Abstract
AIM: To investigate the tryptase and histamine release ability of human colon mast cells upon IgE dependent or independent activation and the potential mechanisms.
METHODS: Enzymatically dispersed cells from human colons were challenged with anti-IgE or calcium ionophore A23187, and the cell supernatants after challenge were collected. Both concentration dependent and time course studies with anti-IgE or calcium ionophore A23187 were performed. Tryptase release was determined with a sandwich ELISA procedure and histamine release was measured using a glass fibre-based fluorometric assay.
RESULTS: Both anti-IgE and calcium ionophore were able to induce dose dependent release of histamine from colon mast cells with up to approximately 60% and 25% net histamine release being achieved with 1 μg/mL calcium ionophore and 10 μg/mL anti-IgE, respectively. Dose dependent release of tryptase was also observed with up to approximately 19 ng/mL and 21 ng/mL release of tryptase being achieved with 10 μg/mL anti-IgE and 1 μg/ mL calcium ionophore, respectively. Time course study revealed that both tryptase and histamine release from colon mast cells stimulated by anti-IgE initiated within 10 sec and reached their maximum release at 6 min following challenge. Pretreatment of cells with metabolic inhibitors abolished the actions of anti-IgE as well as calcium ionophore. Tryptase and histamine release, particularly that induced by calcium ionophore was inhibited by pretreatment of cells with pertussis toxin.
CONCLUSION: Both anti-IgE and calcium ionophore are able to induce significant release of tryptase and histamine from colon mast cells, indicating that this cell type is likely to contribute to the pathogenesis of colitis and other mast cell associated intestinal diseases.
INTRODUCTION
Increased numbers of mast cells have been found in the epithelium of intestine of the patients with ulcerative colitis and Crohn’s disease[1,2]. Through releasing its proinflammatory mediators including tryptase, histamine, heparin, and other preformed or newly synthesized mast cell products[3], mast cells actively participate in the pathogenesis of inflammatory bowel diseases[4].
Tryptase is a tetrameric serine proteinase that constitutes some 20% of the total protein within human mast cells and is stored almost exclusively in the secretory granules of mast cells[5] in a catalytically active form[6]. Upon degranulation, tryptase is released from mast cells along with chymase, histamine, and other mast cell products. In recent years, evidence has been emerging that this major secretory product of human mast cells may be a key mediator of allergic inflammation and a promising target for therapeutic intervention[3] as it has been found to be able to induce microvascular leakage in the skin of guinea pig[7], bronchoconstriction[8] in allergic sheep airways, inflammatory cell accumulation in peritoneum of mouse[9] and release of IL-8 from epithelial cells[10].
For more than four decades, histamine has been widely used as a marker of mast cell degranulation in vitro, and numerous anti-allergic drugs such as sodium cromoglycate, lodoxamide, salbutamal, ketotifen, terfenadine and cetirizine[11,12] and salmeteral[13] were reported to be able to inhibit anti-IgE induced histamine release from human tonsil, skin or lung mast cells. However, little is known of the properties of tryptase release from human mast cells, and even histamine release properties of human colon mast cells were relatively less studied. We therefore parallelly investigated the tryptase and histamine release properties of human colon mast cells by using the same preparations of challenged mast cells in the current study.
MATERIALS AND METHODS
Reagents
The following compounds were purchased from Sigma (St. Louis, USA): collagenase (type I), hyaluronidase (type I), bovine serum albumin (BSA, fraction V), penicillin and streptomycin, calcium ionophore A23187, antimycin A, 2-deoxy-D-glucose, pertussis toxin. Goat anti-human IgE (inactivated) was from Serotec (Kidlington, Oxford, UK). FCS and minimum essential medium (MEM) containing 25 mM N-2-hydroxylethylpiperazine-N’-2-ethane sulphonic acid (HEPES) were from Gibco (Paisley, Renfrewshire, UK). A polyclonal antibody and a monoclonal antibody against tryptase were donated by Dr Andrew F. Walls (University of Southampton, UK). Histamine plate was from RefLab (Copenhagen, Denmark). HEPES and all other chemicals were of analytical grade.
Dispersion of mast cells
Human colon tissue was obtained from patients with carcinoma of colon at colectomy. Only macroscopically normal tissue was used for the study. After removing fat, tissue was washed and chopped finely with scissors into fragments of 0.5-2.0 mm3, and then incubated with 1.5 mg/ml collagenase and 0.75 mg/ml hyaluronidase in MEM containing 2% fetal calf serum (1 g colon/10 ml buffer) for 70 min at 37 °C. Dispersed cells were separated from undigested tissue by filtration through nylon gauze (pore size 100 µm diameter), washed and maintained in MEM (containing 10% FCS, 200 U/ml penicillin, 200 µg/ml streptomycin) on a roller overnight at room temperature. Mast cell purity, as determined by light microscopy after stained by alcine blue, ranged from 3.5% to 5.4%.
Mast cell challenge
Dispersed cells were resuspended in HEPES buffered salt solution (HBSS, pH 7.4) with CaCl2 and MgCl2 (complete HBSS), and 100 µl aliquots containing 4-6 × 103 mast cells were added to a 50 µl anti-IgE, calcium ionophore or control buffer alone and incubated for 15 min at 37 °C. The reaction was terminated by the addition of 150 µl ice cold incomplete HBSS and the tubes were centrifuged immediately (500 g, 10 min, 4 °C). All experiments were performed in duplicate. For the experiments with pertussis toxin, cells were incubated with 0.1 or 1.0 µg/ml pertussis toxin for four hours at 37 °C, and then washed with HBSS before adding stimulus. Similarly, for the experiments with metabolic inhibitors, cells were incubated with 2-deoxy-D-glucose (10 mM) and antimycin A (1 µM) for 40 min at 37 °C before challenged with stimulus. For the measurement of total histamine concentration, in certain tubes the suspension was boiled for 6 min. Supernatants were stored at -20 °C until use.
Tryptase and histamine measurement
Tryptase concentrations were measured with a sandwich ELISA procedure with a specific polyclonal antibody against human tryptase as the capture antibody and AA5 a monoclonal antibody specific for human tryptase as the detecting antibody[14]. Histamine concentrations were determined using a glass fibre-based fluorometric assay[15].
Statistical analysis
Data are shown as mean ± SEM for the number of experiments (n) indicated, and the paired Student’s t test was applied to evaluate two independent samples. In all analyses P < 0.05 was taken as statistically significant.
RESULTS
Effect of anti-IgE and calcium ionophore on tryptase and histamine release from colon mast cells
Both anti-IgE and calcium ionophore were able to induce a dose dependent release of histamine from colon mast cells with up to approximately 60% and 25% net histamine release being achieved with 1 μg/mL calcium ionophore and 10 μg/mL anti-IgE, respectively. Increasing the concentrations of calcium ionophore up to 10 μg/mL and anti-IgE up to 100 μg/mL failed to provoke any further release of histamine from colon mast cells (Figure 1). Dose dependent release of tryptase was also observed when dispersed colon mast cells were incubated with calcium ionophore or anti-IgE. Up to approximately 19 ng/mL and 21 ng/mL release of tryptase were achieved with 10 μg/mL anti-IgE and 1 μg/mL calcium ionophore, respectively. Only as little as 0.1 μg/mL calcium ionophore and 1 μg/mL anti-IgE were required to elicit a significant release of tryptase. Similar to histamine release, increasing the concentrations of calcium ionophore to more than 1 μg/mL and anti-IgE more than 10 μg/mL did not stimulate more tryptase release from colon mast cells (Figure 2). There was a significant correlation between the quantities of histamine and tryptase released in response to anti-IgE (Pearson correlation: r = 0.939, P = 0.005) and calcium
Figure 1.
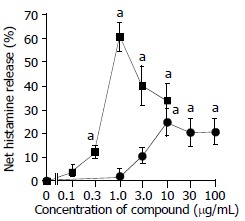
Anti-IgE and calcium ionophore induced histamine re-lease from colon mast cells. The values shown are mean ± SEM for four separate experiments. Stimulus or HBSS alone was incubated with cells for 15 min before termination of the reactions. aP < 0.05 compared with spontaneous release group (paired Student’s t test).
Figure 2.
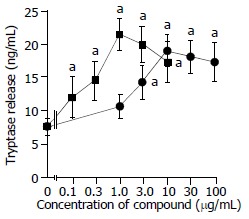
Anti-IgE and calcium ionophore induced tryptase re-lease from colon mast cells. The values shown are mean ± SEM for four separate experiments. Stimulus or HBSS alone was incubated with cells for 15 min before termination of the reactions. aP < 0.05 compared with spontaneous release group (paired Student’s t test).
Time course study revealed that both tryptase and histamine release from colon mast cells stimulated by anti-IgE initiated within 10 sec when cells were incubated with the stimulus, the release was then steadily increased while incubation periods were prolonged. Tryptase and histamine reached their maximum release at 6 min following incubation. Prolonging the incubation period from 6 min to 15 min had little effect on the release of tryptase and histamine (Figure 3). The time course pattern of tryptase release provoked by calcium ionophore was similar to that induced by anti-IgE with approximately 45% tryptase released within 10 sec. This was different from the time course for calcium ionophore induced histamine release, which showed as much as approximately 70% of histamine released within 10 sec of challenge (Figure 4).
Figure 3.
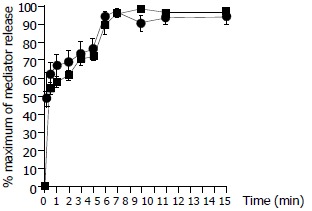
Time course for anti-IgE (10 µg/ml) induced release of tryptase (─■─) and histamine (─●─) from colon mast cells. Data shown are mean ± SEM of four separate experiments.
Figure 4.
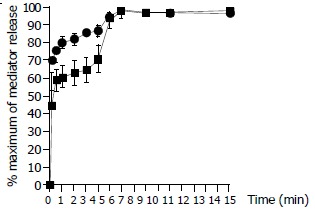
Time course for calcium ionophore (1 µg/ml) induced release of tryptase (─■─) and histamine (─●─) from colon mast cells. Data shown are mean ± SEM of four separate experiments.
Inhibition of anti-IgE and calcium ionophore induced tryptase and histamine release by pertussis toxin and metabolic inhibitors
Pretreatment of cells with metabolic inhibitors abolished the actions of anti-IgE as well as calcium ionophore. Tryptase and histamine release, particularly that induced by calcium ionophore was inhibited by pretreatment of cells with pertussis toxin (Table 1, Table 2).
Table 1.
Effects of pertussis toxin and metabolic inhibitors on anti-IgE induced histamine and tryptase release from colonic mast cells
| Treatment |
Net mediator release (ng/mL) |
||
| Tryptase | Histamine | ||
| Untreated | 11.4 ± 2.7 | 14.0 ± 3.2 | |
| Pertussis toxin | 0.1 μg/mL | 3.2 ± 1.3a | 5.4 ± 1.7a |
| 1.0 μg/mL | 4.0 ± 1.6a | 4.0 ± 1.9a | |
| Metabolic inhibitors | -2.1 ± 1.7a | -2.6 ± 1.7a | |
The values shown aremean±SEMof four separate experiments performed in duplicate.
P < 0.05 compared with the untreated group (paired Students t test). Metabolic inhibitors=1 μMantimycin A+10 mM 2-deoxy-D-glucose.
Table 2.
Effects of pertussis toxin and metabolic inhibitors on calcium ionophore induced tryptase and histamine release from colonic mast cells
| Treatment |
Mediator release (ng/mL) |
||
| Tryptase | Histamine | ||
| Untreated | 13.8 ± 2.4 | 34.3 ± 3.4 | |
| Pertussis toxin | 0.1 μg/mL | 4.0 ± 1.6a | 2.7 ± 2.8a |
| 1.0 μg/mL | 1.4 ± 1.8a | -0.1 ± 1.3a | |
| Metabolic inhibitors | 2.0 ± 1.4a | 1.8 ± 2.6a | |
The values shown aremean±SEMof four separate experiments performed in duplicate.
P < 0.05 compared with the untreated group (paired Students t test). Metabolic inhibitors=1 μMantimycin A+10 mM 2-deoxy-D-glucose.
The values shown are mean ± SEM of four separate experiments performed in duplicate. aP < 0.05 compared with the untreated group (paired Student’s t test). Metabolic inhibitors = 1 mM antimycin A + 10 mM 2-deoxy-D-glucose.
DISCUSSION
We have reported for the first time the in vitro tryptase release ability of human colon mast cells. This is particularly important when tryptase and histamine, the two major mast cell mediators were investigated in the same experiments, which would not only prove that the two mediators were released from mast cells unnecessarily at a constantly parallel ratio, but also revealed that histamine was released slightly faster than tryptase upon degranulation.
Approximately 25% histamine and 10% tryptase (presumably 10 pg tryptase per mast cell[16], and 5000 cells in each test tube) were released from colon mast cells in response to IgE dependent provocation, indicating that only a proportion of mast cells was activated or the granule contents in activated mast cells were partially released. The maximum release of histamine and tryptase was induced by 10 mg/mL rather than higher concentrations of anti-IgE, suggesting that colon mast cells would give their maximum response to a sufficient stimulation, but not an excess stimulation, which showed a self-protection mechanism of colon mast cells.
Approximately 45% degranulation occurred within 10 sec of IgE dependent stimulation, suggesting that colon mast cells were able to quickly respond to allergen challenge, but they required about 6 min to reach their full capacity (maximum histamine or tryptase release). The time course also revealed that histamine was released slightly faster than tryptase upon degranulation, and this was particularly evident with calcium ionophore. The peak histamine release induced by anti-IgE and calcium ionophore occurred at 6 min following stimulation of colon mast cells, and appeared much faster than the occurrence of peak histamine release with lung mast cells, which required at least 15 min to reach its peak following anti-IgE and calcium ionophore stimulation[17].
Pretreatment of cells with metabolic inhibitors antimycin A, which blocks the oxidative phosphorylation process of cells, and 2-deoxy-D-glucose which blocks anaerobic metabolism pathway in cells, abolished the actions of anti-IgE as well as calcium ionophore, indicating that the induced release of these two mediators was a non-cytotoxic process, and was dependent on cell energy supply. Tryptase and histamine release, particularly that induced by calcium ionophore was inhibited by pretreatment of cells with pertussis toxin, suggesting that the degranulation process elicited by anti-IgE and calcium ionophore was associated with activation of G-protein coupled receptors.
Over the last decade, a number of functions of mast cell tryptase have been discovered. However, the mechanism by which tryptase acts on cells is still unclear. The finding that proteinase-activated receptor-2 (PAR-2) on cos cells could be a receptor for tryptase[18] suggested that the effects of tryptase on various types of cells were associated with this type of receptor. Since both tryptase[7,15] and PAR-2 agonists[19] were able to activate human mast cells, it is likely that there is a self-amplification mechanism of mast cell degranulation, which would contribute largely to the pathogenesis of mast cell related diseases including inflammatory bowel diseases. The report that induction of intestinal inflammation in mouse by activation of PAR-2[20] and PAR-2 as an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis[21] suggested the potential roles of PAR-2 in inflammatory bowel diseases.
Histamine has long been accepted as a specific marker of mast cell degranulation. Tryptase however, is seldom used in the same way though it has been employed as a specific marker of mast cells since 1980’s. Based on the results in the current study and the evidence that these two mediators are located in the same secretory granules of mast cells, we believe that human tryptase can serve as an indicator of mast cell degranulation in vitro. In conclusion, both anti-IgE and calcium ionophore are able to induce a significant release of tryptase and histamine from colon mast cells, indicating that this cell type is likely to contribute to the pathogenesis of colitis and other mast cell associated intestinal diseases.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30140023, and the Li Ka Shing Foundation, Hong Kong, China, No. C0200001
Edited by Wang XL Proofread by Zhu LH
References
- 1.Sasaki Y, Tanaka M, Kudo H. Differentiation between ulcerative colitis and Crohn's disease by a quantitative immunohistochemical evaluation of T lymphocytes, neutrophils, histiocytes and mast cells. Pathol Int. 2002;52:277–285. doi: 10.1046/j.1440-1827.2002.01354.x. [DOI] [PubMed] [Google Scholar]
- 2.Furusu H, Murase K, Nishida Y, Isomoto H, Takeshima F, Mizuta Y, Hewlett BR, Riddell RH, Kohno S. Accumulation of mast cells and macrophages in focal active gastritis of patients with Crohn's disease. Hepatogastroenterology. 2002;49:639–643. [PubMed] [Google Scholar]
- 3.Walls AF, He SH, Buckley MG, McEuen AR. Roles of the mast cell and basophil in asthma. Clin Exp Allergy Review. 2001;1:68–72. [Google Scholar]
- 4.Stoyanova II, Gulubova MV. Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta Histochem. 2002;104:185–192. doi: 10.1078/0065-1281-00641. [DOI] [PubMed] [Google Scholar]
- 5.Abraham WM. Tryptase: potential role in airway inflammation and remodeling. Am J Physiol Lung Cell Mol Physiol. 2002;282:L193–L196. doi: 10.1152/ajplung.00429.2001. [DOI] [PubMed] [Google Scholar]
- 6.McEuen AR, He S, Brander ML, Walls AF. Guinea pig lung tryptase. Localisation to mast cells and characterisation of the partially purified enzyme. Biochem Pharmacol. 1996;52:331–340. doi: 10.1016/0006-2952(96)00211-0. [DOI] [PubMed] [Google Scholar]
- 7.He S, Walls AF. Human mast cell tryptase: a stimulus of microvascular leakage and mast cell activation. Eur J Pharmacol. 1997;328:89–97. doi: 10.1016/s0014-2999(97)83033-6. [DOI] [PubMed] [Google Scholar]
- 8.Molinari JF, Scuri M, Moore WR, Clark J, Tanaka R, Abraham WM. Inhaled tryptase causes bronchoconstriction in sheep via histamine release. Am J Respir Crit Care Med. 1996;154:649–653. doi: 10.1164/ajrccm.154.3.8810600. [DOI] [PubMed] [Google Scholar]
- 9.He S, Peng Q, Walls AF. Potent induction of a neutrophil and eosinophil-rich infiltrate in vivo by human mast cell tryptase: selective enhancement of eosinophil recruitment by histamine. J Immunol. 1997;159:6216–6225. [PubMed] [Google Scholar]
- 10.Cairns JA, Walls AF. Mast cell tryptase is a mitogen for epithelial cells. Stimulation of IL-8 production and intercellular adhesion molecule-1 expression. J Immunol. 1996;156:275–283. [PubMed] [Google Scholar]
- 11.Okayama Y, Church MK. Comparison of the modulatory effect of ketotifen, sodium cromoglycate, procaterol and salbutamol in human skin, lung and tonsil mast cells. Int Arch Allergy Immunol. 1992;97:216–225. doi: 10.1159/000236122. [DOI] [PubMed] [Google Scholar]
- 12.Okayama Y, Benyon RC, Lowman MA, Church MK. In vitro effects of H1-antihistamines on histamine and PGD2 release from mast cells of human lung, tonsil, and skin. Allergy. 1994;49:246–253. doi: 10.1111/j.1398-9995.1994.tb02657.x. [DOI] [PubMed] [Google Scholar]
- 13.Butchers PR, Vardey CJ, Johnson M. Salmeterol: a potent and long-acting inhibitor of inflammatory mediator release from human lung. Br J Pharmacol. 1991;104:672–676. doi: 10.1111/j.1476-5381.1991.tb12487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley MG, Walters C, Wong WM, Cawley MI, Ren S, Schwartz LB, Walls AF. Mast cell activation in arthritis: detection of alpha- and beta-tryptase, histamine and eosinophil cationic protein in synovial fluid. Clin Sci (Lond) 1997;93:363–370. doi: 10.1042/cs0930363. [DOI] [PubMed] [Google Scholar]
- 15.He S, Gaça MD, Walls AF. A role for tryptase in the activation of human mast cells: modulation of histamine release by tryptase and inhibitors of tryptase. J Pharmacol Exp Ther. 1998;286:289–297. [PubMed] [Google Scholar]
- 16.Schwartz LB, Irani AM, Roller K, Castells MC, Schechter NM. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J Immunol. 1987;138:2611–2615. [PubMed] [Google Scholar]
- 17.Holgate ST, Burns GB, Robinson C, Church MK. Anaphylactic- and calcium-dependent generation of prostaglandin D2 (PGD2), thromboxane B2, and other cyclooxygenase products of arachidonic acid by dispersed human lung cells and relationship to histamine release. J Immunol. 1984;133:2138–2144. [PubMed] [Google Scholar]
- 18.Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie JA, Schechter N, Woolkalis M, Brass LF. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 19.He SH, Xie H, He YS. [Effect of a proteinase-activated receptor-2 (PAR-2) agonist on tryptase release from human mast cells] Sheng Li Xue Bao. 2002;54:531–534. [PubMed] [Google Scholar]
- 20.Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, Wallace JL, Hollenberg MD, Bunnett NW, Garcia-Villar R, et al. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol. 2002;161:1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorucci S, Mencarelli A, Palazzetti B, Distrutti E, Vergnolle N, Hollenberg MD, Wallace JL, Morelli A, Cirino G. Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proc Natl Acad Sci USA. 2001;98:13936–13941. doi: 10.1073/pnas.241377298. [DOI] [PMC free article] [PubMed] [Google Scholar]


