Abstract
AIM: To determine whether Saiko-keishi- to (TJ-10), a Japanese herbal medicine, could protect liver injury induced by gut ischemia/reperfusion (I/R), and to investigate the role of NO.
METHODS: Male Wistar rats were exposed to 30-min gut ischemia followed by 60 min of reperfusion. Intravital microscopy was used to monitor leukocyte recruitment. Plasma tumor necrosis factor (TNF) levels and alanine aminotransferase (ALT) activities were measured. TJ- 10 1 g/(kg·d) was intragastrically administered to rats for 7 d. A NO synthase inhibitor was administered.
RESULTS: In control rats, gut I/R elicited increases in the number of stationary leukocytes, and plasma TNF levels and ALT activities were mitigated by pretreatment with TJ-10. Pretreatment with the NO synthase inhibitor diminished the protective effects of TJ-10 on leukostasis in the liver, and the increase of plasma TNF levels and ALT activities. Pretreatment with TJ-10 increased plasma nitrite/nitrate levels.
CONCLUSION: TJ-10 attenuates the gut I/R-induced hepatic microvascular dysfunction and sequential hepatocellular injury via enhancement of NO production.
INTRODUCTION
Herbal medicines that have been used in China for thousands of years are now being manufactured in Japan as drugs containing ingredients of standardized quality and quantity. The clinical efficacy of these medicines has been utilized by Japanese Western-medicine practitioners for more than 20 years and is well recognized. One of the herbal medicines, Saiko-keisi-to (TJ-10) (Chinese name; Chai-Hu-Gui-Zhi-Tang), is a common drug to treat duodenal ulcer, pancreatitis, and chronic liver disease in Japan. It is an oral medicine and consists of 9 herb components: Bupleurum root, Pinellia tuber, Scutellaria root, Jujube fruit, Ginger rhizome, ginseng root, cinnamon bark, peony root and Glycyrrhiza root. Another common herbal medicine, Sho-saiko-to (TJ-9), consists of 7 components of them: Bupleurum root, Pinellia tuber, Scutellaria root, jujube fruit, ginger rhizome, ginseng root, and Glycyrrhiza root. In a double -blind multicenter clinical trial, TJ-9 was reported to reduce the elevated serum activities of aspartate transaminase, alanine transaminase (ALT), and glutamyl transpeptidase in chronic active hepatitis patients[1]. TJ-9 has been shown to improve liver function as well as the symptoms associated with chronic liver disease including digestive discomfort[2]. Although TJ-10 has been often administered to patients with chronic liver disease as well as TJ-9, there are few epidemiological reports[3], and little is known about mechanisms of its cytoprotective effects on liver damage. Recently, because of its major pharmaceutical effects, TJ-10 is presumed to gradually improve biological defense mechanisms. One of the components of TJ -10, Saikosaponin, has been reported to inhibit hepatocyte necrosis induced by galactosamine[4], and Saikosaponin-d has been shown to reduce microsomal lipid peroxidation induced by NADPH and CCl4[5]. However, its mode of action has not been fully elucidated.
Nitric oxide (NO) has been found to be a modulator of the adhesive interactions between leukocytes, platelets, and endothelial cells[6-9] as well as an important modulator of tissue blood flow, arterial pressure, and neurotransmission[8], and NO-dependent cell-cell interactions have been demonstrated in tissues exposed to ischemia and reperfusion (I/R), an injury process in which leukocyte-endothelial cell adhesion plays a critical role. A role of NO in the pathobiology of I/R injury has been supported by observations that inhibition of NO biosynthesis could elicit most of the microvascular alterations observed in tissues exposed to I/R[7,10], and NO -donating compounds have been shown to provide significant protection against the microvascular dysfunction that is normally associated with I/R[7]. We developed a murine model of leukocyte- dependent hepatocellular dysfunction that was elicited by gut I/R[11-13]. The model allows in vivo assessment of the effects of I/R on leukocyte sequestration in sinusoids of different regions of the liver lobule, leukocyte adherence in postsinusoidal venules, and the number of perfused sinusoids. Using this model, we have recently demonstrated that inhibition of both NO synthase and supplementation with exogenous NO could affect the leukocyte rolling, leukocyte adhesion, and sinusoidal perfusion elicited in the liver by gut I/R[14-16].
Some herbal medicines have been reported to have an inducing effect on NO production by non-stimulated macrophages[17]. We have recently reported that TJ-9 could attenuate the gut I/R-induced hepatic microvascular dysfunction and sequential hepatocellular injury via NO[18]. However, little is known about the effect of TJ-10 on plasma NO levels and I/R injury in vivo. TJ-9 is contraindicated for liver cirrhosis, because of its side-effect, causing lung fibrosis. Since TJ-10 has 30% less Saikosaponin compared to TJ-9, it seems to be safer. Therefore, frequency and importance of TJ-10 are expected to increase in Japan. In the present study, we investigated whether TJ-10 modulated the gut I/R-induced microvascular dysfunction in the liver, and the role of NO in the responses by inhibiting NO synthase.
MATERIALS AND METHODS
Animals and surgical procedure
Male Wistar rats (200-250 g) were fed a standard rat chow for 2 wk, and TJ-10 (1 g/kg in saline) or saline alone was then administered for 7 d intragastrically through a tube. Experiments below were performed 18 h after the final dose of TJ-10 or saline. The rats were fasted for 18 h prior to each experiment, and then intraperitoneally anesthetized with pentobarbital sodium (35 mg/kg). The left carotid artery was cannulated, and a catheter was positioned in aortic arch to monitor blood pressure. The left jugular vein was cannulated for drug administration. All experiments were performed according to the criteria outlined in the National Research Council Guide.
Intravital microscopy
After laparotomy, one lobe of the liver was examined through an inverted intravital microscope (TMD-2S, Diaphoto, Nikon, Tokyo, Japan) and images were recorded with a silicon intensified target (SIT) camera (C -2400-08, Hamamatsu photonicus, Shizuoka, Japan). The liver was placed on an adjustable Plexiglas microscope stage and covered with a nonfluorescent coverslip that allowed observation of a 2 cm2 segment of tissue. The liver was carefully positioned to minimize the influence of respiratory movements, and its surface was moistened and covered with cotton gauze soaked with saline. Images of microcirculation at the surface of the liver were observed through consecutive microfluorographs of hepatic microcirculation, that is, those of rhodamine -6G -labeled leukocytes in the sinusoids, were observed at 90 min after the onset of SMA occlusion and recorded on a digital video recorder. The number of stationary leukocytes was determined off-line during playback of videotape images. A leukocyte was considered to be stationary within the microcirculation (sinusoids) if it remained stationary for more than 10 s. The lobule, which had the fewest stationary leukocytes, was selected for observation at the basal condition. Stationary leukocytes were quantified in both the midzonal and pericentral regions of the liver lobule and expressed as the number per field of view (2.1×105 µm2). The percentage of non-perfused sinusoids was calculated as the ratio of the number of non-perfused sinusoids to the total number of sinusiods per microscopic field.
Experimental protocols
We observed the surface of liver for 10 min before ligating the superior mesenteric artery to ensure that all parameters measured on-line were in a steady state. The superior mesenteric artery was then ligated for 0 (sham) or 30 min with a snare created from a polyethylene tube. At the end of the ischemic period, the ligation was gently removed. Leukocyte accumulation and the number of non-perfused sinusoids (NPS) were measured before ischemia, immediately following reperfusion, and every 15 min for 1 h thereafter. In one set of experiments, 7 untreated animals, and 5 TJ-10-treated animals in the control groups (sham gut I/R) and gut I/R groups were used. In another set of experiments in which TJ-10 was administered, the rats were given a NO synthase (NOS) inhibitor, NG-monomethyl-L-arginine (L-NMMA: Sigma, St. Louis, MO) (0.5 mg/kg, i.v.) 30 min before the onset of ischemia. These experiments were performed with 5 animals in each group. In some experiments, the rats were given dexamethasone (2 mg/kg, Sigma, St. Louis, MO) with or without L-NMMA (0.5 mg/kg, i.v.) 30 min before the onset of ischemia. These experiments were performed with 5 animals in each group.
Tumor necrosis factor and endotoxin assay
Sixty min after the onset of reperfusion, blood plasma samples for tumor necrosis factor (TNF) detection were collected from the inferior vena cava at a point proximal to the hepatic vein. Plasma TNF concentration was determined in a microtiter plate using a TNF immunoassay kit (BioSource International, Camarillo, CA) based on enzyme-linked immunosorbent assay (ELISA) as described in our previous study[19]. Plasma endotoxin levels were measured by endospecy (an endotoxin-specific chromogen. Seikagaku Co.,Tokyo, Japan) according to our previous report[20].
Enzyme and nitrite/nitrate assay
Blood samples for enzyme activities were collected from the carotid artery 6 h after the onset of reperfusion. Serum ALT activity was determined by conventional UV methods as previously described[21]. Blood samples for nitrite/nitrate assay were collected from the inferior vena cava 16 h after the last administration of TJ-10 (saline as control). The combined levels of nitrite and nitrate in plasma were determined by a previously reported method[22]. Five separate experiments were performed.
Statistical analysis
The data were analyzed by standard statistical methods, i.e., ANOVA and Scheffe’s (post hoc) test. All values were reported as mean ± SD. P < 0.05 was considered statistically significant.
RESULTS
Figure 1A shows the effects of TJ-10 and/or L-NMMA on the gut I/R induced leukostasis in sinusoids of the midzonal and pericentral (including the terminal hepatic venule [THV]) regions of the liver lobule (panel A), and the entire liver lobule (sinusoids + THV, panel B). In control rats, gut I/R elicited significant increases in the number of stationary leukocytes compared to basal values. Pretreatment with TJ -10 blunted the gut I/R-induced leukostasis in the midzonal (untreated+I/R: 9.6 ± 0.6, TJ-10+I/R: 3.6 ± 0.6, per field) and the pericentral regions (untreated+I/R: 6.0 ± 0.7, TJ-10+I/R: 3.6 ± 0.5, per field). L-NMMA diminished the protective effect of TJ-10 in the pericentral region (5.6 ± 0.2, per field), but did not significantly affect the gut I/R-induced leukostasis in the midzonal region or the entire liver lobule.
Figure 1.
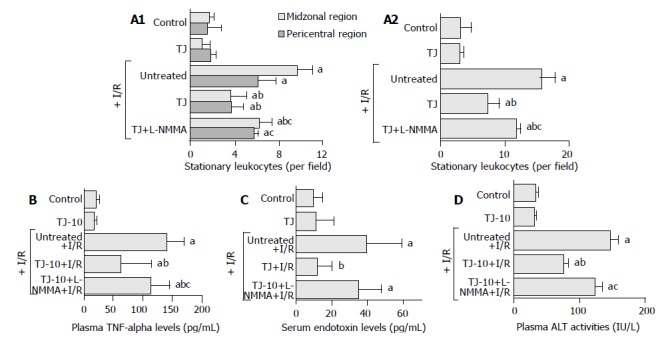
Effects of Saiko-keishi -to (TJ-10) and/or an NO synthase inhibitor (NG- monomethyl-L-arginine, L- NMMA) on the number of stationary leukocytes, plasma TNF-α levels, serum endotoxin levels, and plasma ALT activities. A: In each region (the midzonal and pericentral regions) (panel A) and the entire (combined) liver lobule (panel B) after 30 min of gut ischemia and 60 min of reperfusion; B: Plasma TNF-α levels at 60 min after gut I/R; C: Serum endotoxin levels at 60 min after gut I/R; D: Plasma ALT activities at 6 h after gut I/R.
Figure 1B shows the effect of TJ-10 and/or L-NMMA on the gut I/R-induced elevation of plasma TNF levels. In the control rats, gut I/R elevated the plasma TNF-α levels. Pretreatment with TJ-10, however, blunted the gut I/R-induced elevation of plasma TNF-α levels (untreated+I/R: 140.8 ± 12.6, TJ-10+I/R: 61.7 ± 22.5 ng/L). L-NMMA diminished the protective effects of TJ-10 (113.2 ± 13.7 ng/L).
Figure 1C shows the effect of TJ-10 and/or L-NMMA on the gut I/R-induced elevation of serum endotoxin levels. In the control rats, gut I/R elevated the serum endotoxin levels. Pretreatment with TJ-10, however, blunted the gut I/R-induced elevation of serum endotoxin levels (untreated+I/R: 39.2 ± 8.1, TJ-10+I/R: 11.1 ± 3.3 ng/L). L-NMMA diminished the protective effects of TJ-10 (34.7 ± 5.3 ng/L).
Figure 1D illustrates the effect of TJ-10 and/or L-NMMA on the gut I/R-induced elevation of plasma ALT activities. In the control rats, gut I/R elevated the plasma ALT activities. Pretreatment with TJ-10, however, blunted the gut I/R-induced elevation of plasma ALT levels (untreated + I/R: 146.6 ± 13.0, TJ-9+I/R: 76.0 ± 7.0 IU/L). L-NMMA diminished the protective effects of TJ-10 (123.2 ± 11.9 IU/L).
Table 1 shows the effects of TJ-9 and TJ- 10 on plasma nitrite/nitrate levels. Pretreatment with TJ-10 increased the plasma nitrite/nitrate levels as well as TJ-9.
Table 1.
Effects of Sho-saiko-to (TJ-9) and Saiko-keishi-to (TJ-10) on plasma nitrite/nitrate levels (n = 5)
P<0.05 vs control.
DISCUSSION
Herbal medicines are widely used in Japan. The clinical efficacy of these medicines has been utilized by Japanese Western-medicine practitioners for more than 20 years and is well recognized. In cases of gastroenterological and hepatological diseases, they are often used for patients with chronic liver disease as well as gastrointestinal functional disorder. Although TJ-9 has been the most famous herbal medicine for chronic liver disease in Japan, TJ-10 is another common drug to treat duodenal ulcer, pancreatitis, and chronic liver disease. TJ-10 has effectiveness for more kinds of diseases rather than TJ-9. Indeed, the components of TJ-10 include all of the 7 components of TJ-9. Although TJ-10 has been often administered to patients with chronic liver disease as well as TJ-9, there were few epidemiological reports[3], and little is known about mechanisms of the cytoprotective effects on liver damage.
Previously published work has demonstrated that reperfusion of the ischemic small intestine could elicit an acute inflammatory response both in the intestine and in distant organs, such as the liver[11,12,23] and lung[23,24]. In the liver, the response was characterized by leukocyte plugging of sinusoids, leukocyte adherence in postcapillary venules, a reduction in the number of perfused sinusoids, hepatocellular hypoxia, and leakage of enzymes (ALT) from hepatocytes[11,12,23,25]. Recently, we have reported that TJ-9 could attenuate the gut I/R-induced responses in the liver[18]. In the present study, it was demonstrated that TJ-10 had a similar effect to TJ-9 on the gut I/R-induced responses in the liver. NO has been found to be a modulator of the adhesive interactions between leukocytes, platelets, and endothelial cells[6-9], and NO-dependent cell-cell interactions have been demonstrated in tissues exposed to ischemia and reperfusion (I/R), an injury process in which leukocyte -endothelial cell adhesion plays a critical role. Depletion and/or inactivation of NO has been implicated as a key event in the recruitment of leukocytes in tissues exposed to I/R[26-28]. We previously demonstrated that treatment with L-NMMA resulted in exaggerated leukostasis and cellular injury in the murine liver after gut I/R and that increased delivery/ generation of NO in the liver via a NO donor attenuated the inflammatory responses and microvascular dysfunction elicited in the liver by gut I/R[13]. These results suggest a protective effect of NO on the gut I/R-induced responses in the rat liver. The finding in the present study that L-NMMA diminished the protective effect of TJ-10 on the increase in plasma TNF-α and serum endotoxin levels, leukostasis in the liver, and plasma ALT activities, suggested that TJ-10 could prevent the gut I/R-induced cytokine production and microvascular dysfunction in the liver by elevating sinusoidal NO level. NO could modulate leukocyte- and /or platelet-endothelial cell interactions[6,9,13,14]. Since pretreatment with TJ-10 actually increased plasma nitrite/ nitrate levels in the present study, the increase in NO production by hepatocytes and macrophages after treatment with TJ-10 appeared to be involved in the cytoprotective effects of TJ-10.
In the present study, L-NMMA diminished the protective effect of TJ-10 on the increase in plasma TNF -α and serum endotoxin levels, leukostasis in the liver, and plasma ALT activities. While, in the previous study[18], L-NMMA did not affect the protective effect of TJ-9 on the increase in leukostasis in the midzonal region, the total number of stationary leukocytes, or the plasma ALT activity. One likely interpretation is that a mechanism other than the increase in NO production mediates the protective effects of TJ-9. Steroids have been known to prevent reperfusion injury[29]. The evidence in our previous study[18] that L-NMMA did not affect either TJ-9- or dexamethasone-induced decrease in the gut I/R-elevated plasma ALT activities, raises a possibility that TJ-9-increased blood corticosterone level may prevent the gut I/R-induced microvascular and hepatocellular injury. Since L-NMMA diminished the protective effect of TJ-10 on the gut I/R-induced increase in plasma ALT activities, corticosteroid effect was not involved in the prevention of gut I/R-induced hepatocellular injury by TJ-10. Kampo (herbal medicines) prescriptions affect as the complex. For example, though one of the components in TJ-10, cinnamon bark, which is not included in TJ-9, had a very strong anti-oxidant effect, there was no significant difference between TJ-9 and 10 in the potential for anti-oxidation[30]. Thus, our results suggest that TJ-10 has different characteristics from TJ-9, even including all components of TJ-9.
Recently , the importance of the way of traditional diagnosis “Sho” in the use of Kampo prescriptions has come to be widely described even in package insert drug information pamphlets of Kampo prescriptions. “Sho” is judged comprehensively by a complex of subjective and objective symptoms at a certain point of illness. The process is generally complicated, but we sometimes diagnose by tonus felt on the abdomen. Namely, hypertonus is “Jitsu Sho”, and hypotonus is “Kyo Sho”. General fatigue, sleepiness, sleepless, appetite loss, tiredness of eyes, easily catching cold, dizziness, looking pale, and so on are symptoms of “Kyo Sho”. TJ-10 suits for “Kyo Sho” rather than TJ-9. Moreover, most patients with chronic hepatitis have “Kyo Sho”. TJ-10 prevents microvascular dysfunction in the liver increase in NO production. Symptoms of “Kyo Sho” described above seem to be caused by microvascular dysfunction. Taken together, TJ-10 is recommended for chronic hepatitis patients with “Kyo Sho”.
Although further studies are required to clarify the mechanisms of the protective effects of TJ-10 on reperfusion injury, this study has demonstrated the protective effect of TJ-10 on reperfusion injury via NO.
Footnotes
Supported by the Grants from Tsumura Co. Ltd
Edited by Wang XL and Chen WW Proofread by Xu FM
References
- 1.Hirayama C, Okumura M, Tanikawa K, Yano M, Mizuta M, Ogawa N. A multicenter randomized controlled clinical trial of Shosaiko-to in chronic active hepatitis. Gastroenterol Jpn. 1989;24:715–719. doi: 10.1007/BF02774173. [DOI] [PubMed] [Google Scholar]
- 2.Ogura H, Fujiwara T. Establishment and characterization of a virus-free chick cell line. Acta Med Okayama. 1987;41:141–143. doi: 10.18926/AMO/31758. [DOI] [PubMed] [Google Scholar]
- 3.Itoh T, Shibahara N, Mantani N, Tahara E, Shimada Y, Terasawa K. Effect of kampo treatment on chronic viral hepatitis. J Trad Med. 1997;14:204–210. [Google Scholar]
- 4.Abe H, Sakaguchi M, Yamada M, Arichi S, Odashima S. Pharmacological actions of saikosaponins isolated from Bupleurum falcatum. 1. Effects of saikosaponins on liver function. Planta Med. 1980;40:366–372. doi: 10.1055/s-2008-1074987. [DOI] [PubMed] [Google Scholar]
- 5.Abe H, Orita M, Konishi H, Arichi S, Odashima S. Effects of saikosaponin-d on enhanced CCl4-hepatotoxicity by phenobarbitone. J Pharm Pharmacol. 1985;37:555–559. doi: 10.1111/j.2042-7158.1985.tb03066.x. [DOI] [PubMed] [Google Scholar]
- 6.Kurose I, Kubes P, Wolf R, Anderson DC, Paulson J, Miyasaka M, Granger DN. Inhibition of nitric oxide production. Mechanisms of vascular albumin leakage. Circ Res. 1993;73:164–171. doi: 10.1161/01.res.73.1.164. [DOI] [PubMed] [Google Scholar]
- 7.Kurose I, Wolf R, Grisham MB, Granger DN. Modulation of ischemia/reperfusion-induced microvascular dysfunction by nitric oxide. Circ Res. 1994;74:376–382. doi: 10.1161/01.res.74.3.376. [DOI] [PubMed] [Google Scholar]
- 8.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 9.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 10.Granger DN, Kurose I, Kubes P. Nitric oxide: A modulator of cell-cell adhesion and protein exchange in postcapillary venules. In: Shock, Sepsis, and Organ Failure-Nitric Oxide. G. Schlang, H. Redl, eds. Springer, Heidelberg, Germany. 1994:121–136. [Google Scholar]
- 11.Horie Y, Wolf R, Miyasaka M, Anderson DC, Granger DN. Leukocyte adhesion and hepatic microvascular responses to intestinal ischemia/reperfusion in rats. Gastroenterology. 1996;111:666–673. doi: 10.1053/gast.1996.v111.pm8780571. [DOI] [PubMed] [Google Scholar]
- 12.Horie Y, Wolf R, Anderson DC, Granger DN. Hepatic leukostasis and hypoxic stress in adhesion molecule-deficient mice after gut ischemia/reperfusion. J Clin Invest. 1997;99:781–788. doi: 10.1172/JCI119224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horie Y, Ishii H. Liver dysfunction elicited by gut ischemia-reperfusion. Pathophysiology. 2001;8:11–20. doi: 10.1016/s0928-4680(01)00063-3. [DOI] [PubMed] [Google Scholar]
- 14.Horie Y, Wolf R, Granger DN. Role of nitric oxide in gut ischemia-reperfusion-induced hepatic microvascular dysfunction. Am J Physiol. 1997;273:G1007–G1013. doi: 10.1152/ajpgi.1997.273.5.G1007. [DOI] [PubMed] [Google Scholar]
- 15.Horie Y, Wolf R, Anderson DC, Granger DN. Nitric oxide modulates gut ischemia-reperfusion-induced P-selectin expression in murine liver. Am J Physiol. 1998;275:H520–H526. doi: 10.1152/ajpheart.1998.275.2.H520. [DOI] [PubMed] [Google Scholar]
- 16.Horie Y, Yamagishi Y, Kato S, Kajihara M, Kimura H, Ishii H. Low-dose ethanol attenuates gut ischemia/reperfusion-induced liver injury in rats via nitric oxide production. J Gastroenterol Hepatol. 2003;18:211–217. doi: 10.1046/j.1440-1746.2003.02929.x. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda K. Modulation of nitric oxide production by crude drugs and Kampo medicines. J Traditional Med. 1998;15:22–32. [Google Scholar]
- 18.Horie Y, Kajihara M, Yamagishi Y, Kimura H, Tamai H, Kato S, Ishii H. A Japanese herbal medicine, Sho-saiko-to, prevents gut ischemia/reperfusion-induced hepatic microvascular dysfunction in rats. J Gastroenterol Hepatol. 2001;16:1260–1266. doi: 10.1046/j.1440-1746.2001.02622.x. [DOI] [PubMed] [Google Scholar]
- 19.Horie Y, Wolf R, Russell J, Shanley TP, Granger DN. Role of Kupffer cells in gut ischemia/reperfusion-induced hepatic microvascular dysfunction in mice. Hepatology. 1997;26:1499–1505. doi: 10.1002/hep.510260617. [DOI] [PubMed] [Google Scholar]
- 20.Tamai H, Kato S, Horie Y, Ohki E, Yokoyama H, Ishii H. Effect of acute ethanol administration on the intestinal absorption of endotoxin in rats. Alcohol Clin Exp Res. 2000;24:390–394. [PubMed] [Google Scholar]
- 21.Horie Y, Kato S, Ohki E, Hamamatsu H, Fukumura D, Kurose I, Suzuki H, Suematsu M, Miura S, Ishii H. Effect of lipopolysaccharides on erythrocyte flow velocity in rat liver. J Gastroenterol. 1997;32:783–790. doi: 10.1007/BF02936955. [DOI] [PubMed] [Google Scholar]
- 22.Horie Y, Kimura H, Kato S, Ohki E, Tamai H, Yamagishi Y, Ishii H. Role of nitric oxide in endotoxin-induced hepatic microvascular dysfunction in rats chronically fed ethanol. Alcohol Clin Exp Res. 2000;24:845–851. [PubMed] [Google Scholar]
- 23.Hill J, Lindsay T, Rusche J, Valeri CR, Shepro D, Hechtman HB. A Mac-1 antibody reduces liver and lung injury but not neutrophil sequestration after intestinal ischemia-reperfusion. Surgery. 1992;112:166–172. [PubMed] [Google Scholar]
- 24.Carden DL, Young JA, Granger DN. Pulmonary microvascular injury after intestinal ischemia-reperfusion: role of P-selectin. J Appl Physiol (1985) 1993;75:2529–2534. doi: 10.1152/jappl.1993.75.6.2529. [DOI] [PubMed] [Google Scholar]
- 25.Simpson R, Alon R, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Neutrophil and nonneutrophil-mediated injury in intestinal ischemia-reperfusion. Ann Surg. 1993;218:444–453; discussion 453-454. doi: 10.1097/00000658-199310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma XL, Weyrich AS, Lefer DJ, Lefer AM. Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ Res. 1993;72:403–412. doi: 10.1161/01.res.72.2.403. [DOI] [PubMed] [Google Scholar]
- 28.Siegfried MR, Erhardt J, Rider T, Ma XL, Lefer AM. Cardioprotection and attenuation of endothelial dysfunction by organic nitric oxide donors in myocardial ischemia-reperfusion. J Pharmacol Exp Ther. 1992;260:668–675. [PubMed] [Google Scholar]
- 29.Valen G, Kawakami T, Tähepôld P, Starkopf J, Kairane C, Dumitrescu A, Löwbeer C, Zilmer M, Vaage J. Pretreatment with methylprednisolone protects the isolated rat heart against ischaemic and oxidative damage. Free Radic Res. 2000;33:31–43. doi: 10.1080/10715760000300591. [DOI] [PubMed] [Google Scholar]
- 30.Valen G YoshikawaT. Free radical and Kampo medicines. Kyoto Univer-sity Kampo Seminar. 1995;4:24–37. [Google Scholar]


