Abstract
AIM: To evaluate the clinical effect of high-intensity focused ultrasound (HIFU) in the treatment of patients with liver cancer.
METHODS: HIFU treatment was performed in 100 patients with liver cancer under general anesthesia and by a targeted ultrasound. Evaluation of efficacy was made on the basis of clinical symptoms, liver function tests, AFP, MRI or CT before and after the treatment.
RESULTS: After HIFU treatment, clinical symptoms were relieved in 86.6%(71/82) of patients. The ascites disappeared in 6 patients. ALT (95 ± 44) U/L and AST (114 ± 58) U/L before HIFU treatment were reduced to normal in 83.3%(30/36) and 72.9%(35/48) patients, respectively, after the treatment. AFP was lowered by more than 50% in 65.3%(32/49) patients. After HIFU treatment, MRI or CT findings indicated coagulation necrosis and blood supply reduction or disappearance of tumor in the target region.
CONCLUSION: HIFU can efficiently treat the patients with liver cancer. It will offer a significant noninvasive therapy for local treatment of liver tumor.
INTRODUCTION
Since 1942, Lynn et al[1] firstly brought forward the conception of HIFU, i.e. high intensity focused ultrasound. Fry[2] applied HIFU technology to experimentally treat nervous system diseases, and suggested the potential of HIFU in surgical operations. As a result, he found that ultrasound beam could form a preferable focal field at a special depth inside body, and destroy target tissue through focus pointing without damaging neighboring tissues. In 1956, Burov proposed that effect of short-time high-intensity ultrasound irradiation was better than low-intensity ultrasound. With research progress on HIFU technology, this technology has been used in clinic over the years[3-6]. The present study makes an assessment on clinic effect of HIFU in treating liver cancer.
MATERIALS AND METHODS
Materials
We performed HIFU treatments in 100 patients (80 male, 20 female, ranging 30-74 years with mean age of 56 years) with liver cancer from July 2001 to July 2003, totally 130 HIFU treatments, 1.30 times per patient. Patients included 62 primary liver cancers and 38 metastatic liver cancers. Sixty-eight patients were with single nodule, 22 with two nodules, 10 with three nodules. Totally 36 tumor nodules with a diameter less than 5 cm, 76 with a diameter between 5-10 cm, 30 with a diameter more than 10 cm were involved. All cases were investigated and verified by pathohistology or an obvious increase of serum AFP and positive imaging, and conformed to diagnostic standard of National Cooperation Conference and Hepatic Carcinoma Prevention and Treatment in 1997. Eighteen patients were in stage I, 48 patients in stage II, and 34 patients in stage III.
Instrument
JC type focused ultrasound tumor therapeutic system was designed by Chongqing HIFU Technology Co, Ltd. Chongqing, China. It includes two main parts, i.e. ultrasound real-time orientation monitor device and HIFU three- dimensional combination scanning therapeutic device. Under the control of a computer, it can orient to preassigned tumor target zone automatically, determine range of therapy.
The main parameters included therapy frequency 0.8 MHz, mean diameter of focal field 1.1 mm, length of focal field 9.8 mm, focus distance 135 mm, therapy power 140-240 W.
Methods
Routine examinations and preoperative preparation were conducted according to the principle of surgery. Based on the result of image and ultrasound examination, the therapeutic scheme of HIFU was constituted. HIFU treatment was performed with patient fixed properly. The tumor position and size, therapeutic layers and therapeutic range of every layer were determined by ultrasound diagnostic probe. Then the therapeutic probe treated tumor tissue of every layer from outside body, and in terms of order of layer, from spot to line, and from line to area, leading whole tumor to coagulation necrosis. During the therapeutic process, through changes of graphics of target field and echo of tissue between before and after therapy at every layer, real-time estimate of HIFU therapeutic effect by computer processing image system was carried out, and with feedback, ultrasound therapeutic dosage estimated in the therapy scheme was controlled according to changes of ultrasound photograph. The therapeutic method was divided into complete coverage and local coverage. Twenty-eight cases used complete coverage, including whole tumor focus and normal liver tissue within 2 cm away from edge of tumor, the other cases used local coverage due to reasons, such as large tumor volume, rib overlap or close-by, or involvement of liver tube or cholecyst, etc.
Observatory parameters
The following aspects were observed: Improvement of clinic symptoms; changes of liver function 2 d and 2 wk before and after therapy; changes of AFP 2 wk before and after therapy; changes in range and blood supply of coagulation necrosis of tumor focus, also shrink of tumor through re-examination of MRI or CT before and after therapy.
RESULTS
One hundred liver cancer patients were treated by using HIFU in this group, of which 82 exhibited clinic symptoms. Seventy-one patients were improved obviously after HIFU treatment: appetite increased, weight gained, discomfort or pain on liver region relieved. Remission rate for symptoms was 86.6%(71/82). For 6 patients who had had mild ascites, ascites disappeared after HIFU treatment. For patients with abnormal liver function (ALT 95 ± 44 U/L, AST 114 ± 58 U/L), 2 d after HIFU treatment, liver enzymes rose slightly, but no obvious statistic difference was found compared with pre -treatment. The patients’ liver enzymes fell to normal level after 2 wk, respectively, ALT 83.3%(30/36), AST 72.9%(35/48). For the patients whose AFP was increased, AFP was 50% less than original level 2 wk after HIFU treatment, in 65.3%(32/49), only one patient’s AFP rose continuously after HIFU treatment, and multi-bone metastases were found by ECT examination. Self-comparison of MRI showed that T1-weighted images and T2-weighted images of tumor therapeutic region were high signal and low signal respectively after HIFU treatment. Enhanced MRI did not show enhanced signal, indicating that coagulation necrosis had occurred in the tumor therapeutic region, blood supply of tumor was diminished or eliminated, tumor of some patients had shrunk obviously after countercheck MRI (Figures 1-3).
Figure 1.
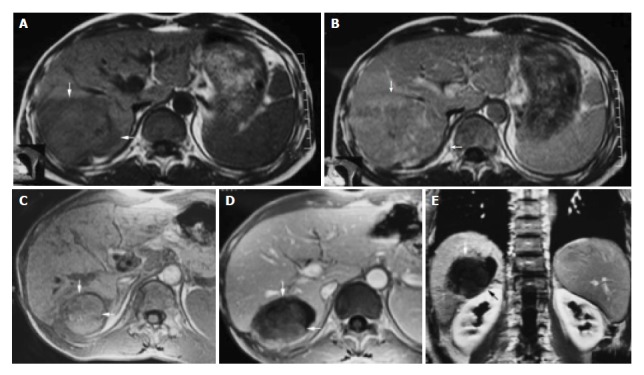
A 42-year-old male with hepatocellular carcinoma. A: MRI T1-weighted images before HIFU treatment showed that liver tumor of right-posterior lobe was 115 mm × 100 mm × 66 mm; B: MRI enhanced imaging before HIFU revealed that enhancement of mass in liver right-posterior lobe was higher than that of surrounding normal liver tissues; C: MRI T1-weighted imaging 11 mo after HIFU revealed that liver tumor of right-posterior lobe obviously decreased in size (50 mm × 55 mm × 60 mm); D, E: MRI enhanced imaging (cross section and coronal section) 11 mo after HIFU revealed that liver tumor had shrunk obviously, blood supply of tumor was eliminated, the tumor therapeutic region had coagulation necrosis.
Figure 3.
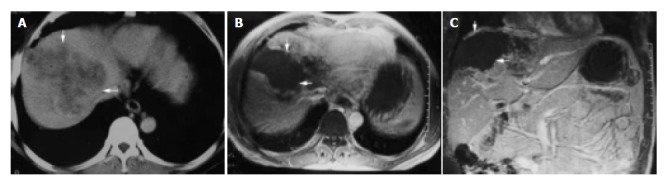
A 54-year-old man with primary carcinoma of liver in right lobe. A: CT enhanced imaging before HIFU treatment revealed that enhancement of mass in liver right-posterior lobe was higher than that of surrounding normal liver tissues (AFP: 5516 μg/L); B, C: MRI enhanced imaging (cross section and coronal section) 2 mo after HIFU revealed that necrotic tissue in the therapeutic region of the tumor was not enhanced (AFP: 183 μg/L).
Figure 2.
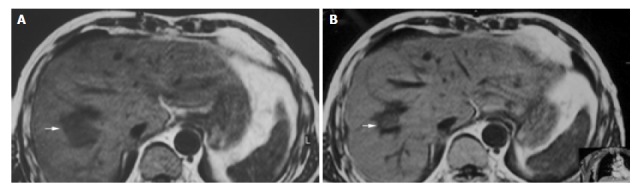
A 55-year-old man with metastatic hepatocarcinoma in sigmoid cancer postoperation. A: MRI images before HIFU treatment showed that liver tumor of right-posterior lobe was 40 mm × 30 mm × 30 mm; B: MRI images 2 wk after HIFU revealed that liver tumor of right-posterior lobe obviously decreased in size (20 mm × 20 mm × 15 mm).
DISCUSSION
HIFU is a high-tech developed successfully in the 1990’s, a local way of treating tumor without any damages. It utilizes the physics characteristics of ultrasound beam with assemble and penetration, to focus low energy outside body on inner tumor target field, through instantaneous high temperature effect, cavitate effect, making tumor target tissue of focal zone coagulation necrosis, without destroying surrounding tissues[7-14].
HIFU has the following characteristics in therapy of malignant tumors. Firstly, noninvasiveness. HIFU treats inner tumor without damaging outside body. Previous research on animal angiography indicated that after treating liver tumor of Morris rat using HIFU, nourishing blood vessels which diameter was less than 200 µm of irradiation zone closed, but they were normal for blood vessels which diameter was more than 200 µm[15]. Secondly, accuracy. HIFU can pass through tissues and accurately damage target tissues inside organisms. The boundary between therapy zone and un-therapy zone is clear, tissue beyond target zone is hardly destroyed or without damage[16,17]. Ultrasound Therapy Section of London Emperor Hospital, Britain found that only six cells existed between cells killed completely and cells without damage[18]. Thirdly, real-time therapy. For whole process of therapy, it is a real-time targeting and monitoring process, real -time estimating therapeutic effect and adjusting dosage[19]. Fourthly, suitable therapy. According to the size and shape of tumor, it determines therapy range of tumor target zone, overlays tumor target zone. Therefore, treatment of malignant tumor using HIFU has many advantages such as less pain, no damage, fewer influences on splanchnic function, faster recovery for body and no increase of tumor metastasis chance[20], etc.
It is shown that in this research, 100 liver cancer patients had obvious improvement in symptoms and signs after being treated by using HIFU. Short-term effect of HIFU therapy was obvious and affirmative in liver cancer. But for liver cancer patients who had huge block or multifocal big nodules, to make tumor completely occur coagulation necrosis, to gain the purpose of “ablation” tumor integrity, we performed treatment for two or three times. Firstly, we treated deeper tumor parts, to induce coagulation necrosis of the tumor, so as to make it easy to treat remaining superficial parts next time. Conversely, if at first, it makes superficial parts occur coagulation necrosis, and then treats deep tumor tissue, because of change of the impedance dispersion and absorb coefficient of sound, the attenuation of penetrating tissue with ultrasound will aggravate. Meanwhile, focus energy in focal field can not reach ideal degree, as a result it takes more time to make tumor tissue coagulation necrosis.
If tumor locates on the edge of liver with poor blood supply, during therapy of HIFU, We estimate every therapeutic effect by using real-time ultrasound imaging to monitor the change of gray value. If gray value is changed, it indicates that tumor tissue must occur coagulation necrosis. However, tissues with coagulation necrosis do not always exhibit changes of gray value. For tumor in deep part, during real-time monitor, most changes of gray value are not distinct or lightly increased with suffusion. Through quantitative analysis and comparison of gray value of ultrasound imaging before and after therapy, we can find that the difference of gray rank before and after therapy is obvious. This indicates that local tumor tissues have no reversible coagulation necrosis.
For liver cancer patients with abundant blood supply relatively, we should first perform transcatheter arterial chemoembolization before using HIFU, so as to make inner tumor tissue have more iodised oil deposition, which not only is convenient to ultrasound orientation, but also changes the impedance dispersion and absorb coefficient of sound of tumor zone. Accordingly, it is convenient to energy sediment of focal zone, exerts cooperative function of raising temperature, excites high temperature to get to purpose of destruct therapy at local and makes tumor tissue coagulation necrosis[21,22].
Yang et al[15] used HIFU to treat Morris rat hepatoma implanted in the liver. Treatment was administered with a lens-focused 4- MHz transducer that created a focused beam of 550 W/cm2 at peak intensity. One hundred and twelve rats with liver tumors were divided into two groups of 56 each. Group 1 received HIFU therapy while group 2 (the control group) did not. All rats were killed immediately or 1, 3, 7, 14, 21, 28 d after treatment. Eight rats in each group were killed at each interval for pathologic and biochemical studies. Significant inhibition of the tumor growth was seen in the HIFU-treated group, with tumor growth inhibition rates of 65.4-93.1% from on d 3 to 28 after treatment. Ultrasound -treated tumors showed direct thermal cytotoxic necrosis and fibrosis. An additional 56 ACl rats with liver tumors were divided into 4 groups of 14 each. Group 1 received doxorubicin hydrochloride intraperitoneally and HIFU therapy; group 2, HIFU therapy; group 3, doxorubicin hydrochloride; and group 4 (the control group), neither HIFU nor doxorubicin hydrochloride. Significantly improved survival rates were noted in HIFU-treated animals (groups 1 and 2) compared with those of groups 3 and 4.
Research results of home and abroad using animals showed that HIFU can safely and effectively destroy inner liver tissue or liver transplant tumor[23-30]. Clinic results showed that HIFU can also treat liver cancer securely and effectively[11,31,32].
HIFU is a new method with definite therapeutic effect on tumor without damage and poisonous to normal tissues. With improvement of HIFU technology, accumulation of clinic application experience and further research on mechanism of HIFU, HIFU, as a new local therapy, would be applied more extensively to treat liver cancer.
Footnotes
Edited by Zhu LH and Chen WW Proofread by Xu FM
References
- 1.Lynn JG, Putnam TJ. Histology of Cerebral Lesions Produced by Focused Ultrasound. Am J Pathol. 1944;20:637–649. [PMC free article] [PubMed] [Google Scholar]
- 2.FRY WJ, FRY FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans Med Electron. 1960;ME-7:166–181. doi: 10.1109/iret-me.1960.5008041. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Wang Z, Wu F, Bai J, Zhu H, Zou J, Li K, Xie F, Wang Z. [High intensity focused ultrasound alone for malignant solid tumors] Zhonghua Zhongliu Zazhi. 2002;24:278–281. [PubMed] [Google Scholar]
- 4.Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu H, Wang ZB. Tumor vessel destruction resulting from high-intensity focused ultrasound in patients with solid malignancies. Ultrasound Med Biol. 2002;28:535–542. doi: 10.1016/s0301-5629(01)00515-4. [DOI] [PubMed] [Google Scholar]
- 5.Yang R, Sanghvi NT, Rescorla FJ, Kopecky KK, Grosfeld JL. Liver cancer ablation with extracorporeal high-intensity focused ultrasound. Eur Urol. 1993;23 Suppl 1:17–22. doi: 10.1159/000474674. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy JE, Ter Haar GR, Cranston D. High intensity focused ultrasound: surgery of the future. Br J Radiol. 2003;76:590–599. doi: 10.1259/bjr/17150274. [DOI] [PubMed] [Google Scholar]
- 7.ter Haar GR. High intensity focused ultrasound for the treatment of tumors. Echocardiography. 2001;18:317–322. doi: 10.1046/j.1540-8175.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 8.Paek BW, Vaezy S, Fujimoto V, Bailey M, Albanese CT, Harrison MR, Farmer DL. Tissue ablation using high-intensity focused ultrasound in the fetal sheep model: potential for fetal treatment. Am J Obstet Gynecol. 2003;189:702–705. doi: 10.1067/s0002-9378(03)00664-1. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Bai J, Li F, Du Y, Wen S, Hu K, Xu G, Ma P, Yin N, Chen W, et al. Study of a "biological focal region" of high-intensity focused ultrasound. Ultrasound Med Biol. 2003;29:749–754. doi: 10.1016/s0301-5629(02)00785-8. [DOI] [PubMed] [Google Scholar]
- 10.ter Haar G. High intensity ultrasound. Semin Laparosc Surg. 2001;8:77–89. [PubMed] [Google Scholar]
- 11.Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu H, Wang ZB. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med Biol. 2001;27:1099–1106. doi: 10.1016/s0301-5629(01)00389-1. [DOI] [PubMed] [Google Scholar]
- 12.Bailey MR, Couret LN, Sapozhnikov OA, Khokhlova VA, ter Haar G, Vaezy S, Shi X, Martin R, Crum LA. Use of overpressure to assess the role of bubbles in focused ultrasound lesion shape in vitro. Ultrasound Med Biol. 2001;27:695–708. doi: 10.1016/s0301-5629(01)00342-8. [DOI] [PubMed] [Google Scholar]
- 13.Prat F, Ponchon T, Berger F, Chapelon JY, Gagnon P, Cathignol D. Hepatic lesions in the rabbit induced by acoustic cavitation. Gastroenterology. 1991;100:1345–1350. [PubMed] [Google Scholar]
- 14.Huo YM, Chen YZ. [Comparative study of ultrasound transducers in HIFU] Zhongguo Yiliao Qixie Zazhi. 2000;24:97–101. [PubMed] [Google Scholar]
- 15.Yang R, Reilly CR, Rescorla FJ, Faught PR, Sanghvi NT, Fry FJ, Franklin TD, Lumeng L, Grosfeld JL. High-intensity focused ultrasound in the treatment of experimental liver cancer. Arch Surg. 1991;126:1002–1009; discussion 1002-1009;. doi: 10.1001/archsurg.1991.01410320088012. [DOI] [PubMed] [Google Scholar]
- 16.Wang ZB, Wu F, Wang ZL, Zhang Z, Zou JZ, Liu C, Liu YG, Cheng G, Du YH, He ZC, et al. Targeted damage effects of high intensity focused ultrasound (HIFU) on liver tissues of Guizhou Province miniswine. Ultrason Sonochem. 1997;4:181–182. doi: 10.1016/s1350-4177(97)00028-x. [DOI] [PubMed] [Google Scholar]
- 17.Yang R, Sanghvi NT, Rescorla FJ, Galliani CA, Fry FJ, Griffith SL, Grosfeld JL. Extracorporeal liver ablation using sonography-guided high-intensity focused ultrasound. Invest Radiol. 1992;27:796–803. doi: 10.1097/00004424-199210000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Dorr LN, Hynynen K. The effects of tissue heterogeneities and large blood vessels on the thermal exposure induced by short high-power ultrasound pulses. Int J Hyperthermia. 1992;8:45–59. doi: 10.3109/02656739209052878. [DOI] [PubMed] [Google Scholar]
- 19.Vaezy S, Shi X, Martin RW, Chi E, Nelson PI, Bailey MR, Crum LA. Real-time visualization of high-intensity focused ultrasound treatment using ultrasound imaging. Ultrasound Med Biol. 2001;27:33–42. doi: 10.1016/s0301-5629(00)00279-9. [DOI] [PubMed] [Google Scholar]
- 20.Oosterhof GO, Cornel EB, Smits GA, Debruyne FM, Schalken JA. Influence of high-intensity focused ultrasound on the development of metastases. Eur Urol. 1997;32:91–95. [PubMed] [Google Scholar]
- 21.Jin CB, Wu F, Wang ZB, Chen WZ, Zhu H. [High intensity focused ultrasound therapy combined with transcatheter arterial chemoembolization for advanced hepatocellular carcinoma] Zhonghua Zhongliu Zazhi. 2003;25:401–403. [PubMed] [Google Scholar]
- 22.Zhao J, Wang Z, Guo D, Yu C, Xie W, Li G. [CT appearance and its diagnosis value in liver cancer after transcatheter oily chemoembolization combining with high intensity focused ultrasound therapy] Zhonghua Ganzangbing Zazhi. 2001;9 Suppl:61–63. [PubMed] [Google Scholar]
- 23.Sibille A, Prat F, Chapelon JY, abou el Fadil F, Henry L, Theilliere Y, Ponchon T, Cathignol D. Characterization of extracorporeal ablation of normal and tumor-bearing liver tissue by high intensity focused ultrasound. Ultrasound Med Biol. 1993;19:803–813. doi: 10.1016/0301-5629(93)90096-7. [DOI] [PubMed] [Google Scholar]
- 24.Adams JB, Moore RG, Anderson JH, Strandberg JD, Marshall FF, Davoussi LR. High-intensity focused ultrasound ablation of rabbit kidney tumors. J Endourol. 1996;10:71–75. doi: 10.1089/end.1996.10.71. [DOI] [PubMed] [Google Scholar]
- 25.Sibille A, Prat F, Chapelon JY, Abou el Fadil F, Henry L, Theillère Y, Ponchon T, Cathignol D. Extracorporeal ablation of liver tissue by high-intensity focused ultrasound. Oncology. 1993;50:375–379. doi: 10.1159/000227213. [DOI] [PubMed] [Google Scholar]
- 26.Righetti R, Kallel F, Stafford RJ, Price RE, Krouskop TA, Hazle JD, Ophir J. Elastographic characterization of HIFU-induced lesions in canine livers. Ultrasound Med Biol. 1999;25:1099–1113. doi: 10.1016/s0301-5629(99)00044-7. [DOI] [PubMed] [Google Scholar]
- 27.Gignoux BM, Scoazec JY, Curiel L, Beziat C, Chapelon JY. [High intensity focused ultrasonic destruction of hepatic parenchyma] Ann Chir. 2003;128:18–25. doi: 10.1016/s0003-3944(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 28.Yang R, Kopecky KK, Rescorla FJ, Galliani CA, Wu EX, Grosfeld JL. Sonographic and computed tomography characteristics of liver ablation lesions induced by high-intensity focussed ultrasound. Invest Radiol. 1993;28:796–801. [PubMed] [Google Scholar]
- 29.Cheng SQ, Zhou XD, Tang ZY, Yu Y, Wang HZ, Bao SS, Qian DC. High-intensity focused ultrasound in the treatment of experimental liver tumour. J Cancer Res Clin Oncol. 1997;123:219–223. doi: 10.1007/BF01240318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishikawa T, Okai T, Sasaki K, Umemura S, Fujiwara R, Kushima M, Ichihara M, Ichizuka K. Functional and histological changes in rat femoral arteries by HIFU exposure. Ultrasound Med Biol. 2003;29:1471–1477. doi: 10.1016/s0301-5629(03)00951-7. [DOI] [PubMed] [Google Scholar]
- 31.Li CX, Xu GL, Li JJ, Luo GY. [High intensity focused ultrasound for liver cancer] Zhonghua Zhongliu Zazhi. 2003;25:94–96. [PubMed] [Google Scholar]
- 32.Wu F, Wang Z, Chen W. [Pathological study of extracorporeally ablated hepatocellular carcinoma with high-intensity focused ultrasound] Zhonghua Zhongliu Zazhi. 2001;23:237–239. [PubMed] [Google Scholar]


