Abstract
AIM: To compare the prevalence of Helicobacter pylori (H pylori) IgG and IgA antibodies between adult subjects, with defined gastric diseases, nondefined gastric disorders and those representing the population.
METHODS: Data on H pylori IgG and IgA antibodies, determined by enzyme immunoassay, were analyzed in 3 252 subjects with DGD including 482 patients with gastric ulcer, 882 patients with duodenal ulcer, 1 525 patients with chronic gastritis only and 363 subjects with subsequent gastric cancer, 19 145 patients with NoDg and 4 854 POPUL subjects. The age-adjusted prevalences were calculated for 1- and 20-year age cohorts.
RESULTS: The prevalences of IgG antibodies were equally high (89-96%) in all 20-year age cohorts of the DGD groups, whereas the prevalences of IgG antibodies were lower and increased by age in the POPUL and NoDg groups. The prevalences of IgA antibodies were also higher in the DGD groups; among them CA (84-89%) and GU groups (78-91%) showed significantly higher prevalences than DU (68-77%) and CG patients (59-74%) (OR 2.49, 95%CI 1.86-3.34 between the GU and DU groups). In the CA, GU, and DU groups, the IgA prevalences showed only minor variation according to age, while they increased by age in the CG, POPUL, and NoDg groups (P≤0.0001). The IgA response, but not the IgG response, was associated with an increased risk of CA (OR 2.41, 95%CI 1.79-3.53) and GU (OR 2.57, 95%CI 1.95-3.39) in comparison with CG patients.
CONCLUSION: An IgA antibody response during H pylori infection is significantly more common in CA and GU patients as compared with CG patients.
Keywords: Helicobacter pylori, IgA antibodies, Gastric cancer, Gastric ulcer, Duodenal ulcer, Chronic gastritis
INTRODUCTION
Helicobacter pylori (H pylori), the causative agent of chronic gastritis[1], is also the most important risk factor for peptic ulcer disease and distal gastric cancer[4,5]. The presence of H pylori antibodies signify this chronic infection and their prevalence increases with age in all populations, mainly due to the birth of cohort phenomenon[6,7]. The optimal serological tests for IgG antibodies to H pylori show a sensitivity and a specificity of over 95%[8-10]. Antibodies of the IgA class are usually detected in combination with elevated IgG antibodies in approximately two-thirds of infected subjects[8,11,12]. They are diagnostically useful in the 2-7% of H pylori patients who do not have elevated IgG levels[7,8,12-15]. IgA antibodies have been shown to be a sensitive indicator of an increased risk for gastric cancer[14]. In this context, it may be important that subjects with CagA antibodies have more often H pylori antibodies of the IgA class as compared with those who are CagA antibody-negative[15], since CagA-positive infections have been associated with an increased risk of both peptic ulcer disease and gastric cancer[16,17].
In the present study, we analyzed the prevalences of H pylori antibodies determined in our laboratory from 1986 to 2000 in clinical samples taken from patients with endoscopically verified or undefined gastric disorders and in samples collected from the Finnish population.
MATERIALS AND METHODS
Study subjects
Serum samples for this study were obtained from 1986 to 2000 from the following patient groups: 3 252 patients with defined gastric diseases (DGD), including 482 patients with an endoscopically confirmed gastric ulcer (GU) (mean age 60.79 years, SD±12.59 years), 882 patients with an endoscopically confirmed duodenal ulcer (DU) (mean age 53.80 years, SD±13.64 years), 1 525 patients with a histologically verified chronic gastritis (CG) (mean age 50.58 years, SD±15.95 years) and 363 subjects with subsequent gastric cancer (CA) (mean age at the time of the serum sampling 57.23 years, SD±10.91 years). Sera from GU, DU, and CG patients were collected on the day of the endoscopy, those from CA patients between 2 wk to 24 years before the diagnosis of cancer was made (reported in part earlier[5,14]). In the GU, DU, and CG groups, patients who had prior successful eradication therapy were excluded from the study. In addition, serum samples were obtained from 4 854 subjects participating in a population study in Vammala, Finland (POPUL) (mean age 41.73 years, SD±20.60 years), reported in part earlier[7] and from 19 145 patients whose sera were sent by general practitioners, Municipal Health Centers or Hospitals to our diagnostic laboratory for H pylori antibody tests without any information on possible gastric disorders (NoDg) (mean age 51.47 years, SD±16.97 years).
Ethics
The study was approved by the Ethics Committee for Epidemiology and Public Health of the Helsinki and Uusimaa Hospital district.
Laboratory assessment
H pylori IgG and IgA antibody titers were determined by in-house enzyme immunoassays[8,10].The antigen used was an acid glycine extract from H pylori strain NCTC 11637. During the study period, the sensitivity and specificity of the IgG test were 95-99% and 93-97%, respectively, and those of the IgA test were 64-67% and 92-98%, respectively, as determined in patients in whom the presence of H pylori infection had been verified by culture and histology of gastric biopsies[8,10].
Statistical analysis
The trend in changes in the prevalences of IgG and IgA antibodies by age was studied using the linear trend test. The comparisons of prevalences of IgA and IgG antibodies between the groups were analyzed using the logistic regression model adjusting for age based on 1-year age cohorts. For an overview, the prevalences were determined for 20-year age-adjusted cohorts (15-34, 35-54, 55-74, and 75-94 years), each including at least 50 subjects. The association of IgA and IgG responses with the risk of serious complications (CA, GU, and DU) was analyzed using a logistic regression model by comparing the number of subjects in each antibody response and complication category to that in CG patients, who are regarded to present the basic disease caused by H pylori. Statistical analyses were carried out using the SPSS 12.0 software package (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
RESULT
Of the 27 251 subjects, 13 939 (51.2%) were positive for H pylori antibodies. Of the antibody-positive subjects, 61.8% were positive for both IgG and IgA antibodies, 34.9% for IgG antibodies only and 3.3% for IgA antibodies only.
IgG antibody prevalences
Among the subjects in the DGD groups, 88.6-95.7% had H pylori antibodies of the IgG class in all 20-year age cohorts (Figure 1). In contrast, among the subjects representing the POPUL and NoDg groups, significantly lower seroprevalences were observed (OR 19.73, 95%CI 16.15-24.10 and OR 14.11, 95%CI 12.28-16.21, respectively) (Figure 1). Furthermore, the prevalence was seen to increase by age from 12% in the youngest cohort to 63% in the 55-74-year-old cohort in the POPUL group (P<0.0001; trend test), and from 27% to 62%, respectively, in the NoDg patients (P<0.0001; trend test). The prevalence of IgG antibodies was significantly higher in the NoDg patients than in the POPUL group (OR 2.18, 95%CI 2.00-2.36) (Figure 1).
Figure 1.
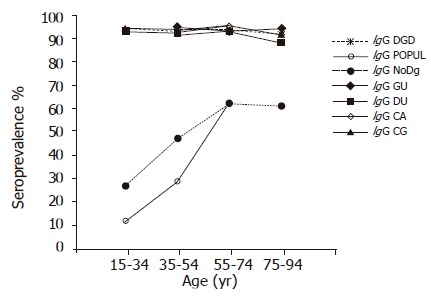
Prevalence of H pylori IgG antibodies by 20-year age cohorts in the Finnish population and patients with different gastric disorders. (Only cohorts including at least 50 subjects are shown).
Within the DGD group, the prevalences did not differ between the GU, DU, CG, and CA groups, nor did they show any significant variation by age (trend test).
IgA antibody prevalences
The prevalence of IgA antibodies group in all age cohorts was significantly higher in the DGD group than in the POPUL (OR 9.61; 95%CI 8.20-11.26) and NoDg groups (OR 5.00; 95%CI 4.59-5.44) (Figure 2). Within the DGD group, the highest prevalences were found in the GU and CA groups in all 20-year cohorts (77.7-90.7% and 84.3-88.6%, respectively) (Figure 2) without a significant difference between these two groups (OR 1.09, 95%CI 0.75-1.58). Although the GU patients showed a small decrease of IgA-positive subjects by increasing age (P = 0.016; trend test), the prevalence was markedly higher than in DU (68.4-77.4%, OR 2.49; 95%CI 1.86-3.34) and CG patients (58.7-74.2%, OR 2.57, 95%CI 1.95-3.39). In the DU patients, the IgA prevalence showed no significant trend by age (trend test), whereas a significantly increased trend by age was found in CG patients (P = 0.0001; trend test); the overall prevalences did not differ significantly between these two groups (OR 1.13; 95%CI 0.95-1.35) (Figure 2, Table 1).
Figure 2.
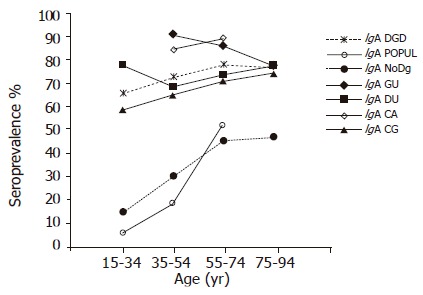
Prevalence of H pylori IgA antibodies by 20-year age cohorts in the Finnish population and patients with different gastric disorders. (Only cohorts including at least 50 subjects are shown.)
Table 1.
Association of H pylori IgA and IgG antibodies with the risk of CA, GU or DU in comparison to CG
| Subjects |
IgA |
IgG |
||
| with | OR | 95%CI | OR | 95%CI |
| CG | 1 | 1 | ||
| CA | 2.41 | 1.79–3.53 | 1.28 | 0.81–2.02 |
| GU | 2.57 | 1.95–3.39 | 0.69 | 0.46–1.03 |
| DU | 1.13 | 0.95–1.35 | 0.72 | 0.55–0.99 |
In the subjects representing the POPUL and NoDg groups, the prevalence of IgA antibodies increased by age from the lowest rates (6.5% and 15.1%, respectively) to significantly higher rates in the 55-74-year-old cohorts (52.1% and 45.6%, respectively; P<0.0001; trend test) (Figure 2). The overall prevalence of IgA antibodies was higher in the NoDg patients than that in the POPUL group (OR 1.93, 95%CI 1.73-2.10).
Association of IgG and IgA responses with the risk of CA, GU, and DU in comparison with CG
IgA response was more common in CA and GU groups as compared with CG patients (OR 2.41, 95%CI 1.79-3.53 and OR 2.57, 95%CI 1.95-3.39, respectively); however, this difference was not significant in DU patients (Table 1). The number of IgG responders in CA and GU groups did not differ significantly as compared with the CG patients (Table 1), whereas it was even slightly lower in DU patients as compared with the CG patients (OR 0.72, 95%CI 0.55-0.99).
DISCUSSION
In the present study, we analyzed, according to age cohorts, a large body of serological data collected during a 15-year period. DGD subjects with gastric disorders known to be associated with H pylori infection showed a high and rather a constant prevalence of H pylori IgG antibodies in all the 20-year age cohorts. Based on the prevalence of IgA antibodies, the DGD group could be divided into two categories: in GU-CA-category, the age-adjusted IgA prevalences ranged from 78% to 91%; whereas in DU-CG-category, the age-adjusted IgA prevalences remained significantly lower. With the exception of CG patients, the IgA antibody rates also remained rather constant throughout the age range. In contrast, the infected subjects in the POPUL and NoDg groups showed significantly lower IgG and IgA rates than those in the DGD group and that increased significantly by age. The CG patients formed a special intermediate group with overall IgG and IgA antibody prevalences at the same level as those of the DU patients, but with a significantly increasing trend by age in the prevalence of IgA antibodies.
The importance of the IgA response increases when considered in connection with our earlier findings showing the association of H pylori antibodies of the IgA class with a CagA-positive infection[15], as well as with other reports showing an increased risk of peptic ulcer disease and gastric cancer in CagA-positive infection[16,17]. The present results imply that an IgA response during H pylori infection might be regarded as an indicator of an increased risk not only for gastric cancer[14] but also for gastric ulcer disease. In these comparisons, that we carried out using the data from patients with chronic gastritis as baseline values, we found that the higher IgA response rate seen in DU patients did not reach significance.
By using the data obtained in prospective gastric cancer studies, we wanted to avoid the bias caused by severe atrophic gastritis, regarded as a precancerous process[18]. Severe atrophic gastritis may progress to a disease stage when Helicobacters first gradually decrease in number, then disappear and finally also Helicobacter antibodies, the longest lasting indicators of the infection, fall to a normal level[19]. In particular, in elderly subjects with non-cardia cancer, there may be several individuals who at the time of diagnosis may have lost all direct indicators of their burnt out Helicobacter infection.
Our large materials and the high sensitivity and specificity of our antibody tests also gave an opportunity to compare the prevalence of H pylori antibodies between the POPUL and NoDg groups. It is tempting to speculate that the higher H pylori prevalence in the two youngest cohorts (by 15% and 18% units in antibodies of IgG class in the order of increasing age) in NoDg patients would preferentially reflect the strength of gastric symptoms driving patients to clinical consultations.
In conclusion, irrespective of age, practically all DGD subjects have H pylori IgG antibody. The prevalence of IgA antibodies highest in CA and GU patients, second highest in DU and CG patients, and lowest in the NoDg patients and POPUL subjects. An IgA response is associated with serious sequelae of H pylori infection.
Footnotes
Supported by the University of Helsinki, the Helsinki University Central Hospital and the Finnish Cancer Organisations, Helsinki, Finland
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
References
- 1.Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch's postulates for pyloric Campylobacter. Med J Aust. 1985;142:436–439. doi: 10.5694/j.1326-5377.1985.tb113443.x. [DOI] [PubMed] [Google Scholar]
- 2.Rauws EA, Tytgat GN. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990;335:1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- 3.Marshall BJ. Helicobacter pylori. Am J Gastroenterol. 1994;89:S116–S128. [PubMed] [Google Scholar]
- 4.Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177–240. [PMC free article] [PubMed] [Google Scholar]
- 5.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talley NJ, Noack KB. The woldwide prevalence of Helicobacter pylori: Asymptomatic infection and clical states associated with infection in adults. In: Goodwin CS, Worsley BW, eds , editors. Helicobacter pylori: Biology and clinical practice. Boca Raton, Florida: CRC Press Inc; 1993. pp. 63–83. [Google Scholar]
- 7.Kosunen TU, Aromaa A, Knekt P, Salomaa A, Rautelin H, Lohi P, Heinonen OP. Helicobacter antibodies in 1973 and 1994 in the adult population of Vammala, Finland. Epidemiol Infect. 1997;119:29–34. doi: 10.1017/s0950268897007565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosunen TU, Seppälä K, Sarna S, Sipponen P. Diagnostic value of decreasing IgG, IgA, and IgM antibody titres after eradication of Helicobacter pylori. Lancet. 1992;339:893–895. doi: 10.1016/0140-6736(92)90929-w. [DOI] [PubMed] [Google Scholar]
- 9.Feldman RA, Deeks JJ, Evans SJ. Multi-laboratory comparison of eight commercially available Helicobacter pylori serology kits. Helicobacter pylori Serology Study Group. Eur J Clin Microbiol Infect Dis. 1995;14:428–433. doi: 10.1007/BF02114899. [DOI] [PubMed] [Google Scholar]
- 10.Oksanen A, Veijola L, Sipponen P, Schauman KO, Rautelin H. Evaluation of Pyloriset Screen, a rapid whole-blood diagnostic test for Helicobacter pylori infection. J Clin Microbiol. 1998;36:955–957. doi: 10.1128/jcm.36.4.955-957.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosunen TU, Höök J, Rautelin HI, Myllylä G. Age-dependent increase of Campylobacter pylori antibodies in blood donors. Scand J Gastroenterol. 1989;24:110–114. doi: 10.3109/00365528909092247. [DOI] [PubMed] [Google Scholar]
- 12.Andersen LP, Rosenstock SJ, Bonnevie O, Jørgensen T. Seroprevalence of immunoglobulin G, M, and A antibodies to Helicobacter pylori in an unselected Danish population. Am J Epidemiol. 1996;143:1157–1164. doi: 10.1093/oxfordjournals.aje.a008694. [DOI] [PubMed] [Google Scholar]
- 13.Jaskowski TD, Martins TB, Hill HR, Litwin CM. Immunoglobulin A antibodies to Helicobacter pylori. J Clin Microbiol. 1997;35:2999–3000. doi: 10.1128/jcm.35.11.2999-3000.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aromaa A, Kosunen TU, Knekt P, Maatela J, Teppo L, Heinonen OP, Härkönen M, Hakama MK. Circulating anti-Helicobacter pylori immunoglobulin A antibodies and low serum pepsinogen I level are associated with increased risk of gastric cancer. Am J Epidemiol. 1996;144:142–149. doi: 10.1093/oxfordjournals.aje.a008901. [DOI] [PubMed] [Google Scholar]
- 15.Rautelin HI, Oksanen AM, Karttunen RA, Seppälä KM, Virtamo JR, Aromaa AJ, Kosunen TU. Association of CagA-positive infection with Helicobacter pylori antibodies of IgA class. Ann Med. 2000;32:652–656. doi: 10.3109/07853890009002036. [DOI] [PubMed] [Google Scholar]
- 16.Cover TL, Glupczynski Y, Lage AP, Burette A, Tummuru MK, Perez-Perez GI, Blaser MJ. Serologic detection of infection with cagA+ Helicobacter pylori strains. J Clin Microbiol. 1995;33:1496–1500. doi: 10.1128/jcm.33.6.1496-1500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Correa P, Haenszel W, Cuello C, Zavala D, Fontham E, Zarama G, Tannenbaum S, Collazos T, Ruiz B. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50:4737–4740. [PubMed] [Google Scholar]
- 19.Kokkola A, Kosunen TU, Puolakkainen P, Sipponen P, Harkonen M, Laxen F, Virtamo J, Haapiainen R, Rautelin H. Spontaneous disappearance of Helicobacter pylori antibodies in patients with advanced atrophic corpus gastritis. APMIS. 2003;111:619–624. doi: 10.1034/j.1600-0463.2003.1110604.x. [DOI] [PubMed] [Google Scholar]


