ABSTRACT
Influenza remains a major global health burden. Seasonal vaccines offer protection but can be rendered less effective when the virus undergoes extensive antigenic drift. Antibodies that target the highly conserved hemagglutinin stalk can protect against drifted viruses, and vaccine constructs designed to induce such antibodies form the basis for a universal influenza virus vaccine approach. In this study, we analyzed baseline and postvaccination serum samples of children (6 to 59 months), adults (18 to 49 years), and elderly individuals (≥65 years) who participated in clinical trials with a recombinant hemagglutinin-based vaccine. We found that baseline IgG and IgA antibodies against the H1 stalk domain correlated with the ages of patients. Children generally had very low baseline titers and did not respond well to the vaccine in terms of making stalk-specific antibodies. Adults showed the highest induction of stalk-specific antibodies, but the elderly had the highest absolute antibody titers against the stalk. Importantly, the stalk antibodies measured by enzyme-linked immunosorbent assay (ELISA) showed neutralizing activity in neutralization assays and protected mice in a passive-transfer model in a stalk titer-dependent manner. Finally, we found similar patterns of stalk-specific antibodies directed against the H3 and influenza B virus hemagglutinins, albeit at lower levels than those measured against the H1 stalk. The relatively high levels of stalk-specific antibodies in the elderly patients may explain the previously reported low influenza virus infection rates in this age group. (This study has been registered at ClinicalTrials.gov under registration no. NCT00336453, NCT00539981, and NCT00395174.)
IMPORTANCE
The present study provides evidence that titers of broadly neutralizing hemagglutinin stalk-reactive antibodies increase with age, possibly due to repeated exposure to divergent influenza viruses. These relatively high levels of antistalk titers may be responsible for lower circulation rates of influenza viruses in older individuals. Our findings suggest that the level of antistalk antibodies is a good surrogate marker for protection against influenza virus infection. In addition, the levels of antistalk antibodies might determine the breadth of protection against different drifted strains.
INTRODUCTION
Seasonal influenza virus infections cause significant global morbidity and mortality every year (1, 2). In addition, influenza A viruses cause pandemics in irregular intervals. Current influenza virus vaccines are efficacious but are very strain specific and protect against viruses well matched with the vaccine formulation (3). Immunity induced by these conventional vaccines is mostly directed to the immuno-dominant globular head domain of the hemagglutinin (HA), the major surface glycoprotein of the virus. This part of the HA has a high plasticity and allows the virus to escape the immune response, a mechanism called antigenic drift (4). This phenomenon makes it necessary to update vaccines on a regular (annual) basis (5). Antibodies against the conserved, immuno-subdominant stalk domain of the HA are usually not induced to high titers by seasonal influenza virus vaccines (6–8). However, such antibodies have been shown to be broadly protective and efficacious against multiple subtypes of influenza virus HAs (9–16). Universal influenza virus vaccine candidates aiming to induce stalk-reactive antibodies are currently under development (17–25). Here we investigate the prevalence of anti-HA stalk antibodies in different age groups. Using reagents based on chimeric HAs (cHAs) (26, 27), we determined titers for the group 1, group 2, and influenza B virus HA stalk domains in children (6 to 59 months), adults (18 to 49 years), and elderly individuals (≥65 years). Immunity was measured pre- and postvaccination with a licensed recombinant-protein-based influenza virus vaccine (28, 29). Furthermore, we characterized the functionality of group 1 stalk-reactive antibodies in vitro and in vivo.
RESULTS
Titers of anti-HA stalk IgG antibodies increase with age.
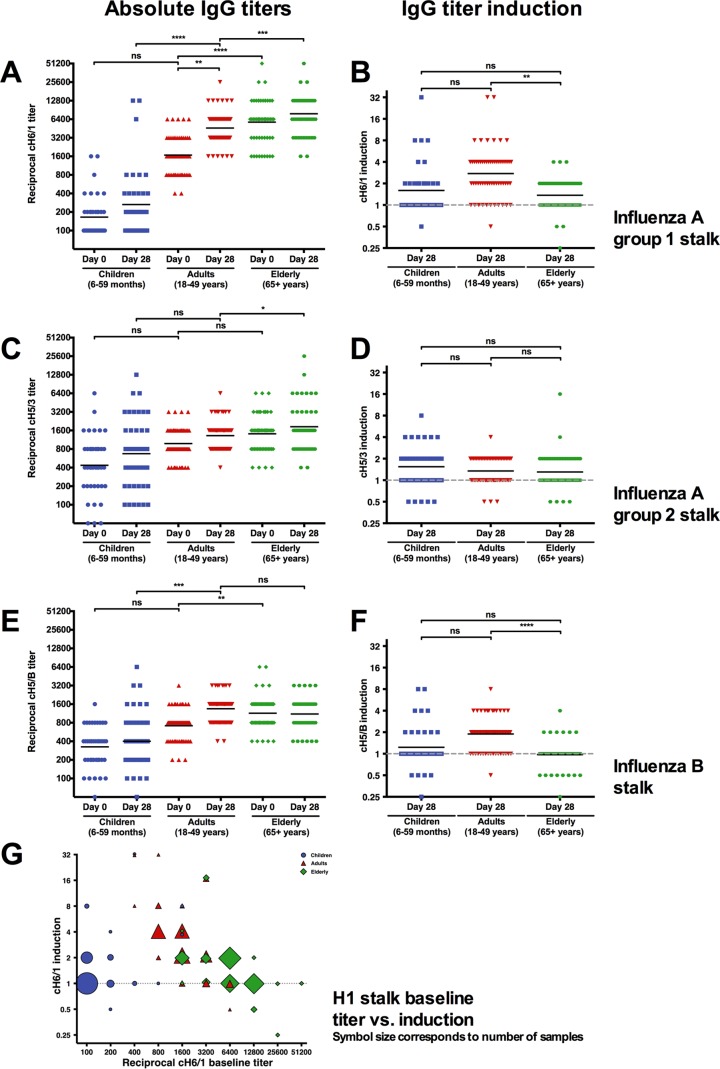
A recent report has shown that the titer of antistalk antibodies can rise over time in individuals (30). Here we analyze baseline and postvaccination serum samples of children (6 to 59 months), adults (18 to 49 years), and elderly individuals (≥65 years) who participated in various clinical trials conducted with Flublok (manufactured by Protein Sciences Corporation), a novel recombinant-HA (rHA)-based vaccine (28, 29, 31). Recombinant cHA proteins consisting of exotic head domains (avian origin H5 and H6 heads) and stalk domains of H1, H3, or influenza B virus HAs were used to analyze the immune response to the HA stalk domain. Humans are usually naive to these exotic head domains, and we have previously shown that cHA constructs can be used to measure stalk-reactive antibodies in human serum without interference from head-reactive antibodies (27, 32, 33). Group 1 HA/H1 baseline stalk titers increased significantly with age from geometric mean titers of 165.3 in children to 1674.4 in adults and 5740.6 in the elderly (Fig. 1A). Interestingly, vaccination with rHA moderately boosted antistalk titers in adults (who had medium baseline stalk titers) 2.8-fold but boosted them less in children (low baseline titers, 1.6-fold) or the elderly (high baseline titers, 1.4-fold) (Fig. 1B). These results were in good agreement with reactivity to heterosubtypic H5 HA (also group 1) from a highly pathogenic avian H5N2 isolate (H5NX strain A/Northern pintail/Washington [WA]/40964/14) demonstrating the breadth of the antistalk response (see Fig. S1 in the supplemental material). Interestingly, group 1/H1 stalk titers correlated with hemagglutination inhibition titers in children, but this correlation was lost in elderly individuals (Fig. S2). Group 2/H3 stalk titers and B HA stalk titers followed the same trend, with significantly higher stalk titers in the elderly, moderate titers in middle-aged adults, and low titers in children. Baseline titers against group 2- and B virus-specific HA stalks were in general low in all age groups (Fig. 1C to F). Importantly, we also found that prevaccination sera of adults and the elderly competed strongly with a characterized, neutralizing antistalk antibody (Fig. S3).
FIG 1 .
Titers of antistalk antibodies are age dependent. (A) Group 1/H1 stalk antibodies increase with age. Mean baseline titers for children (165.3) are lower than for adults (1674.4) and are significantly higher in elderly individuals (5740.6, P < 0.0001). (B) Induction of group 1/H1 stalk antibodies after vaccination with a recombinant HA vaccine is highest in adults (2.7) and relatively low in children (1.6) and the elderly (1.4). (C) Group 2/H3 baseline stalk antibody levels are higher (436.2) than H1 antibodies in children but do not increase as steadily with age (adults, 981.6; elderly, 1412.3). (D) Induction of group 2/H3 stalk antibodies postvaccination is not significantly higher in children (1.5) than in adults (1.3) and elderly individuals (1.3). (E) Influenza B baseline stalk antibody titers increase with age in a manner similar to that seen with H1 and H3 stalk antibodies. They are low for children (324.9), higher for adults (714.1), and highest in elderly individuals (1139.1). (F) Influenza B stalk antibodies increase slightly in adults (1.9) after vaccination but barely for children (1.2) and not at all in elderly individuals (1.0). (G) Baseline titers for group 1/H1 stalk are plotted against fold induction after vaccination. The sizes of the symbols correspond to the numbers of samples. Induction is generally low for individuals with baseline titers lower than 800 and higher than 12,800. Most of the adult population consists of high inducers, while children show mostly low titers and low induction and elderly individuals have high titers and low induction.
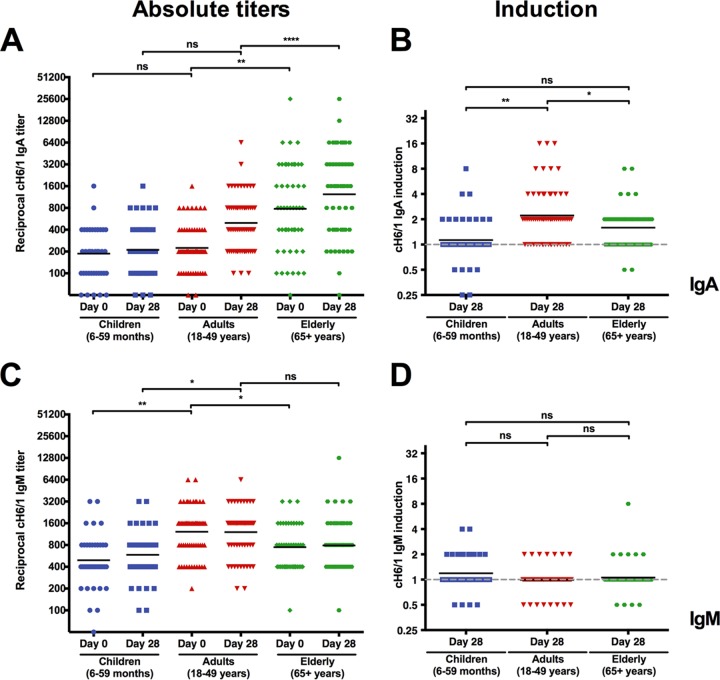
IgA, but not IgM, antibodies against the H1 stalk were increased after vaccination.
Next, we assessed whether other antibody subtypes, like IgA and IgM, followed similar trends. We were interested in the IgA response since IgA stalk antibodies have been shown to have greater neutralizing potency than IgG antistalk antibodies (34). Group 1/H1 antistalk IgA antibodies had lower baseline titers than IgG in all age groups (Fig. 2A). However, they followed the same trend as IgG, with lowest titers in children, medium titers in middle-aged adults, and the highest titers in the elderly (Fig. 2A). Interestingly, there was much more variation in the IgA baseline responses—specifically in the elderly—than in the IgG titers. Again, the response to the vaccine was best (2.2-fold induction) in the middle-aged adult group (Fig. 2B). IgM baseline titers were higher than IgA baseline titers (albeit lower than IgG baseline titers). However, IgM titers 28 days postvaccination were not significantly different from baseline titers (Fig. 2C and D). This phenomenon might be explained by the late sampling time point, which might have missed the IgM peak. Alternatively, it might also indicate that stalk responses originate from memory B cell pools and are not necessarily the result of de novo responses by naive B cells.
FIG 2 .
IgA but not IgM anti-group 1/H1 stalk titers are induced postvaccination. (A) IgA baseline titers against the H1 stalk domain follow a pattern similar to (but lower than) that shown for IgG titers. Children start at a low titer (186.6), while adults show slightly higher titers (224.1) and elderly individuals have the highest titers (778.5). (B) Adults show the strongest induction for IgA against the H1 stalk domain (2.2), while children remain close to baseline (1.1) and elderly individuals increase slightly (1.6). (C) IgM titers against the H1 stalk domain are higher in adults (1,218.1) than in children (492.5) and elderly individuals (746.4). (D) No induction of IgM is seen in adults and elderly individuals 28 days after vaccination and very low induction is seen in children (1.2).
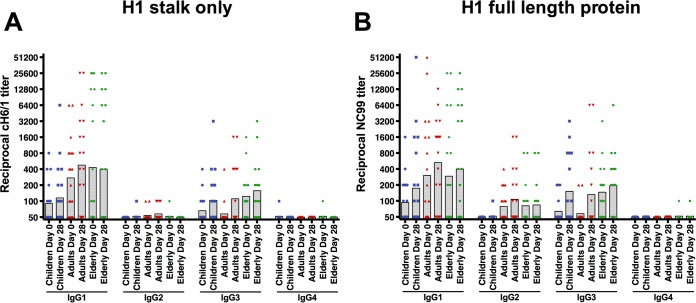
IgG titers against the H1 stalk consist predominantly of IgG1 and IgG3 and not IgG2 or IgG4.
In addition to analyzing the response to different immunoglobulin subtypes, we examined the different IgG isotypes that exist in humans. We randomly selected 20 individuals for each age group and tested their sera for all 4 IgG subclasses against the H1 stalk, as well as reactivity against a full-length H1 HA (A/New Caledonia/20/1999 virus). Both pre- and postvaccination titers against the HA stalk were driven by IgG1 and IgG3. IgG2 and IgG4 titers were mostly below the limit of detection and were also not boosted by vaccination (Fig. 3A). Similarly, the response against full-length H1 HA was mostly driven by IgG1 and IgG3. However, we also detected IgG2 titers—a clear difference from the antibody response against the HA stalk (Fig. 3B). These findings are consistent with earlier studies that showed that antibody responses against the HA are dominated by IgG1 and IgG3 (35) in healthy adults.
FIG 3 .
Isotype composition of the group 1/H1 antistalk response. (A) IgG antibodies against the group 1/H1 stalk domain are predominantly of the IgG1 and IgG3 subclasses, while there is very low reactivity for IgG2 and no reactivity for IgG4. (B) Responses against the full-length H1 protein used in the vaccine. Interestingly, adults and the elderly seem to have low IgG2 reactivity to the H1 head domain.
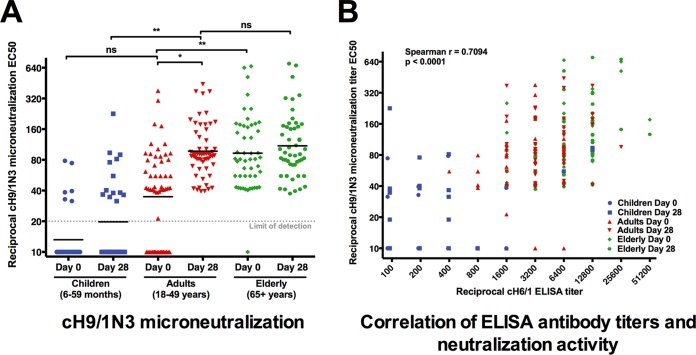
H1 stalk-reactive antibody titers measured by ELISA correlate with in vitro neutralization.
To assess the in vitro functionality of anti-group 1 stalk-reactive antibodies, we performed a microneutralization assay with a virus that expresses a cH9/1 HA and an N3 neuraminidase (NA). The stalk domain of this virus is derived from H1, but both the head domain and the neuraminidase are of avian origin and will not be bound by sera from humans who are naive to H9 and N3 (27, 32). Therefore, the assay measures only the neutralizing activities of group 1/H1 stalk-reactive antibodies. We found that neutralization baseline titers were lowest in children (geometric mean titer, 13.2), followed by adults (34.8) and the elderly (93.0) (Fig. 4A). Induction of neutralization titers postvaccination was highest in middle-aged individuals. These results reflect the titers measured by enzyme-linked immunosorbent assay (ELISA). A correlation analysis showed that there was in fact good correlation between antistalk ELISA titers and anti-chimeric H9/1N3 (anti-cH9/1N3) neutralization titers (Fig. 4B).
FIG 4 .
Antistalk neutralization titers. (A) Neutralizing titers against a virus with an H1 stalk domain show a pattern of age-related titer increases similar to that for the ELISA titers. The mean of the IC50 values is very low in children (13.2) but increases after vaccination (19.7). The adults start with medium titers (34.8) and increase to a titer of 97.3. Elderly individuals start at a titer of 93.0 and increase slightly to a titer of 109.8 after vaccination. (B) The microneutralization IC50s against a virus containing the H1 stalk domain correlate very well with the H1 titers measured by ELISA (Spearman r = 0.7094, P < 0.0001).
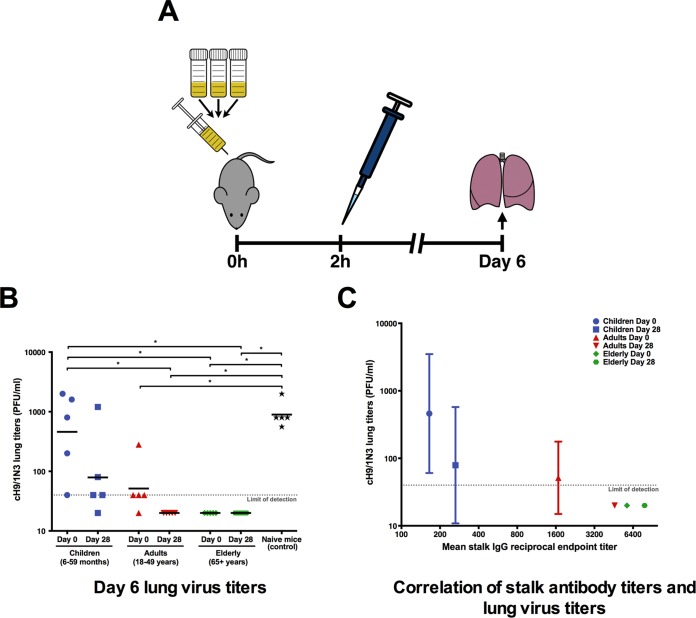
H1 stalk-reactive antibodies are protective in an in vivo mouse challenge model.
To test the in vivo functionality and protection provided by group 1/H1 stalk-reactive antibodies in humans, a passive serum transfer challenge experiment was performed with BALB/c mice. Pools of prevaccination and postvaccination serum samples were generated separately for all age groups. The serum was then intraperitoneally injected into five mice per serum pool. Two hours after serum transfer, the mice were challenged with 9,000 PFU of cH9/1N3 virus. As discussed above, this virus has an exotic HA head domain (H9) and an irrelevant neuraminidase. Therefore, a protective effect by these human sera can be attributed to antibodies that react with the H1 stalk domain. Six days postchallenge, the mouse lungs were extracted and homogenized, and the amount of remaining infectious virus particles was measured in a plaque assay. Interestingly, the virus titers on day 6 postchallenge were inversely correlated to the stalk-reactive antibody titers measured by ELISA (Fig. 5). Children had the lowest baseline stalk antibody titers, and mice that received this serum had the highest remaining infectious virus particles in their lungs (Fig. 5). Furthermore, the small increase in antibodies postvaccination had a big impact in the in vivo experiment and lowered the mean virus titer from 459 PFU to 79 PFU. Mice receiving the adult prevaccination serum had a mean virus titer of 51 PFU, but there was no detectable infectious virus in the lungs of the adult postvaccination group. Mice receiving serum from elderly individuals had no detectable virus levels for both the pre- and postvaccination pools (Fig. 5). While the virus dose used was nonlethal and did not induce significant weight loss in the control mice, these results show that antistalk titers measured by ELISA or by microneutralization assay exhibit significant biological activity in an in vivo assay in a dose-dependent manner.
FIG 5 .
In vivo efficacy of antistalk antibodies. (A) Day 0 and day 28 serum samples were pooled separately for each age group and intraperitoneally injected into five 6- to 8-week-old BALB/c mice for each pool. Two hours later, mice where challenged with the cH9/1N3 virus. Six days later, lungs were harvested and virus titers in lungs were measured. (B) The virus titers in the lungs of mice that received the children’s prevaccination serum were almost as high as in the naive challenged mice. The titers were lower in the mice receiving the postvaccination children’s serum. There was virus detectable in the mice that received adult prevaccination sera but not in those receiving adult postvaccination sera. Both the pre- and the postvaccination sera of the elderly individuals were protective in mice. (B) The virus lung titers negatively correlate with the IgG titers against the H1 stalk.
DISCUSSION
A majority of the human population has low titers of antibodies with specificity to the HA stalk domains. These antibody titers are most likely generated by natural infection (27, 36–38) or exposure to very divergent influenza virus vaccines (32, 33, 39, 40). Regular seasonal inactivated influenza virus vaccines usually do not induce these types of antibodies at significant levels (6–8). While strain-specific antihead antibodies might have higher neutralizing potency than stalk-reactive antibodies in vitro (34, 41), it has been shown that stalk-reactive antibodies can confer robust protection in vivo (11, 14, 15, 41, 42). In our cross-sectional study, we found that children (6 to 59 months) had the lowest baseline titers of stalk-reactive antibodies. Middle-aged individuals (18 to 49 years) had intermediate baseline titers, and elderly individuals (≥65) had relatively high titers of stalk-reactive antibodies. Our findings correlate very well with a recent longitudinal study that found an increase in antistalk titers in individuals over time (30). The trends were similar for group 1, group 2, and influenza B HA stalk antibodies. However, group 1 stalk titers were in general higher than group 2 antibody or B virus antistalk titers. Again, this is not surprising since humans have been repeatedly exposed to divergent group 1 HAs (H1N1, H2N2, and pandemic H1N1 viruses and potentially also the 1976 H1N1 swine influenza virus vaccine). It is known that sequential exposure to divergent influenza HAs from the same group is able to boost stalk-reactive antibodies (27, 30, 32, 33, 38–40, 43, 44). In contrast, only one group 2 HA virus (H3N2) has been circulating in humans in the last 100 years. For influenza B viruses, the situation is more complex, since two divergent lineages cocirculate. However, these two lineages express very similar HA proteins with a maximum amino acid divergence of approximately 10%. This small difference is most likely not enough to efficiently drive antistalk antibody induction.
Influenza viruses are the cause of an estimated 5 million cases of severe illness and 500,000 deaths annually worldwide. The majority of influenza deaths, up to 90%, are within the population older than 65 years (45). Importantly, this high influenza-related mortality rate in the elderly is not reflective of the overall infection attack rates. Influenza viruses circulate predominantly in children, less so in adults, and to only a very low extent in the elderly population (46–48). A recent study in the Netherlands determined the H1N1 attack rates in children to be 35%, in 20- to 39-year-old individuals to be 6.6%, and in ≥40-year-old individuals to be 2.8% (48). This means that elderly people are not likely to become ill with influenza virus infections, but if they do, they have a high risk of mortality (48), mostly due to immuno-senescence and a higher incidence of comorbidities in this age group. Our current study provides a possible explanation for the reduced attack rates in the elderly population. Attack rates in the elderly are low for H1N1, and the rates for H3N2 are moderate (46, 47, 49). Baseline titers of antistalk antibodies against the group 1 stalk (e.g., H1) are high in the elderly populations, while stalk titers against group 2 (e.g., H3) are comparatively lower. Stalk titers and attack rates inversely correlate, and high titers of antistalk antibodies in the elderly population may be one of the factors that provide protection and contribute to lower attack rates. Stalk antibodies against influenza B virus HA are also higher in the elderly, and the attack rate of influenza B virus declines with age (46). As an example, a study by Monto and Sullivan showed that infection rates during an influenza B outbreak were 28 to 35% in children (5 to 14 years) but were much lower in adults (20 to 59 years, 2.5 to 5.2%) and the elderly (≥60 years, 3.1%) (47). However, many other factors might influence the relative protection of the elderly population. The remaining question is whether elderly individuals who get infected with influenza viruses are the ones who have lower-than-average levels of stalk antibodies or whether there might be other confounding factors, like immuno-senescence or a higher frequency of underlying disease, that will cause this population cohort to get sick. An alternative explanation may be that IgA stalk titers are important, and these show much higher variation than IgG titers (although they follow the same trend as IgG in terms of age). Individuals with low titers of IgA antistalk antibodies may have a higher risk of contracting a severe influenza virus infection. In contrast to the elderly, children have low baseline stalk titers and also show the highest attack rates. Vaccination of children with a vaccine that induces high antistalk titers might reduce the circulation of the virus in that age group and would most likely indirectly protect the elderly as well (50, 51). Importantly, a strategy in which individuals are vaccinated at a young age to induce high and long-lasting antistalk immune responses would also circumvent the problem of low seroconversion rates in immuno-senescent elderly vaccinees. If individuals maintain their protective titers over time, they will not have to be vaccinated at a later point in their life, when their response to the vaccine would already be suboptimal.
We have shown that the measured antistalk antibodies are functional in vitro, they neutralize virus, and they are also protective in vivo. Importantly, ELISA stalk titers, neutralizing activity, and in vivo protection correlate well. The majority of the induced antistalk antibodies were IgG and IgA antibodies. IgM levels were relatively low and did not—in contrast to IgG and IgA levels—increase with age. IgG1 and IgG3 represented the majority of the stalk-reactive IgG response and IgG2 and IgG4 were mostly below the limit of detection. Interestingly, a portion of the study subjects mounted an IgG2 antibody response against full-length NC99 H1 HA, while such a response was absent for the HA stalk. Furthermore, middle-aged individuals had the best stalk response to the vaccine, while children (low stalk titers) and the elderly (high stalk titers) showed very low induction. It is likely that a solid priming for the stalk domain enhances the ability of the immune system to boost an antistalk response. This priming might be absent in children. In contrast, the lack of a boost in the elderly might be a sign of immuno-senescence. The other possibility is that the ceiling for an antistalk response is already reached. However, anti-group 2 and anti-influenza B HA stalk titers were significantly lower than anti-group 1 stalk titers, making this possibility very unlikely. Interestingly, there was no IgM response to the vaccine in any age group, but it is unlikely that the peak of the IgM response was captured on day 28 postvaccination.
It was surprising that middle-aged individuals mounted a moderately strong response against the stalk domain upon vaccination with a seasonal influenza virus vaccine. A possible explanation may be the nature of the vaccine used in this study. The formulation contained highly purified influenza virus HA protein at a dose three times higher than that in the standard vaccine dose (29). First, the stalk domain might be more exposed in purified HA vaccines than in split-virion vaccines, and this might facilitate interactions between antigen and B cell receptors. Second, the higher vaccine dose given might have enhanced the stalk response as well. Third, the recombinant HA was produced in insect cells, and these cells attach smaller N-linked glycans to proteins than do mammalian cells or avian cells. It has been shown that smaller glycans on HA antigens correlate with a broader immune response, possibly due to a more accessible stalk domain (2, 52–54). All three factors might have contributed to the observed effect.
Finally, these findings are of importance for the development of a stalk-based universal influenza virus vaccine. Many stalk-based vaccine approaches build on preexisting immunity (55, 56). The presence of baseline antistalk immunity present in the middle-aged population suggests that these individuals have been primed and that their antistalk antibodies can potentially be boosted with the right vaccine. In contrast, children might need a prime vaccination with, e.g., a live attenuated influenza virus vaccine before administering a stalk-based vaccine.
In conclusion, we find that stalk-reactive antibody levels increase with age, are protective in vitro and in vivo, and might be associated with lower influenza virus attack rates in elderly individuals. Our findings warrant further investigation of antistalk antibody titers as a possible correlate of protection.
MATERIALS AND METHODS
Cells, viruses, and proteins.
Madin Darby canine kidney (MDCK) cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco). DMEM was supplemented with fetal bovine serum (FBS, 10%; HyClone) and a penicillin-streptomycin antibiotics mix (100 U/ml of penicillin, 100 µg/ml streptomycin; Gibco). BTI-TN5B1-4 cells were propagated in serum-free SFX medium (HyClone) in the presence of antibiotics (100 U/ml of penicillin, 100 µg/ml streptomycin; Gibco). The cH9/1N3 virus was grown in 10-day-old embryonated eggs (Charles River Laboratories) as described before (27, 32), and the titer was determined on MDCK cells. This virus features an H9 globular head domain from influenza A/guinea fowl/Hong Kong/WF10/99 virus combined with an H1 stalk domain from A/Puerto Rico/8/34 virus (PR8), an N3 NA from A/swine/Missouri/4296424/06 virus, and the internal genes from PR8. Recombinant proteins—including H1 from A/New Caledonia/20/99 virus, H5 from A/Northern pintail/WA/40964/14 virus, cH6/1 HA (H6 head from A/mallard/Sweden/81/02 virus combined with an H1 PR8 stalk), cH5/3 HA (H5 head from A/Vietnam/1203/1204 virus combined with an H3 A/Perth/16/09 stalk) and cH5/B HA (H5 head from A/Vietnam/1203/1204 virus with the stalk domain from the B/Florida/04/06 HA)—were expressed in the baculovirus expression system as described before (8, 17, 57, 58).
Human serum samples.
Human serum samples were provided by Protein Sciences Corporation from trivalent recombinant seasonal influenza HA vaccine trials. The trials were randomized, double-blind, multicenter trials comparing levels of safety and immunogenicity of Flublok recombinant influenza vaccine versus inactivated influenza vaccine or a placebo. All participants received a total of 135 µg of recombinant HA (45 µg per strain). The details for the clinical trials can be found on ClinicalTrials.gov under the following identifiers: NCT00336453 (children, 6 to 59 months, 40 individuals were included [59]), NCT00539981 (adults, 18 to 49 years, 61 individuals [60]), NCT00395174 (elderly, ≥65 years, 51 individuals [61]). The youngest cohort included 40 children between 6 and 59 months of age (mean, 29 months; 40% female and 60% male), the middle cohort included 61 adults between 18 and 49 years of age (mean, 34 years; 57% female and 43% male), and the oldest cohort included 51 adults 65 years of age and older (mean, 73 years; 55% female and 45% male). Additional information is shown in Table S1 in the supplemental material. Serum samples before vaccination (baseline) and 28 days after vaccination were retrieved sequentially by subject identification number, dependent on the availability of both time points, and analyzed at the Icahn School of Medicine at Mount Sinai, NY, under the Sinai exempt HS number 15-00126 (not a human research determination).
ELISA.
Microtiter plates (96 wells) were coated with 50 µl recombinant protein per well diluted in carbonate buffer (0.1 M Na2CO3-NaHCO3, pH 9.4, 50 µl/well) at a concentration of 2 µg/ml overnight at 2 to 5°C. The next day, plates were blocked with phosphate-buffered saline containing 0.1% Tween 20 (PBS-T), 3% goat serum (Life Technologies), and 0.5% milk powder (blocking solution) for 1 h at room temperature. Serum samples were diluted to a 1:100 starting concentration, followed by 2-fold serial dilutions in blocking solution. After a 2-h incubation, the plates were washed 3 times with PBS-T and 50 µl of secondary antibody diluted in blocking solution added to each well. After 1 h, plates were washed 4 times with PBS-T. Plates were developed for 10 min with SigmaFast o-phenylenediamine dihydrochloride (OPD) (Sigma), and then the reaction was stopped with 3 M hydrochloric acid. Plates were read at an optical density (OD) at 490 nm, and data were analyzed in Microsoft Excel. Endpoint titers were determined when the reactivity of the diluted sample reached background levels. The following secondary antibodies were used: anti-human IgG (Fab specific)-horseradish peroxidase (HRP) antibody (Sigma A0293, 1:3,000), anti-human IgA (α-chain-specific)-HRP antibody (Sigma A0295, 1:3,000), anti-human IgM (μ-chain-specific)-HRP antibody (Sigma A6907, 1:3,000), anti-human IgG1 Fc-HRP (SouthernBiotech 9054-05, 1:3,000), anti-human IgG2 Fc-HRP (SouthernBiotech 9060-05, 1:3,000), anti-human IgG3hinge-HRP (SouthernBiotech 9210-05, 1:3,000), and anti-human IgG4 Fc-HRP (SouthernBiotech 9200-05, 1:10,000). The complete set of serum samples was analyzed in group 1, group 2, and group B. To find differences in IgG subtype specificities of antihemagglutinin head and stalk antibodies, a subset of 20 individuals of each age group were randomly selected and tested for all IgG subtypes against H1 stalk and a full-length H1 protein. For competition ELISAs, we followed the protocol described above, with the modification that plates were incubated after the initial blocking step with murine stalk MAb KB2 (26, 62) as the competitor (100 µl/well at 10 µg/ml) for 1 h. Plates with and without KB2 competition were run side by side, and the areas under the curve were compared.
Microneutralization assay.
Serum samples with sufficient residual volume for analysis (33 children, 55 adults, 49 elderly individuals) were inactivated by treatment with receptor-destroying enzyme (RDE; Denka Seiken) for 18 h at 37°C. Sodium citrate was added, and RDE was inactivated by heating the solution to 56°C for 30 min. Inactivated serum samples were 2-fold diluted in minimal essential medium with tosyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (infection medium) at a concentration of 1 µg/ml in 96-well cell culture plates. Chimeric 9/1N3 virus was diluted to a concentration of 100 PFU per 50 µl in infection medium. Fifty microliters of diluted sera was incubated with 50 µl of virus for 1 h at room temperature. MDCK cells were washed once with PBS, and 100 µl of serum-virus mixture was added onto cells. Cells were incubated at 37°C for 1 h, washed once with PBS, and 50 µl of diluted serum and 50 µl of infection medium were added to each well. Infected cells were incubated for 48 h at 37°C and washed with PBS, and the reaction was stopped with ice-cold 80% acetone. Cells were washed three times with PBS-T and incubated for 30 min with 3% hydrogen peroxide. Hydrogen peroxide was replaced with blocking solution (PBS-T plus 3% milk powder), and cells were blocked for 30 min. Cells were incubated for 1 h at room temperature with 50 µl of blocking solution containing a biotinylated anti-NP antibody (Millipore; MAB8257, 1:2,000). Plates were washed three times with PBS-T and incubated with 50 µl of blocking solution containing HRP-labeled streptavidin (Millipore; 18-152, 1:5,000) for 1 h. Plates were washed three times with PBS-T and developed with 100 µl of SigmaFast OPD for 30 min. Developing was stopped with 50 µl of 3 M hydrochloric acid, and plates were read at an OD of 490 nm. Fifty-percent inhibitory concentrations (IC50s) were calculated in GraphPad Prism.
Passive-transfer challenge experiments in mice.
Pre- and postvaccination serum samples were pooled for all age groups separately (from all available serum samples per cohort), and 250 µl of serum was intraperitoneally transferred into 6- to 8-week-old female BALB/c mice (n = 5 per group) per serum pool. Two hours later, mice were challenged with 9,000 PFU of cH9/1N3 virus (an approximately 0.1 50% lethal dose [LD50]), and their lungs were harvested on day 6 postchallenge. The lungs were homogenized and spun down, and the supernatant was aliquoted and frozen at −80°C. Virus titers were determined in a plaque assay on MDCK cells as previously described (32). All procedures were performed in accordance with the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee guidelines.
Statistical analysis.
Statistical analysis was performed in GraphPad Prism. Age groups were compared in a one-way analysis of variance (ANOVA) with Tukey multiple-comparison tests. Correlation analysis was performed with a Spearman correlation test. Data are presented as geometric means. Competition ELISA results were compared using a paired t test. Significance is indicated as follows: not significant (ns), P > 0.05; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; and ****, P ≤ 0.0001.
SUPPLEMENTAL MATERIAL
Age, gender, and HAI titers of study participants.
Titers of anti-H5 antibodies are age dependent and in good agreement with results from group 1 stalk ELISAs. (A) Anti-H5 antibodies increase with age. Mean baseline titers for children are lower than for adults and are significantly higher in elderly individuals. (B) Induction of H5 antibodies after vaccination with a recombinant HA vaccine is highest in adults and relatively low in children and the elderly. These data are in good agreement with the antistalk titers shown in Fig. 1, for which an H1 stalk was used as the substrate. Both H1 and H5 belong phylogenetically to group 1 HAs. The recombinant H5 HA used in this assay is from H5N2 strain A/Northern pintail/WA/40964/14, a highly pathogenic H5NX isolate. Download
Correlation of group 1 antistalk titers and HA inhibition (HAI) titers. The correlations for children (A), for adults (B), and for the elderly (C) are shown. While antistalk titers and HAI titers correlate well for children, no correlation is observed in the elderly. Adults show an intermediate phenotype. Download
Stalk-reactive antibody KB2 competes with prevaccination sera from adults (A) and the elderly (B) for stalk binding. The data are shown as areas under the curve. Download
ACKNOWLEDGMENTS
We thank Irina Margine for generating preliminary data for this project and Ariana Hirsh for excellent technical assistance.
Funding was provided in part by the NIH Centers of Excellence in Influenza Virus Research and Surveillance (CEIRS; contract no. HHSN272201400008C to F.K. and P.P.) and grant U19 AI109946-01 (to F.K. and P.P.).
Footnotes
Citation Nachbagauer R, Choi A, Izikson R, Cox MM, Palese P, Krammer F. 2016. Age dependence and isotype specificity of influenza virus hemagglutinin stalk-reactive antibodies in humans. 7(1):e01996-15. doi:10.1128/mBio.01996-15.
REFERENCES
- 1.Jayasundara K, Soobiah C, Thommes E, Tricco AC, Chit A. 2014. Natural attack rate of influenza in unvaccinated children and adults: a meta-regression analysis. BMC Infect Dis 14:670. doi: 10.1186/s12879-014-0670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krammer F, Palese P. 2015. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov 14:167–182. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- 3.Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, Tashkandi M, Bauch CT, Loeb M. 2013. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med 11:153. doi: 10.1186/1741-7015-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heaton NS, Sachs D, Chen CJ, Hai R, Palese P. 2013. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc Natl Acad Sci U S A 110:20248–20253. doi: 10.1073/pnas.1320524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerdil C. 2003. The annual production cycle for influenza vaccine. Vaccine 21:1776–1779. doi: 10.1016/S0264-410X(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 6.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng N, Mays I, Garman L, Helms C, James J, Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng N, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, Whitesides JF, Drinker MS, Amos JD, Gurley TC, Eudailey JA, Foulger A, DeRosa KR, Parks R, Meyerhoff RR, Yu J, Kozink DM, Barefoot BE, Ramsburg EA, Khurana S, Golding H, Vandergrift NA, Alam SM, Tomaras GD, Kepler TB, Kelsoe G, Liao HX, Haynes BF. 2011. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One 6:e25797. doi: 10.1371/journal.pone.0025797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, García-Sastre A, Palese P, Treanor JJ, Krammer F. 2013. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J Virol 87:4728–4737. doi: 10.1128/JVI.03509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Throsby M, van den Brink E, Jongeneelen M, Poon LLM, Alard P, Cornelissen L, Bakker A, Cox F, van Deventer E, Guan Y, Cinatl J, ter Meulen JT, Lasters I, Carsetti R, Peiris M, de Kruif J, Goudsmit J. 2008. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One 3:e3942. doi: 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekiert DC, Friesen RHE, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJWM, Brandenburg B, Vogels R, Brakenhoff JPJ, Kompier R, Koldijk MH, Cornelissen LAHM, Poon LLM, Peiris M, Koudstaal W, Wilson IA, Goudsmit J. 2011. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333:843–850. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry Dunand CJ, Leon PE, Kaur K, Tan GS, Zheng N, Andrews S, Huang M, Qu X, Huang Y, Salgado-Ferrer M, Ho IY, Taylor W, Hai R, Wrammert J, Ahmed R, García-Sastre A, Palese P, Krammer F, Wilson PC. 2015. Preexisting human antibodies neutralize recently emerged H7N9 influenza strains. J Clin Invest 125:1255–1268. doi: 10.1172/JCI74374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A. 2011. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 13.Friesen RHE, Lee PS, Stoop EJM, Hoffman RMB, Ekiert DC, Bhabha G, Yu W, Juraszek J, Koudstaal W, Jongeneelen M, Korse HJWM, Ophorst C, Brinkman-van der Linden ECM, Throsby M, Kwakkenbos MJ, Bakker AQ, Beaumont T, Spits H, Kwaks T, Vogels R, Ward AB, Goudsmit J, Wilson IA. 2014. A common solution to group 2 influenza virus neutralization. Proc Natl Acad Sci U S A 111:445–450. doi: 10.1073/pnas.1319058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan GS, Lee PS, Hoffman RMB, Mazel-Sanchez B, Krammer F, Leon PE, Ward AB, Wilson IA, Palese P. 2014. Characterization of a broadly neutralizing monoclonal antibody that targets the fusion domain of group 2 influenza A virus hemagglutinin. J Virol 88:13580–13592. doi: 10.1128/JVI.02289-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan GS, Krammer F, Eggink D, Kongchanagul A, Moran TM, Palese P. 2012. A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J Virol 86:6179–6188. doi: 10.1128/JVI.00469-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krammer F, Hai R, Yondola M, Tan GS, Leyva-Grado VH, Ryder AB, Miller MS, Rose JK, Palese P, García-Sastre A, Albrecht RA. 2014. Assessment of influenza virus hemagglutinin stalk-based immunity in ferrets. J Virol 88:3432–3442. doi: 10.1128/JVI.03004-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krammer F, Pica N, Hai R, Margine I, Palese P. 2013. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol 87:6542–6550. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneemann A, Speir JA, Tan GS, Khayat R, Ekiert DC, Matsuoka Y, Wilson IA. 2012. A virus-like particle that elicits cross-reactive antibodies to the conserved stem of influenza virus hemagglutinin. J Virol 86:11686–11697. doi: 10.1128/JVI.01694-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanekiyo M, Wei C, Yassine HM, McTamney PM, Boyington JC, Whittle JRR, Rao SS, Kong W, Wang L, Nabel GJ. 2013. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 499:102–106. doi: 10.1038/nature12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei CJ, Boyington JC, McTamney PM, Kong WP, Pearce MB, Xu L, Andersen H, Rao S, Tumpey TM, Yang Z-, Nabel GJ. 2010. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 329:1060–1064. doi: 10.1126/science.1192517. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Welsh JP, Swartz JR. 2014. Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc Natl Acad Sci U S A 111:125–130. doi: 10.1073/pnas.1308701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bommakanti G, Lu X, Citron MP, Najar TA, Heidecker GJ, ter Meulen J, Varadarajan R, Liang X. 2012. Design of Escherichia coli-expressed stalk domain immunogens of H1N1 hemagglutinin that protect mice from lethal challenge. J Virol 86:13434–13444. doi: 10.1128/JVI.01429-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallajosyula VVA, Citron M, Ferrara F, Lu X, Callahan C, Heidecker GJ, Sarma SP, Flynn JA, Temperton NJ, Liang X, Varadarajan R. 2014. Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc Natl Acad Sci U S A 111:E2514–E2523. doi: 10.1073/pnas.1402766111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wohlbold TJ, Nachbagauer R, Margine I, Tan GS, Hirsh A, Krammer F. 2015. Vaccination with soluble headless hemagglutinin protects mice from challenge with divergent influenza viruses. Vaccine 33:3314–3321. doi: 10.1016/j.vaccine.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steel J, Lowen AC, Wang TT, Yondola M, Gao Q, Haye K, García-Sastre A, Palese P. 2010. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio 1:e00018-10. doi: 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, Margine I, Albrecht RA, Palese P. 2012. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J Virol 86:5774–5781. doi: 10.1128/JVI.00137-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, García-Sastre A, Palese P. 2012. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A 109:2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox MMJ, Hollister JR. 2009. FluBlok, a next generation influenza vaccine manufactured in insect cells. Biologicals 37:182–189. doi: 10.1016/j.biologicals.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Yang LPH. 2013. Recombinant trivalent influenza vaccine (Flublok®): a review of its use in the prevention of seasonal influenza in adults. Drugs 73:1357–1366. doi: 10.1007/s40265-013-0103-6. [DOI] [PubMed] [Google Scholar]
- 30.Miller MS, Gardner TJ, Krammer F, Aguado LC, Tortorella D, Basler CF, Palese P. 2013. Neutralizing antibodies against previously encountered influenza virus strains increase over time: a longitudinal analysis. Sci Transl Med 5:198ra107. doi: 10.1126/scitranslmed.3006637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox MMJ, Hashimoto Y. 2011. A fast track influenza virus vaccine produced in insect cells. J Invertebr Pathol 107(Suppl):S31–S41. doi: 10.1016/j.jip.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Nachbagauer R, Wohlbold TJ, Hirsh A, Hai R, Sjursen H, Palese P, Cox RJ, Krammer F. 2014. Induction of broadly reactive anti-hemagglutinin stalk antibodies by an H5N1 vaccine in humans. J Virol 88:13260–13268. doi: 10.1128/JVI.02133-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellebedy AH, Krammer F, Li GM, Miller MS, Chiu C, Wrammert J, Chang CY, Davis CW, McCausland M, Elbein R, Edupuganti S, Spearman P, Andrews SF, Wilson PC, García-Sastre A, Mulligan MJ, Mehta AK, Palese P, Ahmed R. 2014. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc Natl Acad Sci U S A 111:13133–13138. doi: 10.1073/pnas.1414070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He W, Mullarkey CE, Duty JA, Moran TM, Palese P, Miller MS. 2015. Broadly neutralizing anti-influenza virus antibodies: enhancement of neutralizing potency in polyclonal mixtures and IgA backbones. J Virol 89:3610–3618. doi: 10.1128/JVI.03099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen GK, Höschler K, Øie Solbak SM, Bredholt G, Pathirana RD, Afsar A, Breakwell L, Nøstbakken JK, Raae AJ, Brokstad KA, Sjursen H, Zambon M, Cox RJ. 2014. Serum IgG titres, but not avidity, correlates with neutralizing antibody response after H5N1 vaccination. Vaccine 32:4550–4557. doi: 10.1016/j.vaccine.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Krammer F, Pica N, Hai R, Tan GS, Palese P. 2012. Hemagglutinin stalk-reactive antibodies are boosted following sequential infection with seasonal and pandemic H1N1 influenza virus in mice. J Virol 86:10302–10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krammer F, Margine I, Hai R, Flood A, Hirsh A, Tsvetnitsky V, Chen D, Palese P. 2014. H3 stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J Virol 88:2340–2343. doi: 10.1128/JVI.03183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O’Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. 2011. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med 208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li G-M, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng N-, Lee J-, Huang M, Qu X, Edupuganti S, Mulligan M, Das SR, Yewdell JW, Mehta AK, Wilson PC, Ahmed R. 2012. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A 109:9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halliley JL, Khurana S, Krammer F, Fitzgerald T, Coyle EM, Chung KY, Baker SF, Yang H, Martínez-Sobrido L, Treanor JJ, Subbarao K, Golding H, Topham DJ, Sangster MY. 2015. High-affinity H7 head and stalk domain-specific antibody responses to an inactivated influenza H7N7 vaccine after priming with live attenuated influenza vaccine. J Infect Dis 212:1270–1278. doi: 10.1093/infdis/jiv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dilillo DJ, Tan GS, Palese P, Ravetch JV. 2014. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat Med 20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wohlbold TJ, Chromikova V, Tan GS, Meade P, Amanat F, Comella P, Hirsh A, Krammer F. 28 October 2015. Hemagglutinin stalk- and neuraminidase-specific monoclonal antibodies protect against lethal H10N8 influenza virus infection in mice. J Virol doi: 10.1128/JVI.02275-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller MS, Tsibane T, Krammer F, Hai R, Rahmat S, Basler CF, Palese P. 2013. 1976 and 2009 H1N1 influenza virus vaccines boost anti-hemagglutinin stalk antibodies in humans. J Infect Dis 207:98–105. doi: 10.1093/infdis/jis652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sangster MY, Baer J, Santiago FW, Fitzgerald T, Ilyushina NA, Sundararajan A, Henn AD, Krammer F, Yang H, Luke CJ, Zand MS, Wright PF, Treanor JJ, Topham DJ, Subbarao K. 2013. B cell response and hemagglutinin stalk-reactive antibody production in different age cohorts following 2009 H1N1 influenza virus vaccination. Clin Vaccine Immunol 20:867–876. doi: 10.1128/CVI.00735-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 46.Monto AS, Koopman JS, Longini IM. 1985. Tecumseh study of illness. XIII. Influenza infection and disease, 1976–1981. Am J Epidemiol 121:811–822. [DOI] [PubMed] [Google Scholar]
- 47.Monto AS, Sullivan KM. 1993. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect 110:145–160. doi: 10.1017/S0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steens A, Waaijenborg S, Teunis PFM, Reimerink JHJ, Meijer A, van der Lubben M, Koopmans M, van der Sande MAB, Wallinga J, van Boven M. 2011. Age-dependent patterns of infection and severity explaining the low impact of 2009 influenza A (H1N1): evidence from serial serologic surveys in the Netherlands. Am J Epidemiol 174:1307–1315. doi: 10.1093/aje/kwr245. [DOI] [PubMed] [Google Scholar]
- 49.Bedford T, Riley S, Barr IG, Broor S, Chadha M, Cox NJ, Daniels RS, Gunasekaran CP, Hurt AC, Kelso A, Klimov A, Lewis NS, Li X, McCauley JW, Odagiri T, Potdar V, Rambaut A, Shu Y, Skepner E, Smith DJ, Suchard MA, Tashiro M, Wang D, Xu X, Lemey P, Russell CA. 2015. Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature 523:217–220. doi: 10.1038/nature14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halloran ME, Longini IM. 2006. Public health. Community studies for vaccinating schoolchildren against influenza. Science 311:615–616. doi: 10.1126/science.1122143. [DOI] [PubMed] [Google Scholar]
- 51.Longini IM. 2012. A theoretic framework to consider the effect of immunizing schoolchildren against influenza: implications for research. Pediatrics 129(Suppl 2):S63–S67. doi: 10.1542/peds.2011-0737D. [DOI] [PubMed] [Google Scholar]
- 52.Magadán JG, Khurana S, Das SR, Frank GM, Stevens J, Golding H, Bennink JR, Yewdell JW. 2013. Influenza A virus hemagglutinin trimerization completes monomer folding and antigenicity. J Virol 87:9742–9753. doi: 10.1128/JVI.00471-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J-R, Yu Y-H, Tseng Y-C, Chiang W-L, Chiang M-F, Ko Y-A, Chiu Y-K, Ma H-H, Wu C-Y, Jan J-T, Lin K-I, Ma C, Wong C-H. 2014. Vaccination of monoglycosylated hemagglutinin induces cross-strain protection against influenza virus infections. Proc Natl Acad Sci U S A 111:2476–2481. doi: 10.1073/pnas.1323954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C-C, Chen J-R, Tseng Y-C, Hsu C-H, Hung Y-F, Chen S-W, Chen C-M, Khoo K-H, Cheng T-J, Cheng Y-SE, Jan J-T, Wu C-Y, Ma C, Wong C-H. 2009. Glycans on influenza hemagglutinin affect receptor binding and immune response. Proc Natl Acad Sci U S A 106:18137–18142. doi: 10.1073/pnas.0909696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krammer F, Palese P. 2013. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol 3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krammer F, Palese P. 2014. Universal influenza virus vaccines: need for clinical trials. Nat Immunol 15:3–5. doi: 10.1038/ni.2761. [DOI] [PubMed] [Google Scholar]
- 57.Krammer F, Margine I, Tan GS, Pica N, Krause JC, Palese P. 2012. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS One 7:e43603. doi: 10.1371/journal.pone.0043603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Margine I, Palese P, Krammer F. 2013. Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the baculovirus expression system. J Vis Exp 81:e51112. doi: 10.3791/51112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.King JC, Cox MM, Reisinger K, Hedrick J, Graham I, Patriarca P. 2009. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy children aged 6–59 months. Vaccine 27:6589–6594. doi: 10.1016/j.vaccine.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 60.Treanor JJ, El Sahly H, King J, Graham I, Izikson R, Kohberger R, Patriarca P, Cox M. 2011. Protective efficacy of a trivalent recombinant hemagglutinin protein vaccine (FluBlok®) against influenza in healthy adults: a randomized, placebo-controlled trial. Vaccine 29:7733–7739. [DOI] [PubMed] [Google Scholar]
- 61.Keitel WA, Treanor JJ, El Sahly HM, Gilbert A, Meyer AL, Patriarca PA, Cox MM. 2009. Comparative immunogenicity of recombinant influenza hemagglutinin (rHA) and trivalent inactivated vaccine (TIV) among persons ≥65 years old. Vaccine 28:379–385. doi: 10.1016/j.vaccine.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 62.Heaton NS, Leyva-Grado VH, Tan GS, Eggink D, Hai R, Palese P. 2013. In vivo bioluminescent imaging of influenza A virus infection and characterization of novel cross-protective monoclonal antibodies. J Virol 87:8272–8281. doi: 10.1128/JVI.00969-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Age, gender, and HAI titers of study participants.
Titers of anti-H5 antibodies are age dependent and in good agreement with results from group 1 stalk ELISAs. (A) Anti-H5 antibodies increase with age. Mean baseline titers for children are lower than for adults and are significantly higher in elderly individuals. (B) Induction of H5 antibodies after vaccination with a recombinant HA vaccine is highest in adults and relatively low in children and the elderly. These data are in good agreement with the antistalk titers shown in Fig. 1, for which an H1 stalk was used as the substrate. Both H1 and H5 belong phylogenetically to group 1 HAs. The recombinant H5 HA used in this assay is from H5N2 strain A/Northern pintail/WA/40964/14, a highly pathogenic H5NX isolate. Download
Correlation of group 1 antistalk titers and HA inhibition (HAI) titers. The correlations for children (A), for adults (B), and for the elderly (C) are shown. While antistalk titers and HAI titers correlate well for children, no correlation is observed in the elderly. Adults show an intermediate phenotype. Download
Stalk-reactive antibody KB2 competes with prevaccination sera from adults (A) and the elderly (B) for stalk binding. The data are shown as areas under the curve. Download