Abstract
Studies in systematic palaeontology are greatly aided when numerous, well-preserved specimens are available so that quantitative methods can be used to substantiate qualitative observations. This is often not the case for fossil decapod crustaceans due to their relatively low preservation potential. Here, we examined primarily two large collections of the well-preserved ghost shrimp Glypturus from the Holo-Pleistocene of Panama and the late Miocene of Florida. Using descriptive, bivariate, multivariate and geometric morphometric methods, two new species are described based on appendage material: Glypturus panamacanalensis sp. nov. and G. sikesi sp. nov. New characters are identified, and size-related and intraspecific variation are assessed for these taxa and modern G. acanthochirus. Taxonomic placement of single specimens from other localities was confirmed by multivariate methods. Furthermore, Glypturus is revised, especially with regard to Western Atlantic species that inhabited both carbonate and siliciclastic environments. Callianassa anguillensis, C. latidigata, and Neocallichirus? quisquellanus are referred to as Glypturus sp. until more material is available to determine the validity of these species. Diversity within Glypturus may thus be underestimated, thereby also impacting the assessment of phylogenetic relationships. Minor propodi appear under-represented relative to major propodi, suggesting a taphonomic bias. Single specimens of interest include a specimen of G. panamacanalensis sp. nov. exhibiting a peculiar swelling in the fixed finger and another showing damage on the propodal upper margin, suggesting failed predation or antagonistic behaviour. Glypturus is first found in the Oligocene in the Western Atlantic and may have expanded its palaeobiogeographical range since the Miocene. The genus was still present on the Pacific side of the Isthmus of Panama in the Holo-Pleistocene, but is only known from the Western Atlantic today, suggesting a relatively recent extinction on the Pacific side.
Keywords: Cenozoic, shrimp, biogeography, growth, systematics, intraspecific variation
Introduction
For fossil decapod crustaceans, a limited number of specimens are typically available for study because of their limited preservation potential compared to other marine invertebrates (e.g. Kidwell & Flessa 1995; Foote & Sepkoski 1999; Stempien 2005). As a result, the systematics of fossil decapods is typically based on qualitative or marginally quantitative descriptions. In addition, the small sample sizes typical of many fossil decapod species hamper rigorous testing of interspecific, intraspecific and size-related variation in morphology. Consequently, few studies address these critical systematic issues in the palaeontological literature devoted to decapods (for exceptions, see Starzyk et al. 2011; Klompmaker et al. 2012a, b; Jones 2013). The flipside is that species delineation may appear easier because variation is necessarily limited, provided that the preservation of the specimen(s) is adequate. Parataxonomies exist as decapods consist of multi-component exoskeletons (e.g. Jagt et al. 2010; Fraaije et al. 2013), but this does not appear to pose substantial problems with respect to synonymies, at least for the Cretaceous (Klompmaker 2013). For ghost shrimp systematics parataxonomies are not problematical because, typically, only the appendages are preserved, especially the relatively strongly calcified chelipeds, on which taxonomy of fossil taxa is primarily based. No possible synonymies of this kind were encountered among Cretaceous callianassid shrimp (Klompmaker 2013). Since (a) parataxonomies are limited for ghost shrimp; (b) their propodi are well calcified; (c) they can occur in high numbers locally; and (d) their burrowing nature allows for preferential preservation (Bishop & Williams 2005), major propodi of fossil ghost shrimp are often common and can be used for assessing interspecific, intraspecific (Hyžný & Hudáčková 2012) and size-related variations. Also, biogeographical patterns can be reconstructed because of the abundance of fossils, as in this example using extant and fossil specimens of Glypturus Stimpson, 1866.
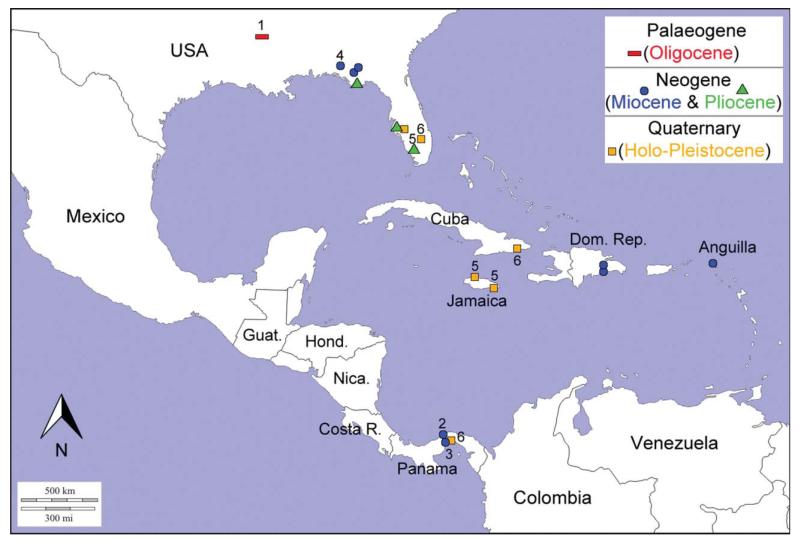
Today, specimens of Glypturus inhabit the Western Atlantic [G. acanthochirus Stimpson, 1866; Glypturus sp. = Glypturus rabalaisae sensu Sakai, 2005], the Indian Ocean and the south-west Pacific [G. armatus (A. Milne-Edwards, 1870)], and the Red Sea [G. laurae (de Saint Laurent in de Vaugelas & de Saint Laurent, 1984)] (Hyžný & Müller 2012, fig. 11). Furthermore, Glypturus has a fairly extensive fossil record that commences in the Eocene (Hyžný & Müller 2012; Hyžný et al. 2013). Mostly resembling modern species distributions of the genus, fossil species have been found in the Indo-West Pacific, the Tethys region and the Western Atlantic. Currently, the taxonomy of fossil Glypturus spp. is based on the presence/absence and extent of tuberculation on the lateral sides of the major propodus (Hyžný & Müller 2012; Hyžný et al. 2013), characters which also can be applied to extant Glypturus spp. (Hyžný & Müller 2012).
Recently, two large assemblages of fossil Glypturus spp., one from the Holo-Pleistocene of Panama and the other from the late Miocene of Florida, were discovered and collected, respectively. Additionally, many isolated Cenozoic specimens from a variety of localities in the Western Atlantic have been recognized. The main goals of this paper are to assess the species-level taxonomic placement of the large collections of fossil and extant specimens of Glypturus, explore the utility of quantitative methods in systematic studies of fossil decapod crustaceans, comment on the sedimentary environment and phylogeny of Glypturus, and explore the Western Atlantic palaeobiogeography of Glypturus through time.
Geological setting
Specimens identified below as Glypturus panamacanalensis sp. nov. were collected on Amador and Farfan beaches near the entrance to the Panama Canal, Panama City, in 1959 by M. D. Burkenroad, who identified at least one specimen as ‘Callianassa acanthochirus’. They were most likely dredged before being dumped onto the beaches, suggesting that the provenance of the material is from or near the Pacific entrance of the Panama Canal. The age of these decapods, based on a co-occurring, well-preserved mollusc fauna, is Holo-Pleistocene (undifferentiated). All faunal elements were found in irregularly shaped siliciclastic concretions and/or burrows of sandstone and siltstone. Bivalves included Anadara nux (Sowerby, 1833a) (UF 233946), Anadara concinna (Sowerby, 1833a) (UF 233945), Trachycardium procerum (Sowerby, 1833b) (UF 233997), Caryocorbula ovulata (Sowerby, 1833c) (UF 233956), Lirophora mariae (d’Orbigny, 1846), Chionopsis amathusia (Philippi, 1844), Tenuicorbula tenuis (Sowerby, 1833c), Tellina regia Hanley, 1844, and Lamelliconcha paytensis (d’Orbigny, 1845) (UF 233957); gastropods included Cosmioconcha modesta (Powys, 1835) (UF 233955) and Prunum sapotilla (Hinds, 1844) (UF 233960). All these molluscs are extant and are known from shallow marine (< 100 m), sandy to muddy environments (Keen 1971; Coan & Valentich-Scott 2012; Portell et al. 2012; Paleo-Biology Database 2013). Co-occurring brachyuran decapods include Hepatus sp. and a portunid. Farfan and Amador beaches are surrounded by Holocene and Pleistocene sediments of the Pacific Muck (e.g. Jones 1950; Woodring 1957), further supporting a Holo-Pleistocene age and consistent with the age determination of Portell et al. (2012), who suggested a Holo–late Pleistocene age for fossils found from nearby Bique and other beaches.
Specimens identified as Glypturus sikesi sp. nov. below were collected at University of Florida’s Sikes Sand Mine 02 locality in Washington County, Florida, USA. These shrimp remains, found in fine phosphatic, micaceous, sandy clay, are believed to be part of the upper Miocene (Tortonian–Messinian) Choctawhatchee Formation of the Alum Bluff Group. First described by Matson & Clapp (1909) from a ravine near the Choctawhatchee River, south-east of the town of Redbay, the Choctawhatchee Formation has subsequently been revised, renamed and restricted; no longer included are the mollusc-rich beds described by Mansfield (1930, 1932), which are now referred to the Jackson Bluff Formation. For a comprehensive discussion of the Choctawhatchee Formation, see Huddlestun (1976; see also Portell et al. 2006, for a brief summary of the nomenclatural history of this and other fossiliferous Miocene deposits of the Florida Panhandle). Based on planktonic foraminifera, Huddlestun (1976) considered the formation to be of late Miocene age. Associated invertebrate taxa from Sikes Sand Mine 02 include the calcitic bivalve genera Nodipecten Dall, 1898 and Amusium Röding, 1798, and internal moulds of the gastropod genera Persististrombus Kronenberg & Lee, 2007 and Conus Linnaeus, 1758. Fragments of echinoid tests, rare valves of the brachiopod genus Glottidia Dall, 1870, and common remains of portunid, calappid and especially hexapodid crabs were also collected.
Examined specimens of Glypturus toulai (Rathbun, 1919) originated from the middle–upper Miocene (Serravallian–Tortonian) Gatun Formation of Panama (see Hendy 2013 for detailed stratigraphy and associated mollusc fauna), the formation from which they were first described. This species has been reported from the lower (Hyžný et al. 2013) and middle (Todd & Collins 2005) Gatun Formation. Typically, siliciclastic sediments were deposited on an inner shelf at an estimated depth of < 75 m (Hendy 2013).
Material and methods
The collections of the Division of Invertebrate Paleontology at the Florida Museum of Natural History (FLMNH) were searched for and expanded with specimens of fossil Glypturus spp. as a result of fieldwork at the Sikes Sand Mine 02 in Washington County, Florida. Collections in invertebrate zoology in the same museum and the Naturhistorisches Museum, Wien were used for modern G. acanthochirus. The latter museum also yielded specimens of G. toulai including the types. The number of specimens per species is detailed in Supplemental Table 1. The three assemblages containing the most Glypturus specimens are: Sikes Sand Mine 02, Farfan and Amador beaches near Panama City (Panama), and specimens of modern G. acanthochirus from a variety of sites in the Western Atlantic.
Institutional abbreviations used here are: NHMW: Naturhistorisches Museum Wien, Vienna, Austria; UF: Florida Museum of Natural History at the University of Florida, Gainesville, Florida, USA; BMNPH PI IC and NHM UK In: Department of Earth Sciences, Natural History Museum, London, UK; MNHNCu-P: National Museum of Natural History, Paleontological collection, Havana, Cuba; USNM: US National Museum, Smithsonian Institution, Washington, DC, USA; MNHN: Muséum national d’Histoire naturelle, Paris, France.
Statistical analyses were performed in PAST 2.17c (Hammer et al. 2001) and R 3.0.1 (R Development Team). A significance level of 5% was used for all tests.
In an attempt to distinguish the major propodi of the three assemblages mentioned above and to explore possible taxonomic placement of isolated specimens of other localities, the following data were obtained: (a) length of the manus at the outer side as measured from the notch above the proximalmost part of the finger to the rim of the manus at the centre of the concavity (Fig. 1); (b) height of the manus as measured perpendicular to the axis of the propodus from the proximalmost spine on the upper margin (Fig. 1); (c) the areas occupied by tubercles on the inner and outer sides; and (d) the number of tubercles on both sides. The tubercles on the margin toward the dactylus were not included for (c) or (d). The measurements and areas were obtained using ImageJ 1.46r. All tubercles were counted. Initially, bivariate analyses were carried out separately for the inner and outer sides to explore whether the two sides showed similar growth-related trends in length/height ratios, percentage of coverage by tubercles, and density of tubercles. The precision of measurement errors was estimated by measuring 10 times the length, height and area occupied by tubercles on the outer side of the manus of the same image of a specimen (UF 248937 from the Holo-Pleistocene of Panama). Furthermore, 10 images taken from a specimen (UF 248936) from the same locality were analysed using the same variables to explore the combined effect of measurement error and the possibly slightly different angles of the specimen as the specimen was positioned anew for every photograph. A Sony DSC-R1 (10 MP) was used for most digital macrophotography.
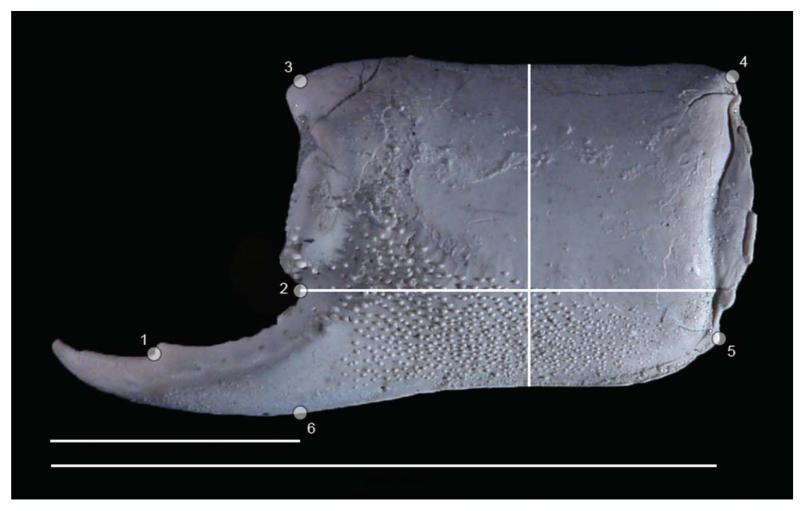
Figure 1.
Placement of six landmarks on the outer side of the propodus of Glypturus sikesi sp. nov. (UF 235154, flipped horizontally). Method of measuring manus length, height and length of the fixed finger, and total propodus length are also shown. Lowermost scale bar width: 27.1 mm.
To test whether the percentage of tubercle coverage of the manus varied across the three assemblages, a non-parametric Kruskal–Wallis test was applied to the percentage estimates obtained separately for the outer and inner sides of the mani. Subsequently, Mann–Whitney tests were used (with and without a Bonferroni correction for multiple tests) to assess whether tubercle coverage differed significantly for any given pair of samples. However, because tubercle coverage may vary with growth, the relationship between specimen size (a proxy of ontogenetic age) and the percentage coverage of tubercles was evaluated using simple linear regression (both ordinary least square (OLS) and Reduced Major Axis regression models yielded comparable outcomes and only OLS results are reported here). When this correlation was significant, Mann–Whitney tests were carried out on restricted data to make samples comparable in terms of size range of specimens included in the analysis. The Kruskal–Wallis and Mann–Whitney tests are based on ranks rather than absolute values of the observations and thus require less stringent statistical assumptions than the equivalent parametric tests (ANOVA [analysis of variance] and t-test, respectively).
The significance of the regression lines of plots of the length/height ratios versus length for the three assemblages was determined for the outer and inner side of the propodi. Subsequently, the slopes of the l/h ratios versus length plots of assemblages from the late Miocene of Florida and the Holo-Pleistocene of Panama were compared using a one-way ANCOVA (analysis of covariance). A Mann–Whitney test was performed to assess whether l/h ratios of these two assemblages were significantly different for the data restricted to specimens within the length range 13.1–18.0 mm based on the outer part of the manus. Sufficient samples were present in this range and a size-related change of the l/w ratio does not appear substantial.
Bivariate analyses were also carried out for the number of tubercles per 10 mm2 (tubercle density) versus the length of the manus to explore trends within and among assemblages. This was done for the outer and inner sides separately.
To compare the shape of the major propodus of the assemblages from the late Miocene of Florida and the Holo-Pleistocene of Panama, six landmarks were defined. Using standard schemes (Bookstein 1997; Zelditch et al. 2004) these landmarks can be classified as follows: Landmark 1, type II, at the distal base of the tooth on the fixed finger; Landmark 2, type II, at the notch marking the start of the fixed finger; Landmark 3, type II, at the base of the distalmost spine on the upper margin of the propodus, usually best visible on the inner side; Landmark 4, type II, at the proximal corner of the upper margin; Landmark 5, type II, at the proximal corner of the lower margin; Landmark 6, type III, on the lower margin straight below Landmark 2 using a propodal orientation as in Figure 1. Specimens with the fixed finger located to the right in outer lateral view were flipped horizontally in Adobe Photoshop CS5, so that all specimens had their fixed finger oriented to the left. Left and right major propodi did not differ in shape visually, implying that they could be put into one sample. Initially, 16 specimens from the Holo-Pleistocene of Panama and 14 from the late Miocene of Florida were used for morphometric analyses. Tps Utility program (tpsUtil) 1.56 and tpsDig 1.40 were used to generate .tps files containing landmark data. The average shape of the three smallest specimens per assemblage was calculated to use as references to determine the relative Procrustes distance for other specimens (see Webster 2007; Vergara-Solana et al. 2013). This distance was obtained in MorphoJ 1.05f (Klingenberg 2011) using canonical variate analysis (CVA) after a Procrustes fit aligned by principal axes (superposition of landmarks) and after classifying the specimens (smallest three specimens arranged in one group). As there was a positive, significant relationship between the Procrustes distance and the log-10 of the centroid size of the specimens from the late Miocene of Florida, indicating an allometric effect (see below), specimens with a centroid size of 19–27 (= 1.28–1.43 log10 centroid size) were selected for both assemblages for further analyses to compare the shape of the two assemblages while minimizing allometric effects (see Vergara-Solana et al. 2013). There was no allometric effect observed for the Procrustes distances for specimens from the Holo-Pleistocene of Panama. Exclusion of specimens outside the range reduced the final dataset to seven specimens from the late Miocene of Florida and six from the Holo-Pleistocene of Panama. These specimen groups were further analysed using CVA (in MorphoJ with permutation tests) and a Generalized Goodall F-test (using tpsRegr 1.38).
To compare further the two assemblages the six non-landmark variables as defined above were used for multivariate analyses. To compare results to the abundant modern species in the Western Atlantic, five specimens of G. acanthochirus, 12 specimens of the late Miocene of Florida (Sikes Sand Mine 02), 13 specimens from the Holo-Pleistocene of Panama, and two specimens of G. toulai from the Miocene Gatun Formation were analysed. Additionally, five well-preserved specimens of Glypturus from other localities were included to determine to which species they could be ascribed. The data were obtained for only the best preserved specimens. Consequently, specimens from the lower Miocene Culebra Formation attributed to G. toulai (see Hyžný et al. 2013) were not included, as tubercles were not sufficiently preserved and the specimens are from a different age and assemblage than G. toulai from the Gatun Formation. As the morphometric variables used here are not all expressed in the same units, principal component analysis based on a correlation matrix was used to explore the ordination of the specimens of each assemblage on the log10-transformed data. Because strong size-dependence is suggested by the fact that PC1 explains most of the variance (71.3%) and all variables show strong positive correlations with PC1 (PCA loadings: 0.69–0.93), the within-group allometric size component was minimalized by regressing all log10-transformed variables on PC1 (see Kowalewski et al. 1997). The residuals of this regression were used to perform multivariate tests including CVA and discriminant analysis (DA). Multivariate normality required for the reliable performance of DA was tested using the Dornik and Hansen omnibus test in PAST.
Using the same six non-landmark variables from above, allometric coefficients were computed per variable in PAST for the 12 specimens from the late Miocene of Florida and 13 specimens from the Holo-Pleistocene of Panama separately (> 10 specimens available), as well as for all specimens combined. The square root was taken from values of the two variables involving area before the data were log-transformed, after which the coefficient for each variable was estimated by dividing the PC1 loading for that variable by the average PC1 loading for all variables (Kowalewski et al. 1997). The 95% confidence intervals for the coefficients were calculated by bootstrapping (2000 replicates).
The same variables were used to determine the a posteriori error rate (= probability of misclassification) by the proportion of observations misclassified by DA (Kowalewski et al. 1997). Data were log-transformed after multiple 0-values in the data of G. acanthochirus were replaced by 0.0001, resulting in eight specimens instead of five for that species. All classificatory error rates are jackknife-corrected or jackknife cross-validated (e.g. Holdener 1994; Kowalewski & Novack-Gottshall 2010). The minimum required sample size needed for correct classification of a sample was assessed by resampling with replacement (bootstrapping) for n observations (n = 1–5) for all assemblages, except for G. toulai due to its low sample size (n = 2). Centroids of the samples were projected a posteriori (Kowalewski et al. 1997). Analyses were performed in R.
Since not a single minor propodus of Glypturus has been confirmed from the fossil record to date, we identified some of the characters that might be useful to distinguish minors from majors. First, the presence of tubercles on the minor was checked for all three extant species based on collections at FLMNH and NHMW, especially for G. acanthochirus, the species living in the West Atlantic region today. These minor propodi were also checked for the presence of spines on the upper margin. Third, the length of the fixed finger relative to the length of the propodus was determined for modern specimens of G. acanthochirus. A Mann–Whitney test was performed on the percentages of the relative fixed finger length of the minors and majors to determine whether the relative lengths of the fixed fingers differed statistically. Well-preserved specimens of the large assemblage from the late Miocene of Florida were used to explore whether potential minors were present in the sample using the same measurements. Specimens from the Holo-Pleistocene of Panama were not used as the tip of the fixed finger was rarely preserved.
Results
Statistical and morphometric analyses
The dataset including all numerical measurements is found in Supplemental Appendix 1. Repeated measurements of the same specimen indicate relatively high precision: the standard deviations of repeated estimates of the length, height and area occupied by tubercles at the outer side of the manus were typically < 2% of the means (Supplemental Appendix 2). Thus, measurement errors are unlikely to have substantially influenced the results reported below.
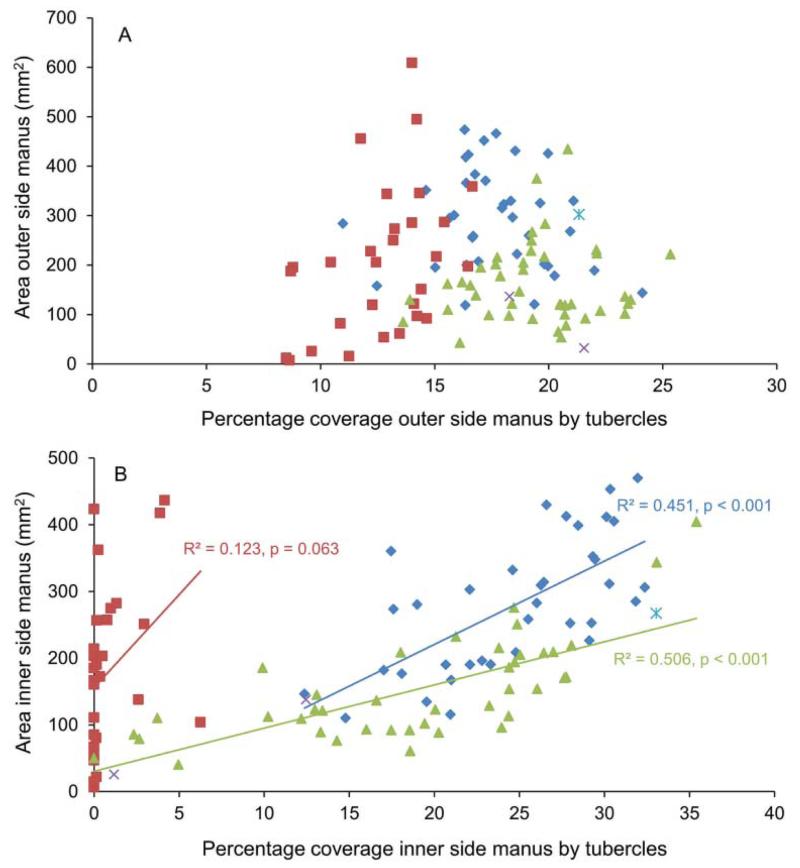
The percentage of tubercle coverage on the outer side of the manus of Glypturus spp. does not increase with increasing size of the specimen (Fig. 2A). In contrast, for the inner side of the manus, larger specimens have a significantly higher percentage of tubercle coverage for the fossil species (Fig. 2B). The slope of the relation differs significantly for Glypturus spp. from the late Miocene of Florida when compared to the assemblage from the Holo-Pleistocene of Panama (one-way ANCOVA, p = 0.01).
Figure 2.
The influence of growth on the percentage of tubercle coverage of the manus of the propodus for species of Glypturus. A, outer side; B, inner side. Squares (red) = modern Glypturus acanthochirus; diamonds (blue) = G. panamacanalensis sp. nov. (Holo-Pleistocene of Panama); triangles (green) = G. sikesi sp. nov. (late Miocene of Florida, USA); cross (purple) = G. toulai (late Miocene of Panama); cross with vertical line (blue) = G. berryi (Oligocene of Mississippi, USA).
The Kruskal–Wallis test on the percentages of tubercle coverage of the outer side of the manus for the three large assemblages of Glypturus (modern, Holo-Pleistocene of Panama, and late Miocene of Florida) indicates that the medians are statistically different (p = 1.595 × 10−12). The results are also highly significant for all pairwise assemblage comparisons of the outer side of the manus (Mann–Whitney tests; Table 1). In contrast, percentage based tubercle coverage of the inner side of the mani of the two Glypturus assemblages from the Holo-Pleistocene of Panama and the late Miocene of Florida is not significantly different (Mann–Whitney test: p = 0.83). Here, the surface area of mani was restricted to 120–285 mm2 to minimize growth effects on tubercle coverage (relatively more tubercles with size, see above). Mann–Whitney tests of these two assemblages with G. acanthochirus separately indicate that tubercle coverage is significantly different (p = 0.0001) for both pairs, suggesting a lower coverage of tubercles on the inner side for G. acanthochirus. There appears to be a trend towards decreasing tubercle coverage of the outer side of the manus through geological time (Fig. 2A). The inner side (Fig. 2B) seems to show a similar trend, where similar-sized specimens show a decrease in tubercle coverage from the oldest to the youngest assemblage.
Table 1.
The p-values of the Mann–Whitney pairwise comparison tests on the percentages of tubercle coverage of the outer side of the manus for three assemblages. Upper right: without a Bonferroni correction; lower left: with a Bonferroni correction.
|
Glypturus acanthochirus (modern) |
G. panamacanalensis sp. nov. (Holo-Pleistocene, Panama) |
G. sikesi sp. nov. (late Miocene, Florida) |
|
|---|---|---|---|
| Glypturus acanthochirus (modern) | — | 3.43 × 10−9 | 4.58 × 10−11 |
|
G. panamacanalensis sp. nov. (Holo-Pleistocene, Panama) |
1.03 × 10−8 | — | 0.00712 |
| G. sikesi sp. nov. (late Miocene, Florida) | 1.37 × 10−10 | 0.02136 | — |
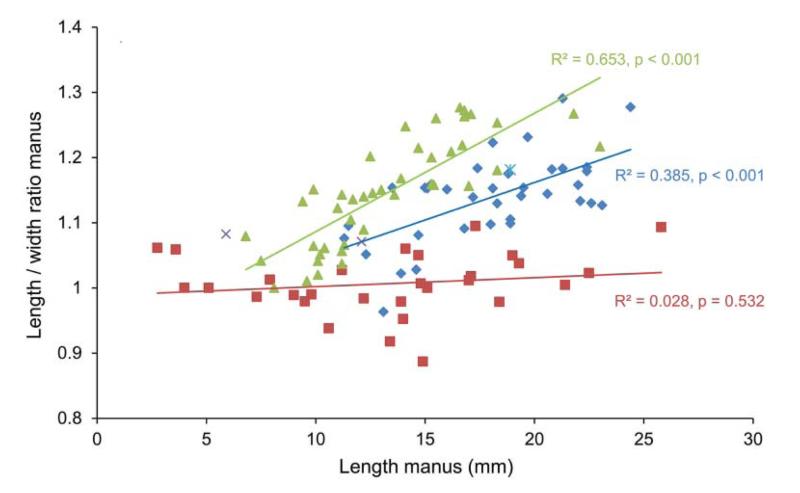
Length/height ratios versus length based on the outer side of the manus show that there is no significant size-related increase of l/h ratios for modern G. acanthochirus, whereas the fossil assemblages (late Miocene of Florida and Holo-Pleistocene of Panama) do show an increase in the l/h ratio as specimen size increases (Fig. 3). The slopes of the relationship between l/h ratio and size differ significantly for the two fossil assemblages (one-way ANCOVA, p = 0.04). The l/h ratios for the late Miocene assemblage are generally higher than those from the Holo-Pleistocene assemblage for a restricted size range. For example, for the length range of 13.1–18.0 mm, a Mann–Whitney test shows that the two assemblages significantly differ (p = 9.9 × 10−5). Similar results were obtained for measurements on the inner side of the manus, where the length of specimens is typically smaller compared to the outer side. Length/height ratios appear to increase faster throughout growth in geologically older assemblages (Fig. 3).
Figure 3.
Length/height ratios throughout growth based on measurements on the outer side of the manus for species of Glypturus. Symbols as in Figure 2.
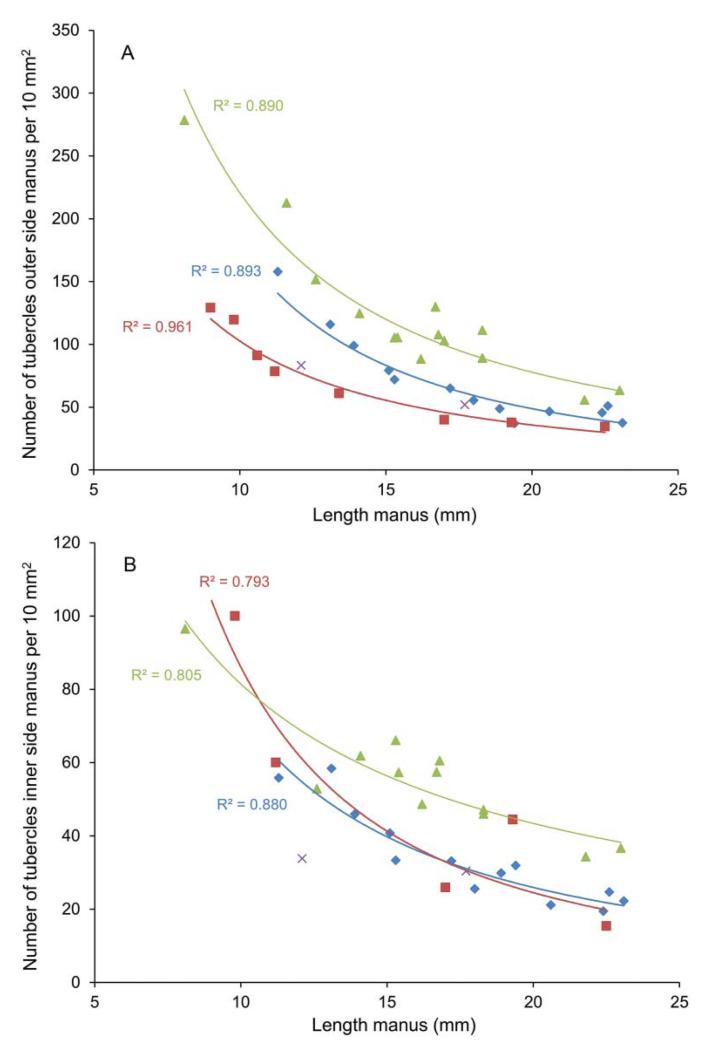
Although the number of tubercles increases throughout growth, the density of tubercles per unit area decreases as specimen size increases regardless of the species of Glypturus (Fig. 4). The trends closely follow a power function. All trend lines in Figure 4A are statistically significant (p < 0.05) when the y-data is log10-transformed and a linear trend line is chosen (all R2 values ≥ 0.77). The spacing between the centres of the tubercles thus increases as specimens grow. Although not quantified here, tubercle size appears to increase throughout growth. Density of tubercles varies with geological age (Fig. 4A): the specimens from the oldest assemblage display highest tubercle densities on the outer side of the manus, whereas specimens of the modern G. acanthochirus exhibit lowest tubercle densities. Patterns are less obvious for the inner side of the manus, where Holo-Pleistocene assemblages from Panama and G. acanthochirus overlap (Fig. 4B).
Figure 4.
Tubercle density (number of tubercles per 10 mm2) versus length of the manus of Glypturus spp. A, outer side; B, inner side. Symbols as in Figure 2.
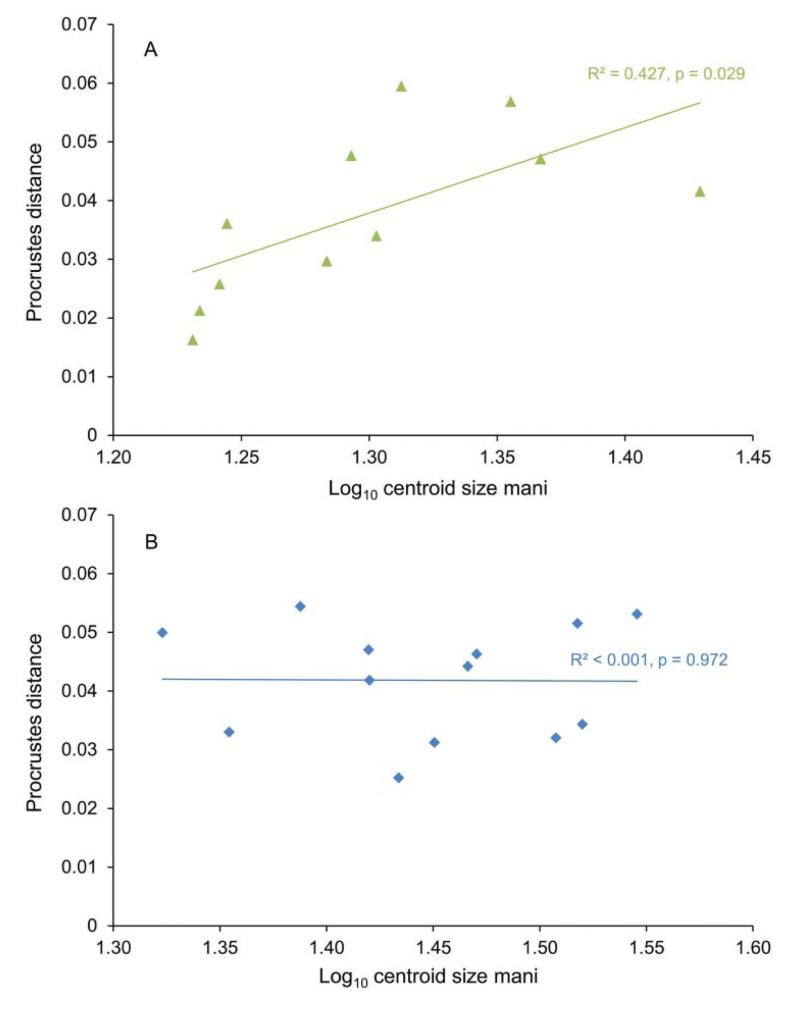
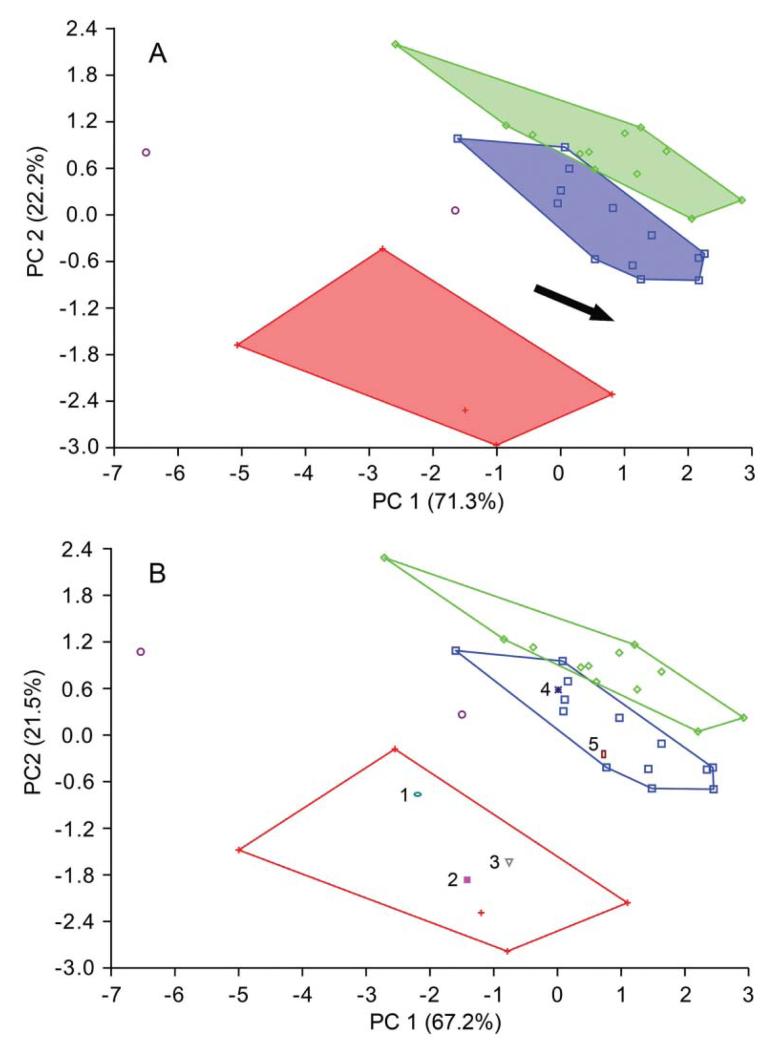
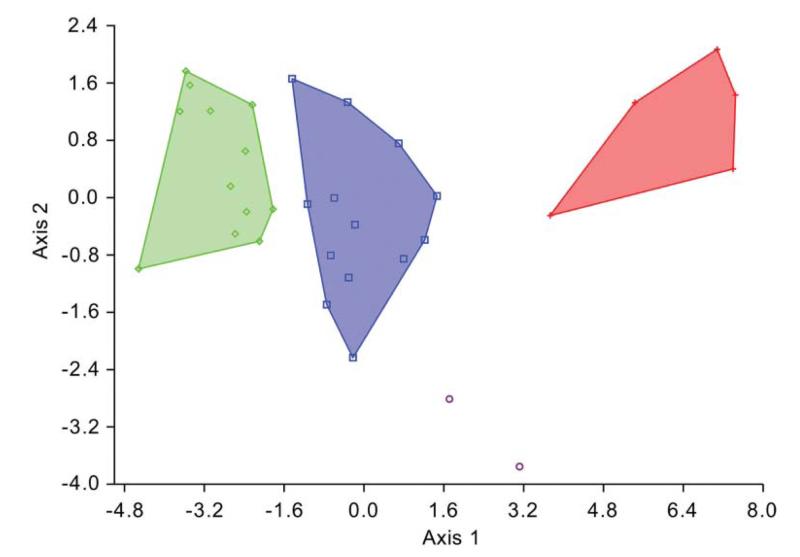
After minimizing the allometric effect on the shape of the assemblage from the late Miocene of Florida (Fig. 5), by restricting the size range to 1.28–1.43 log10 centroid size for the assemblages from the Holo-Pleistocene of Panama and the late Miocene of Florida, the propodal shape of these assemblages were statistically distinguishable (Mahalanobis distance = 5.343; permutation test with 10,000 runs on Mahalanobis distance: p = 0.0002). Other tests (generalized Goodall F-test, permutation test on Procrustes distance) also returned significant differences. PCA on the log10-transformed dataset based on six non-landmark variables showed separation between G. acanthochirus and other species of Glypturus (Fig. 6A; Supplemental Appendix 3). Specimens of the assemblages from the Holo-Pleistocene of Panama and the late Miocene of Florida assemblage do not overlap substantially. The two specimens of G. toulai do not fall within any of the convex hulls. After minimizing the effect of size by regressing all variables on PC1, CVA on the residuals shows separation of all analysed Glypturus species (Fig. 7). Importantly, even the 95% confidence ellipses of assemblages from the Holo-Pleistocene of Panama and the late Miocene of Florida do not include the two specimens of G. toulai. Discriminant analysis separates the assemblages from the Holo-Pleistocene of Panama and the late Miocene of Florida (Hotelling’s T2 = 135.53; F = 17.45; p = 0.000002). The data do not depart significantly from a multivariate normal distribution (Dornik and Hansen omnibus Ep = 10.89; p = 0.53). DA also separates the assemblage from the late Miocene of Florida and G. toulai (Hotelling’s T2 = 191.02; F = 6.08; p = 0.035; multivariate normality: Ep = 15.43, p = 0.22), and may distinguish the assemblage from the Holo-Pleistocene of Panama and G. toulai (Hotelling’s T2 = 72.95; F = 7.48; p = 0.006), although the compared datasets appear to not to follow a normal distribution (Ep = 33.78, p = 0.0007).
Figure 5.
Procrustes distance relative to the three smallest specimens of each assemblage versus centroid size of the mani. A, Glypturus sikesi sp. nov. (late Miocene, Florida, USA); B, Glypturus panamacanalensis sp. nov. (Holo-Pleistocene, Panama).
Figure 6.
Principal component analysis on the log10-transformed dataset based on six variables extracted from the mani of Glypturus spp. A, PCA based on four collections; B, PCA based on four collections and five additional specimens from various localities (1–5). Red plus-sign = modern G. acanthochirus; blue squares = G. panamacanalensis sp. nov. (Holo-Pleistocene, Panama); green diamonds = G. sikesi sp. nov. (upper Miocene of Florida, Choctawhatchee Formation); purple circles = G. toulai (lower Miocene of Panama, Gatun Formation). Assemblages with > 2 individuals are surrounded by convex hulls. The arrow in A estimates the direction of size increase based on the shape of the convex hulls in conjunction with underlying specimen size. Numbers in B represent: 1, specimen from the upper Pliocene–lower Pleistocene Tamiami Formation (Pinecrest beds) of Florida (Mule Pen) (UF 42451); 2, specimen from the upper Pleistocene Jaimanitas Formation of Cuba (Good Road 01) (UF 222839); 3, specimen from the Holo-Pleistocene of Jamaica (Falmouth 01) (UF 232383); 4, specimen from the upper Pleistocene Jaimanitas Formation of Cuba (Good Road 01) (UF 248008); 5, specimen from the middle Pleistocene Bermont Formation of Florida (Belle Glade 01) (UF 131471).
Figure 7.
Canonical variate analysis on the residuals of the log10-transformed variables extracted from the mani of Glypturus spp. Symbols as in Figure 6. Assemblages with > 2 individuals are surrounded by convex hulls.
In summary, the assemblages from the Holo-Pleistocene of Panama and the late Miocene of Florida differ in the following aspects:
per cent coverage of tubercles on the outer side of the manus;
statistically different slopes of the regressions lines of (a) l/h versus length manus and (b) area manus versus percentage of inner side manus covered by tubercles;
higher l/h ratios for specimens from the late Miocene assemblage of Florida;
specimens from the late Miocene assemblage of Florida exhibit a higher tubercle density (outer and inner sides manus);
shape significantly differs using geometric morphometric analyses;
separation using multivariate analyses: PCA, size-independent CVA, and DA.
Additionally, two minor morphological differences were found. The distalmost of the two dactylar teeth tends to be more pronounced (higher) in specimens from the Holo-Pleistocene assemblage of Panama for similar-sized specimens. Furthermore, the carpi of the Holo-Pleistocene assemblage of Panama exhibit tubercles near the lower margin of the outer side; tubercles are absent near the lower margin of the outer side of carpi from the late Miocene assemblage of Florida.
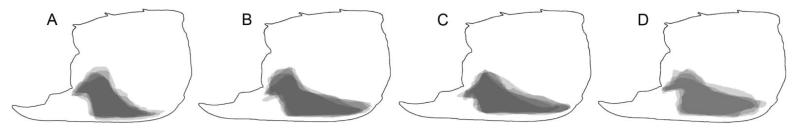
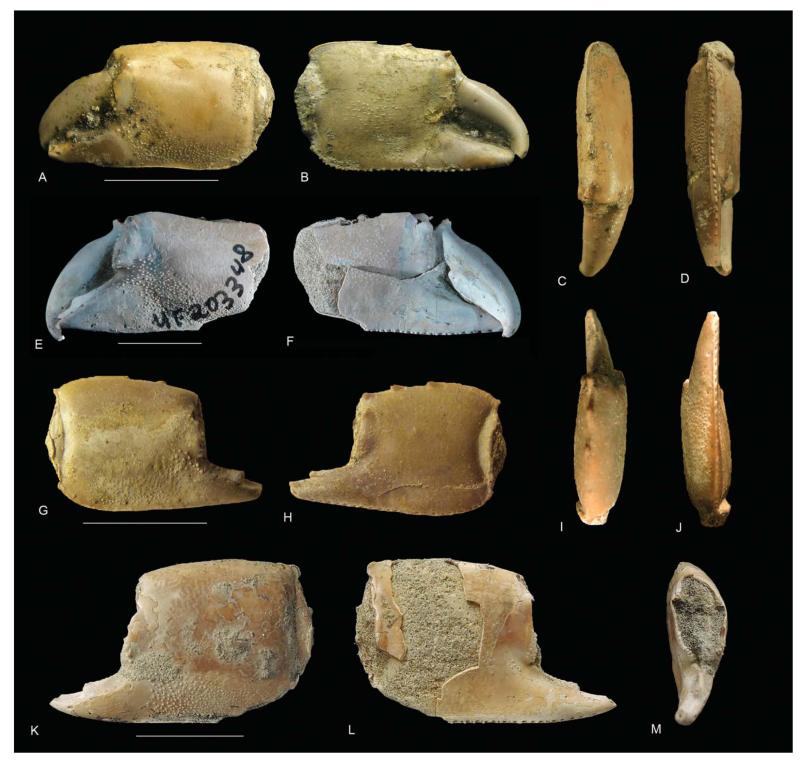
Differences with Glypturus toulai, the late Miocene species from Panama, roughly resembling the latter two in terms of tubercular coverage on the outer side of the propodus (Fig. 2A), are less numerous because of the lack of sufficient specimens precluding rigorous statistical testing of length/height ratios, percentage of tubercle coverage, and geometric morphometric analysis. Only two specimens from the Gatun Formation were available for multivariate analyses. Nevertheless, their location in PCA ordination space (Fig. 6A) and in size-independent CVA (Fig. 7) does suggest that the assemblages from the Holo-Pleistocene of Panama and the late Miocene of Florida differ from G. toulai. This seems further supported by DA. Morphological differences can also be found. For instance, the tuberculation pattern on the outer side shows a clearer re-entrant close to the proximalmost part of the fixed finger in the case of G. toulai compared to other younger Cenozoic Glypturus spp. (Fig. 8). Additionally, the tubercular extent on the inner side seems to differ in that only the central portion of this side of G. toulai appears covered (Fig. 9), whereas the lower extent of the tubercular area of the assemblages from the Holo-Pleistocene of Panama and the late Miocene of Florida typically run diagonally towards the lower-proximal corner of the manus in medium- to large-sized specimens (Fig. 9). Although incomplete, the largest specimen of G. toulai from the Gatun Formation (UF 203348) does not show a clear trend of the lower tubercular margin directed toward the lower-proximal corner (Fig. 9; Hyžný et al. 2013, fig. 5). Lastly, the number of tubercles per 10 mm2 appears lower for medium-sized specimens of G. toulai for both sides compared to the assemblages from the Holo-Pleistocene of Panama and the late Miocene of Florida (Fig. 4). Given the numerous differences between the latter two assemblages and other congeneric species (see below), these two species are referred to as G. panamacanalensis sp. nov. and G. sikesi sp. nov., respectively, in the text from here onwards.
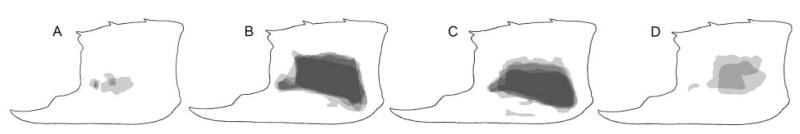
Figure 8.
Tubercular extent on the outer side of the propodus highlighted by a grey, translucent shade for Glypturus spp. A, G. acanthochirus (modern); B, G. panamacanalensis sp. nov. (Holo-Pleistocene, Panama); C, G. sikesi sp. nov. (late Miocene, Florida, USA); D, G. toulai (late Miocene, Panama). All specimens were scaled to the same size. Five specimens were randomly selected for A–C. For G. toulai from the Gatun Formation only four specimens were available (NHMW 1933/0018/0159, NHMW 1933/0018/0160, UF 203348 and BMNPH PI IC 395). Possible tubercles directly above the notch above the fixed finger were not included.
Figure 9.
Tubercular extent on the inner side of the propodus highlighted by a grey, translucent shade for Glypturus spp. for specimens with a length of ≥ 20 mm including the fixed finger as tubercular patterns are better developed at larger sizes for the inner side. A, G. acanthochirus (modern); B, G. panamacanalensis sp. nov. (Holo-Pleistocene, Panama); C, G. sikesi sp. nov. (late Miocene, Florida, USA); D, G. toulai (late Miocene, Panama). All specimens were scaled to the same size. Five specimens were randomly selected for A–C. For G. toulai from the Gatun Formation only two specimens showed the inner tubercle pattern with this size restriction (NHMW 1933/0018/0159 and UF 203348), of which the latter was incomplete.
Taxonomic placement of isolated specimens
The tuberculation patterns on two specimens, UF 42451 from the late Pliocene–early Pleistocene of Florida and UF 232383 from the Holo-Pleistocene of Jamaica (see below), suggest they can be ascribed to G. acanthochirus. This is supported by their placement in the PCA ordination space within the area of modern G. acanthochirus (Fig. 6B). Another specimen (UF 131471, middle Pleistocene of Florida) morphologically resembles G. panamacanalensis sp. nov. from the Holo-Pleistocene of Panama. This is confirmed by the PCA ordination, which is why this specimen is referred to G. panamacanalensis sp. nov. The same applies for a specimen from the late Pleistocene of Cuba (UF 248008) (Fig. 6B). Another specimen (UF 222839) from the late Pleistocene of Cuba (see below), but from a different locality, plots in the space of G. acanthochirus, but does not show the typical tuberculation pattern on the outer side of the propodus. It is conservatively referred to Glypturus sp. here as not all tubercles may be preserved on the outer side or this may represent an anomalous specimen. The alternative is that both G. acanthochirus and G. panamacanalensis sp. nov. are present in the late Pleistocene of Cuba, which would not be surprising given the overlapping age ranges of the species (Supplemental Table 1).
Allometric coefficients and multiple-specimen classification
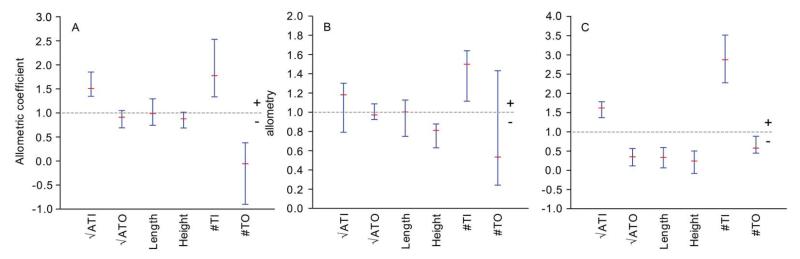
Figure 10 shows variables with negative (< 1.0) and positive allometry (> 1.0) relative to the PC1-based latent size proxy based on all six variables. Figure 10A shows positive allometry for the variables measured on the inner side and negative allometry for the number of tubercles on the outer side. The other variables do not display significant allometric departures from isometry. Isometry is observed for four variables in Figure 10B, whereas height and the number of tubercles on the inner side exhibit negative and positive allometry, respectively. For all specimens combined (Fig. 10C), the majority of the variables shows negative allometries, whereas positive allometries are seen for the variables measured on the inner side. Thus, the ratio of the number of tubercles and √ATI to manus size increases as manus size increases.
Figure 10.
Allometric coefficients for six variables including 95% confidence intervals. ATI = area tubercles on inner side manus (mm2); ATO = area tubercles on outer side manus (mm2); length = length of manus (mm); height = height of manus (mm); #TI = number of tubercles on inner side manus; #TO = number of tubercles on outer side manus. A, Glypturus panamacanalensis sp. nov., 81.0% of variation in PC1; B, G. sikesi sp. nov., 90.8% of variation in PC1; C, All groups combined, 91.0% of variation in PC1.
The jackknife-corrected a posteriori classification using DA (Table 2) indicates that > 90% of specimens are classified correctly for the new species. When all taxa are combined > 85% of specimens are classified correctly. This is in line with the separation of the four taxa in Figures 6 and 7. The error rates for the three analysed groups decrease with increasing sample size (Table 3), to zero for a sample size of n = 5. Thus, a reliable classification of specimens should be possible for samples consisting of five or more well-preserved specimens for which all six non-landmark variables can be measured.
Table 2.
Jackknife-corrected a posteriori classification of specimens of Glypturus using DA.
| Number of misclassified specimens |
||||||
|---|---|---|---|---|---|---|
| Taxon | Total number of specimens |
% classified correctly |
G. panamacanalensis sp. nov. |
G. sikesi sp. nov. |
G. acanthochirus | G. toulai |
| G. panamacanalensis sp. nov. | 13 | 92.3 | — | 1 | 0 | 0 |
| G. sikesi sp. nov. | 12 | 91.7 | 1 | — | 0 | 0 |
| G. acanthochirus | 8 | 75.0 | 1 | 0 | — | 1 |
| G. toulai | 2 | 50.0 | 1 | 0 | 0 | — |
| Total | 35 | 85.7 | 3 | 1 | 0 | 1 |
Table 3.
Error rates as a function of sample size (n) in the Glypturus study.
| Total | G. panamacanalensis sp. nov. | G. sikesi sp. nov. | G. acanthochirus | |
|---|---|---|---|---|
| n = 1 | 0.143 | 0.077 | 0.083 | 0.25 |
| n = 2 | 0 | 0 | 0 | 0 |
| n = 3 | 0.048 | 0 | 0 | 0.25 |
| n = 4 | 0.056 | 0 | 0 | 0.333 |
| n = 5 | 0 | 0 | 0 | 0 |
Major versus minor propodi in Glypturus
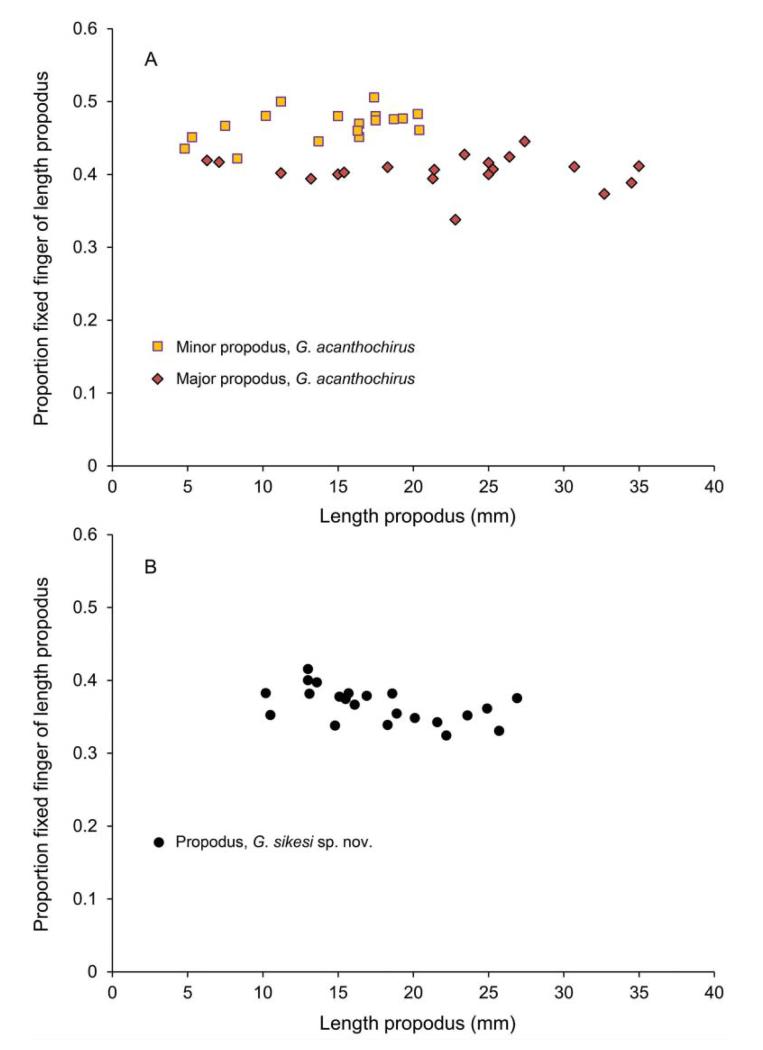
The minor propodi of the three extant species (Glypturus acanthochirus, G. armatus and G. laurae) based on specimens in collections at the FLMNH and NHMW contain no tubercles. Spines were always present on the upper margin of the minor and major propodus. The fixed fingers of G. acanthochirus are relatively large in minors compared to majors, and this proportion appears not to be influenced by growth (Fig. 11A). A Mann–Whitney test on the percentages shows that the relative lengths are significantly different (p < 0.001). Modern specimens of G. acanthochirus and specimens of G. sikesi sp. nov. from the late Miocene of Florida show no obvious size-related change (Fig. 11A, B). Data used for modern and late Miocene specimens are stored in Supplemental Appendix 4.
Figure 11.
Proportion of the fixed finger of the total propodus length versus length propodus. A, modern Glypturus acanthochirus; B, late Miocene G. sikesi sp. nov. from Florida, USA.
Systematic palaeontology
Order Decapoda Latreille, 1802
Infraorder Axiidea de Saint Laurent, 1979
Family Callianassidae Dana, 1852
Subfamily Callichirinae Manning & Felder, 1991
Genus Glypturus Stimpson, 1866
Type species
Glypturus acanthochirus Stimpson, 1866, by monotypy.
Included species
(M = modern, F = fossil). Glypturus acanthochirus Stimpson, 1866 (M + F); G. armatus (A. Milne-Edwards, 1870) (= G. motupore Poore & Suchanek, 1988) (M + F); G. laurae (de Saint Laurent in de Vaugelas & de Saint Laurent, 1984) (M); Glypturus sp. (= Glypturus rabalaisae sensu Sakai, 2005) (M); G. berryi (Rathbun, 1935) (F); G. fraasi (Noetling, 1885) (F); G. munieri (Brocchi, 1883) (F); G. panamacanalensis sp. nov. (F); G. persicus Hyžný et al., 2013 (F); G. pugnax (Böhm, 1922) (F); G. sikesi sp. nov. (F); G. spinosus (Lőrenthey, 1897) (F); G. toulai (Rathbun, 1919) (F).
Diagnosis
Carapace with rostral spine (anterior margin of carapace with three spines, median extending to cornea). Cornea dorsal, subterminal, disk-shaped. Al peduncle not longer and stouter than A2 peduncle. Mxp 3 without exopod, ischium-merus subpediform; merus not projecting beyond articulation with carpus. Chelipeds unequal, major without meral hook; [propodus usually with three distally directed spines near or on upper margin; lower margins of carpus and merus with spines; upper margin of merus with spines; keel present on outer side merus; tubercles usually present on outer side propodus and merus.] Plp l slender and uniramous, Plp 2 slender and biramous, Plp 3–5 foliaceous and biramous in both sexes; appendices intemae finger-like on Plp2 in both sexes, stubby, projecting from endopod of Plp 3–5 in both sexes (modified from Manning & Felder 1991, p. 778). [changes and/or additions in brackets]
Remarks
Manning & Felder (1991) provided a useful diagnosis that can be applied especially to modern species. However, other criteria are useful for palaeontologists and have been added here including tuberculation, spination, and a keel on the merus. Manning & Felder (1991, p. 778) stated “palm [= manus] with 3 spines on upper margin.” This was modified as Hyžný & Müller (2012) mentioned exceptions (2–5 spines), and we found a specimen with only one spine (UF 248037, G. sikesi sp. nov.).
Until this study, the extent of tuberculation on the outer and inner sides of the propodus were sufficient to distinguish species of Glypturus (Hyžný & Müller 2012; Hyžný et al. 2013), and this character remains useful. However, as shown in Figures 8 and 9 the extent of the tuberculation on the propodus is not sufficient to distinguish between the new species, but other criteria clearly show they must be considered different taxa (see above).
The tubercular pattern of Glypturus sp. (= G. rabalaisae sensu Sakai, 2005) was neither figured nor described and, thus, cannot be compared to the new species below. A search did not result in locating the type material: the only specimen number mentioned in Rabalais et al. (1981) is USNM 172310, but this number does not correspond to a specimen of Glypturus in the USNM collection (http://collections.nmnh.si.edu/search/; Rafael Lemaitre, pers. comm. 22 January 2014). Moreover, the same database does not yield specimens of Glypturus from the type locality as defined in Sakai (2005, 2011). An attempt to locate the un-numbered specimens including the types, probably stored in the University of Texas Marine Science Institute, was unsuccessful.
Glypturus panamacanalensis sp. nov.
Figure 12.
Glypturus panamacanalensis sp. nov. from: A–Y, Holo-Pleistocene of Panama (Farfan and Amador beaches); Z, A’, middle Pleistocene Bermont Formation of Florida (Belle Glade 01); B’, C’, upper Pleistocene Jaimanitas Formation in Cuba (Good Road 01). A, B, UF 126585, paratype: outer and inner sides of left dactylus, propodus, and carpus; C–E, UF 248035, paratype: outer side, inner side, and upper margin of right propodus; F–H, UF 248033, holotype: outer side, inner side, and upper margin of left propodus; I–L, UF 248032, paratype: outer side, inner side, lower margin, and upper margin of right propodus; M, N, UF 246095: occlusal surface and outer side of right dactylus; O, P, UF 248030: occlusal surface and outer side of right dactylus; Q, R, UF 126719, paratype: occlusal surface and outer side of right dactylus; S–U, UF 246099, paratype: outer side, inner side, and lower margin of left carpus; V–X, UF 246097: outer side, inner side, and lower margin of right carpus; Y, UF 246078, paratype: part of outer side right merus; Z, A’, UF 131471: outer and inner sides of right propodus; B’, C’, UF 248008: outer and inner sides of right propodus. Scale bar widths: 10.0 mm. Note that images C–L were taken before specimens received new UF catalogue numbers.
Figure 13.
Anomalous specimens of Glypturus panamacanalensis sp. nov. A–D, outer side, inner side, upper margin, and lower margin of a specimen exhibiting a swelling in the fixed finger (UF 126570); E–G, upper part of a manus showing concavity on the margin, inner side near margin showing a subcircular indent, and a concave ridge on the outer side near the margin (UF 246071). Scale bar widths: 10.0 mm.
Diagnosis
Lateral tuberculation on outer propodal side from base of fixed finger diagonally to lower margin and proximal lower corner. Lateral tuberculation on inner propodal side covering large lower part of manus in medium to large specimens with lower margin of tuberculated area diagonally crossing from area of articulation of dactylus toward (but not reaching) proximal lower corner, tuberculation in small specimen more variable but mostly as in larger specimens. Occlusal margin of dactylus with two elongated, strong teeth exhibiting smooth top. Outer side carpus with tubercles near lower margin; inner side with tubercles on lower ~75%.
Derivation of name
Derived from the location where the type specimens were found: at beaches near the entrance of the Panama Canal near Panama City, Panama. The name also acknowledges the tremendous efforts in constructing, maintaining, and expanding the Panama Canal.
Material
Holotype: UF 248033 (Fig. 12F–H). Para-types: UF 126585 (Fig. 12A, B), 248035 (Fig. 12C–E), 248032 (Fig. 12I–L), 126719 (Fig. 12Q, R), 246099 (Fig. 12S–U) and 246078 (Fig. 12Y).
Other material (P = propodus; D = dactylus; C = carpus; M = merus; FF = fixed finger): UF 246095 (Fig. 12M, N), 248030 (Fig. 12O, P), 246097 (Fig. 12V–X), 131471 (Fig. 12Z, A’), 248008 (Fig. 12B’, C’), 124236 (1P1C), 126571 (8D), 126572 (1D), 126573 (2P), 126574 (3P), 126575 (1D), 126576 (1FF), 126577 (2P), 126578 (13P), 126581 (1P), 126582 (2P), 126583 (1C), 126584 (2C), 126594 (5P), 126595 (8P), 126645–126646 (23P each), 126647 (16P), 126648 (28P), 126649 (23P), 126650 (24P), 126651 (19P), 126652 (20P), 126653 (13P), 126654–126655 (4FF each), 126663 (15P), 126664 (13P), 126665 (2FF), 126685–126686 (18P each), 126687 (11P), 126688 (20P), 126689 (21P), 126717 (4P), 126718 (1P), 126739 (3FF), 126740 (1FF), 126741 (12P), 126742 (2P), 230782 (1P), 230816–230841 (1P each), 246064 (2P), 246065–246070 (1P each), 246072–246076 (1P each), 246077 (1FF), 246079 (2P), 246080–246086 (1P each), 246087 (1C), 246088–246090 (1P each), 246091 (2P), 246092 (1P), 246093–246094 (1FF each), 246096 (1C), 246098 (1C), 246100–246101 (1C each), 248050–248053 (1P each), 248936–248937 (1P each), 250088 (2P), 251703–251725 (1P each), 251802–251803 (3P each), 251804 (1P), 251805–251807 (2P each), 251830–251833 (1P each), 251834 (7P), 251835 (4P), 251836–251839 (1P each), 251840 (2P), 251841 (3P), 251842–251843 (1P each) and 251844 (4P).
Occurrence
Collected (ex situ) on Amador and Farfan beaches (type locality) at the entrance of the Panama Canal near Panama City, Panama. The age is Holo-Pleistocene (see Geological setting); the formation is unknown. Additionally, the species is known from the middle Pleistocene Bermont Formation of Florida (Belle Glade 01 locality) and the upper Pleistocene Jaimanitas Formation of Cuba (Good Road 01 locality).
Description
Manus up to ~25 mm long and ~20 mm tall, length/height ratio ~1.0–1.3. Upper margin curving inward distally, proximally keeled, typically bearing three prominent spines pointing distally, proximalmost spine around mid-margin, keel terminating in blunt corner proximally. Lower margin sharp and keeled, lined with a row of setal pits on inner lateral side. Proximal margin convex on outer face, concave on inner; heightwise groove near proximal margin concave on inner side, concave to straight on outer side. Distal margin with protrusion just above fixed finger, hinge point with dactylus expressed as notch. Lateral tuberculation on outer side from base of fixed finger diagonally to lower margin and proximal lower corner. Lateral tuberculation on inner side covering large lower part of manus in medium to large specimens with lower margin of tuberculated area diagonally crossing from area of articulation of dactylus toward (but not reaching) proximal lower corner, tuberculation in small specimens more variable but mostly as in larger specimens. Fixed finger curving inward, triangular, sharply pointed, with distinct blunt tooth on occlusal margin around mid-length, tooth pointing distally. Dactylus stout; occlusal margin with two elongated, strong teeth exhibiting smooth top; tip hooked, curving downward. Carpus trapezoid, slightly taller than long; upper margin keeled and smooth with setal pits on inner part margin; lower margin keeled, shows bases of spines on inner side; distal margin straight to slightly concave; proximal margin concave on inner side, convex on outer side; outer side with tubercles near lower margin only; inner side with tubercles on lower ~75%. Outer side merus with longitu dinal keel in centre, tuberculated below keel, spinose and convex lower margin. Other parts of species not preserved.
Remarks
Intraspecific variation is observed in the strength of the teeth on the dactylus (Fig. 12A, B, M–R) and the tooth on the fixed finger (compare Fig. 12C to 12F, I). Furthermore, the number of spines on the upper margin of the propodus is typically three, but some (< 10%) specimens exhibit two (UF 248053) or four spines (UF 248050–248052). Size-related variation is particularly expressed in the tuberculation pattern on the inner side of small specimens as opposed to the more stable coverage in larger specimens, an increasing length/height ratio with size, and an increasing coverage of tubercles on the inner side with size. Although a higher number of major propodi (defined here: length manus ≥ 15 mm) are right-handed (193 versus 159 left-handed), this is not statistically significant (χ2 = 3.28; p = 0.07).
Glypturus panamacanalensis sp. nov. differs from G. acanthochirus, G. armatus, G. laurae, G. persicus, G. munieri and G. pugnax in that the tubercles cover a substantially greater area on the outer side of the propodus. Similarly, the inner side of the propodus of G. panamacanalensis sp. nov. has a greater coverage of tubercles compared to G. acanthochirus, G. armatus and G. laurae. Conversely, tubercular coverage on the inner side of G. fraasi is greater than in G. panamacanalensis sp. nov. The tubercles on the outer side of G. berryi reach above the lower proximal corner, which is not the case in G. panamacanalensis sp. nov. Glypturus spinosus bears much stronger spines on the upper margin compared to the new species, although spines may be variable (see Hyžný & Müller 2012, p. 982). Tubercular patterns are more difficult to use to distinguish G. toulai and G. sikesi sp. nov. from the new species, but sufficient differences were found to erect G. panamacanalensis sp. nov. (see above).
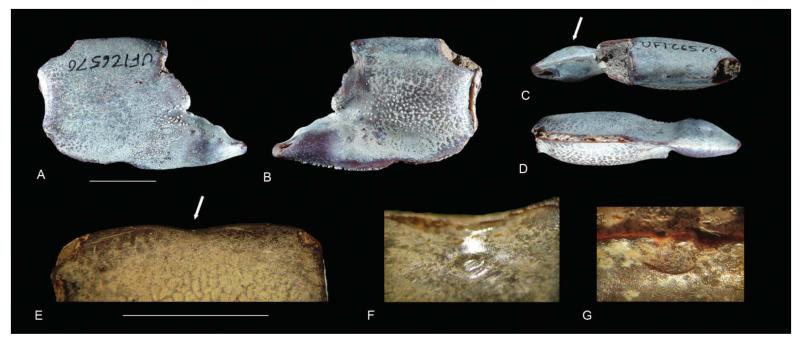
One specimen (UF 126570) exhibits a swelling in the fixed finger (Fig. 13A–D). Isopod-induced swellings are known to occur in the branchial region of the carapace of decapod crustaceans including shrimp (Franţescu 2014; Klompmaker et al. 2014), but not in the fixed finger. These ichnofossils were named Kanthyloma crusta Klompmaker et al., 2014. The origin of this particular swelling is unclear, but it may represent an example of a bacterial infection (Christopher Boyko, pers. comm. 11 February 2013). Another specimen (UF 246071) shows a concavity on the upper margin of the propodus accompanied by a subcircular indent on the inner side of the margin and a concave ridge on the outer side (Fig. 13E–G). The keel on the upper margin is somewhat offset. There are no signs of a fracture. It is speculated here that this could be a failed predation attempt or an attack by a conspecific individual (antagonistic behaviour), perhaps directly after the moulting phase when the cuticle was not fully hardened, which may explain why no fracture is observed. Antagonistic behaviour is known from modern ghost shrimps (e.g. Nihonotrypaea Manning & Tamaki, 1998), whereby the major cheliped is protruded and used to grapple with the chelipeds of another individual (Shimoda et al. 2005). Predators of fossil decapods are numerous (e.g. Klompmaker et al. 2013, table 1); those of modern ghost shrimp include fish, crabs, birds and juvenile grey whales (e.g. Posey 1986; Dworschak et al. 2012).
Glypturus sikesi sp. nov.
(Fig. 14)
Figure 14.
Glypturus sikesi sp. nov. from the upper Miocene Choctawhatchee Formation of Florida (Sikes Sand Mine 02). A–D, UF 235355, paratype, outer side, inner side, lower margin and upper margin of left propodus; E–G, UF 235154, paratype, outer side, inner side and upper margin of right propodus; H–J, UF 235152, holotype, outer side, inner side and upper margin of right propodus; K–M, UF 235370, paratype, outer side, inner side and upper margin of left propodus; N–Q, UF 235401, paratype, outer side, inner side, lower margin and upper margin of left propodus; R, S, UF 248044, occlusal surface and outer side of right dactylus; T, U, UF 235166, paratype, occlusal surface and outer side of right dactylus; V, W, UF 235420, outer and inner sides of right carpus; X–Z, UF 248029, paratype, outer side, inner side and lower margin of right carpus; A’–C’, UF 248038, paratype, outer side, inner side and upper margin of left merus; D’, E’, UF 248041, paratype, outer and lower sides of partial propodus and dactylus; F’, UF 248028, part of inner side or plate of right merus; G’, UF 248042, paratype, inner side carpus, and outer sides of merus and propodus (from left to right); H’, UF 248042, paratype, inner sides of propodus and partially exposed merus, and outer side of carpus (from left to right). Scale bar widths: 10.0 mm.
Diagnosis
Lateral tuberculation on outer propodal side from base of fixed finger diagonally to lower margin and proximal lower corner. Lateral tuberculation on inner propodal side covering large lower part of manus in medium to large specimens with lower margin of tuberculated area diagonally crossing from area of articulation of dactylus toward (but not reaching) proximal lower corner, tuberculation in small specimens more variable but mostly as in larger specimens. Occlusal margin dactylus without teeth or exhibiting two weak to medium teeth. Outer side carpus without tubercles; inner side with tubercles on lower ~75%.
Derivation of name
Named after the owner (Mr Lamar Sikes) of the Sikes Sand Mine in Florida, from which all currently known specimens originated.
Material
Holotype: UF 235152 (Fig. 14H–J); Para-types: UF 235355 (Fig. 14A–D), 235154 (Fig. 14E–G), 235370 (Fig. 14K–M), 235401 (Fig. 14N–Q), 235166 (Fig. 14T, U), 248029 (Fig. 14X–Z), 248038 (Fig. 14A’–C’), 248041 (Fig. 14D’, E’) and 248042 (Fig. 14G’, H’).
Other material (P = propodus; D = dactylus; C = carpus; M = merus; FF = fixed finger): UF 248044 (Fig. 14R, S), 235420 (Fig. 14V, W), 248028 (Fig. 14F’), 232621 (1D), 232622–232632 (1P each), 232633 (14P), 232660 (1P), 232661 (8P), 232662 (2P), 235153 (1P), 235155–235164 (1P each), 235165 (1D), 235167–235168 (10P), 235169 (7P), 235356–235369 (1P each), 235371 (3P), 235372–235373 (15P each), 235374 (10P), 235399–235400 (1P each), 235402–235403 (1D each), 235404 (1FF), 235405–235419 (1P each), 235421–235432 (1P each), 235433 (33P), 248034 (1P), 248037 (1P), 248039 (1M), 248040 (4C), 248043 (10D), 248045–248049 (1P each), 251651 (1P), 251652 (1C), 251653–251658 (1P each) and 251901 (1C).
Occurrence
All specimens were collected at Sikes Sand Mine 02 (type locality) in Washington County, Florida, USA, and come from the upper Miocene Choctawhatchee Formation (see Geological setting).
Description
Manus up to ~23 mm long and ~19 mm tall, length/height ratio ~1.0–1.3. Upper margin curving inward distally, proximally keeled, typically bearing three prominent spines pointing distally, proximalmost spine around mid-margin, keel terminating in blunt corner proximally. Lower margin sharp and keeled, lined with a row of setal pits on inner lateral side. Proximal margin convex on outer face, concave on inner; heightwise groove near proximal margin concave on inner side, concave to straight on outer side. Distal margin with protrusion just above fixed finger, hinge point with dactylus expressed as notch. Lateral tuberculation on outer side from base of fixed finger diagonally to lower margin and proximal lower corner. Lateral tuberculation on inner side covering large lower part of manus in medium to large specimens with lower margin of tuberculated area diagonally crossing from area of articulation of dactylus toward (but not reaching) proximal lower corner, tuberculation in small specimens less prominent, largest specimens contain additional tubercles on lower margin below two distalmost spines on upper margin. Fixed finger curving inward, triangular, sharply pointed, with distinct blunt tooth on occlusal margin around mid-length, tooth pointing distally. Occlusal margin dactylus without teeth or exhibiting two weak to medium teeth with proximalmost one being largest; tip dactylus hooked, curving downward. Carpus subrectangular with rounded distalmost lower corner on outer side, slightly taller than wide; upper margin keeled and smooth with setal pits on inner part margin; lower margin keeled, exhibits spines on inner side; distal margin straight to slightly concave; proximal margin concave on inner side, convex on outer side with one tooth approximately one-third the distance from upper margin directed proximally; distalmost lower corner with one spine directed distally; outer side without tubercles; inner side with tubercles on lower ~75%. Outer side merus with longitudinal keel in centre; tuberculated below keel, especially proximally; spinose and convex lower margin; upper margin exhibiting three distally directed spines. Inner side merus flattened with tubercles on lower half and proximally. Other parts of species not preserved.
Remarks
Intraspecific variation is observed in the strength of the teeth on the dactylus (Fig. 14R–U, D’, E’) and the tooth on the fixed finger. Furthermore, the number of spines on the upper margin of the propodus is typically three, but some (< 10%) specimens exhibit more (four: UF 235424; five: UF 248034) or fewer spines (one: UF 248037; two: UF 232625, UF 235408). Size-related variation is particularly expressed in the tuberculation pattern on the inner side of small specimens as tubercle coverage is more variable in these specimens, an increasing length/height ratio with size, and an increasing coverage of tubercles on the inner side with size. A nearly equal number of major propodi (defined here: length manus ≥ 15 mm) is left-handed (34) and right-handed (36) (χ2 = 0.06; p = 0.81).
The differences between Glypturus sikesi sp. nov. and G. acanthochirus, G. armatus, G. berryi, G. fraasi, G. laurae, G. persicus, G. munieri and G. pugnax are the same as described for G. panamacanalensis sp. nov. since the tubercular patterns of G. sikesi sp. nov. and G. panamacanalensis sp. nov. are nearly identical on the propodus. Glypturus spinosus bears much stronger spines on the upper margin compared to the new species. Tubercular patterns are more difficult to use to distinguish G. toulai and G. panamacanalensis sp. nov. from the new species, but sufficient differences were found to justify erection of G. sikesi sp. nov. (see above).
Glypturus acanthochirus Stimpson, 1866
(Fig. 15)
Figure 15.
Modern and fossil specimens of Glypturus acanthochirus. A–D, NHMW 15340, outer side, inner side, upper margin and lower margin of right propodus of a modern specimen from Belize (Twin Cays, mangrove channel); E, F, NHMW 24967, outer and inner sides of left dactylus, propodus, carpus, merus and ischium of a modern specimen from Belize (Twin Cays) (modified from Hyžný & Müller 2012, fig. 2; courtesy of the Palaeontological Association); G–I, UF 42451, outer side, inner side and upper margin of left propodus from the upper Pliocene-lower Pleistocene Tamiami Formation (Pinecrest beds) of Florida (Mule Pen); J–M, UF 232383, outer side, inner side, upper margin and lower margin of right propodus from the Holo-Pleistocene of Jamaica (Falmouth 01). Scale bar widths: 10.0 mm.
*1866 Glypturus acanthochirus Stimpson, 46.
2012 Glypturus acanthochirus Stimpson; Hyžný & Müller: 971, figs 1, 2A–C, 3A–C, I, 4C (cum syn.).
Diagnosis
Front trispinous. Manus of large cheliped usually with three well-developed spines along distal half of dorsal margin. Endopod of uropod elongate oval, about twice as long as wide. Outer side major propodus with tubercles arranged on lower half (~8–17% of manus), not reaching proximal corner. Tubercles on inner side major propodus absent to rare (< ~6% manus). Carpi without tubercles on outer side; part of specimens exhibit tubercles near lower-distal margin on inner side (< ~5% coverage). Minor chelipeds without tubercles (expanded from Biffar 1971, p. 655).
Material
F = fossil (propodi), remainder = modern (entire specimens). NHMW 6766 (Twin Cays, Belize), 6768–6770 (Twin Cays, Belize), 15338 (Twin Cays, Belize), 15340 (Fig. 15A–D; Twin Cays, Belize), 15341–15342 (Twin Cays, Belize), 19624 (Twin Cays, Belize), 19625 (South Water Caye, Belize), 19626–19627 (Twin Cays, Belize), 24967 (Fig. 15E, F; Twin Cays, Belize), 24968 (Twin Cays, Belize), 25261 (Pos Chiquito, Aruba), 25266 (Cayo Muerto, Morrocoy, Venezuela), 25635 (near STRI station, Bocas del Toro, Panama), 25636 (Panama, Bocas del Toro), 25637 (Panama, Bocas del Toro, San Cristobal, Punta Coco), 25638 (Panama, Bocas del Toro), 25639–25640 (Panama, Isla Grande). MNHN Th-1593 (Venezuela, Isla Margarita, Guamache). UF 32050 (French Antilles, Saint Martin, Le Galion), 32479 (French Antilles, Saint Martin, Little Key), 32113 (French Antilles, Saint Martin, Le Galion), 32131 (French Antilles, Saint Martin, Baie de l’Embrouchure), 32483 (French Antilles, Saint Martin, Little Key), 42451 (F, Fig. 15G–I; upper Pliocene–lower Pleistocene Tamiami Formation, Pinecrest beds, of Florida, Mule Pen), 232383 (F, Fig. 15J–M; Holo-Pleistocene of Jamaica, Falmouth 01), 232384–232385 (two fixed fingers, Holo-Pleistocene, Jamaica, Falmouth 01).
Occurrence
Western Atlantic region: Gulf of Mexico, Caribbean region, and northern part of the east coast of South America (Dworschak 1992; Sakai 2005; Hyžný & Müller 2012). Fossil specimens known from the Pleistocene and Holo-Pleistocene of Jamaica, and the late Pliocene–early Pleistocene of Florida (Collins et al. 1996; herein) (Supplemental Table 1).
Description
Additional to Biffar (1971, pp. 655–660): major propodus exhibiting tooth on fixed finger; tooth on fixed finger of minor reduced to absent. Upper margin minor and major propodus exhibit usually three forwardly oriented spines. Outer side major propodus with tubercles arranged on lower half (~8–17% of manus), not reaching proximal corner. Tubercles on inner side major propodus absent to rare (< ~6% manus). Carpi without tubercles on outer side; part specimens exhibit tubercles near lower-distal margin on inner side (< ~5% coverage). Meri with tubercles below longitudinal keel (especially proximally) on outer side of major propodus for larger specimens; inner side exhibiting tubercles, especially proximally and on lower margin inner plate for larger specimens. Minor chelipeds without tubercles. Fixed finger of minor comprises a statistically greater portion of propodal length compared to fixed finger of major (Fig. 11A).
Remarks
Biffar (1971, pp. 655–660) provided a detailed description of the entire body of the modern species. Details of tuberculation patterns, known to be important for fossil Glypturus spp., were not described in detail. Therefore, the description of the species is augmented.
Glypturus toulai (Rathbun, 1919)
(Fig. 16)
Figure 16.
Glypturus toulai from the upper Miocene Gatun Formation of Panama. A–D, NHMW 1933/0018/0159, lectotype, outer side, inner side, upper margin and lower margin of left propodus; E, F, UF 203348, outer and inner sides of left propodus (from Hyžný et al. 2013, fig. 5; permission of Springer Science and Business Media); G–J, NHMW 1933/0018/0160, outer side, inner side, upper margin and lower margin of right propodus; K–M, BMNPH PI IC 395, outer side, inner side and frontal view of left propodus. Scale bar widths: A–F, K–M, 10.0 mm; G–J, 5 mm.
*1919 Callianassa toulai Rathbun, 146.
2013 Glypturus toulai (Rathbun); Hyžný et al.: 132, figs 4, 5 (cum syn.).
Diagnosis
Moderately tuberculated Glypturus; lateral tuberculation on outer side of propodus extending from near base of fixed finger diagonally to lower margin and reaching proximal lower corner; lateral tuberculation on inner side of the propodus restricted mainly to area of articulation with dactylus in small specimens, but covering [central portion of inner side of manus in large specimens.] (modified from Hyžný et al. 2013, pp. 132–133).
Material
UF 203348 (Fig. 16E, F). NHMW 1933/0018/0159 (Fig. 16A–D), 1933/0018/0160 (Fig. 16G–J + 3 fixed fingers). BMNPH PI IC 395 (Fig. 16K–M).
Occurrence
This species is known from the upper Miocene Gatun Formation in Panama (Supplemental Table 1).
Description
Manus length exceeding height (length/height ratio ~1.1). Upper margin converging distally, proximally keeled, bearing three prominent spines pointing distally, proximalmost spine around mid-margin, keel terminating in blunt corner. Lower margin sharp and keeled, in larger specimens denticulate, lined with a row of setal pits on inner lateral side. Proximal margin convex on outer face, concave on inner, [heightwise groove near proximal margin concave on inner side, concave to straight on outer side]. Distal margin weakly convex. Lateral tuberculation on outer side from base of fixed finger diagonally to lower margin and proximal lower corner. Lateral tuberculation on inner side of propodus restricted mainly to area of articulation with dactylus in small specimens, but covering [central portion of inner side of manus in large specimens]. Fixed finger triangular, sharply pointed, with distinct blunt tooth on occlusal margin around mid-length, tooth pointing distally. Dactylus stout, occlusal margin [with or] without tooth [around mid-length], tip hooked (modified from Hyžný et al. 2013, pp. 134–135).
Glypturus aff. G. toulai (Rathbun, 1919)
2013 Glypturus toulai (Rathbun); Hyžný et al.: 132, fig. 6.
Remarks
Hyžný et al. (2013) ascribed specimens from the lower Miocene Culebra Formation in Panama to G. toulai based on the extent of the tuberculation on the propodus. The limited number of specimens and their inadequate preservation does not allow rigorous bi- and multivariate analyses to reassess their placement. One possible difference with G. toulai from the upper Miocene Gatun Formation in Panama is the extent of the tubercles on the inner side of the propodus (Hyžný et al. 2013, fig. 6). Also given the long interval between deposition of the Gatun and Culebra formations (~10 Ma, see Coates 1999; Kirby et al. 2008) relative to the other, younger species its ascription to G. toulai must be regarded as preliminary. Therefore, we herein propose the more conservative Glypturus aff. G. toulai until more material is studied.
Glypturus sp.
(Fig. 17)
Figure 17.
Western Atlantic Glypturus sp. A, MNHNCu-P5116, outer side of propodus of Glypturus sp. (= Neocallichirus? quisquellanus Schweitzer et al., 2006) from the lower–middle Miocene Yanigua Formation of the Dominican Republic (Río Camarón) (refigured from Schweitzer et al. 2006, fig. 3D; permission of the Carnegie Museum of Natural History); B, C, USNM MO166941, outer side and upper margin of propodus of Glypturus sp. (= Callianassa anguillensis Rathbun, 1919, holotype) from the lower–middle Miocene Anguilla Formation of Anguilla (Crocus Bay) (courtesy of the Smithsonian Institution); D, E, USNM MO324470, outer and inner sides of fixed finger of Glypturus sp. (= Callianassa latidigata Rathbun, 1919) from the early Miocene of the Dominican Republic (Santo Domingo) (courtesy of the Smithsonian Institution); F, G, NHM UK In 23770, outer and inner sides of propodus of Glypturus sp. (= Callianassa anguillensis) from the lower–middle Miocene Anguilla Formation of Anguilla (Cartouche Bay) (courtesy of the Natural History Museum, London); H, NHM UK In 23784, outer side of dactylus of Glypturus sp. (= Callianassa anguillensis) from the lower–middle Miocene Anguilla Formation of Anguilla (Cartouche Bay) (courtesy of the Natural History Museum, London); I, J, NHM UK In 23774, outer and inner sides of propodus of Glypturus sp. (= Callianassa anguillensis) from the lower–middle Miocene Anguilla Formation of Anguilla (Cartouche Bay) (courtesy of the Natural History Museum, London); K–M, NHM UK In 23777, upper margin and outer and inner sides of propodus of Glypturus sp. (= Callianassa anguillensis) from the lower–middle Miocene Anguilla Formation in Anguilla (Cartouche Bay) (courtesy Natural History Museum, London); N–Q, UF 222839, outer side, inner side, upper margin and lower margin of left propodus from the upper Pleistocene Jaimanitas Formation in Cuba (Good Road 01); R, S, UF 248031, outer and inner sides of left propodus from the lower Pleistocene Caloosahatchee Formation in Florida (De Soto Shell Pit 04); T, UF 246508, outer side of propodus of Glypturus sp. from the lower Miocene Chipola Formation in Florida (Tenmile Creek 12); U, V, UF 77426, outer and inner sides of left propodus from the upper Pleistocene Port Morant Formation in Jamaica (Port Morant Bay 01). All scale bar widths are 10.0 mm, except for D and E, which are 2 mm. Note that images R and S were taken before specimen received new UF catalogue number.
1919 Callianassa anguillensis Rathbun: 164, pl. 1.1–1.7.
1919 Callianassa latidigata Rathbun: 165, pl. 9.10, 9.11.
1924 Callianassa anguillensis Rathbun; Withers: 226, pl. 4.3–4.5.
2006 Neocallichirus? quisquellanus Schweitzer et al.: 115, fig. 3D.
non 2008 ?Neocallichirus? quisquellanus Schweitzer et al.; Schweitzer et al.: 4, fig. 2.
Material
UF 222839 (Fig. 17N–Q), 248031 (Fig. 17R, S), 246508 (Fig. 17T), 77426 (Fig. 17U, V), 222383 (partial propodus, upper Pleistocene Jaimanitas Formation of Cuba, Caravela Road Fill Pit 01), 238826 (propodus, Pliocene Intracoastal Formation of Florida, Pickett Bay 01), 251883 (seven propodi, lower Miocene Chattahoochee Formation, Florida, Jim Woodruff Dam), 109960 & 109946 (propodus and two fixed fingers, lower Pleistocene Caloosahatchee Formation, Florida, De Soto Shell Pit 04), 35055 (fixed finger, upper Pliocene Tamiami Formation, lower Pinecrest beds, Florida, Macasphalt Shell Pit), 96809 (dactylus, Plio-Pleistocene, Florida, Macasphalt Shell Pit). MNHNCu-P5116 (Fig. 17A). USNM MO166941–166943 (in part Fig. 17B, C), MO324470 (Fig. 17D, E). NHM UK In 23770–23784 (in part Fig. 17F–M).
Remarks
Callianassa anguillensis Rathbun, 1919, from the Miocene of Anguilla (see Supplemental Table 1 for details), shows tubercles near the lower margin of the outer side of the propodus (Rathbun 1919, pl. 1.1, 1.3), tubercles in the centre of the inner side (pl. 1.2), and a spine is visible on the upper margin of the incompletely preserved holotype (pl. 1.4). Rathbun (1919, p. 164) mentioned that this spine (“tubercle”) is directed forward and noted another spine at the distal extremity. A third spine on the upper margin, as in the far majority of well-preserved specimens of Glypturus spp., was not observed, but the upper margin is incomplete. A tubercle on the upper margin is also noted for an incompletely preserved paratype (Rathbun 1919, pl. 1.2, 1.3). A finger appears to show a tooth (pl. 1.6). All these features suggest placement in Glypturus. Given the incomplete nature of the specimens, we refrain from a specific assignment, and refer the assemblage to Glypturus sp. Puzzling, however, are some of the tubercles shown near the upper margin of the outer side of the holotype (pl. 1.4), which would be unique for Glypturus. More specimens are needed to confirm whether or not this is an anomaly, but a paratype does not seem to show this feature (pl. 1.3). Five years later, Withers (1924) reported on 15 specimens (12 propodi, three dactyli) from a locality within a few kilometres of the type locality of the species (Crocus Bay), of which the best-preserved specimens are figured here (Fig. 17F–M). He did not mention a lithostratigraphical unit, but these specimens are very likely to have also originated from the Miocene Anguilla Formation. This was also assumed by Collins et al. (2009) and supported by outcrops of this formation in that part of Anguilla (e.g. Christman 1953; Budd et al. 1995). None of the specimens is well preserved on both lateral sides, which makes comparisons with other species difficult. Tubercles near the upper margin were not observed on these specimens. Well-preserved material is needed to determine whether this is a separate species or can be synonymized with an existing species. However, some preliminary suggestions can be made. The specimens seem different from the late Miocene G. sikesi sp. nov., given the smaller tubercles in the latter, and appear different from the late Miocene G. toulai in that the tubercles on the inner side appear to extend to the lower proximal corner.
Rathbun (1919) also described Callianassa latidigata from the early Miocene of the Dominican Republic based on a fixed finger and a dactylus. The fixed finger shows a tooth and tubercles (Rathbun 1919, pl. 9.11), consistent with Glypturus. Given the preservation and limited number of specimens we refer them to Glypturus sp. Withers (1924) suggested that this species resembled Callianassa anguillensis, but conclusive evidence cannot be provided.
Schweitzer et al. (2006) erected the species Neocallichirus? quisquellanus based on a Miocene specimen from the Dominican Republic (see also Supplemental Table 1). The presence of tubercles on the lower part of the outer side of the propodus, the tooth on the fixed finger and the presence of at least one spine on the upper margin (Schweitzer et al. 2006, fig. 3D) allows it to be ascribed to Glypturus with confidence. Given the preservation and the single photo provided showing only the outer side, specific assignment is premature and could be better addressed with additional specimens. Therefore, we refer N.? quisquellanus to Glypturus sp. The holotype and sole type specimen (MNHNCu-P511) could not be located in the Museo Nacional de Historia Natural (Havana, Cuba) when requested and may be lost. Later, Schweitzer et al. (2008) questionably referred specimens from the Oligocene of Puerto Rico to N.? quisquellanus. The notch above the fixed finger is much larger than the type specimen of N.? quisquellanus. Moreover, there is no evidence of tubercles on one of the figured sides and no obvious evidence of spines on the upper margin are visible (Schweitzer et al. 2008, fig. 2), which could also be related to the poor preservation. Nevertheless, given the major difference in the size of the notch, the specimen is unlikely to be conspecific to the Miocene specimen from the Dominican Republic and, thus, assignment to Glypturus is not favoured here.
We refrained from referring these three taxa to nomen dubium so that the names remain available in case better-preserved material becomes available from the respective localities, which may justify or refute these species names.
Discussion
Diversity and use of characters
Distinguishing between species of Glypturus using the extent of the tuberculation pattern on the outer and inner lateral sides of the propodi has worked well and will continue to be useful to distinguish most species. However, two taxa studied herein are different species (G. sikesi sp. nov. and G. panamacanalensis sp. nov., see above), but the extent of the tuberculation on both sides is insufficient to distinguish them. The implication is that looking solely at the tubercular extent can underestimate species richness. The poor preservation and limited number of specimens from a variety of localities (Supplemental Table 1) severely hampers assessment of the true diversity and several existing taxa may be either synonyms or separate species (see Glypturus sp.). Since new, well-preserved specimens are needed for reliably evaluating the identity of these taxa, diversity within Glypturus may be underestimated further.
Use of geometric morphometric and multivariate methods
Traditional multivariate methods (based on non-landmark variables such as linear measurements) have been used in a variety of systematic studies to delineate new taxa and/or assess previously defined taxa (e.g. Hageman 1991, for Mississippian bryozoans; Geary 1992, for Miocene gastropods; Ausich & Meyer 1994, for Mississippian crinoids; Goldman 1995, for Ordovician graptolites; Kowalewski et al. 1997, for modern lingulide brachiopods; Graham et al. 2012, for modern scorpions). Landmark-based geometric morphometrics has been also used to distinguish among species (e.g. Budd et al. 1994, for modern corals; Vergara-Solana et al. 2013, for modern fish; Webster 2007, for Cambrian trilobites). However, the application of traditional multivariate morphometrics and/or geometric morphometrics in decapod palaeontology is unknown to us (except discriminant analysis in Starzyk et al. 2012). Furthermore, these methods have rarely been used for the systematics of extant decapods. The dearth of such studies is, at least in part, due to insufficient sample sizes and poorly preserved specimens. This study highlights the usefulness of these methods when sample sizes are sufficient and specimens are well preserved.
Where are the minors?
Palaeontological research on Glypturus has focused on the major propodus (Hyžný & Müller 2012; Hyžný et al. 2013). Hyžný & Müller (2012, p. 982) mentioned that the minor chela usually lack tuberculation in extant G. acanthochirus. We found that spines on the upper margin of the minor were present, no evidence of tubercles on the minor, and a significant difference of the proportion of the propodus length of the fixed finger comparing the major and the minor for G. acanthochirus. Minors may also be expected in the fossil record in large samples. They should be smaller than the majors (< ~20 mm propodus length), may have no tubercles, and may have a relatively long fixed finger compared to the major by analogy with modern G. acanthochirus. However, none of the several hundred specimens of G. sikesi sp. nov. from the late Miocene of Florida lacks tubercles and no specimen has a much longer fixed finger than other specimens (Fig. 11B), although some small specimens appear to have a higher proportion of the propodus represented by the fixed finger. Whether these specimens represent true minors is challenging to prove given that a marked increase in variance towards smaller specimens as shown in Figure 11A seems absent. Unless minors and majors exhibit exactly the same shape and tuberculation pattern for this species, which may be unlikely given the differences in the modern species, the implication is that minors are rarely preserved. This taphonomic bias is likely due to their small size and relative fragility compared to the majors of the same specimens, and perhaps also by the handling of moults by the animal. Schäfer (1972) noted that the moult of Callianassa Leach, 1814 is taken outside the burrow except for the major claw, decreasing the preservation potential of minors. However, small-sized majors appear to be present in the sample (Fig. 11B). If all are majors, then minors are under-represented, possibly suggesting that their cuticle is thinner than that of the majors of equal size. This is supported by the multiple functions of major chelipeds such as for feeding purposes, digging and fighting of ghost shrimp (e.g. Shimoda et al. 2005; Dworschak et al. 2006). To our knowledge, no neontological study is known that determines possible differences in the thickness of equal-sized majors and minors from the same taxon.
Sedimentary environment
Hyžný & Müller (2012, p. 984) concluded that “extinct species of Glypturus are found in settings that are typical for modern relatives, that is, tropical to subtropical, near-shore carbonates of normal salinity.” Our compilation of Western Atlantic specimens (Supplemental Table 1) indicates that Glypturus is also found frequently in siliciclastic sediments. In fact, the largest assemblages studied herein originated from siliciclastic settings and most localities contained siliciclastic sediments. Thus, Glypturus lived in a variety of sedimentary settings, at least in the Western Atlantic.
Biogeography
Prior to this research describing new occurrences and reassigning taxa to Glypturus, fossil specimens of this genus were only known from the USA, Jamaica and Panama (Fig. 18) in the Western Atlantic. This research thus expands the number of occurrences of the genus in the Western Atlantic to the extent that preliminary suggestions may be made about biogeographical patterns through time. As shown in Figure 18, the first known occurrence of Glypturus in the Western Atlantic region is in the USA (Mississippi) during the Oligocene. By the Miocene, occurrences are known from all over the Western Atlantic, perhaps suggesting a biogeographical expansion of the genus. Pliocene occurrences are only known from the USA (Florida), which may be related to limited collecting in other places. This is supported by the wider distribution in the Holo-Pleistocene all over the Western Atlantic and the modern distribution (Hyžný & Müller 2012, fig. 11). Today, Glypturus is only described from the Atlantic side of the Americas including Panama (e.g. Dworschak 1992; Sakai 2005; Hyžný & Müller 2012; herein). Importantly, this may not have been so in the recent past as Holo-Pleistocene specimens of G. panamacanalensis sp. nov. were found primarily on the Amador and Farfan beaches near Panama City, and their provenance is very likely sediments from the Pacific side (see Geological setting). Thus, Glypturus may have gone extinct on the Pacific side in the Holo-Pleistocene. This also suggests that Glypturus migrated into the Pacific prior to closure of the Central American Isthmus during the Cenozoic (e.g. Keigwin 1978; Coates et al. 1992; Montes et al. 2012). Interestingly, G. panamacanalensis sp. nov. was also found in the Pleistocene of Florida and Cuba on the Atlantic side of the isthmus, well after the closure isthmus. This suggests a wide distribution of the species prior to its relatively recent apparent extinction.
Figure 18.
Known Cenozoic occurrences of fossil Glypturus spp. in the Western Atlantic by epoch. 1. G. berryi, 2. G. toulai, 3. G. aff. toulai, 4. G. sikesi sp. nov., 5. G. acanthochirus, 6. G. panamacanalensis sp. nov. Unnumbered dots represent occurrences of Glypturus sp. Note that G. acanthochirus from the Mule Pen Quarry in Florida is colour-coded as Pliocene, but the more conservative age is late Pliocene–early Pleistocene. For details of localities and more specific ages see Supplemental Table 1. Basemap from http://d-maps.com/carte.php?num_car=1389&lang=en.
Phylogenetic relationships and morphological trends
Glypturus has been included in several phylogenetic analyses of extant ghost shrimps (Tudge et al. 2000; Felder & Robles 2009; Robles et al. 2009), but not all recognized taxa, implying that species-level phylogenetic relationships within Glypturus are unknown. Tudge et al. (2000) worked with a broadly defined Glypturus and the genus was resolved as a paraphyletic group. Following the concept of Glypturus as originally proposed by Stimpson (1866) and further emended by Manning (1987), Felder & Robles (2009) included two Glypturus species in their analyses, both from the Western Atlantic (G. acanthochirus and G. sp. = G. rabalaisae sensu Sakai, 2005). The sister group relationship of these two species resulting from their analysis is not surprising given their close geographical distribution. Robles et al. (2009) analysed relationships among the ‘thalassinidean’ families and several members of callianassid ghost shrimps were included. Glypturus laurae was among them, however, without any other congeners.
Assuming a Tethyan origin of Glypturus and further division into several independent lineages (Hyžný & Müller 2012), G. armatus from the Indo-West Pacific and G. laurae from the Red Sea form a different lineage than the Western Atlantic species G. acanthochirus and G. sp. (see Hyžný & Müller 2012, fig. 11). Phylogenetic analyses evaluating members of both supposed lineages are desirable, if these are possible given the limited number of characters on fossil material.
Hyžný et al. (2013) noted an evolutionary trend in Glypturus with the most tuberculated species dating from the Palaeogene and less tuberculated species from today. As an example they mentioned the possible Western Atlantic lineage consisting of G. berryi–G. toulai–G. acanthochirus. However, the two new species described herein complicate the picture, as they are younger than G. toulai but clearly more tuberculated. In the Tethyan region, G. fraasi from the Eocene of Spain, Italy and Hungary is more tuberculated than G. munieri from the Miocene of Hungary, Austria and Malta (see Hyžný & Müller 2012, for details). Furthermore, Glypturus persicus from the Miocene of Iran possesses tubercles only on its inner propodal side (Hyžný et al. 2013, fig. 7d) and may be part of a lineage towards modern G. laurae and G. armatus. These species are less tuberculated (G. laurae) than G. acanthochirus, or have no tubercles at all (G. armatus). The pattern is not straightforward here either. The fossil record of the Indo-West Pacific region is patchy and the only known fossil species, G. pugnax from the early Miocene of Indonesia, is clearly more tuberculated than the slightly younger G. persicus from the middle–late Miocene of Iran (Hyžný et al. 2013).
Supposedly, the roots of the possible Western Atlantic lineage are in the Eocene assemblages of Europe (Hyžný & Müller 2012, fig. 13), i.e. its evolution could be independent from the Tethyan lineage, at least since the Oligocene from which the oldest Western Atlantic species is known. Another possibility is that there were several introductions of Glypturus into the Western Atlantic region via trans-Atlantic migrations during the Miocene (Harzhauser et al. 2002; Hyžný & Müller 2012). In such a case more tuberculated species (G. sikesi sp. nov. and G. panamacanalensis sp. nov.) would be more closely related to Tethyan G. munieri than to the roughly coeval G. toulai. Thorough analysis of the tuberculation pattern of G. munieri may resolve this issue in the future, as well as the documentation of Glypturus from more localities. Similarly, the analysis of the tuberculation pattern of G. sp. (= G. rabalaisae sensu Sakai, 2005) may shed light on the relationships within the Western Atlantic Glypturus species.
Results indicate that the length/height ratios increased faster throughout growth in geologically older assemblages based on three main assemblages (modern G. acanthochirus, Holo-Pleistocene G. panamacanalensis sp. nov. and late Miocene G. sikesi sp. nov.). This could represent an example of heterochrony, specifically paedomorphosis (e.g. McNamara 2001). However, phylogenetic relationships within Glypturus are unresolved and the oldest known specimen from the Western Atlantic, G. berryi from the Oligocene, does not plot above G. sikesi sp. nov. in Figure 3.
Supplementary Material
Acknowledgements
Peter Dworschak (Naturhistorisches Museum Wien) is thanked for useful information on modern Glypturus; the FLMNH Division of Invertebrate Zoology for access to modern specimens of G. acanthochirus; Alexis Rojas and Sahale Casebolt (both FLMNH) for information on geometric morphometrics; Austin Hendy (FLMNH) for literature and access to some Panamanian specimens and comments on part of the geological setting; Peter Ng (National University of Singapore) for important nomenclatural advice; and Manuel Iturralde-Vinent (Museo Nacional de Historia Natural, Havanna, Cuba) for attempting to locate the holotype of Neocallichirus? quisquellanus. We further acknowledge: Rafael Lemaitre and Thomas Jorstad (both Smithsonian Institution) for information on related specimens and permission to use Figure 17B–E, respectively; John Wible (Carnegie Museum of Natural History) for permission to use Figure 17A; Carrie Schweitzer (Kent State University) for providing the high resolution image used in Figure 17A; and Claire Mellish (Natural History Museum, London) for providing photos of G. anguillensis as described in Withers (1924). Field assistance at the Sikes Sand Mine 02 was provided by Harley Means (Florida Geological Survey), Alex Kittle and Sean Roberts (both FLMNH), and Jon Bryan (Northwest Florida State College). Paul Huddlestun (retired stratigrapher from Georgia Geological Survey) visited the Sikes Sand Mine 02 and kindly offered his insights into the geology of the quarry and surrounding area. Fieldwork at Guantanamo Bay, Cuba by R.W.P. and James K. Toomey (FLMNH volunteer) was possible with the assistance, support and hospitality of José B. Montalvo (Natural Resource Officer) and Michael R. McCord (Public Works Department – Environmental). Emily Vokes is thanked for facilitating the transfer of the Tulane University palaeontological collections. We also thank two anonymous reviewers for their useful comments that improved this manuscript substantially. This is University of Florida Contribution to Paleobiology 668. Disclosure statement: the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This work was supported by the Jon L. and Beverly A. Thompson Endowment Fund to A.A.K.; National Science Foundation [grant 0966884, Partnerships for International Research and Education], the Florida Museum of Natural History (McGinty and Invertebrate Paleontology) endowments, and private funding by James K. Toomey to R.W.P.; the National Science Foundation [grant DBI 0645865] for curating the Tulane University Collection (including the Burkenroad Collection) into the collections of the FLMNH; the Austrian Science Fund [FWF; Lise Meitner Program M 1544-B25], [Scientific Grant Agency of the Slovak Republic VEGA 2/0068/11]; and the Slovak Research and Development Agency [under contracts no. APVV-0644-10; APVV-0436-12] to M.H.
Footnotes
Supplemental material for this article can be accessed online: http://dx.doi.org/10.1080/14772019.2015.1009505.
References
- Ausich WI, Meyer DL. Hybrid crinoids in the fossil record (Early Mississippian, Phylum Echinodermata) Paleobiology. 1994;20:362–367. [Google Scholar]
- Biffar TA. The genus Callianassa (Crustacea, Decapoda, Thalassinidea) in South Florida, with keys to the western Atlantic species. Bulletin of Marine Science. 1971;21:637–715. [Google Scholar]
- Bishop GA, Williams AB. Taphonomy and preservation of burrowing thalassinidean shrimps. Proceedings of the Biological Society of Washington. 2005;118:218–236. [Google Scholar]
- Böhm J. Crustacea. In: Martin K, editor. Die Fossilien von Java. I. Band, 2 Abteilung. E. J. Brill; Leiden: 1922. pp. 521–538. [Google Scholar]
- Bookstein FL. Morphometric tools for landmark data: geometry and biology. Cambridge University Press; Cambridge: 1997. p. 435. [Google Scholar]
- Brocchi P. Notes sur les Crustacés fossiles des terres tertiaires de la Hongrie. Annales des Sciences Géologiques. 1883;14(2):1–8. pls 4, 5. [Google Scholar]
- Budd AF, Johnson KG, Potts DC. Recognizing morphospecies in colonial reef corals: I. Landmark-based methods. Paleobiology. 1994;20:484–505. [Google Scholar]
- Budd AF, Johnson KG, Edwards JC. Caribbean reef coral diversity during the early to middle Miocene: An example from the Anguilla Formation. Coral Reefs. 1995;14:109–117. [Google Scholar]
- Christman RA. Geology of St. Bartholomew, St. Martin, and Anguilla, Lesser Antilles. Geological Society of America Bulletin. 1953;64:65–96. [Google Scholar]
- Coan EV, Valentich-Scott P. Bivalve seashells of tropical west America. Marine bivalve mollusks from Baja California to Peru. Santa Barbara Museum of Natural History, Santa Barbara, California, Monographs. 2012;6:1–1258. [Google Scholar]
- Coates AG. Lithostratigraphy of the Neogene strata of the Caribbean coast from Limon, Costa Rica, to Colon, Panama. Bulletins of American Paleontology. 1999;357:5–16. [Google Scholar]
- Coates AG, Jackson JBC, Collins LS, Cronin TM, Dowsett HJ, Bybell LM, Jung P, Obando JA. Closure of the Isthmus of Panama: The near-shore marine record of Costa Rica and western Panama. Geological Society of America Bulletin. 1992;104:814–828. [Google Scholar]
- Collins JSH, Donovan SK, Dixon HL. Crabs and barnacles (Crustacea: Decapoda & Cirripedia) from the late Pleistocene Port Morant Formation of southeast Jamaica. Bulletin of the Mizunami Fossil Museum. 1996;23:51–63. [Google Scholar]
- Collins JSH, Portell RW, Donovan SK. Decapod crustaceans from the Neogene of the Caribbean: diversity, distribution and prospectus. Scripta Geologica. 2009;138:55–111. [Google Scholar]
- Dall WH. A revision of the Terebratulidae and Lingulidae, with remarks and descriptions of some recent forms. American Journal of Conchology. 1870;6:88–168. [Google Scholar]
- Dall WH. Contributions to the Tertiary fauna of Florida, with especial reference to the Silex beds of Tampa and the Pliocene beds of the Caloosahatchie River, including in many cases a complete revision of the generic groups treated of and their American species. Part IV. I. Prionodesmacea. II. Teleodesmacea: Teredo to Ervilia. Transactions of the Wagner Free Institute of Science of Philadelphia. 1898;3(4):viii, 571–947. pls 23–35. [Google Scholar]
- Dana JD. Crustacea. Part I. United States Exploring Expedition, during the years 1838, 1839, 1840, 1841, 1842, under the command of Charles Wilkes, U.S.N.13. C. Sherman; Philadelphia: 1852. p. viii.p. 685. [PMC free article] [PubMed] [Google Scholar]
- Dworschak PC. The Thalassinidea in the Museum of Natural History, Vienna; with some remarks on the biology of the species. Annalen des Naturhistorischen Museums in Wien, B. 1992;93:189–238. [Google Scholar]
- Dworschak PC, Koller H, Abed-Navandi D. Burrow structure, burrowing and feeding behaviour of Corallianassa longiventris and Pestarella tyrrhena (Crustacea, Thalassinidea, Callianassidae) Marine Biology. 2006;148:1369–1382. [Google Scholar]
- Dworschak PC, Felder DL, Tudge CC. Chapter 69. Infraorders Axiidea de Saint Laurent, 1979 and Gebiidea de Saint Laurent, 1979 (formerly known collectively as Thalassinidea) In: Schram FR, von Vaupel Klein JC, Forest J, Charmantier-Daures M, editors. Treatise on Zoology – Anatomy, Taxonomy, Biology. The Crustacea. Complementary to the volumes translated from the French of the Traité de Zoologie [founded by P.-P. Grassé], Volumee 9 Part B. Eucarida: Decapoda: Astacidea p.p. (Enoplometopoidea, Nephropoidea), Glypheidea, Axiidea, Gebiidea, and Anomura. Brill; Leiden: 2012. pp. 109–219. [Google Scholar]
- Felder DL, Robles R. Molecular phylogeny of the family Callianassidae based on preliminary analysis of two mitochondrial genes. In: Martin JW, Crandall KA, Felder DL, editors. Decapod crustacean phylogenetics. Taylor & Francis/CRC Press; Boca Raton: 2009. pp. 327–342. [Google Scholar]
- Foote M, Sepkoski JJ. Absolute measures of the completeness of the fossil record. Nature. 1999;398:415–417. doi: 10.1038/18872. [DOI] [PubMed] [Google Scholar]
- Fraaije RHB, Artal P, Van Bakel BWM, Jagt JWM, Klompmaker AA. An array of sixth abdominal tergite types of paguroid anomurans (Crustacea) from the mid-Cretaceous of Navarra, northern Spain. Netherlands Journal of Geosciences. 2013;92:109–117. [Google Scholar]
- Franţescu OD. Fossil mudshrimps (Decapoda: Axiidea) from the Pawpaw Formation (Cretaceous: Albian), northeast Texas, USA. Bulletin of the Mizunami Fossil Museum. 2014;40:13–22. [Google Scholar]
- Geary DH. An unusual pattern of divergence between two fossil gastropods: ecophenotypy, dimorphism, or hybridization? Paleobiology. 1992;18:93–109. [Google Scholar]
- Goldman D. Taxonomy, evolution, and biostratigraphy of the Orthograptus quadrimucronatus species group (Ordovician, Graptolithina) Journal of Paleontology. 1995;69:516–540. [Google Scholar]
- Graham MR, Ayrey RF, Bryson RW., Jr Multivariate methods support the distinction of a new highland Vaejovis (Scorpiones: Vaejovidae) from the Sierra de los Ajos, Mexico. Journal of Arachnology. 2012;40:281–290. [Google Scholar]
- Hageman SJ. Approaches to systematic and evolutionary studies of perplexing groups: An example using fenestrate Bryozoa. Journal of Paleontology. 1991;65:630–647. [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica. 2001;4(1):1–9. art. 4. [Google Scholar]
- Hanley SCT. Descriptions of new species of the genus Tellina, chiefly collected by H. Cuming, Esq., in the Philippine Islands and Central America. Proceedings of the Zoological Society of London. 1844–5;1844:59–64. [Google Scholar]
- Harzhauser M, Piller WE, Steininger FF. Circum-Mediterranean Oligo-Miocene biogeographic evolution – the gastropods’ point of view. Palaeogeography, Palaeoclimatology, Palaeoecology. 2002;183:103–133. [Google Scholar]
- Hendy AJW. Spatial and stratigraphic variation of marine paleoenvironments in the middle-upper Miocene Gatun Formation, Isthmus of Panama. Palaios. 2013;28:210–227. [Google Scholar]
- Hinds RB. The zoology of the voyage of H. M. S. Sulphur, under the command of Capt. Sir Edward Belcher, …during the years 1836–42, vol. 2 [Mollusca] Smith, Elder & Co.; London: 1844–5. p. 72. 21 pls. [Google Scholar]
- Holdener EJ. Numerical taxonomy of fenestrate bryozoans: evaluation of methodologies and recognition of intraspecific variation. Journal of Paleontology. 1994;68:1201–1214. [Google Scholar]
- Huddlestun PF. Unpublished PhD thesis. Florida State University; Tallahassee: 1976. The Neogene stratigraphy of the central Florida Panhandle; p. 209. [Google Scholar]
- Hyžný M, Hudáčková N. Redescription of two ghost shrimps (Decapoda: Axiidea: Callianassidae) from the Middle Miocene of the Central Paratethys: systematics, intraspecific variation, and in situ preservation. Zootaxa. 2012;3210:1–25. [Google Scholar]
- Hyžný M, Müller PM. The fossil record of Glypturus Stimpson, 1866 (Crustacea, Decapoda, Axiidea, Callianassidae) revisited, with notes on palaeoecology and palaeobiogeography. Palaeontology. 2012;55:967–993. [Google Scholar]
- Hyžný M, Bahrami A, Klompmaker AA, Yazdi M, Portell RW, Neumann C. The fossil record of Glypturus (Decapoda: Axiidea: Callianassidae) revisited with additional observations and description of a new species. Swiss Journal of Palaeontology. 2013;132:129–139. [Google Scholar]
- Jagt JWM, Fraaije RHB, Van Bakel BWM, Artal P. Necrocarcinus ornatissimus Forir, 1887, and Prehepatus werneri Fraaye and Collins, 1987 (Upper Maastrichtian, the Netherlands) revisited, with notes on other Cretaceous dynomenid crabs (Decapoda, Brachyura) Crustaceana Monographs. 2010;11:173–195. [Google Scholar]
- Jones SM. Geology of Gatun Lake and Vicinity, Panama. Geological Society of America Bulletin. 1950;61:893–922. [Google Scholar]
- Jones A. MS thesis. Kent State University; Ohio: 2013. Population dynamics of Dakoticancer overanus from the Pierre Shale, South Dakota; p. 117. [Google Scholar]
- Keen AM. Sea shells of tropical West America: marine mollusks from Baja California to Peru. Stanford University Press; Stanford: 1971. p. 1064. [Google Scholar]
- Keigwin LD. Pliocene closing of the Isthmus of Panama, based on biostratigraphic evidence from nearby Pacific Ocean and Caribbean Sea cores. Geology. 1978;6:630–634. [Google Scholar]
- Kidwell SM, Flessa KW. The quality of the fossil record: Populations, species, and communities. Annual Review of Ecology and Systematics. 1995;24:433–464. [Google Scholar]
- Kirby MX, Jones DS, MacFadden BJ. Lower Miocene stratigraphy along the Panama Canal and its bearing on the Central American Peninsula. PLOS ONE. 2008;3(7):e2791. doi: 10.1371/journal.pone.0002791. doi:10.1371/journal.pone.0002791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources. 2011;11:353–357. doi: 10.1111/j.1755-0998.2010.02924.x. [DOI] [PubMed] [Google Scholar]
- Klompmaker AA. Extreme diversity of decapod crustaceans from the mid-Cretaceous (late Albian) of Spain: Implications for Cretaceous decapod paleoecology. Cretaceous Research. 2013;41:150–185. [Google Scholar]
- Klompmaker AA, Feldmann RM, Schweitzer CE. A hotspot for Cretaceous goniodromitids (Decapoda: Brachyura) from reef associated strata in Spain. Journal of Crustacean Biology. 2012a;32:780–801. [Google Scholar]
- Klompmaker AA, Feldmann RM, Robins CM, Schweitzer CE. Peak diversity of Cretaceous galatheoids (Crustacea, Decapoda) from northern Spain. Cretaceous Research. 2012b;36:125–145. [Google Scholar]
- Klompmaker AA, Karasawa H, Portell RW, Fraaije RHB, Ando Y. An overview of predation evidence found on fossil decapod crustaceans with new examples of drill holes attributed to gastropods and octopods. Palaios. 2013;28:599–613. [Google Scholar]
- Klompmaker AA, Artal P, Van Bakel BWM, Fraaije RHB, Jagt JWM. Parasites in the fossil record: A Cretaceous fauna with isopod-infested decapod crustaceans, infestation patterns through time, and a new ichnotaxon. PLOS ONE. 2014;9(3):e92551. doi: 10.1371/journal.pone.0092551. doi:10.1371/journal.pone.0092551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalewski M, Dyreson E, Marcot JD, Vargas J, Flessa KW, Hallman DP. Phenetic discrimination of biometric simpletons: paleobiological implications of morphospecies in the lingulide brachiopod Glottidia. Paleobiology. 1997;23:444–469. [Google Scholar]
- Kowalewski M, Novack-Gottshall P. Resampling methods in paleontology. Paleontological Society Papers. 2010;16:19–54. [Google Scholar]
- Kronenberg GC, Lee HG. Genera of American strombid gastropods (Gastropoda: Strombidae) and remarks on their phylogeny. The Veliger. 2007;49:256–264. [Google Scholar]
- Latreille PA. Histoire naturelle, générale et particulière des crustacés et des insectes. Tome 3. Dufart; Paris: 1802. p. 467. [Google Scholar]
- Leach WE. Crustaceology. In: Brewster D, editor. The Edinburgh Encyclopedia. Blackwood; Edinburgh: 1814. pp. 383–437. [Google Scholar]
- von Linnaeus C. Systema Naturae per Regna tria Naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. ed. 10. Laurentii Salvii, Holmiae; Stockholm: 1758. pp. 1–824. 1. [Google Scholar]
- Lőrenthey E. Adatok Magyarország harmadkorú rákfaunájához. Mathematikai és Természettudományi Értesito. 1897;15:149–169. [Google Scholar]
- Manning RB. Notes on western Atlantic Callianassidae (Crustacea: Decapoda: Thalassinidea) Proceedings of the Biological Society of Washington. 1987;100:386–401. [Google Scholar]
- Manning RB, Felder DL. Revision of the American Callianassidae (Crustacea: Decapoda: Thalassinoidea) Proceedings of the Biological Society of Washington. 1991;104:764–792. [Google Scholar]
- Manning RB, Tamaki A. A new genus of ghost shrimp from Japan (Crustacea: Decapoda: Callianassidae) Proceedings of the Biological Society of Washington. 1998;111:889–892. [Google Scholar]
- Mansfield WC. Miocene gastropods and scaphopods of the Choctawhatchee Formation. Florida Geological Survey Bulletin. 1930;3:1–189. [Google Scholar]
- Mansfield WC. Miocene pelecypods of the Choctawhatchee Formation. Florida Geological Survey Bulletin. 1932;8:1–240. [Google Scholar]
- Matson GG, Clapp FG. A preliminary report on the geology of Florida with special reference to the stratigraphy. Florida Geological Survey Annual Report. 1909;2:25–173. [Google Scholar]
- McNamara KJ. Importance of heterochrony. In: Briggs DEG, Crowther PR, editors. Palaeobiology II. Wiley-Blackwell; Malden: 2001. pp. 180–188. [Google Scholar]
- Milne-Edwards A. Révision du genre Callianassa (Leach) et description de plusieurs espèces nouvelles de ce groupe faisant partie de la collection du muséum. Nouvelles Archives du Muséum d’Histoire naturelle, Paris. 1870;6:75–101. [Google Scholar]
- Montes C, Cardona A, McFadden R, Morón SE, Silva CA, Restrepo-Moreno S, Ramírez DA, Hoyos N, Wilson J, Farris D, Bayona GA, Jaramillo CA, Valencia V, Bryan J, Flores JA. Evidence for middle Eocene and younger land emergence in central Panama: Implications for Isthmus closure. Geological Society of America Bulletin. 2012;124:780–799. [Google Scholar]
- Noetling F. Über Crustaceen aus dem Tertiär Aegyptens. Sitzungsberichte der Königlichen Preussischen Akademie der Wissenschaften. 1885;26:487–500. pl. 4. [Google Scholar]
- d’Orbigny A. Mollusques. In: de la Sagra R, editor. Histoire naturelle de l’ile de Cuba. Vol. 2. Bertrand; Paris: 1845. p. 380. [Google Scholar]
- d’Orbigny A. Voyage dans l’Amérique Méridionale. Mollusques. Bertrand; Paris: 1846. p. 758. [Google Scholar]
- PaleoBiology Database [accessed 16 November 2013];2013 http://fossilworks.org.
- Philippi RA. Abbildungen und Beschreibungen neuer oder wenig gekannter Conchylien. 5. Vol. 1. Fischer; Cassel: 1844. Venus Tab. II; pp. 5–7. pl. 2. [Google Scholar]
- Poore GC, Suchanek TH. Glypturus motupore, a new callianassid shrimp (Crustacea: Decapoda) from Papua New Guinea with notes on its ecology. Records of the Australian Museum. 1988;40:197–204. [Google Scholar]
- Posey MH. Predation on a burrowing shrimp: distribution and community consequences. Journal of Experimental Marine Biology and Ecology. 1986;103:143–161. [Google Scholar]
- Powys WL. Characters of new species of shells collected by Mr. Cuming. Proceedings of the Zoological Society of London. 1835;3:94–96. [Google Scholar]
- Portell RW, Polites GL, Schmelz GW. Mollusca – Shoal River Formation (middle Miocene) Florida Fossil Invertebrates. 2006;9:1–52. [Google Scholar]
- Portell RW, Luque J, Hendy AJW, Christy J. Fidelity of marine invertebrate death and fossil assemblages in a coastal marine ecosystem, Bahia Bique, Panama. Geological Society of America, Abstracts with Programs. 2012;44(7):268. [Google Scholar]
- Rabalais NN, Holt SA, Flint RW. Mud shrimps (Crustacea, Decapoda, Thalassinidea) of the northwestern Gulf of Mexico. Bulletin of Marine Science. 1981;31:96–115. [Google Scholar]
- Rathbun MJ. West Indian Tertiary decapod crustaceans. Carnegie Institution of Washington Publication. 1919;219:159–184. pls 1–9. [Google Scholar]
- Rathbun MJ. Fossil Crustacea of the Atlantic and Gulf Coastal Plain. Geological Society of America, Special Paper. 1935;2:i–viii. 1–160. [Google Scholar]
- Robles R, Tudge CC, Dworschak PC, Poore GCB, Felder DL. Molecular phylogeny of the Thalassinidea based on nuclear and mitochondrial genes. In: Martin JW, Crandall KA, Felder DL, editors. Decapod crustacean phylogenetics. Taylor & Francis/CRC Press; Boca Raton: 2009. pp. 309–326. [Google Scholar]
- Röding PF. Museum Boltenianum sive catalogus cimeliorum e tribus regnis naturæ quæ olim collegerat Joa. Fried Bolten, M. D. p. d. per XL. annos proto physicus Hamburgensis. Pars secunda continens conchylia sive testacea univalvia, bivalvia & multivalvia. Typis Johan Christi Trapii; Hamburg: 1798. p. viii.p. 119. [Google Scholar]
- de Saint Laurent M. Sur la classification et la phylogénie des Thalassinides: définitions de la superfamille des Axioidea, de la sous-famille des Thomassiniinae et de deux genres nouveaux (Crustacea Decapoda) Comptes Rendus de l’Académie des Sciences, Paris, Série D. 1979;288:1395–1397. [Google Scholar]
- Sakai K. Callianassoidea of the world (Decapoda: Thalassinidea) Crustaceana Monographs. 2005;4:1–285. [Google Scholar]
- Sakai K. Axioidea of the world and a reconsideration of the Callianassoidea (Decapoda, Thalassinidea, Callianassida) Crustaceana Monographs. 2011;13:1–520. [Google Scholar]
- Schäfer W. In: Ecology and Paleoecology of Marine Environments. Oertel I, Craig GY, translators. University of Chicago Press; Chicago: 1972. p. 568. [Google Scholar]
- Schweitzer CE, Iturralde-Vinent M, Hetler JL, Velez-Juarbe J. Oligocene and Miocene decapods (Thalassinidea and Brachyura) from the Caribbean. Annals of Carnegie Museum. 2006;75:111–136. [Google Scholar]
- Schweitzer CE, Velez-Juarbe J, Martinez M, Hull AC, Feldmann RM, Santos H. New Cretaceous and Cenozoic Decapoda (Crustacea: Thalassinidea, Brachyura) from Puerto Rico, United States Territory. Bulletin of the Mizunami Fossil Museum. 2008;34:1–15. [Google Scholar]
- Shimoda K, Wardiatno Y, Kubo K, Tamaki A. Intraspecific behaviors and major cheliped sexual dimorphism in three congeneric callianassid shrimp. Marine Biology. 2005;146:543–557. [Google Scholar]
- Sowerby GB. Characters of new species of shells from the collection formed by Mr. Cuming on the western coast of South America, and among the islands of the South Pacific Ocean. Proceedings of the Zoological Society of London. 1833a;1833:16–22. [Google Scholar]
- Sowerby GB. Characters of new species of shells forming part of the collection made by Mr. Cuming on the western coast of South America, and among the islands of the South Pacific Ocean. Proceedings of the Zoological Society of London. 1833b;1833:82–85. [Google Scholar]
- Sowerby GB. Characters of new species of shells from the collection formed by Mr. Cuming on the western coast of South America and among the islands of the South Pacific Ocean. Proceedings of the Zoological Society of London. 1833c;1833:34–38. [Google Scholar]
- Starzyk N, Krzemińska E, Krzemiński W. Intraspecific variation in the Jurassic crab Bucculentum bucculentum (Decapoda: Homolodromioidea: Bucculentidae) Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2011;260:203–210. [Google Scholar]
- Starzyk N, Krzemińska E, Krzemiński W. A new crab species from the Oxfordian of Poland (Decapoda: Brachyura: Goniodromitidae) Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2012;263:143–153. [Google Scholar]
- Stempien JA. Brachyuran taphonomy in a modern tidal-flat environment: Preservation potential and anatomical bias. Palaios. 2005;20:400–410. [Google Scholar]
- Stimpson W. Descriptions of new genera and species of macrurous Crustacea from the coasts of North America. Proceedings of the Chicago Academy of Sciences. 1866;1:46–48. [Google Scholar]
- Todd AJ, Collins JSH. Neogene and Quaternary crabs (Crustacea, Decapoda) collected from Costa Rica and Panama by members of the Panama Paleontology Project. Bulletin of the Mizunami Fossil Museum. 2005;32:53–85. [Google Scholar]
- Toula F. Die jungtertiäre Fauna von Gatun am Panamakanal. Jahrbuch der kaiserlich-königlichen Geologischen Reichanstalt Wien. 1911;61:487–530. [Google Scholar]
- Tudge CC, Poore GCB, Lemaitre R. Preliminary phylogenetic analysis of generic relationships within the Callianassidae and Ctenochelidae (Decapoda: Thalassinidea: Callianassoidea) Journal of Crustacean Biology. 2000;20(Special Issue 2):129–149. [Google Scholar]
- de Vaugelas J, de Saint Laurent M. Premières données sur l’écologie de Callichirus laurae de Saint Laurent sp. nov. (Crustacé décapode Callianassidae): son action bioturbatrice sur les formations sédimentaires du golfe d’Aqaba (Mer Rouge) Comptes Rendus des Séances de l’Académie des Sciences, Paris, Série 3, Sciences de la Vie. 1984;298(6):147–152. [Google Scholar]
- Vergara-Solana FJ, García-Rodríguez FJ, De La Cruz-Agüero J. Interspecific comparison of growth and shape within Diapterus (Gerreidae: Perciformes) using geometric morphometrics. Journal of Ichthyology. 2013;53:445–454. [Google Scholar]
- Webster M. Ontogeny and evolution of the Early Cambrian trilobite genus Nephrolenellus (Olenelloidea) Journal of Paleontology. 2007;81:1168–1193. [Google Scholar]
- Withers TH. Decapod crustaceans from the Oligocene of Anguilla. (Series 9).Annals and Magazine of Natural History. 1924;14:225–233. pl. 6. [Google Scholar]
- Woodring WP. Geology and paleontology of Canal Zone and adjoining parts of Panama. U.S. Geological Survey Professional Paper. 1957;306(A):1–145. [Google Scholar]
- Zelditch ML, Swiderski DL, Sheets HD, Fink WL. Geometric morphometrics for biologists: a primer. Elsevier Academic Press; San Diego: 2004. p. 443. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.