ABSTRACT
Recent studies suggest small intestine bacterial overgrowth (SIBO) is common among developing world children. SIBO’s pathogenesis and effect in the developing world are unclear. Our objective was to determine the prevalence of SIBO in Bangladeshi children and its association with malnutrition. Secondary objectives included determination of SIBO’s association with sanitation, diarrheal disease, and environmental enteropathy. We performed a cross-sectional analysis of 90 Bangladeshi 2-year-olds monitored since birth from an impoverished neighborhood. SIBO was diagnosed via glucose hydrogen breath testing, with a cutoff of a 12-ppm increase over baseline used for SIBO positivity. Multivariable logistic regression was performed to investigate SIBO predictors. Differences in concomitant inflammation and permeability between SIBO-positive and -negative children were compared with multiple comparison adjustment. A total of 16.7% (15/90) of the children had SIBO. The strongest predictors of SIBO were decreased length-for-age Z score since birth (odds ratio [OR], 0.13; 95% confidence interval [CI], 0.03 to 0.60) and an open sewer outside the home (OR, 4.78; 95% CI, 1.06 to 21.62). Recent or frequent diarrheal disease did not predict SIBO. The markers of intestinal inflammation fecal Reg 1β (116.8 versus 65.6 µg/ml; P = 0.02) and fecal calprotectin (1,834.6 versus 766.7 µg/g; P = 0.004) were elevated in SIBO-positive children. Measures of intestinal permeability and systemic inflammation did not differ between the groups. These findings suggest linear growth faltering and poor sanitation are associated with SIBO independently of recent or frequent diarrheal disease. SIBO is associated with intestinal inflammation but not increased permeability or systemic inflammation.
IMPORTANCE
A total of 165 million children worldwide are considered stunted, which is associated with increased risk of death prior to age 5 years and cognitive disability. Stunting has, in part, been attributed to the presence of environmental enteropathy. Environmental enteropathy is a poorly understood condition leading to chronic intestinal inflammation. It has been postulated that small intestine bacterial overgrowth contributes to the pathogenesis of environmental enteropathy as overgrowth has been associated with intestinal inflammation and micronutrient malabsorption when it develops in other clinical contexts. This study confirms the finding that overgrowth occurs at high rates in the developing world. This is the first study to show that overgrowth is associated with intestinal inflammation and linear growth delay in this setting and is the first to examine why children with no known gastrointestinal dysfunction develop overgrowth from the developing world environment.
INTRODUCTION
Small intestine bacterial overgrowth (SIBO) is defined as greater than 105 CFU/ml upper intestinal aspirate as assessed by both anaerobic and aerobic cultures (1). SIBO can be measured by culture of endoscopically obtained upper gastrointestinal (GI) aspirates or noninvasively by hydrogen breath testing. Traditionally, SIBO has been considered a secondary condition that develops in the setting of altered intestinal anatomy, slowed intestinal motility, or aberrant gastrointestinal function. SIBO in this setting has been associated with poor nutritional outcomes, including steatorrhea with loss of fat-soluble vitamins (excluding vitamin K) (2–9), carbohydrate malabsorption (10–13), and a protein-losing enteropathy (14–18). Deficiencies in cobalamin (19–21), thiamine (22), riboflavin (18), pyridoxine (18), and nicotinamide (23) have been documented. SIBO has also been shown to lead to increased GI permeability (24, 25) and alteration of mucosal immunity, including an increase in IgA plasma cells and increased mucosal interleukin-6 (IL-6) (26, 27). SIBO has recently been recognized as an underdiagnosed condition in children in the developed world with gastrointestinal symptoms (28).
Recently, preliminary studies have suggested that children in the lower socioeconomic strata of developing world countries may develop SIBO at significantly higher rates than their more privileged counterparts, with a prevalence of up to 30% in slum-dwelling children (29–31). SIBO in children from low-income countries has been associated with poor carbohydrate absorption and underperformance of an oral cholera vaccine (32–34). The pathogenesis of SIBO in developing world children with no underlying intestinal pathology remains unclear. It is also unclear what role SIBO plays in environmental enteropathy (EE), an inflammatory intestinal disorder of the developing world that has been implicated in growth failure and poor neurocognitive outcomes (35–38).
In this study, we sought to test the prevalence of SIBO and its potential association with malnutrition, sanitation, and diarrheal disease. We also ought to determine SIBO’s association with concomitant gut inflammation, intestinal permeability, and systemic inflammation. It was our primary hypothesis that development of SIBO in this setting would be associated with poor nutrition, poor sanitation, and recent or frequent diarrheal episodes. Our secondary hypothesis was that SIBO was associated with intestinal inflammation, increased intestinal permeability, and systemic inflammation.
RESULTS
Enrollment characteristics.
A total of 103 children were assessed for SIBO testing. Of note, none had known chronic gastrointestinal disease. Nine children were excluded for a weight-for-age Z (WAZ) score of ≤−3 standard deviations (SD) and were referred for nutritional therapy. One parent refused testing. Three children were unable to complete testing for the 3-h test period and were excluded from analysis. A total of 90 children successfully competed SIBO testing. The average age of children tested was 24.6 months (range, 24.3 to 25.1 months). Of the children tested, 46 (51%) were female. Birth demographics of the children showed an average estimated gestational age of 37.3 weeks with an average length and weight at enrollment of 48.9 cm and 2.8 kg, respectively. Mothers had an average age of 24.5 years, and 82 (91%) considered themselves housewives. The average monthly household income was 13,913 Bangladeshi taka (approximately $179 U.S. dollars). The average number of people living per room in homes was 3.8. There was no significant difference in the enrollment characteristics between SIBO-positive and SIBO-negative children (Table 1) or between these 90 children and the 700 children in the full PROVIDE cohort (data not shown). Of note, data on estimated gestational age were not collected for the entire cohort of 700 children and thus were unavailable for comparison.
TABLE 1 .
Enrollment characteristics of the study cohort stratified by SIBO positivity
| Parameter | Result fora: |
P valueb | |
|---|---|---|---|
| SIBO negative (n = 75) | SIBO positive (n = 15) | ||
| Female, no. (%) | 37 (49) | 9 (60) | 0.58* |
| Estimated gestational age, wk | 37.3 ± 1.3 | 37.5 ± 1.4 | 0.67** |
| Characteristic at enrollment | |||
| Age, days | 5.0 ± 1.8 | 4.7 ± 1.9 | 0.53** |
| Length, cm | 48.7 ± 1.6 | 49.5 ± 1.9 | 0.11** |
| Wt, kg | 2.8 ± 0.4 | 2.9 ± 0.4 | 0.73** |
| Maternal age, yr | 24.4 ± 4.1 | 25.3 ± 4.1 | 0.40** |
| Mother as housewife, no. (%) | 67 (89) | 15 (100) | 0.34* |
| Income, taka | 13,283 ± 9,539 | 17,067 ± 12,759 | 0.94** |
| People/room living in home, no. | 3.9 ± 1.3 | 3.4 ± 0.9 | 0.24** |
Data are expressed as the mean ± SD for continuous measures and count (percentage) for discrete measures.
*, Exact Pearson chi-square test; **, Mann-Whitney U test.
SIBO was associated with growth faltering and lack of sanitation.
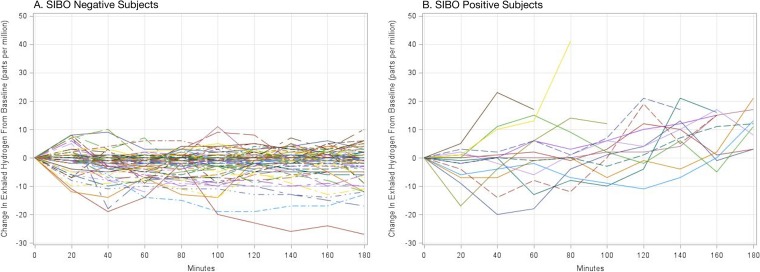
The prevalence of SIBO in our cohort was 16.7% (15/90). Figure 1 shows the glucose hydrogen breath test results from both SIBO-positive and SIBO-negative children. Children with SIBO had significantly worse linear growth (stunting) from enrollment to age 2 years compared to children without SIBO. A 1-U drop in length-for-age Z (LAZ) score from enrollment to 2 years conferred an odds ratio (OR) of 7.69 (95% confidence interval [CI], 1.67 to 33.33) for the development of SIBO.
FIG 1 .
Glucose-hydrogen breath testing results for 90 2-year-olds in Dhaka, Bangladesh, with the change in exhaled breath hydrogen from the patient’s baseline shown on the y axis and time shown on the x axis. (A) Results for small intestine bacterial overgrowth (SIBO)-negative subjects demonstrate no increase in breath hydrogen greater than 12 ppm of exhaled hydrogen over the patient’s own baseline. (B) Results for SIBO-positive subjects demonstrate a peak in exhaled breath hydrogen over the subject’s baseline by >12 ppm.
The odds of developing SIBO were increased by the presence of an open drain/sewer outside the home (OR, 4.78; 95% CI, 1.06 to 21.62). Odds of developing SIBO were also increased by the household’s drinking water being obtained from a source other than the municipal water supply and by the mother cutting her fingernails less than once a month. Because of the low number of households with a water source other than the municipal supply and the low number of mothers who did not cut their nails at least once a month (1 per group and 2 per group, respectively), the 95% CI for these OR was extremely large, making estimation of odds unreliable despite their significance.
Weight faltering (negative change in WAZ score from enrollment to 2 years), socioeconomic status (income), and diarrheal disease (number of diarrheal episodes in the child’s life or presence of a diarrheal episode in the 30 days prior to SIBO testing) were not significant predictors of SIBO (Table 2).
TABLE 2 .
Predictors of SIBO positivity at 2 years of age in Bangladeshi infants
| Parameter | Result fora: |
P value | OR (95% CI) | |
|---|---|---|---|---|
| SIBO negative (n = 75) | SIBO positive (n = 15) | |||
| Income, takab | 13,394.6 ± 9,554.5 | 17,066.7 ± 14,758.6 | 0.15 | 1.00 (0.99, 1.01) |
| Diarrheal episodes in child’s life, no. | 6.1 ± 5.0 | 4.9 ± 4.3 | 0.15 | 0.88 (0.74, 1.05) |
| At least 1 diarrheal episode in 30 days prior to SIBO testing, no. (%) | 12 (16) | 2 (13) | 0.97 | 0.97 (0.12, 7.83) |
| ΔWAZ score from enrollment to 2 yr of age | 0.03 ± 1.0 | −0.27 ± 0.9 | 0.19 | 1.99 (0.71, 5.56) |
| ΔLAZ score from enrollment to 2 yr of age | −0.36 ± 0.9 | −0.86 ± 0.7 | 0.01 | 0.13 (0.03, 0.60) |
| Presence of open drain/sewer outside home, no. (%) | 24 (32) | 8 (53) | 0.04 | 4.78 (1.06, 21.62) |
| Water source other than municipal supply, no. (%) | 1 (1) | 1 (6) | 0.01 | —c |
| Mother cuts her fingernails <1 time per mo, no. (%) | 2 (3) | 2 (13) | 0.03 | — |
Data are expressed as the mean ± SD for continuous measures and count (percentage) for discrete measures. Homer-Lemeshow goodness of fit, χ2 = 11.38 and P = 0.18.
One United States dollar = 77 to 82 Bangladeshi taka for the duration of this study.
—, insufficient sample size to report a reliable OR.
SIBO was associated with concomitant intestinal inflammation but not increased intestinal permeability or systemic inflammation.
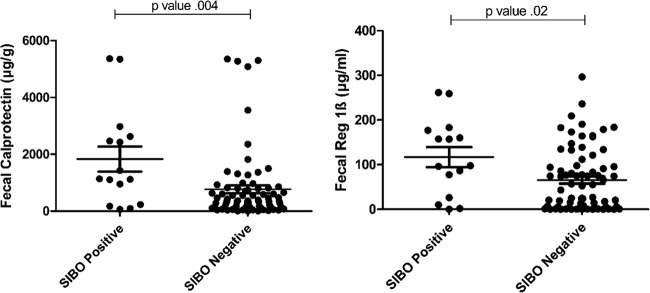
Mean fecal Reg 1β was 116.8 µg/ml in the SIBO-positive group compared to 65.6 µg/ml in SIBO-negative children (P = 0.02). Fecal calprotectin was also significantly increased in SIBO-positive children compared to the SIBO-negative group (mean of 1,834.6 versus 766.7 µg/g, respectively; P = 0.004) (Fig. 2).
FIG 2 .
Inflammatory markers in small intestine bacterial overgrowth (SIBO)-positive and -negative subjects. (A) Calprotectin is a neutrophil-derived protein that, when elevated in the stool, indicates intestinal inflammation. The mean fecal calprotectin level in SIBO-positive subjects was 1,834.6 µg/g compared to 766.7 µg/g in SIBO-negative children (P = 0.004). (B) Reg 1β is a proproliferative, antiapoptotic protein secreted by damaged epithelial cells. When elevated in the stool, it indicates damage to the intestinal epithelium. Children with SIBO had a mean Reg 1β level of 116.8 µg/ml compared to 65.6 µg/ml in SIBO-negative children (P = 0.02).
While SIBO-positive children had significantly increased markers of intestinal inflammation, there was no difference in lactose/mannitol (L/M) ratios between SIBO-positive and SIBO-negative children. Likewise, there was no difference in C-reactive protein or any of the 17 cytokines measured between the groups (data not shown). Anthropometry results at the time of SIBO testing were also not significantly different in SIBO-positive children compared to SIBO-negative children.
When the false discovery rate (FDR) correction was applied, fecal Reg 1β and fecal calprotectin remained significant, with FDR-adjusted values of 0.25 and 0.1, respectively.
DISCUSSION
Our study demonstrates that SIBO in a developing world setting is associated with growth faltering, poor sanitation, and intestinal inflammation. Poor growth and intestinal inflammation are endemic in children from low-income countries, and the importance of this work derives from its identification of SIBO as a potentially treatable contributor to these problems of child morbidity. Of note, our findings are contradictory to other investigations which found an increase in intestinal permeability in adults with SIBO in the developed world (24, 25, 39). However, patients in previous studies had underlying gastrointestinal disease that may have predisposed them to increased intestinal permeability. The lack of increased L/M ratios in SIBO is also interesting because L/M ratios were increased in previous studies of EE (38, 40). This may indicate that SIBO and EE are separate but concomitant conditions or that EE is actually a syndrome encompassing a heterogeneous group of environmentally derived intestinal inflammatory conditions with variable manifestations but common outcomes. Furthermore, even SIBO-negative children in our cohort had fecal calprotectin levels that were elevated from those reported in asymptomatic children in the developed world, suggesting that the majority of children in our study population had some degree of EE (41).
This work is the first to investigate factors that may predispose developing world children with no known gastrointestinal pathology to SIBO. The significance of growth stunting in our model demonstrates an association between linear growth delay and overgrowth independent of diarrheal disease and sanitation. However, in the absence of longitudinal analysis, the details of this association remain unclear. Given the known nutritional consequences of SIBO in other settings, it is biologically plausible that SIBO plays a causative role in growth stunting. It is also possible that a variable we did not measure in our analysis leads to both SIBO and declining LAZ and thus is acting as a confounder in our model.
We designed our regression model to investigate two competing hypotheses on why children would develop SIBO from their environment. The first hypothesis was that children develop SIBO due to lumenal stasis secondary to gastroparesis or ileus after recent or recurrent enteric infection. This is biologically plausible given that children in Bangladesh have a mean of 4.7 diarrheal episodes and a median of 3.3 enteric pathogens identified in nondiarrheal stools in the first year of life alone (42). The second hypothesis that has been suggested is that children develop a functional motility disorder with a hypoactive migrating motor complex and decreased intestinal contractility due to constant lipopolysaccharide exposure from an unsanitary environment (43). This phenomenon has been shown to occur in animal models and in women with late radiation enteropathy (44–46). Our analysis suggests that recent or frequent enteric infection does not predispose to SIBO, while measures of environmental contamination do. Although we did not test motility in this study, our results suggest this endeavor might be fruitful in future investigation.
To date, literature on environmentally derived SIBO in the developing world is sparse. It has been shown that SIBO occurs in the lower socioeconomic strata of developing world countries with increased prevalence compared to countries with greater financial means (29–31). SIBO in this setting has been shown to lead to poor carbohydrate absorption and oral vaccine underperformance (32–34). It has also been shown to have increased prevalence in severe malnutrition (47). Much of our understanding of the pathogenesis of SIBO comes from studies in the developed world in patients with underlying gastrointestinal pathology. While these studies are extremely informative, it is important to understand that the pathogenesis and pathophysiology of SIBO in developing world children with no underlying gastrointestinal disease may be different from those of patients in industrialized countries.
Our study has several important limitations. First, the natural history of overgrowth in the developing world setting is unknown. It may be present until treated as in underlying gastrointestinal disease or may have a waxing and waning course. Thus, a cross-sectional analysis that detects children with SIBO only at the time of testing may miss children who have had recent or prolonged SIBO in their lifetime. Second, we did not collect data on recent antibiotic use that may be a confounder in our analysis. Third, a cutoff of 12 ppm for the hydrogen breath test used to diagnose SIBO is based on adult studies in the developed world and may not be optimal for our pediatric and developing world study environment. Currently, there is no evidence-based cutoff for SIBO diagnosis specific to developing world children. Fourth, our sample size was too low to obtain reliable odds ratios for several of the covariates tested, despite their significance. Finally, the inability to test children with a WAZ of ≤−3 SD may introduce selection bias into our study given that we did not evaluate children with the most severe outcomes.
Strengths of this study include duplicate measures intestinal and systemic inflammation. Use of the PROVIDE database for data on predictive variables also provided for duplicate measures of sanitation. This database allowed for accurate data on all diarrheal illnesses over the course of the children’s lifetime.
Conclusion.
SIBO was observed in 2-year-old Bangladeshi children living under unsanitary conditions and was associated with malnutrition, poor sanitation, and markers of intestinal inflammation. Future study is needed to prospectively determine whether SIBO itself has a detrimental impact on growth of developing world children. This should include longitudinal study of SIBO’s natural history to better understand the burden of this disease and to identify the environmental exposures that cause SIBO. Based on this study, these efforts should focus on the role of environmental contamination’s ability to cause a functional intestinal disorder that may predispose to SIBO and the development of EE.
MATERIALS AND METHODS
Data source and design.
We conducted a cross-sectional analysis of 90 2-year-old children from a cohort of 700 children followed since birth in Dhaka, Bangladesh. All children were previously enrolled in the PROVIDE study, which was a randomized clinical trial with two vaccine interventions designed to investigate causes of oral vaccine underperformance in the developing world. The materials and methods of the PROVIDE study have been described elsewhere (48). The children assessed for SIBO testing were the final 103 children enrolled in PROVIDE. This study was approved by the Research and Ethical Review Committees of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) as well as the Institutional Review Boards of the University of Vermont and the University of Virginia.
Population and setting.
The study took place in the urban neighborhood of Mirpur in Dhaka, Bangladesh. Mirpur was settled primarily by ethnic Bihari after Bangladesh obtained its independence in 1971. The neighborhood is densely populated, with an average of 5 people living in 1.5 rooms. The average monthly household income is 12,700 taka (approximately 163 U.S. dollars). Ninety-six percent of construction is tin or mud brick. Open sewers flow throughout the area and are directly adjacent to 59% of homes. Overall, our study participants tended to come from the lowest socioeconomic strata of the Mirpur community due to the area in which recruitment occurred and the location of our study clinic.
Measures. (i) Primary outcome variable.
The primary dichotomous outcome was the presence or absence of SIBO at the 2-year-old study visit. SIBO was measured via glucose hydrogen breath testing using a QuinTron BreathTracker SC gas chromatograph (QuinTron Instrument Company, Inc., Milwaukee, WI). Patients fasted for 3 h prior to the onset of testing. Children with WAZ score of ≤−3 SD were not tested due to concern by the Research and Ethical Review Committees that fasting was unsafe in this vulnerable group. Children meeting this criteria were referred for nutritional therapy at the icddr,b nutritional rehabilitation unit. This was the only additional exclusion criteria added to those used in selection of the original PROVIDE cohort (48). Children with a WAZ score of > −3 SD were tested at baseline and then given a glucose solution of 100 g glucose in 500-ml sterile water administered at 5 ml/kg body weight. Breath was collected via the QuinTron infant bag collection system with an age-appropriate anesthesia mask attached at 20-min intervals for 3 h. CO2 was measured to ensure sampling of alveolar gas. Children were allowed water during the fast and testing but not other food or drink. Presence of SIBO was defined as an increase of at least 12 ppm in breath hydrogen over baseline at any measurement within the 3-h test period, in accordance with expert consensus based on adult patients (49).
(ii) Covariates in logistic regression to predict presence of SIBO at 2 years of age.
Covariates were selected to represent competing hypotheses on why children with no underlying gastrointestinal pathology would develop SIBO from environmental exposure. Income was included as a socioeconomic marker. The changes in LAZ and WAZ scores from enrollment to the time of SIBO testing at 2 years of age were included as markers of overall health and nutrition. The number of diarrheal episodes from birth to 2 years of age and presence of a diarrheal illness within the last 30 days were included as SIBO can develop under conditions of delayed intestinal motility, and postinfectious gastroparesis or ileus is common in children. Indicators of environmental fecal contamination included a primary drinking water source other than the municipal supply, the presence of an open drain/sewer directly outside the home, and a mother who trims her fingernails less than once a month (50). Data on covariates were collected from the PROVIDE database.
(iii) Intestinal inflammation, intestinal permeability, and systemic inflammation.
To assess intestinal inflammation, both fecal Reg 1β and fecal calprotectin were measured on the same day as SIBO testing via enzyme-linked immunosorbent assay. Reg 1β is a proproliferative antiapoptotic protein secreted by damaged intestinal epithelial cells. It has been shown to be elevated in stool in the setting of environmental enteropathy and predictive of growth failure in developing world children (51). Fecal calprotectin is a neutrophil-derived protein also shown to be elevated in states of intestinal inflammation (52, 53). Intestinal permeability was measured via a L/M ratio as assessed via urinary analysis after ingestion of a lactose-mannitol solution. L/M ratio testing was not conducted on the same day as SIBO testing to prevent interference between separate carbohydrate substrates used for the two tests but was performed within 7 days of SIBO testing. To assess systemic inflammation, C-reactive protein and a 17-plex Luminex cytokine panel (granulocyte colony-stimulating factor [G-CSF], granulocyte-macrophage colony-stimulating factor [GM-CSF], gamma interferon [IFN-γ], interleukin-1β [IL-1β], IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 [p70], IL-13, IL-17, monocyte chemoattractant protein 1 [MCP-1], macrophage inflammatory protein 1β [MIP-1β], and tumor necrosis factor alpha [TNF-α]) were conducted on plasma obtained on the day of SIBO testing. Cytokines were dichotomized to ≤50th percentile (baseline) and >50th percentile of the tested cohort for this analysis.
Statistical analysis.
The primary analysis to determine risk factors for SIBO development in the developing world was performed using multivariable logistic regression with the presence of SIBO at 2 years of age as the outcome measure. A Hosmer-Lemeshow statistic was produced for the regression model to ensure stability. A nonsignificant χ2 statistic was generated (P > 0.05), ensuring goodness of fit for our model. The secondary analysis of the difference between SIBO-positive and SIBO-negative children for the measures of inflammation and intestinal permeability was calculated via either Mann-Whitney U test or Fisher’s exact test, as appropriate. Correction for multiple comparisons was conducted via FDR calculation, with an FDR of 30% set for determination of importance. SPSS Statistics version 22 (IBM, Armonk, NY) and SAS version 9.4 (SAS Institute, Cary, NC) were used for all statistical analyses.
ACKNOWLEDGMENTS
We gratefully acknowledge the field workers and staff of the PROVIDE clinic for their conduct of the study and the families and community of Mirpur, Dhaka, Bangladesh, for participation and support.
This research was funded by grants to W.P. from the Bill and Melinda Gates Foundation and the NIH (grant 5R01 AI043596), and to J.D. from The Pediatric Scientist Development Program (5K12HD000850).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Citation Donowitz JR, Haque R, Kirkpatrick BD, Alam M, Lu M, Kabir M, Kakon SH, Islam BZ, Afreen S, Musa A, Khan SS, Colgate ER, Carmolli MP, Ma JZ, Petri WA, Jr. 2016. Small intestine bacterial overgrowth and environmental enteropathy in Bangladeshi children. mBio 7(1):e02102-15. doi:10.1128/mBio.02102-15.
REFERENCES
- 1.O’Mahony S, Shanahan F. 2010. Enteric microbiota and small intestinal bacterial overgrowth, p 1769 In Feldman M, Friedman L, Brandt L (ed), Sleisenger and Fordtran’s gastrointestinal and liver disease: pathophysiology/diagnosis/management, 9th ed. Saunders, Philadelphia, PA. [Google Scholar]
- 2.Tabaqchali S, Hatzioannou J, Booth CC. 1968. Bile-salt deconjugation and steatorrhoea in patients with the stagnant-loop syndrome. Lancet 292:12–16. doi: 10.1016/S0140-6736(68)92888-2. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg IH, Hardison WG, Bull DM. 1967. Abnormal bile-salt patterns and intestinal bacterial overgrowth associated with malabsorption. N Engl J Med 276:1391–1397. doi: 10.1056/NEJM196706222762501. [DOI] [PubMed] [Google Scholar]
- 4.Lee WB, Hamilton SM, Harris JP, Schwab IR. 2005. Ocular complications of hypovitaminosis A after bariatric surgery. Ophthalmology 112:1031–1034. doi: 10.1016/j.ophtha.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 5.Di Stefano M, Veneto G, Malservisi S, Corazza GR. 2001. Small intestine bacterial overgrowth and metabolic bone disease. Dig Dis Sci 46:1077–1082. doi: 10.1023/A:1010722314493. [DOI] [PubMed] [Google Scholar]
- 6.Schjønsby H. 1977. Osteomalacia in the stagnant loop syndrome. Acta Med Scand 603:39–41. [PubMed] [Google Scholar]
- 7.Brin MF, Fetell MR, Green PH, Kayden HJ, Hays AP, Behrens MM, Baker H. 1985. Blind loop syndrome, vitamin E malabsorption, and spinocerebellar degeneration. Neurology 35:338–342. doi: 10.1212/WNL.35.3.338. [DOI] [PubMed] [Google Scholar]
- 8.Ramotar K, Conly JM, Chubb H, Louie TJ. 1984. Production of menaquinones by intestinal anaerobes. J Infect Dis 150:213–218. doi: 10.1093/infdis/150.2.213. [DOI] [PubMed] [Google Scholar]
- 9.Conly JM, Stein K. 1992. The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog Food Nutr Sci 16:307–343. [PubMed] [Google Scholar]
- 10.Jonas A, Flanagan PR, Forstner GG. 1977. Pathogenesis of mucosal injury in the blind loop syndrome. Brush border enzyme activity and glycoprotein degradation. J Clin Invest 60:1321–1330. doi: 10.1172/JCI108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giannella RA, Rout WR, Toskes PP. 1974. Jejunal brush border injury and impaired sugar and amino acid uptake in the blind loop syndrome. Gastroenterology 67:965–974. [PubMed] [Google Scholar]
- 12.Toskes PP, Giannella RA, Jervis HR, Rout WR, Takeuchi A. 1975. Small intestinal mucosal injury in the experimental blind loop syndrome. Gastroenterology 68:1193–1203. [PubMed] [Google Scholar]
- 13.Riepe SP, Goldstein J, Alpers DH. 1980. Effect of secreted Bacteroides proteases on human intestinal brush border hydrolases. J Clin Invest 66:314–322. doi: 10.1172/JCI109859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maxwell JD, Ang L, Cleeve HJ, McGouran RC. 1982. Intestinal bypass in the rat: a model for growth failure, liver disease, and jejunal bacterial overgrowth in marasmus and kwashiorkor. J Pediatr Gastroenterol Nutr 1:417–426. doi: 10.1097/00005176-198201030-00024. [DOI] [PubMed] [Google Scholar]
- 15.Jones EA, Craigie A, Tavill AS, Franglen G, Rosenoer VM. 1968. Protein metabolism in the intestinal stagnant loop syndrome. Gut 9:466–469. doi: 10.1136/gut.9.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutgeerts L, Mainguet P, Tytgat G, Eggermont E. 1974. Enterokinase in contaminated small-bowel syndrome. Digestion 10:249–254. doi: 10.1159/000197543. [DOI] [PubMed] [Google Scholar]
- 17.King CE, Toskes PP. 1981. Protein-losing enteropathy in the human and experimental rat blind-loop syndrome. Gastroenterology 80:504–509. [PubMed] [Google Scholar]
- 18.Parlesak A, Klein B, Schecher K, Bode JC, Bode C. 2003. Prevalence of small bowel bacterial overgrowth and its association with nutrition intake in nonhospitalized older adults. J Am Geriatr Soc 51:768–773. doi: 10.1046/j.1365-2389.2003.51259.x. [DOI] [PubMed] [Google Scholar]
- 19.Brandt LJ, Bernstein LH, Wagle A. 1977. Production of vitamin B12 analogues in patients with small-bowel bacterial overgrowth. Ann Intern Med 87:546–551. [DOI] [PubMed] [Google Scholar]
- 20.Welkos SL, Toskes PP, Baer H. 1981. Importance of anaerobic bacteria in the cobalamin malabsorption of the experimental rat blind loop syndrome. Gastroenterology 80:313–320. [PubMed] [Google Scholar]
- 21.Giannella R, Broitman S, Zamcheck N. 1972. Competition between bacteria and intrinsic factor for vitamin B 12: implications for vitamin B 12 malabsorption in intestinal bacterial overgrowth. Gastroenterology 62:255–260. [PubMed] [Google Scholar]
- 22.Lakhani SV, Shah HN, Alexander K, Finelli FC, Kirkpatrick JR, Koch TR. 2008. Small intestinal bacterial overgrowth and thiamine deficiency after Roux-en-Y gastric bypass surgery in obese patients. Nutr Res 28:293–298. doi: 10.1016/j.nutres.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Tabaqchali S, Pallis C. 1970. Reversible nicotinamide-deficiency encephalopathy in a patient with jejunal diverticulosis. Gut 11:1024–1028. doi: 10.1136/gut.11.12.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riordan SM, McIver CJ, Thomas DH, Duncombe VM, Bolin TD, Thomas MC. 1997. Luminal bacteria and small-intestinal permeability. Scand J Gastroenterol 32:556–563. doi: 10.3109/00365529709025099. [DOI] [PubMed] [Google Scholar]
- 25.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. 2009. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 26.Riordan SM, McIver CJ, Wakefield D, Duncombe VM, Bolin TD, Thomas MC. 1996. Mucosal cytokine production in small-intestinal bacterial overgrowth. Scand J Gastroenterol 31:977–984. doi: 10.3109/00365529609003117. [DOI] [PubMed] [Google Scholar]
- 27.Riordan SM, McIver CJ, Wakefield D, Duncombe VM, Thomas MC, Bolin TD. 2001. Small intestinal mucosal immunity and morphometry in luminal overgrowth of indigenous gut flora. Am J Gastroenterol 96:494–500. doi: 10.1111/j.1572-0241.2001.03533.x. [DOI] [PubMed] [Google Scholar]
- 28.Jones HF, Davidson GP, Brooks DA, Butler RN. 2011. Is small-bowel bacterial overgrowth an underdiagnosed disorder in children with gastrointestinal symptoms? J Pediatr Gastroenterol Nutr 52:632–634. doi: 10.1097/MPG.0b013e31820d5c16. [DOI] [PubMed] [Google Scholar]
- 29.dos Reis JC, de Morais MB, Oliva CA, Fagundes-Neto U. 2007. Breath hydrogen test in the diagnosis of environmental enteropathy in children living in an urban slum. Dig Dis Sci 52:1253–1258. doi: 10.1007/s10620-006-9288-9. [DOI] [PubMed] [Google Scholar]
- 30.Pereira SP, Khin-Maung-U, Bolin TD, Nyunt-Nyunt-Wai, Duncombe VM, Myo-Khin, Linklater JM. 1991. A pattern of breath hydrogen excretion suggesting small bowel bacterial overgrowth in Burmese village children. J Pediatr Gastroenterol Nutr 13:32–38. doi: 10.1097/00005176-199107000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Mello CS, Tahan S, Melli LC, Rodrigues MS, de Mello RM, Scaletsky IC, de Morais MB. 2012. Methane production and small intestinal bacterial overgrowth in children living in a slum. World J Gastroenterol 18:5932. doi: 10.3748/wjg.v18.i41.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khin-Maung-U, Pereira SP, Bolin TD, Duncombe VM, Myo-Khin, Nyunt-Nyunt-Wai, Linklater JM. 1990. Malabsorption of carbohydrate from rice and child growth: a longitudinal study with the breath-hydrogen test in Burmese village children. Am J Clin Nutr 52:348–352. [DOI] [PubMed] [Google Scholar]
- 33.Khin-Maung-U, Bolin TD, Duncombe VM, Myo-Khin, Nyunt-Nyunt-Wai, Pereira SP, Linklater JM. 1992. Epidemiology of small bowel bacterial overgrowth and rice carbohydrate malabsorption in Burmese (Myanmar) village children. Am J Trop Med Hyg 47:298–304. [DOI] [PubMed] [Google Scholar]
- 34.Lagos R, Fasano A, Wasserman SS, Prado V, Martin SO, Abrego P, Losonsky GA, Alegria S, Levine MM. 1999. Effect of small bowel bacterial overgrowth on the immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR. J Infect Dis 180:1709–1712. doi: 10.1086/315051. [DOI] [PubMed] [Google Scholar]
- 35.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. 2002. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet 359:564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- 36.Jiang NM, Tofail F, Moonah SN, Scharf RJ, Taniuchi M, Ma JZ, Hamadani JD, Gurley ES, Houpt ER, Azziz-Baumgartner E, Haque R, Petri WA. 2014. Febrile illness and pro-inflammatory cytokines are associated with lower neurodevelopmental scores in Bangladeshi infants living in poverty. BMC Pediatr 14:50. doi: 10.1186/1471-2431-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendez MA, Adair LS. 1999. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J Nutr 129:1555–1562. [DOI] [PubMed] [Google Scholar]
- 38.Korpe PS, Petri WA Jr. 2012. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med 18:328–336. doi: 10.1016/j.molmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer TM, Schwacha H, Steinbrückner B, Brinkmann FE, Ditzen AK, Aponte JJ, Pelz K, Berger D, Kist M, Blum HE. 2002. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol 97:2364–2370. doi: 10.1111/j.1572-0241.2002.05791.x. [DOI] [PubMed] [Google Scholar]
- 40.Lunn PG, Northrop-Clewes CA, Downes RM. 1991. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet 338:907–910. doi: 10.1016/0140-6736(91)91772-M. [DOI] [PubMed] [Google Scholar]
- 41.Carroccio A, Iacono G, Cottone M, Di Prima L, Cartabellotta F, Cavataio F, Scalici C, Montalto G, Di Fede G, Rini G, Notarbartolo A, Averna MR. 2003. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: a prospective study in adults and children. Clin Chem 49:861–867. doi: 10.1373/49.6.861. [DOI] [PubMed] [Google Scholar]
- 42.Taniuchi M, Sobuz SU, Begum S, Platts-Mills JA, Liu J, Yang Z, Wang XQ, Petri WA Jr, Haque R, Houpt ER. 2013. Etiology of diarrhea in Bangladeshi infants in the first year of life analyzed using molecular methods. J Infect Dis 208:1794–1802. doi: 10.1093/infdis/jit507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donowitz JR, Petri WA. 2015. Pediatric small intestine bacterial overgrowth in low-income countries. Trends Mol Med 21:6–15. doi: 10.1016/j.molmed.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cullen JJ, Caropreso DK, Ephgrave KS. 1995. Effect of endotoxin on canine gastrointestinal motility and transit. J Surg Res 58:90–95. doi: 10.1006/jsre.1995.1015. [DOI] [PubMed] [Google Scholar]
- 45.Husebye E, Hellström PM, Sundler F, Chen J, Midtvedt T. 2001. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol 280:G368–G380. [DOI] [PubMed] [Google Scholar]
- 46.Husebye E, Skar V, Høverstad T, Iversen T, Melby K. 1995. Abnormal intestinal motor patterns explain enteric colonization with Gram-negative bacilli in late radiation enteropathy. Gastroenterology 109:1078–1089. doi: 10.1016/0016-5085(95)90565-0. [DOI] [PubMed] [Google Scholar]
- 47.Omoike IU, Abiodun PO. 1989. Upper small intestinal microflora in diarrhea and malnutrition in Nigerian children. J Pediatr Gastroenterol Nutr 9:314–321. [DOI] [PubMed] [Google Scholar]
- 48.Kirkpatrick BD, Colgate ER, Mychaleckyj JC, Haque R, Dickson DM, Carmolli MP, Nayak U, Taniuchi M, Naylor C, Qadri F, Ma JZ, Alam M, Walsh MC, Diehl SA, PROVIDE Study Teams, Petri WA Jr. 2015. The “Performance of Rotavirus and Oral Polio Vaccines in Developing Countries” (PROVIDE) study: description of methods of an interventional study designed to explore complex biologic problems. Am J Trop Med Hyg 92:744–751. doi: 10.4269/ajtmh.14-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gasbarrini A, Corazza GR, Gasbarrini G, Montalto M, Di Stefano M, Basilisco G, Parodi A, Usai-Satta P, Vernia P, Anania C, Astegiano M, Barbara G, Benini L, Bonazzi P, Capurso G, Certo M, Colecchia A, Cuoco L, Di Sario A, Festi D, Lauritano C, Miceli E, Nardone G, Perri F, Portincasa P, Risicato R, Sorge M, Tursi A, 1st Rome H2-Breath Testing Consensus Conference Working Group . 2009. Methodology and indications of H2-breath testing in gastrointestinal diseases: the Rome Consensus Conference. Aliment Pharmacol Ther 29:1–49. doi: 10.1111/j.1365-2036.2009.03951.x. [DOI] [PubMed] [Google Scholar]
- 50.Mahmud MA, Spigt M, Bezabih AM, Pavon IL, Dinant GJ, Velasco RB. 2015. Efficacy of handwashing with soap and nail clipping on intestinal parasitic infections in school-aged children: a factorial cluster randomized controlled trial. PLOS Med 12:e1001837. doi: 10.1371/journal.pmed.1001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peterson KM, Buss J, Easley R, Yang Z, Korpe PS, Niu F, Ma JZ, Olortegui MP, Haque R, Kosek MN, Petri WA Jr. 2013. REG1B as a predictor of childhood stunting in Bangladesh and Peru. Am J Clin Nutr 97:1129–1133. doi: 10.3945/ajcn.112.048306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fagerberg UL, Lööf L, Myrdal U, Hansson LO, Finkel Y. 2005. Colorectal inflammation is well predicted by fecal calprotectin in children with gastrointestinal symptoms. J Pediatr Gastroenterol Nutr 40:450–455. doi: 10.1097/01.MPG.0000154657.08994.94. [DOI] [PubMed] [Google Scholar]
- 53.Konikoff MR, Denson LA. 2006. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis 12:524–534. doi: 10.1097/00054725-200606000-00013. [DOI] [PubMed] [Google Scholar]