ABSTRACT
Metabolism consists of biochemical reactions that are combined to generate a robust metabolic network that can respond to perturbations and also adapt to changing environmental conditions. Escherichia coli and Salmonella enterica are closely related enterobacteria that share metabolic components, pathway structures, and regulatory strategies. The synthesis of thiamine in S. enterica has been used to define a node of the metabolic network by analyzing alternative inputs to thiamine synthesis from diverse metabolic pathways. To assess the conservation of metabolic networks in organisms with highly conserved components, metabolic contributions to thiamine synthesis in E. coli were investigated. Unexpectedly, we found that, unlike S. enterica, E. coli does not use the phosphoribosylpyrophosphate (PRPP) amidotransferase (PurF) as the primary enzyme for synthesis of phosphoribosylamine (PRA). In fact, our data showed that up to 50% of the PRA used by E. coli to make thiamine requires the activities of threonine dehydratase (IlvA) and anthranilate synthase component II (TrpD). Significantly, the IlvA- and TrpD-dependent pathway to PRA functions in S. enterica only in the absence of a functional reactive intermediate deaminase (RidA) enzyme, bringing into focus how these closely related bacteria have distinct metabolic networks.
IMPORTANCE
In most bacteria, including Salmonella strains and Escherichia coli, synthesis of the pyrimidine moiety of the essential coenzyme, thiamine pyrophosphate (TPP), shares enzymes with the purine biosynthetic pathway. Phosphoribosylpyrophosphate amidotransferase, encoded by the purF gene, generates phosphoribosylamine (PRA) and is considered the first enzyme in the biosynthesis of purines and the pyrimidine moiety of TPP. We show here that, unlike Salmonella, E. coli synthesizes significant thiamine from PRA derived from threonine using enzymes from the isoleucine and tryptophan biosynthetic pathways. These data show that two closely related organisms can have distinct metabolic network structures despite having similar enzyme components, thus emphasizing caveats associated with predicting metabolic potential from genome content.
INTRODUCTION
In living cells, a discrete number of enzymes encoded in the genome act in concert to produce diverse characteristic behaviors (phenotypes). In microbes in particular, the metabolic network responsible for these behaviors is robust and can respond to perturbations by reconfiguring the network to maintain the overall function of the organism (1–9). A fundamental question in metabolic systems biology is whether knowledge of the metabolic components can be extrapolated to predict the existence of a specific pathway or network structure. The presence and conservation of metabolic components is easily ascertained by genome analyses and often used to describe the metabolic potential of an organism. Genome annotation that identifies component enzymes has proven valuable for describing central metabolic pathways. It is unclear, however, whether this approach can be extended to describe the higher metabolic network structure that includes atypical metabolites and recruited pathways, many of which provide network plasticity and the resulting robustness. Definition of the higher-order metabolic structure and a solid understanding of the rules that govern its formation are key to elucidating the dynamic roles of metabolic components in different organisms and to refining the information gleaned from genome annotation.
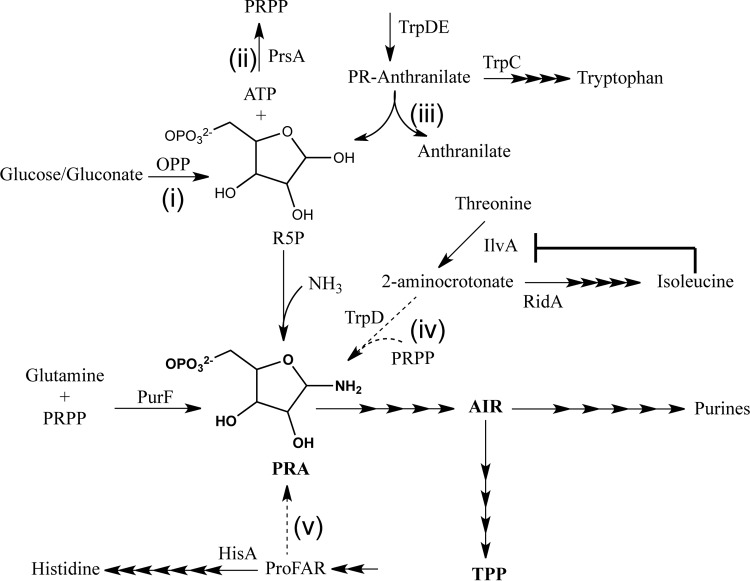
The comparison of thiamine synthesis in the closely related and genetically tractable organisms Escherichia coli and Salmonella enterica provides an opportunity to address the fundamental question of whether the presence of component enzymes can accurately predict metabolic network structure. The essential cofactor thiamine pyrophosphate (TPP) can be synthesized de novo by microbes and plants (10, 11). In most bacteria, including E. coli and S. enterica, the thiamine biosynthetic pathway shares enzymes with the purine biosynthetic pathway (Fig. 1). Phosphoribosylpyrophosphate amidotransferase, encoded by the purF gene, generates phosphoribosylamine (PRA) and is considered the first enzyme in the biosynthesis of purines and the pyrimidine moiety of thiamine in both S. enterica and E. coli (10, 12–14). Biochemical genetic studies in S. enterica described the network surrounding thiamine synthesis (specifically PRA) as one in which metabolites can be made by promiscuous enzymes from other metabolic pathways or by enzyme-independent chemical reactions (5, 6, 8, 15–17). S. enterica requires PurF for thiamine synthesis on glucose medium, but this requirement can be bypassed by growing this bacterium on different media or by the presence of mutations, both of which allow PRA synthesis (4–6, 8, 15, 18, 19). The alternative mechanisms for the synthesis of PRA and, thus, thiamine, can be activated by (i) increasing cellular ribose-5-phosphate (R5P) levels, (ii) the accumulation of the histidine biosynthetic intermediate 1-(5-phosphoribosyl)-5-[(5-phosphoribosylamino)methylideneamino]imidazole-4-carboxamide (ProFAR), or (iii) the elimination of the RidA deaminase. The former involved the nonenzymatic synthesis of PRA from R5P and was facilitated by the high levels of ammonium in the medium (19). Increased cellular R5P levels that allow PRA synthesis could be generated from (i) the oxidative pentose phosphate (OPP) pathway (18) or (ii) by compromising the activities of ribose-phosphate pyrophosphokinase (PrsA) (7) or indole-3-glycerol phosphate synthase/phosphoribosylanthranilate isomerase (TrpC) (6). Lack of RidA allowed PRA to be formed from threonine and phosphoribosylpyrophosphate (PRPP) in a novel pathway that recruited the enzymes IlvA and TrpD from the isoleucine and tryptophan biosynthetic pathways, respectively (4, 15, 16). RidA deaminates enamines, including 2-aminocrotonate, and in its absence, these reactive metabolites accumulate (17). The mechanisms that generate PRA in S. enterica are illustrated in Fig. 1. Studies in E. coli have presumed that PurF catalyzes the first step in both purine and thiamine synthesis but have not specifically addressed the requirement of this enzyme for thiamine synthesis.
FIG 1 .
Mechanisms of PRA synthesis. Metabolic mechanisms capable of contributing to thiamine synthesis (via PRA formation) in the absence of PurF are shown. The reactions that form PRA are denoted i to v. Reactions i, ii, and iii are means to increase R5P, which then allows nonenzymatic formation of PRA when ammonia is in excess, as described in the text. Reactions iv and v are independent of the ammonia concentration. The allosteric inhibition of IlvA by isoleucine is depicted to reflect its importance in the reported work. Abbreviations: PRPP, phosphoribosyl pyrophosphate; PRA, phosphoribosylamine; AIR, aminoimidazole ribotide; TPP, thiamine pyrophosphate; OPP, oxidative pentose phosphate pathway; R5P, ribose-5-phosphate; PR-anthranilate, phosphoribosyl anthranilate; ProFAR, 1-(5-phosphoribosyl)-5-[(5-phosphoribosylamino)methylideneamino]imidazole-4 carboximide.
Although S. enterica and E. coli have unique metabolic capacities encoded in their genomes, the metabolic pathways and regulatory paradigms throughout the central metabolism are conserved between the organisms (20). For instance, each of the proteins mentioned in Fig. 1 are conserved between S. enterica and E. coli and are >95% identical at the amino acid level. Although the components are conserved, the higher-order metabolic structure derived from these components that could generate metabolic robustness has not been queried in E. coli. This study was initiated to determine whether the metabolic network connected to thiamine synthesis was conserved between S. enterica and E. coli. The work reported herein demonstrated that, in E. coli, an IlvA- and TrpD-dependent mechanism of PRA synthesis actively contributes to the thiamine pool despite the presence of functional PurF and RidA proteins. Our results provide evidence that a metabolic network configuration cannot be predicted solely by the presence of the component parts.
RESULTS
Synthesis of PRA for thiamine in E. coli.
S. enterica and E. coli had indistinguishable thiamine requirements, as measured by the growth of thiamine auxotrophs (Table 1). purF mutant strains of both E. coli and S. enterica required a source of purines for growth, but their requirements for thiamine differed. The data in Fig. 2A show the previously characterized growth defect of a purF mutant of S. enterica on glucose-plus-adenine medium, which is eliminated with exogenous thiamine. In contrast to S. enterica, the purF mutant of E. coli grew as well as the wild-type strain on glucose-plus-adenine medium. These data showed that under these conditions, PurF contributed to the synthesis of thiamine in S. enterica but was not necessary in E. coli.
TABLE 1 .
E. coli and S. enterica have the same thiamine requirementa
| Strain | Genotype | Growth (avg OD650 ± SD) with thiamine concn of: |
||
|---|---|---|---|---|
| None | 1 nM | 10 nM | ||
| DM95 (S. enterica) | thi-918::MudJ | 0.03 ± 0.01 | 0.32 ± 0.01 | 1.33 ± 0.12 |
| DM14703 (E. coli) | ΔthiCEFSGH::Kan | 0.01 ± 0.01 | 0.29 ± 0.01 | 1.28 ± 0.19 |
Thiamine auxotrophs of S. enterica (DM95) and E. coli (DM14703) were grown in minimal medium with the concentrations of thiamine indicated. The results are from three independent cultures after 18 h of growth.
FIG 2 .
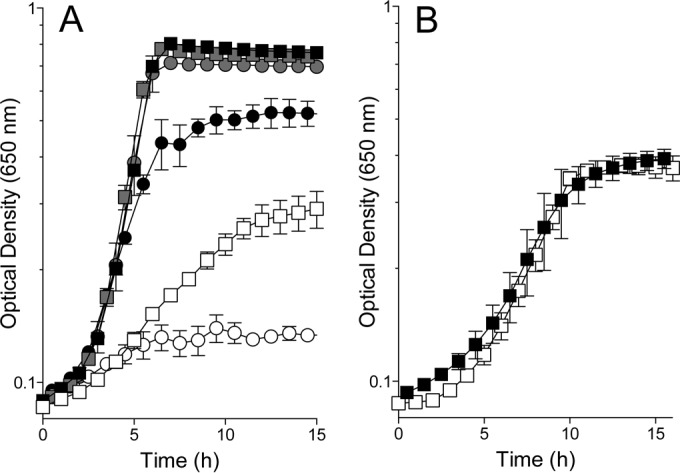
Thiamine-independent growth of S. enterica and E. coli purF and purF gnd mutants. (A) Growth of S. enterica (circles) and E. coli (squares) strains was quantified in glucose-plus-adenine minimal medium. Symbols are filled according to genotype, as follows: gray, wild type (DM1 or DM14520); black, purF mutant (DM1936 or DM14545); white, purF gnd mutant (DM728 or DM14572). The growth of all strains was restored to the wild-type level with the addition of exogenous thiamine (data not shown). (B) The purF gnd mutant of E. coli (DM14572) was grown in glucose-plus-adenine minimal medium (black squares). After 15 h, the mutant was subcultured into identical medium and grown for an additional 15 h (white squares). Error bars show standard deviations.
Blocking the oxidative pentose phosphate pathway (OPP) with a mutation in gnd (6-phosphogluconate dehydrogenase, EC 1.1.1.44) in the purF mutant of S. enterica abolished residual growth in the absence of thiamine (i.e., thiamine synthesis), as previously reported (18). In contrast, the purF gnd mutant of E. coli maintained significant growth in the absence of thiamine, even after subculturing into fresh medium (Fig. 2). The difference in growth with and without a gnd mutation showed that the OPP contributed to PRA synthesis in E. coli. Furthermore, the sustained growth and high final density of the E. coli purF gnd mutant showed that E. coli had a mechanism of PRA synthesis that was independent of both PurF and the OPP. Significantly, this latter mechanism did not require mutational activation as it does in S. enterica.
Ammonia and R5P contribute to PRA formation in E. coli.
The results from two experiments supported the conclusion that the OPP contributed to PRA formation in E. coli by facilitating the nonenzymatic combination of R5P and ammonia. First, when the purF mutant was cultured with glutamine as the sole nitrogen source, its growth on glucose plus adenine decreased to a level comparable to that of the purF gnd mutant (Fig. 3A). Second, when excess ammonia was present, PRA formation in the purF gnd mutant strain was stimulated by exogenous ribose or R5P (data not shown). Taken together, and based on the precedent in S. enterica (19), these data showed that when both R5P and ammonia were in excess, sufficient PRA for thiamine synthesis was synthesized.
FIG 3 .
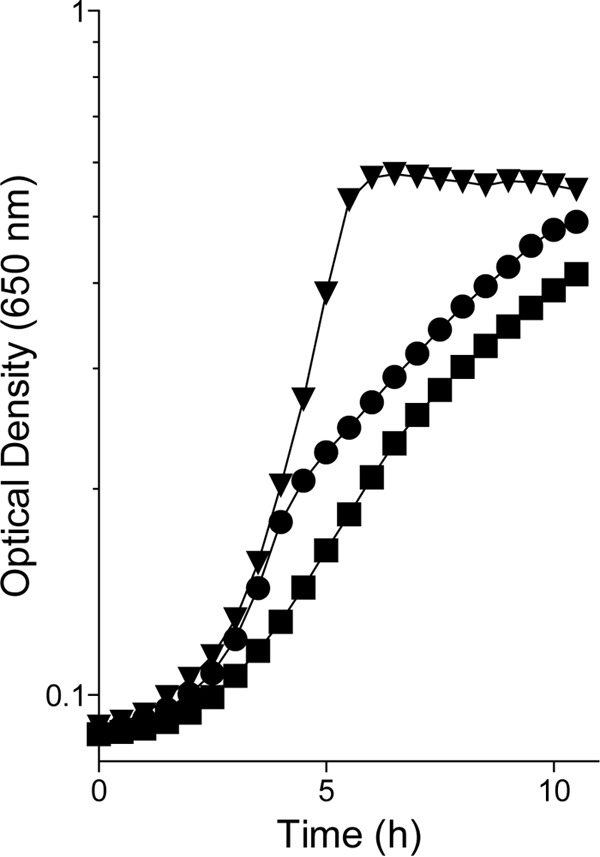
PRA synthesis in E. coli is partially dependent on R5P and ammonia. The growth of the purF mutant (DM14545) in N− C− glucose-plus-adenine minimal medium with 1 mM glutamine as a nitrogen source was quantified with (inverted triangles) or without (circles) thiamine provided. The data for thiamine-independent growth of an E. coli purF gnd mutant (DM14572, squares) are shown for comparison.
The IlvA- and TrpD-dependent pathway for PRA synthesis functions in E. coli.
Nutritional studies with each of the 20 common amino acids provided insights into the mechanism of PRA synthesis in the purF gnd mutant. The results from these studies showed that PRA formation in the purF gnd mutant was stimulated by threonine and inhibited by isoleucine and tryptophan (Fig. 4; Table 2). These nutritional characteristics were reminiscent of the PRA-forming mechanism described in a purF gnd ridA mutant of S. enterica (4, 15, 16). In the relevant mechanism, threonine dehydratase (IlvA) converts threonine to the reactive enamine, 2-aminocrotonate, which is condensed with PRPP by anthranilate synthase component II (TrpD) to form a phosphoribosyl-enamine adduct, the subsequent breakdown of which generates PRA (Fig. 1) (17). Threonine stimulated PRA formation in this mechanism by increasing 2-aminocrotonate synthesis, while isoleucine decreased 2-aminocrotonate production through the allosteric inhibition of IlvA (4, 16). In S. enterica, the 2-aminocrotonate levels needed to support thiamine synthesis occurred only in the absence of RidA, which converts 2-aminocrotonate to 2-ketobutyrate for isoleucine biosynthesis. The utilization of this synthetic mechanism by E. coli, in the presence of a functional RidA, would demand that the metabolic networks of the two organisms are different, despite the presence of conserved, highly identical components.
FIG 4 .
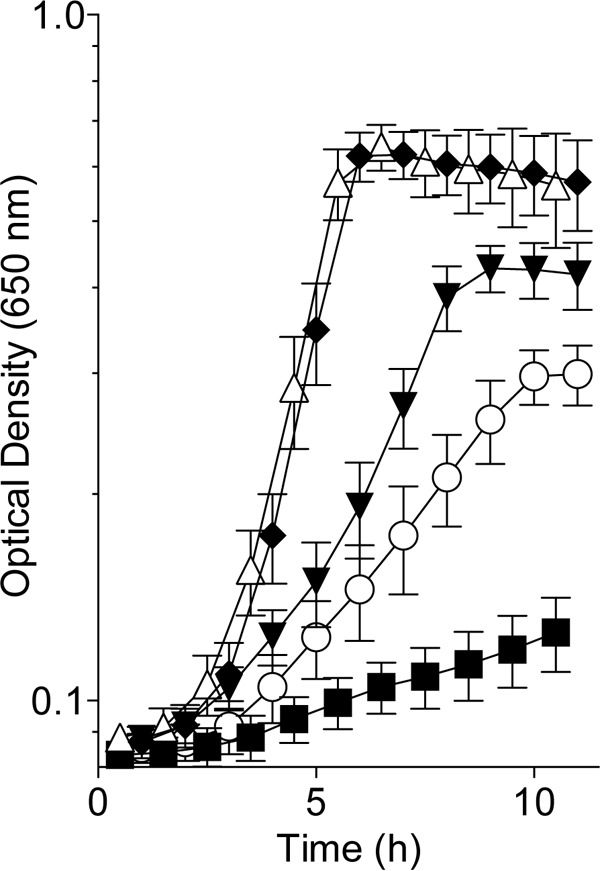
Isoleucine inhibits and threonine stimulates PRA synthesis in E. coli. The growth of a purF gnd mutant strain (DM14572) in glucose-plus-adenine minimal medium (open circles) supplemented with isoleucine (black squares), threonine (black inverted triangles), thiamine (open triangles), or isoleucine and thiamine (black diamonds) is shown. Error bars show standard deviations.
TABLE 2 .
TrpD is required for PRA synthesis in E. coli
| Strain | Relevant genotype | Specific growth rate (μ) (final yield [OD650]) in medium witha: |
||
|---|---|---|---|---|
| Ade | Ade + Trp | Ade + Trp + Thi | ||
| DM14572 | purF gnd | 0.1 (0.2) | NG | 0.7 (0.3) |
| DM14729 | purF gnd trpD | NG | NG | 0.7 (0.4) |
| DM14606 | purF gnd trpR | 0.6 (0.4) | 0.6 (0.4) | 0.6 (0.4) |
| DM14759 | purF gnd trpR trpD | NG | NG | 0.6 (0.4) |
The E. coli strains listed were grown in glucose minimal medium containing the indicated amino acid(s). The results show the average growth rate and final cell density after 16 h (in parentheses) of three independent cultures. Error between cultures was <10%. NG, no growth; indicates the final cell density was <0.15.
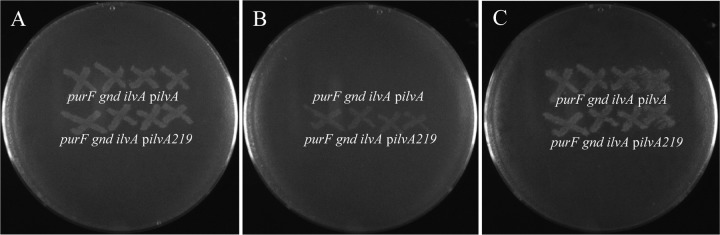
We used genetic approaches to address the role of IlvA in thiamine synthesis in E. coli. For this purpose, we took advantage of the feedback-resistant IlvAL447F variant (with an L-to-F change at position 447) encoded by the ilvA219 allele (21). A purF gnd mutant lacking ilvA was constructed, and either the ilvA+ or the ilvA219 allele was expressed in trans as the only source of IlvA activity. Ectopically produced wild-type IlvA and the IlvAL447F protein restored isoleucine synthesis in the purF gnd ilvA (DM1466) strain, showing that a functional threonine dehydratase activity was associated with both proteins. Both strains were also proficient in thiamine synthesis, as shown by their growth in the absence of thiamine (Fig. 5A). However, when isoleucine was provided in the medium, only the strain expressing the ilvA219 allele in trans (strain DM14684) maintained PRA synthesis that allowed growth in the absence of exogenous thiamine (Fig. 5B). The growth of both strains was restored in the presence of isoleucine when exogenous thiamine was provided (Fig. 5C). These results supported the conclusion that IlvA activity was required for PRA synthesis and that isoleucine inhibited PRA synthesis via allosteric inhibition of IlvA.
FIG 5 .
Isoleucine inhibits PRA synthesis in E. coli by allosteric inhibition of IlvA. The growth of mutant strains purF gnd ilvA pilvA (DM14683) and purF gnd ilvA pilvA219 (DM14684) is shown. Colonies of each strain were patched to nutrient agar containing chloramphenicol and replica printed to glucose-plus-adenine minimal medium plates containing chloramphenicol (A) and further supplemented with isoleucine (B) or isoleucine and thiamine (C).
PRA synthesis in a purF gnd mutant was inhibited by tryptophan, another similarity with the pathway that functions in a purF gnd ridA mutant of S. enterica (Table 2) (15). In the presence of tryptophan, TrpR represses the transcription of the trp operon (15, 22). Eliminating TrpR function in the purF gnd mutant had two effects that supported the involvement of the IlvA- and TrpD-dependent pathway described above. First, a trpR mutation restored the growth of the purF gnd strain to wild-type levels on adenine minimal medium. Interestingly, the trpR mutation also restored growth (i.e., PRA synthesis) on glucose-plus-adenine medium with limiting nitrogen (data not shown). These results suggested that the decrease in PRA synthesis caused by the gnd mutation could be restored not only by increasing nonenzymatic synthesis (from R5P and ammonia) but by increasing the level of a tryptophan enzyme(s). Second, exogenous tryptophan no longer inhibited PRA synthesis in the purF gnd trpR mutant strain (Table 2), confirming that the relevant effect of tryptophan was on transcription. When a trpD mutation was introduced in the purF gnd trpR mutant, the resulting strain failed to synthesize PRA in adenine-plus-tryptophan minimal medium (Table 2). Taken together, these data showed that TrpD was required for PRA synthesis in a purF gnd mutant of E. coli.
IlvA- and TrpD-dependent PRA synthesis contributes to the thiamine pool in wild-type E. coli.
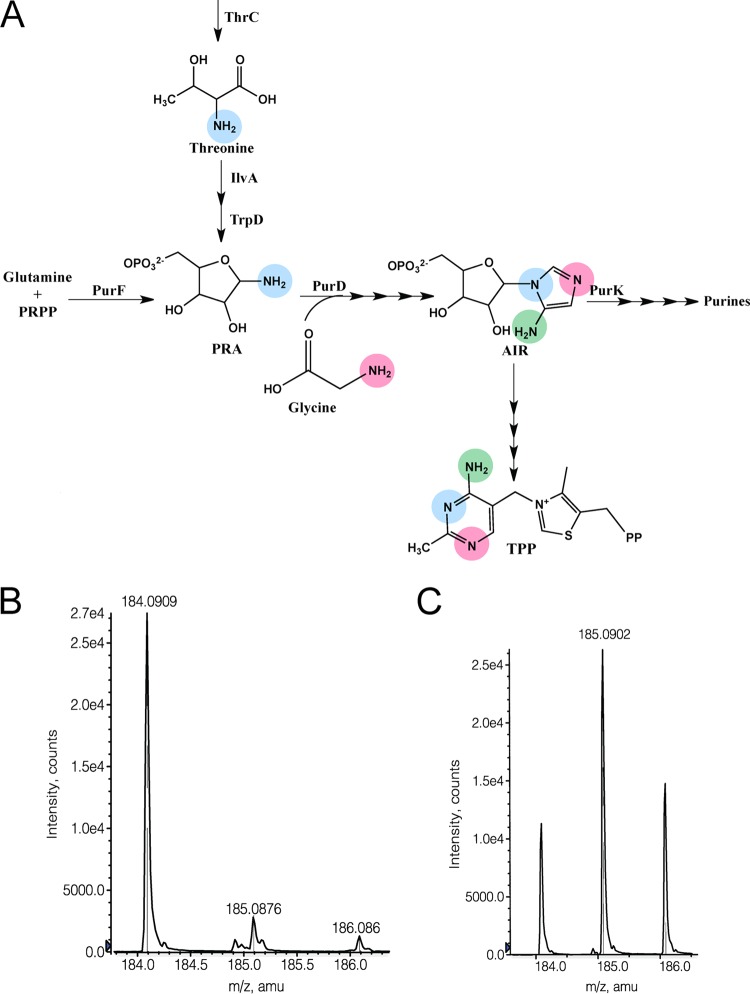
The contribution of IlvA and TrpD to thiamine synthesis in the purF gnd mutant suggested that this pathway could have a role in the wild-type strain. If this were the case, PRA would include the nitrogen of threonine that is ultimately incorporated into the 4-amino-5-hydroxymethyl-2-methylpyrimidin (HMP) moiety of thiamine (Fig. 6A). A strain (DM14893) that carried wild-type alleles of purF and gnd was grown in glucose-plus-adenine minimal medium with 14N- or 15N-labeled threonine. Strain DM14893 carried a thrC mutation to prevent dilution of the labeled threonine by endogenous synthesis and a mutation in purK to divert metabolic flux toward HMP biosynthesis and maximize the yield of thiamine. Figures 6B and C show mass spectrometry (MS) data from positive time of flight (+TOF) MS analysis of derivatized HMP (2-methyl-4-amino-5[(ethylthio)methyl]pyrimidine [ETMP]) isolated from cells grown in each medium. As expected, the HMP purified from the strain grown in the presence of [14N]threonine generated a single ETMP species with an m/z of 184. When the cells were grown in [15N]threonine, three ETMP species were observed, representing unlabeled ETMP (m/z = 184) or ETMP with one (m/z = 185) or two (m/z = 186) heavy (15N) nitrogens. In total, these data showed that threonine could contribute one or two nitrogen atoms to HMP.
FIG 6 .
[15N]HMP was isolated from E. coli cells grown in the presence of [15N]threonine. (A) The IlvA- and TrpD-dependent mechanism of PRA synthesis is incorporated in the purine-thiamine (HMP) biosynthetic pathway. Nitrogen atoms are color coded based on their origin (12, 37, 38). The nitrogen derived from PRA (via PurF or TrpD or nonenzymatically) is blue, the glycine-derived nitrogen (PurD reaction) is pink, and the nitrogen derived from glutamine via PurL is green. (B and C) Mass spectral data from +TOF MS analysis of ETMP isolated from DM14893 (thrC purK) grown in the glucose-plus-adenine minimal medium containing [14N]threonine (B) or [15N]threonine (C) are shown. The expected m/z of unlabeled ETMP in this mode is 184.2819, and [15N]ETMP with one or two heavy nitrogens should be +1 or +2 atomic mass units (amu), respectively.
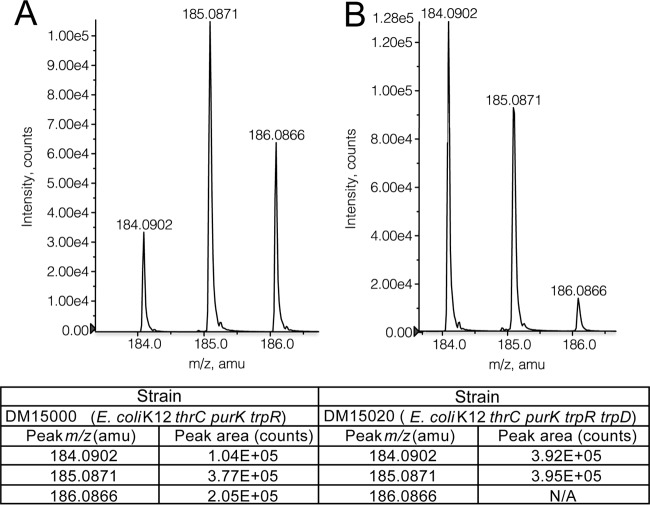
Two strains were generated to probe the contribution of threonine to HMP. A trpR lesion was introduced to eliminate the effect of added tryptophan, which was required to monitor the role of TrpD in this synthesis. Strains DM15000 (thrC purK trpR) and DM15020 (thrC purK trpR trpD) were grown in the adenine-plus-tryptophan minimal medium with [15N]threonine. Figure 7 shows mass spectrometry data from +TOF MS analysis of ETMP isolated from each strain. Three ETMP species were isolated from the first strain (thrC purK trpR), while two ETMP species were present in the strain lacking TrpD. Significantly, in the strain lacking TrpD, the peak with an m/z of 184 was a substantially higher percentage of the total HMP than it was in the strain with a functional TrpD (50% versus 15%, respectively). Qualitatively similar results were obtained in strains with a functional PurK (data not shown). In total, these data supported a scenario in which threonine contributes a nitrogen to HMP via PRA synthesized by the 2-aminocrotonate pathway.
FIG 7 .
IlvA- and TrpD-dependent PRA synthesis is a major pathway for thiamine synthesis in E. coli. Mass spectral data from +TOF MS analysis of ETMP (a derivative of HMP) isolated from DM15000 (thrC purK trpR) (A) and DM15020 (thrC purK trpR trpD) (B) cells grown in the presence of [15N]threonine are shown. The expected m/z of unlabeled ETMP in this mode is 184.2819, and [15N]ETMP with one or two heavy nitrogens should be +1 or +2 amu, respectively. N/A, not applicable.
The presence of a doubly labeled peak in the trpD+ strain and the persistence of a singly labeled peak in the absence of TrpD suggested that the nitrogen from threonine was incorporated into HMP by an additional mechanism. Aminoimidazole ribotide (AIR) contains three nitrogen atoms, one from PRA, one from glutamine, and one from glycine (Fig. 6). Threonine is converted to glycine by poorly characterized pathways but has no direct connection to glutamine (23, 24). Based on these considerations, we favor an explanation where [15N]glycine was derived in vivo from [15N]threonine and served as a precursor for HMP. This scenario was supported by the finding that HMP isolated from DM15020 grown with glycine (in addition to adenine and tryptophan) yielded a single ETMP species with an m/z of 184 (data not shown).
DISCUSSION
S. enterica and E. coli are enteric bacteria that have been model organisms for basic genetic, biochemical, and physiological studies for decades. The prevailing perception was that, a few specific pathways notwithstanding, these organisms were similar in metabolic capacity and regulatory organization. This perception has been supported by countless studies defining proteins, pathways, and regulatory systems that are conserved between the organisms.
The purpose of this study was to address metabolic network structure and determine whether conservation of metabolic components determines the conservation of metabolic network structure and plasticity. The well-described metabolic network structure around the synthesis of PRA and thiamine in S. enterica provided a model network. Significantly, all of the component enzymes and regulatory proteins implicated in this network were >95% identical between S. enterica and E. coli at the amino acid level (Fig. 1).
The data showed that in E. coli, unlike S. enterica, neither phosphoribosylpyrophosphate amidotransferase (PurF) nor 6-phosphogluconate dehydrogenase (Gnd) was required for thiamine synthesis. This result indicated that there was a significant difference between the organisms in the structure of the metabolic networks involving these gene products and the formation of thiamine. The data showed that, in wild-type E. coli, an IlvA- and TrpD-dependent mechanism for PRA formation contributed to thiamine synthesis as significantly as PurF. This pathway was identified and characterized in S. enterica, where it satisfies a thiamine requirement only in the absence of the RidA hydrolase (4, 15–17). Thus, E. coli depended on a recruited pathway that was first characterized in S. enterica. A number of points were taken from this result. First, while the components of this pathway were present in S. enterica, its function was not detectable in a wild-type strain. The fact that this pathway functions in S. enterica only if the RidA hydrolase is absent and yet it functions in E. coli in the presence of RidA suggests that the cellular milieus of the two bacteria are different. The IlvA- and TrpD-dependent synthesis of PRA depends on the unstable enamine, 2-aminocrotonate. Work in Salmonella strains showed that when RidA was present, the levels of 2-aminocrotonate were not sufficient to support PRA synthesis for thiamine. This was presumed to be due to the conversion of 2-aminocrotonate to 2-ketobutyrate by RidA, but the presence of RidA in E. coli means this cannot be the full explanation. In total, the data herein suggested that the metabolic network in E. coli has a characteristic(s) distinct from S. enterica that allows the IlvA-and-TrpD pathway to function in the presence of a functional RidA. An attractive possibility is that the ratio of serine to threonine is different in the two organisms. IlvA uses both amino acids as substrates, and increased availability of threonine could result in higher steady-state levels of 2-aminocrotonate, even in the presence of RidA.
The results of in vivo labeling experiments supported the conclusion that in wild-type E. coli, in the presence of adenine, the IlvA- and TrpD-dependent pathway contributes significantly to thiamine synthesis. This finding was striking in light of the common assumption that dedicated linear pathways are the dominant form of component synthesis. The natural environment of E. coli, the mammalian gut, is rich in purines due to cellular breakdown and release of nucleic acids (25, 26). This correlation makes it reasonable to suggest that aspects of the E. coli metabolic network have evolved to ensure that thiamine is synthesized even when flux through the purine pathway is dramatically reduced by allosteric inhibition of PurF and transcriptional repression by PurR. This study provides an example of how the modulation and/or enhancement of metabolic plasticity can accomplish a necessary metabolic goal that facilitates the inhabiting of specific niches.
When one considers parameters that affect metabolic function, in addition to the components currently identifiable in the genome, a vast number of network architectures are possible. It is worth noting that the identification and understanding of the IlvA- and TrpD-dependent pathway for PRA formation was generated by using a mutant approach to uncover metabolic potential in the wild-type strain. Thus, despite the fact that this pathway does not appear to function in the wild-type S. enterica, its analysis in a ridA mutant strain allowed rapid understanding of the explanation for the E. coli phenotype and, in doing so, suggested significant differences in the cellular milieus of E. coli and S. enterica. The example herein emphasizes the gap in our understanding of how metabolic pieces are organized to generate the complexity and flexibility inherent in the metabolic network, as well as the potential of probing basic metabolic systems to provide fundamental insights into multiple systems.
MATERIALS AND METHODS
Bacterial strains, media, and chemicals.
The strains used in this study are derivatives of S. enterica serovar Typhimurium strain LT2 or E. coli strain K-12 and are described in Table 3. Luria-Bertani (LB) broth and Difco nutrient broth (NB) (8 g/liter) with NaCl (5 g/liter) were used as rich media. No-carbon E medium supplemented with 1 mM MgSO4 (27–29) and trace minerals (30) was used as minimal medium. Nitrogen- and carbon-free (N− C−) salts medium (31) supplemented with 1 mM MgSO4, trace minerals, and 1 (or 5) mM glutamine was used as nitrogen-limiting minimal medium. Glucose (11 mM) or succinate (16.5 mM) was provided as the sole carbon source. Difco BiTek agar was added (15 g/liter) for solid medium. Where indicated in the figure or table legends, additions to the media were used as follows: adenine (0.4 mM), thiamine (100 nM), ribose (20 mM), ribose-5-phosphate (20 mM), glucose-6-phosphate (0.4 mM), isoleucine (0.3 mM), threonine (0.3 mM), serine (10 mM), tryptophan (0.1 mM), methionine (0.3 mM), glycine (0.13 mM), and histidine (0.1 mM). Antibiotics were used at the following concentrations for rich (or minimal) medium: kanamycin, 50 (12.5) µg/ml; chloramphenicol, 20 (5) µg/ml; and ampicillin, 100 (15) µg/ml. [l-15N]threonine was obtained from Cambridge Isotope Laboratories (Tewksbury, MA). All other chemicals were from Sigma (St. Louis, MO).
TABLE 3 .
Bacterial strains used in the study
| Strain | Genotype |
|---|---|
| DM1 | S. enterica LT2 wild type |
| DM95 | S. enterica LT2 thi-918::MudJa |
| DM728 | S. enterica LT2 purF2085 gnd-181 |
| DM1936 | S. enterica LT2 purF2085 |
| DM9890 | S. enterica LT2 purF2085 gnd-181 trpC3620 |
| DM14520 | E. coli K-12 wild type |
| DM14545 | E. coli K-12 ΔpurF723::kan |
| DM14572 | E. coli K-12 purF823 Δgnd-727::kan |
| DM14602 | E. coli K-12 purF823 gnd-827 |
| DM14603 | E. coli K-12 purF823 gnd-827 ΔridA790::kan |
| DM14606 | E. coli K-12 purF823 gnd-827 ΔtrpR789::kan |
| DM14661 | E. coli K-12 purF823 gnd-827 ΔilvA723::kan |
| DM14683 | E. coli K-12 purF823 gnd-827 ΔilvA723::kan pGS22-ilvA |
| DM14684 | E. coli K-12 purF823 gnd-827 ΔilvA723::kan pGS15-ilvA219 |
| DM14703 | E. coli K-12 ΔthiCEFSGH::kan |
| DM14711 | E. coli K-12 purF823 Δgnd-727::kan ΔtrpD1050::cat |
| DM14729 | E. coli K-12 purF823 gnd-827 ΔtrpD1050::cat |
| DM14759 | E. coli K-12 purF823 gnd-827 ΔtrpD1050::cat ΔtrpR789::kan |
| DM14893 | E. coli K-12 thrC824 ΔpurK735::kan |
| DM15000 | E. coli K-12 thrC824 ΔpurK835 ΔtrpR789::kan |
| DM15020 | E. coli K-12 thrC824 ΔpurK835 ΔtrpR789::kan ΔtrpD1050::cat |
MudJ refers to the MudJ1734 transposon (36). pGS22-ilvA and pGS22-ilvA219 contain the loci from S. enterica.
Growth quantification.
Cells from overnight cultures in NB medium were pelleted and resuspended in an equivalent volume of saline (0.85% NaCl), and a 0.1-ml aliquot was inoculated into 5 ml of the appropriate minimal medium. Alternatively, a 5-µl aliquot was used to inoculate 195 µl of medium. Growth was quantified in a microplate reader (model EL808; Bio-Tek Instruments). Unless otherwise stated, cell density was measured as the absorbance at 650 nm and growth was reported as the final cell density at an optical density of 650 nm (OD650), reached after 12 to 24 h of incubation at 37°C with shaking. When nutritional requirements were measured on solid medium, soft agar overlays were used (32).
Genetic techniques.
Bacteriophage P1vir was used for transductional crosses. The preparation of P1 phage lysates and the protocol for transductional crosses have been described previously (33). The published protocol was modified such that the phage and cells were incubated for 1 h, washed twice with 1 ml of M9 buffer, and resuspended in 200 µl of M9 buffer before plating on selective medium. Mutant strains were constructed by transducing the relevant mutations from the Keio collection to the appropriate strain (34). The trpD locus in E. coli K-12 was replaced with the cat element using λ-Red-mediated homologous recombination (35).
Purification of the pyrimidine moiety of thiamine.
A 1- or 0.5-liter culture of the appropriate strain in medium with the appropriate nutrients and 0.5 mM threonine (l-14N or l-15N labeled) was grown to an OD650 of approximately 0.8. The cells were pelleted, and the 4-amino-5-hydroxymethyl-2-methylpyrimidin (HMP) moiety of thiamine was extracted, derivatized, and purified as described previously (23, 24). In brief, the cell pellet (frozen or fresh) was resuspended in 8 ml of 0.1 M HCl and boiled for 20 min. Cell debris was removed by centrifugation, and the thiamine pyrophosphate was cleaved by ethanethiol, resulting in the formation of thiazole diphosphate and 2-methyl-4-amino-5[(ethylthio)methyl]pyrimidine (ETMP). ETMP was purified by repeated extractions with methylene chloride. The extractions were reduced to dryness with a stream of nitrogen, and the residue containing ETMP was suspended in 30 to 100 µl of double-distilled water as needed. The sample was submitted for positive time of flight (+TOF) mass spectral analysis at the University of Wisconsin Biotechnology Center.
ACKNOWLEDGMENT
This work was supported by the competitive grants program at the NSF (grant number MCB 1411672 to D.M.D.).
Footnotes
Citation Bazurto JV, Farley KR, Downs DM. 2016. An unexpected route to an essential cofactor: Escherichia coli relies on threonine for thiamine biosynthesis. mBio 7(1):e01840-15. doi: 10.1128/mBio.01840-15.
REFERENCES
- 1.Ibarra RU, Edwards JS, Palsson BO. 2002. Escherichia coli K-12 undergoes adaptive evolution to achieve in silico predicted optimal growth. Nature 420:186–189. doi: 10.1038/nature01149. [DOI] [PubMed] [Google Scholar]
- 2.Hartman JL, Garvik B, Hartwell L. 2001. Principles for the buffering of genetic variation. Science 291:1001–1004. doi: 10.1126/science.291.5506.1001. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Kershner JP, Novikov Y, Shoemaker RK, Copley SD. 2010. Three serendipitous pathways in E. coli can bypass a block in pyridoxal-5′-phosphate synthesis. Mol Syst Biol 6:436. doi: 10.1038/msb.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enos-Berlage JL, Langendorf MJ, Downs DM. 1998. Complex metabolic phenotypes caused by a mutation in yjgF, encoding a member of the highly conserved YER057c/YjgF family of proteins. J Bacteriol 180:6519–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramos I, Downs DM. 2003. Anthranilate synthase can generate sufficient phosphoribosyl amine for thiamine synthesis in Salmonella enterica. J Bacteriol 185:5125–5132. doi: 10.1128/JB.185.17.5125-5132.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos I, Vivas EI, Downs DM. 2008. Mutations in the tryptophan operon allow PurF-independent thiamine synthesis by altering flux in vivo. J Bacteriol 190:815–822. doi: 10.1128/JB.00582-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koenigsknecht MJ, Fenlon LA, Downs DM. 2010. Phosphoribosylpyrophosphate synthetase (PrsA) variants alter cellular pools of ribose 5-phosphate and influence thiamine synthesis in Salmonella enterica. Microbiology 156:950–959. doi: 10.1099/mic.0.033050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenigsknecht MJ, Lambrecht JA, Fenlon LA, Downs DM. 2012. Perturbations in histidine biosynthesis uncover robustness in the metabolic network of Salmonella enterica. PLoS One 7:e48207. doi: 10.1371/journal.pone.0048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazurto JV, Downs DM. 2011. Plasticity in the purine-thiamine metabolic network of salmonella. Genetics 187:623–631. doi: 10.1534/genetics.110.124362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Begley TP, Downs DM, Ealick SE, McLafferty FW, Van Loon APGM, Taylor S, Campobasso N, Chiu H, Kinsland C, Reddick JJ, Xi J. 1999. Thiamin biosynthesis in prokaryotes. Arch Microbiol 171:293–300. doi: 10.1007/s002030050713. [DOI] [PubMed] [Google Scholar]
- 11.Roje S. 2007. Vitamin B biosynthesis in plants. Phytochemistry 68:1904–1921. doi: 10.1016/j.phytochem.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Le Gal ML, Le Gal Y, Roche J, Hedegaard J. 1967. Purine biosynthesis: enzymatic formation of ribosylamine-5-phosphate from ribose-5-phosphate and ammonia. Biochem Biophys Res Commun 27:618–624. doi: 10.1016/S0006-291X(67)80079-2. [DOI] [PubMed] [Google Scholar]
- 13.Messenger LJ, Zalkin H. 1979. Glutamine phosphoribosylpyrophosphate amidotransferase from Escherichia coli. Purification and properties. J Biol Chem 254:3382–3392. [PubMed] [Google Scholar]
- 14.Newell PC, Tucker RG. 1968. Biosynthesis of the pyrimidine moiety of thiamine. A new route of pyrimidine biosynthesis involving purine intermediates. Biochem J 106:279–287. doi: 10.1042/bj1060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browne BA, Ramos AI, Downs DM. 2006. PurF-independent phosphoribosyl amine formation in yjgF mutants of Salmonella enterica utilizes the tryptophan biosynthetic enzyme complex anthranilate synthase-phosphoribosyltransferase. J Bacteriol 188:6786–6792. doi: 10.1128/JB.00745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambrecht JA, Browne BA, Downs DM. 2010. Members of the YjgF/YER057c/UK114 family of proteins inhibit phosphoribosylamine synthesis in vitro. J Biol Chem 285:34401–34407. doi: 10.1074/jbc.M110.160515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambrecht JA, Downs DM. 2013. Anthranilate phosphoribosyl transferase (TrpD) generates phosphoribosylamine for thiamine synthesis from enamines and phosphoribosyl pyrophosphate. ACS Chem Biol 8:242–248. doi: 10.1021/cb300364k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enos-Berlage JL, Downs DM. 1996. Involvement of the oxidative pentose phosphate pathway in thiamine biosynthesis in Salmonella typhimurium. J Bacteriol 178:1476–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koenigsknecht MJ, Downs DM. 2010. Thiamine biosynthesis can be used to dissect metabolic integration. Trends Microbiol 18:240–247. doi: 10.1016/j.tim.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neidhardt FC, Curtiss R, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE. 1996. Escherichia coli and Salmonella: cellular and molecular biology, vol. 1 and 2, 2nd ed. ASM Press, Washington, DC. [Google Scholar]
- 21.LaRossa RA, Van Dyk TK, Smulski DR. 1987. Toxic accumulation of alpha-ketobutyrate caused by inhibition of the branched-chain amino acid biosynthetic enzyme acetolactate synthase in Salmonella typhimurium. J Bacteriol 169:1372–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balbinder E, Callahan R, McCann PP, Cordaro JC, Weber AR, Smith AM, Angelosanto F. 1970. Regulatory mutants of the tryptophan operon of Salmonella typhimurium. Genetics 66:31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enos-Berlage JL, Downs DM. 1999. Biosynthesis of the pyrimidine moiety of thiamine independent of the PurF enzyme (phosphoribosylpyrophosphate amidotransferase) in Salmonella typhimurium: incorporation of stable isotope-labeled glycine and formate. J Bacteriol 181:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White RH, Rudolph FB. 1979. Biosynthesis of the pyrimidine moiety of thiamine in Escherichia coli: incorporation of stable isotope-labeled glycines. Biochemistry 18:2632–2636. doi: 10.1021/bi00579a031. [DOI] [PubMed] [Google Scholar]
- 25.Salati LM, Gross CJ, Henderson LM, Savaiano DA. 1984. Absorption and metabolism of adenine, adenosine-5′-monophosphate, adenosine and hypoxanthine by the isolated vascularly perfused rat small intestine. J Nutr 114:753–760. [DOI] [PubMed] [Google Scholar]
- 26.Chang D-E, Smalley DJ, Tucker DL, Leatham MP, Norris WE, Stevenson SJ, Anderson AB, Grissom JE, Laux DC, Cohen PS, Conway T. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc Natl Acad Sci U S A 101:7427–7432. doi: 10.1073/pnas.0307888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berkowitz D, Hushon JM, Whitfield HJ, Roth J, Ames BN. 1968. Procedure for identifying nonsense mutations. J Bacteriol 96:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis RW, Botstein D, Roth JR, Cold Spring Harbor Laboratory . 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 29.Vogel HJ, Bonner DM. 1956. Acetylornithase of Escherichia coli: partial purification and some properties. J Biol Chem 218:97–106. [PubMed] [Google Scholar]
- 30.Balch WE, Wolfe RS. 1976. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl Environ Microbiol 32:781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutnick D, Clvo JM, Klopotowaski T, Ames BN. 1969. Compounds which serve as the sole source of carbon or nitrogen for Salmonella typhimurium LT2. J Bacteriol 100:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen S, Zilles JL, Downs DM. 2002. Metabolic flux in both the purine mononucleotide and histidine biosynthetic pathways can influence synthesis of the hydroxymethyl pyrimidine moiety of thiamine in Salmonella enterica. J Bacteriol 184:6130–6137. doi: 10.1128/JB.184.22.6130-6137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willetts NS, Clark AJ, Low B. 1969. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol 97:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castilho BA, Olfson P, Casadaban MJ. 1984. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol 158:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estramareix B, David S. 1990. Conversion of 5-aminoimidazole ribotide to the pyrimidine of thiamin in enterobacteria: study of the pathway with specifically labeled samples of riboside. Biochim Biophys Acta 1035:154–160. doi: 10.1016/0304-4165(90)90110-I. [DOI] [PubMed] [Google Scholar]
- 38.Lawhorn BG, Mehl RA, Begley TP. 2004. Biosynthesis of the thiamin pyrimidine: the reconstitution of a remarkable rearrangement reaction. Organ Biomol Chem 2:2538–2546. doi: 10.1039/b405429f. [DOI] [PubMed] [Google Scholar]