized sections were washed with phosphate-buffered saline (PBS; pH7.4) and incubated in 3% H2O2/methanol for 20 minutes to block endogenous peroxidase. After washed three times in PBS, 5 min each, the sections were heated for 10 min in 0.01 M citrate buffer (pH6.0) using a microwave oven, and then washed three times in PBS, 5 min each, and incubated with a rabbit antibody against human PDGF-BB (Santa Cruz Biotechnology, Inc., Santa Cruz, California, USA) at a dilution of 1:50 in PBS at room temperature for 5 hours. After washed, the immunologic reaction was demonstrated using a kit (Beijing Zhongshan Biotechnology Co., Ltd. Beijing, China) and visualized in a solution containing 3, 3’-diaminobenzidine tetrahydrochloride (DAB). The slides were rinsed in distilled water, counterstained with hematoxylin, dehydrated, air dried, and mounted. PBS was used to substitute for the primary antibody as a negative control.
Sera-assays for hepatic fibrosis
Serum hepatic fibrosis parameters including HA, C-IV, PCIII and LN, were assessed by radioimmunoassays using the kits from Shanghai Navy Medical Institute according to the manufacturer’s instructions.
Statistical analysis
Results were expressed as mean ± SD. Statistical analyses were performed with the one-way ANOVA, Kendall and Spearman rank correlation. Two-tailed tests were done. P < 0.05 was considered statistically significant.
RESULTS
Histological evaluation
The liver tissues of all patients showed various degrees of chronic inflammation and fibrosis. The fibrosis stage was 0 in 7 patients, 1 in 17, 2 in 8, 3 in 6, and 4 in 4. The stage of inflammatory activity was 1 in 13 patients, 2 in 14, 3 in 12, and 4 in 3.
Expression of PDGF-BB in liver biopsy samples
PDGF-BB immunoreactivity was found in a few mesenchymal cells of portal areas and fibrous septa with its products localized diffusely in the cytoplasm compartment. In intralobular areas, some perisinunoidal cells were also immunoreactive in the areas with necroinflammation. The number of positive cells increased with progression of fibrosis (Figures 1, Figures 2, Figures 3, Figures 4, Figures 5) and inflammation. Tables 1, Tables 2, Tables 3 describe PDGF-BB expression levels of different groups respectively. Expression level of PDGF-BB was found to be positively correlated with inflammatory activity, fibrosis stages and grades of histological findings (τ = 0.58, 0.55, 0.55, P < 0.01) in Table 4.
Figure 1.
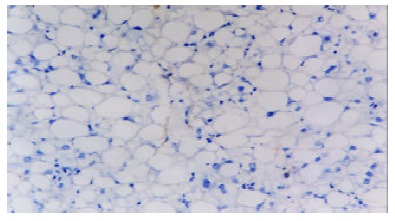
Immunohistochemical detection of PDGF-BB in liver biopsy of a patient with chronic hepatitis B. This case was diagnosed as stage 0. DAB, × 200.
Figure 2.

Immunohistochemical detection of PDGF-BB in liver biopsy of a patient with chronic hepatitis B. This case was diagnosed as stage 1. DAB, × 200.
Figure 3.

Immunohistochemical detection of PDGF-BB in liver biopsy of a patient with chronic hepatitis B. This case was diagnosed as stage 2. DAB, × 200.
Figure 4.
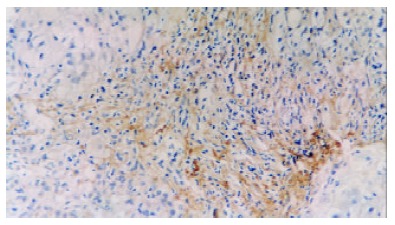
Immunohistochemical detection of PDGF-BB in liver biopsy of a patient with chronic hepatitis B. This case was diagnosed as stage 3. DAB, × 200.
Figure 5.

Immunohistochemical detection of PDGF-BB in liver biopsy of a patient with chronic hepatitis B. This case was diagnosed as stage 4. DAB, × 200.
Table 1.
PDGF-BB expression levels in liver samples with different stages of fibrosis
| Stages of fibrosis | Case numbers | Expression levels (mean ± SD) |
| S0 | 7 | 4.71 ± 1.50 |
| S1 | 17 | 6.47 ± 2.50 |
| S2 | 8 | 11.63 ± 5.66a |
| S3 | 6 | 9.33 ± 2.73a |
| S4 | 5 | 16.50 ± 5.74a |
P < 0.05 vs S0.
Table 2.
PDGF-BB expression levels in liver tissues with dif-ferent grades of necroinflammatory activity
| Grades of inflammatory | Case numbers | Expression levels |
| activity | (mean ± SD) | |
| G1 | 13 | 4.85 ± 1.57 |
| G2 | 14 | 8.21 ± 4.17a |
| G3 | 12 | 10.67 ± 4.12b |
| G4 | 4 | 17.33 ± 6.11b |
P < 0.05 vs G1;
P < 0.01 vs G1.
Table 3.
PDGF-BB expression scores in liver tissues of differ-ent histological grading groups
| Grades of histological finding | Cases numbers | Expression levels |
| (mean ± SD) | ||
| Mild | 27 | 6.59 ± 3.58 |
| Moderate | 10 | 10.20 ± 4.16a |
| Sever | 6 | 15.60 ± 5.37b |
P < 0.05 vs mild group;
P < 0.01 vs mild group.
Table 4.
Relationship between PDGF-BB expression levels in liver tissues of different fibrosis stages, necroinflammatory activity and histological grades
| Coefficients as compared with | |
| PDGF-BB expression levels (τ) | |
| Stages of fibrosis | 0.55b |
| Grades of inflammatory activity | 0.58b |
| And histological findings | 0.55b |
aP < 0.05;
P < 0.01.
Levels of serum hepatic fibrosis
Positive correlations were found between PDGF-BB expression levels and four serum parameters for hepatic fibrosis, including HA, LN, PC III and C-IV, and their coefficient was 0.38, 0.33, 0.32, and 0.40 (P < 0.05), respectively (Table 5).
Table 5.
Relationship between serum fibrosis parameters and PDGF-BB expression levels in liver tissues of different fibrosis stages
| Staging of fibrosis(S) | Cases | PDGF-BB | HA (μg/L) | LN(μg/L) | PCIII(μg/L) | IV-C(μg/L) |
| S0 | 7 | 4.71 ± 1.50 | 102.67 ± 60.38 | 138.23 ± 17.69 | 137.80 ± 35.57 | 58.71 ± 17.40 |
| S1 | 17 | 6.47 ± 2.50 | 168.74 ± 159.76 | 129.97 ± 30.15 | 143.91 ± 51.13 | 64.12 ± 20.26 |
| S2 | 8 | 11.63 ± 5.66 | 434.86 ± 360.58 | 156.39 ± 39.06 | 163.74 ± 70.56 | 86.04 ± 41.94 |
| S3 | 6 | 9.33 ± 2.73 | 430.60 ± 325.37 | 172.65 ± 39.77 | 192.65 ± 40.05 | 115.60 ± 30.62 |
| S4 | 5 | 16.50 ± 5.74 | 392.30 ± 245.53 | 163.25 ± 26.26 | 154.68 ± 22.24 | 122.10 ± 21.34 |
| Coefficients (r) | ... | 0.38a | 0.33a | 0.32a | 0.40b |
P < 0.05;
P < 0.01.
DISCUSSION
Liver fibrosis has been to be resulted from architectural remodeling and progressive deposition of ECM components such as proteoglycans, fibronectin, and collagen molecules[23]. It is one of the major factors affecting the clinical course of chronic liver diseases. Active fibrogenesis is frequently preceded by, and associated with inflammation. Fibrogenic growth factors and cytokines released by inflammatory cells can promote the proliferation of fat-storing cells, and designate HSCs, which were considered to be the main cellular source of matrix proteins in the liver[24,25]. In HSCs isolated from rat, mouse or human liver and activated in culture, the dimeric forms of PDGF, either PDGF-AB or -BB, appeared significantly more potent than that of -AA[26-28]. The results of these in vitro studies implicated that an increased expression of PDGF might occur also in vivo after liver tissue injury. The current study showed that most of the cells immunoreactive for PDGF-BB were located in portal areas. In intralobular areas, the positive cells were seen mainly in the areas with necroinflammation. In addition, the number of cells expressing PDGF-BB was correlated both to the inflammatory activity and to the fibrosis progression. We consider that PDGF may enhance the tissue repair after acute liver injury. In chronic liver diseases, however, the presence of reiterative tissue damage associated with a persistent inflammatory state may cause a sustained release of PDGF involved in the deposition of extracellular matrix. In this context, the prolonged effects of this growth factor on fat-storing cells may contribute to the development of tissue fibrosis rather than to effective tissue repair. Therefore, PDGF-BB may play an essential role in the development and progression of liver fibrosis. The management of PDGF activity by antagonists may prevent aggressive liver fibrosis and improve prognosis of hepatitis B.
It has been well known that collagen type I was the predominant ECM component in fibrosis and cirrhosis, but it required specific procedures to discriminate different collagen fibers on tissue sections[29,30]. We used sirius red stain to subtype the fibers as described by Zhang et al[20]. Under the polarization microscope, collagen types I and III were stained red and green, respectively.
Our data showed that the expression levels of PDGF-BB in the liver were correlative with the proposed serum parameters for hepatic fibrosis, including HA, LN, PC III and C-IV. ECM is a complex of macromolecules that includes collagens, proteoglycans and glycoproteins. In fibrotic liver tissue there is an increase in all of these matrix components, and they increase in serum in patients with chronic hepatitis B or liver cirrhosis. These ECM components have been used as a serum marker of hepatic fibrosis. Therefore, serum levels of connective tissue metabolites are related, to some extent, with the amount of ECM in the liver. Wang et al[31] reported that serum fibrosis markers were fairly well correlated with the staging of fibrosis. Regarding the correlation observed in this study between the serum parameters and the expression of PDGF-BB in liver specimens, tissue PDGF-BB levels may be correlative with the stages of hepatic fibrosis. These findings suggest that measurement of serum PDGF-BB may be useful in estimating the active hepatic fibrogenesis of chronic hepatitis B.
Footnotes
Edited by Su Q and Wang XL
References
- 1.Blomhoff R, Wake K. Perisinusoidal stellate cells of the liver: important roles in retinol metabolism and fibrosis. FASEB J. 1991;5:271–277. doi: 10.1096/fasebj.5.3.2001786. [DOI] [PubMed] [Google Scholar]
- 2.Burt AD. C. L. Oakley Lecture (1993). Cellular and molecular aspects of hepatic fibrosis. J Pathol. 1993;170:105–114. doi: 10.1002/path.1711700203. [DOI] [PubMed] [Google Scholar]
- 3.Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis. 1990;10:1–10. doi: 10.1055/s-2008-1040452. [DOI] [PubMed] [Google Scholar]
- 4.Pinzani M, Gesualdo L, Sabbah GM, Abboud HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989;84:1786–1793. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman SL, Arthur MJ. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989;84:1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinzani M, Knauss TC, Pierce GF, Hsieh P, Kenney W, Dubyak GR, Abboud HE. Mitogenic signals for platelet-derived growth factor isoforms in liver fat-storing cells. Am J Physiol. 1991;260:C485–C491. doi: 10.1152/ajpcell.1991.260.3.C485. [DOI] [PubMed] [Google Scholar]
- 7.Mak KM, Lieber CS. Lipocytes and transitional cells in alcoholic liver disease: a morphometric study. Hepatology. 1998;8:1027–1033. doi: 10.1002/hep.1840080508. [DOI] [PubMed] [Google Scholar]
- 8.Milani S, Grappone C, Pellegrini G, Schuppan D, Herbst H, Calabrò A, Casini A, Pinzani M, Surrenti C. Undulin RNA and protein expression in normal and fibrotic human liver. Hepatology. 1994;20:908–916. doi: 10.1002/hep.1840200420. [DOI] [PubMed] [Google Scholar]
- 9.Maher JJ, McGuire RF. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990;86:1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshimura A, Gordon K, Alpers CE, Floege J, Pritzl P, Ross R, Couser WG, Bowen-Pope DF, Johnson RJ. Demonstration of PDGF B-chain mRNA in glomeruli in mesangial proliferative nephritis by in situ hybridization. Kidney Int. 1991;40:470–476. doi: 10.1038/ki.1991.234. [DOI] [PubMed] [Google Scholar]
- 11.Gesualdo L, Pinzani M, Floriano JJ, Hassan MO, Nagy NU, Schena FP, Emancipator SN, Abboud HE. Platelet-derived growth factor expression in mesangial proliferative glomerulonephritis. Lab Invest. 1991;65:160–167. [PubMed] [Google Scholar]
- 12.Gay S, Jones RE, Huang GQ, Gay RE. Immunohistologic demonstration of platelet-derived growth factor (PDGF) and sis-oncogene expression in scleroderma. J Invest Dermatol. 1989;92:301–303. doi: 10.1111/1523-1747.ep12276895. [DOI] [PubMed] [Google Scholar]
- 13.Reuterdahl C, Tingström A, Terracio L, Funa K, Heldin CH, Rubin K. Characterization of platelet-derived growth factor beta-receptor expressing cells in the vasculature of human rheumatoid synovium. Lab Invest. 1991;64:321–329. [PubMed] [Google Scholar]
- 14.Nagaoka I, Trapnell BC, Crystal RG. Upregulation of platelet-derived growth factor-A and -B gene expression in alveolar macrophages of individuals with idiopathic pulmonary fibrosis. J Clin Invest. 1990;85:2023–2027. doi: 10.1172/JCI114669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilcox JN, Smith KM, Williams LT, Schwartz SM, Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988;82:1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross R, Masuda J, Raines EW, Gown AM, Katsuda S, Sasahara M, Malden LT, Masuko H, Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990;248:1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- 17.Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46:155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- 18.Seifert RA, Hart CE, Phillips PE, Forstrom JW, Ross R, Murray MJ, Bowen-Pope DF. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989;264:8771–8778. [PubMed] [Google Scholar]
- 19.Kelly JD, Haldeman BA, Grant FJ, Murray MJ, Seifert RA, Bowen-Pope DF, Cooper JA, Kazlauskas A. Platelet-derived growth factor (PDGF) stimulates PDGF receptor subunit dimerization and intersubunit trans-phosphorylation. J Biol Chem. 1991;266:8987–8992. [PubMed] [Google Scholar]
- 20.Zhang J, He JW, Wang TL, Zhao JB. Discriminate collagen type I and collagen typeIII by Sirius red stain and polarization microscopy. Zhonghua Binglixue Zazhi. 1996;25:180–181. [Google Scholar]
- 21.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 22.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Bissell DM, Friedman SL, Maher JJ, Roll FJ. Connective tissue biology and hepatic fibrosis: report of a conference. Hepatology. 1990;11:488–498. doi: 10.1002/hep.1840110322. [DOI] [PubMed] [Google Scholar]
- 24.Gressner AM, Bachem MG. Cellular sources of noncollagenous matrix proteins: role of fat-storing cells in fibrogenesis. Semin Liver Dis. 1990;10:30–46. doi: 10.1055/s-2008-1040455. [DOI] [PubMed] [Google Scholar]
- 25.Friedman SL. Cellular sources of collagen and regulation of collagen production in liver. Semin Liver Dis. 1990;10:20–29. doi: 10.1055/s-2008-1040454. [DOI] [PubMed] [Google Scholar]
- 26.Pinzani M, Knauss TC, Pierce GF, Hsieh P, Kenney W, Dubyak GR, Abboud HE. Mitogenic signals for platelet-derived growth factor isoforms in liver fat-storing cells. Am J Physiol. 1991;260:C485–C491. doi: 10.1152/ajpcell.1991.260.3.C485. [DOI] [PubMed] [Google Scholar]
- 27.Pinzani M, Abboud HE, Gesualdo L, Abboud SL. Regulation of macrophage colony-stimulating factor in liver fat-storing cells by peptide growth factors. Am J Physiol. 1992;262:C876–C881. doi: 10.1152/ajpcell.1992.262.4.C876. [DOI] [PubMed] [Google Scholar]
- 28.Pinzani M, Gentilini A, Caligiuri A, De Franco R, Pellegrini G, Milani S, Marra F, Gentilini P. Transforming growth factor-beta 1 regulates platelet-derived growth factor receptor beta subunit in human liver fat-storing cells. Hepatology. 1995;21:232–239. [PubMed] [Google Scholar]
- 29.Alpini G, Elias I, Glaser SS, Rodgers RE, Phinizy JL, Robertson WE, Francis H, Lasater J, Richards M, LeSage GD. gamma-Interferon inhibits secretin-induced choleresis and cholangiocyte proliferation in a murine model of cirrhosis. J Hepatol. 1997;27:371–380. doi: 10.1016/s0168-8278(97)80184-5. [DOI] [PubMed] [Google Scholar]
- 30.Brenner DA, Westwick J, Breindl M. Type I collagen gene regulation and the molecular pathogenesis of cirrhosis. Am J Physiol. 1993;264:G589–G595. doi: 10.1152/ajpgi.1993.264.4.G589. [DOI] [PubMed] [Google Scholar]
- 31.Wang T, Wang B, Liu X. [Correlation of serum markers with fibrosis staging in chronic viral hepatitis] Zhonghua Binglixue Zazhi. 1998;27:185–190. [PubMed] [Google Scholar]


