Abstract
AIM: To determine the risk factors for the development of esophageal adenocarcinoma in these patients with columnar-lined esophagus (CLE).
METHODS: Data collected retrospectively on 597 consecutive patients diagnosed at endoscopy and histology to have CLE at Leeds General Infirmary between 1984 and 1995 were analyzed. Factors evaluated included age, sex, length of columnar segment, smoking, and drinking habits, history of non-steroidal ingestion, presence of endoscopic esophagitis, ulceration or benign strictures and presence of Helicobacter pylori in esophageal biopsies. Univariate and multivariate analyses were performed to identify risk factors for the development of adenocarcinoma.
RESULTS: Forty-four patients presented or developed esophageal adenocarcinoma during follow-up. Independent risk factors for the development of adenocarcinoma in patients with CLE were males (OR 5.12, 95%CI 2.04-12.84, P = 0.0005), and benign esophageal stricture (OR 4.37, 95%CI 2.02-9.45, P = 0.0002). Male subjects and patients who developed benign esophageal stricture constituted 86% (n = 38) of all patients who presented or developed esophageal adenocarcinoma. The presence of esophagitis was associated with a significant reduction in the development of esophageal carcinoma (OR 0.28, 95%CI 0.13-0.57, P = 0.0006). No other clinical characteristics differentiate between the non-malignant and malignant group.
CONCLUSION: In patients with CLE, endoscopic surveillance for the early detection of adenocarcinoma may be restricted to male subjects, as well as patients who develop benign esophageal strictures.
Keywords: Barrett's esophagus, Adenocarcinoma, Risk factors, Esophageal adenocarcinoma, Esophageal stricture
INTRODUCTION
Columnar-lined (Barrett’s) esophagus (CLE) is defined as the replacement of the normal squamous lining of the lower esophagus by a unique metaplastic columnar epithelium usually as a consequence of chronic gastro-esophageal reflux (GER). The prevalence of CLE has been estimated to occur in 1 in 400 of the general population[1], and in 10-16% of patients with reflux esophagitis[2,3]. Cameron et al[4] suggested that there are 20 times as many cases of CLE in the general population as are clinically diagnosed.
Patients with CLE are at increased risk of esophageal adenocarcinoma[5]. The incidence of the latter varies between one in 46 to one in 441 patient-years follow-up[6,7] with an annual incidence of 1%, and is increasing more rapidly than any other type of malignancy[8,9]. Endoscopic surveillance programs are therefore instituted. Nonetheless, the increased cost and workload associated with the surveillance adds to the pressures on available resources. The identification of risk factors for the development of adenocarcinoma in these patients may allow for the selection of patients for intense endoscopic surveillance and a more efficient utilization of health care services and resources.
The current paper examines our experience with 597 consecutive patients diagnosed to have CLE for over a 11-year period. Our aim was to determine clinical factors, which could identify a subgroup of patients who are at a higher risk for the development of adenocarcinoma and who therefore would benefit most from being in a surveillance program. Examining the characteristics of patients with CLE and comparing them between the non-malignant group and both the prevalence and incident cases of adenocarcinoma might serve this purpose.
MATERIALS AND METHODS
Between January 1984 and December 1995, 626 consecutive patients were diagnosed at endoscopy and histology to have a CLE under the care of the Center for Digestive Diseases at the General Infirmary at Leeds. Patients were identified from a computer registry at the Institute of Pathology and their records were retrospectively reviewed. Data, including patient demographic characteristics, endoscopic findings, and histology reports were entered into a computer database.
Columnar-lined esophagus was defined as the presence of columnar-lined epithelium at least 3 cm above the endoscopically determined gastro-esophageal junction or the presence of specialized columnar epithelium (SCE) anywhere in the esophagus. Twenty-nine patients were excluded from this study; 17 patients did not fulfill the definition criteria mentioned above and the medical records of 12 patients could not be located.
Factors that may be associated with increased risk of development of adenocarcinoma were examined. These included age, sex, smoking, regular alcohol use, the ingestion of non-steroidal anti-inflammatory drugs (NSAIDs), length of columnar segment, the presence of hiatul hernia, esophagitis, benign esophageal stricture or ulcers at endoscopy, and the presence of SCE or H pylori in esophageal biopsies. Mean follow-up was 43 (range 1-155 mo) mo. Data related to the size of hiatul hernia and body mass index of the patients were incomplete and excluded from analysis. Data related to the result of the surveillance program for these patients were published elsewhere[6].
Patients were defined as smokers if they regularly smoked more than 10 cigarettes per day for at least one year at any time before the diagnosis of CLE. Patients were defined as regular alcohol users if they have a history of drinking 10 units of alcohol or more weekly for at least one year at any time before the diagnosis of CLE. Patients who have a history of regular ingestion of NSAIDs for at least 6 mo at any time before the diagnosis of CLE were regarded as NSAIDs users. H pylori colonization was determined upon the basis of hematoxylin and eosin and the use of a modified Giemsa stain of esophageal and gastric biopsy specimens.
All cases with malignant stricture were not regarded as cases of benign esophageal stricture and were not considered for the analysis. Only cases where the stricture was away from the cancer and histologically not involved with cancer were regarded as benign esophageal ulcer.
Statistical analysis
Univariate analysis was performed utilizing the χ2 test and the Mann-Whitney U test as appropriate. Statistical significance was accepted at a P<0.05. Stepwise logistic regression analysis was used to identify independent risk factors. Statistical analyses were performed using SPSS for Windows version 10.
RESULTS
Five hundred and ninety-seven patients with histologically confirmed diagnosis of CLE were seen at our institute from 31st January 1984 to 31st January 1995. The number of new patients diagnosed each year during the study period showed an increasing trend (Figure 1). There were 333 (56%) males and 264 (44%) females. The mean age for the total group was 63.4 years (SD 14.86; range 2-94 years). Seventy-three percent of the male patients (244/333) had their diagnosis below the age of 70, while 81% (214/264) of female patients were diagnosed when they were above 60 years of age (Figure 2).
Figure 1.
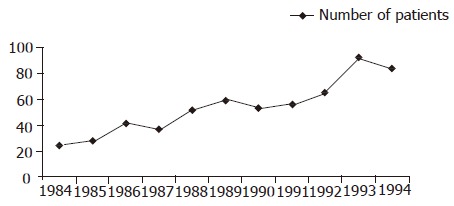
Number of patients diagnosed with columnar-lined esophagus during the study period.
Figure 2.
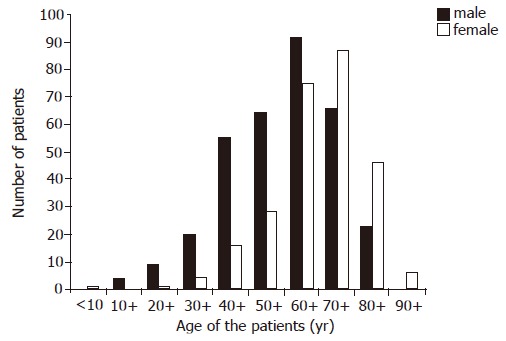
Age of patients at the time of diagnosis of columnar-lined esophagus.
Of the 597 patients included in the analysis, 31 (5.2%) presented or developed adenocarcinoma within 6 mo of initial diagnosis of CLE and 13 (2.2%) developed adenocarcinoma after a mean follow-up of 55 mo (range 8-155 mo). Patients were divided into two groups: Non-malignant group: 553 patients had no esophageal adenocarcinoma either at initial presentation or during follow-up. There were 299 (54%) males and 254 (46%) females. Malignant group: 44 patients presented (31 patients) or developed (13 patients) esophageal adenocarcinoma. There were 34 (77%) males and 10 (23%) females.
Table 1 summarizes the details of patients and the results of the univariate analysis of the risk factors studied. Table 2 summarizes the results of multivariable analysis.
Table 1.
Univariate analysis of potential risk factors for the development of esophageal adenocarcinoma in patients with columnar-lined esophagus
| Non-malignant | Malignant | P | |
| group (n = 553) | group (n = 44) | ||
| Age | |||
| <60 (yr: n, %) | 189 (34.2) | 13 (29.5) | 0.532 |
| >60 (yr: n, %) | 364 (65.8) | 31 (70.5) | |
| Sex: male (%) | 299 (54.1) | 34 (77.3) | 0.003 |
| Smoking+: n (%) | 161 (45.2) | 21 (51.2) | 0.466 |
| Regular alcohol use±: n (%) | 52 (15.2) | 8 (20) | 0.43 |
| NSAIDs: n (%) | 304 (55) | 25 (56.8) | 0.813 |
| Specialized epithelium: n (%) | 467 (84.4) | 42 (95.5) | 0.047 |
| Length of CLE Median (cm) | 5 | 6 | 0.001 |
| Hiatul hernia: n (%) | 314 (56.8) | 22 (50) | 0.383 |
| Esophagitis: n (%) | 330 (59.7) | 13 (29.5) | <0.0001 |
| Esophageal ulcer | 117 (21.2) | 11 (25) | 0.55 |
| Esophageal stricture | 77 (13.9) | 16 (36.4) | <0.0001 |
| Hp in esophageal biopsy | 42 (7.6) | 2 (4.5) | 0.456 |
CLE – columnar-lined esophagus; NSAIDs – non-steroidal anti-inflammatory drugs; Hp – Helicobacter pylori. +Information regarding smoking was available in only 356 patients of the non-malignant group and 41 patients of the malignant group; ±information regarding alcohol consumption was available in only 342 patients of the non-malignant group and 40 patients of the malignant group.
Table 2.
Risk factors associated with the development of adenocarcinoma in patients with CLE; results of multivariable regression analysis
| Risk factors | Odds ratio | 95%CI for Odds ratio | P |
| Age ≥60 yr | 1.65 | 0.75–3.64 | 0.216 |
| Male sex | 5.12 | 2.04–12.84 | 0.0005 |
| Regular alcohol use | 1.15 | 0.46–2.90 | 0.76 |
| NSAIDs | 1.41 | 0.68–2.93 | 0.352 |
| Esophagitis | 0.28 | 0.13–0.57 | 0.0006 |
| Esophageal stricture | 4.37 | 2.02–9.45 | 0.0002 |
CLE – columnar lined esophagus; NSAIDs – non-steroidal anti-inflammatory drugs
Significant independent risk factors for the development of esophageal adenocarcinoma in patients with CLE were male sex, and benign esophageal stricture. Male subjects and patients who developed benign esophageal stricture constituted 86% (n = 38) of all patients who presented or developed esophageal adenocarcinoma. Among the 24 patients who presented with esophageal adenocarcinoma, 9 patients had histologically proven benign peptic stricture above and away from the cancer and the strictures were located at the junction of the columnar mucosa with the squamous epithelium. Among the seven patients who developed esophageal adenocarcinoma within 6 mo after the diagnosis of CLE, four of them had histologically proven benign peptic stricture at the time of diagnosis of CLE. Among the 13 patients who developed esophageal adenocarcinoma after a mean follow-up of 55 (range 8-155 mo) mo, three patients had histologically proven benign peptic stricture at the time of diagnosis of CLE, and these three patients developed adenocarcinoma 17, 24, and 35 mo after the diagnosis of CLE, respectively.
The presence of esophagitis was associated with a significant reduction in the development of esophageal carcinoma. Although univariate analysis identified the length of columnar-lined esophageal segment and the presence of SCE on endoscopic biopsies as significant variables (P = 0.001, P = 0.047 respectively), these did not reach significance on multivariable analysis. Age >60 years, smoking, alcohol consumption, the presence of hiatul hernia or esophageal ulcer, and the presence of H pylori in the esophageal biopsies were insignificant risk factors for malignant progression.
Symptoms as a risk factor
For the purpose of comparison between the two groups, the main principal symptom for each patient was considered at the time of diagnosis, although many patients had more than one symptom. In the non-malignant group, the main symptoms at presentation were as follows: In 259 patients (46.8%), the main symptoms were those of GER (heartburn, regurgitation) or dyspepsia. Anemia or gastro-intestinal bleeding was the main symptom in 137 patients (24.8%). Dysphagia was the main symptom in 111 patients (20%) and chest pain was the main symptom in 26 patients (4.7%). Weight loss was the main symptom in only 20 patients (3.6%). Ninety-four patients (17%) in the non-malignant group had no esophageal symptoms at the time of diagnosis and CLE was diagnosed when endoscopy was performed to investigate iron deficiency anemia. In the malignant group, the main symptom was dysphagia in 21 patients (47.7%). Anemia or gastrointestinal bleeding was the main symptom in nine patients (20.5%). GER symptoms were the main symptoms in 10 patients (22.7%). Weight loss was the main symptom in four patients (9%). Dysphagia rather than reflux symptom was the main complaint of the patients in the malignant group at the time of diagnosis. This was mainly due to dysphagia being prominent in the prevalent adenocarcinoma cases (61%; 19/31) as would be expected; in contrast among the 13 patients who developed adenocarcinoma during follow-up, dysphagia was the main symptom in only two patients (15%; 2/13).
Length of the columnar segment
The length of the columnar segments for all patients is shown in Figure 3. Twenty-three patients had short segment (<3 cm) of SCE; none of this group were adenocarcinoma patients. The mean length of the columnar segment in the non-malignant group was 5.8 cm; range 2-20 cm and for the malignant group was 7.2 cm; range 3-15 cm. There was no correlation between the extent of the columnar segment and the presence or absence of symptoms of GER.
Figure 3.
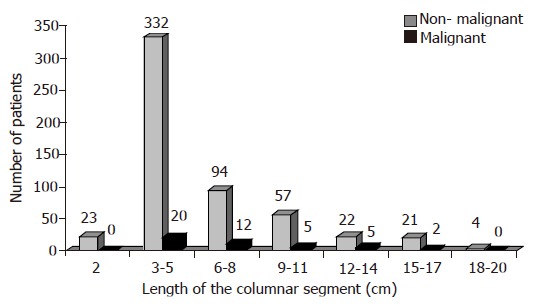
Length of the columnar segments in all patients.
Esophageal and gastric H pylori
Forty-four patients only (7.4%) had H pylori detected in their esophageal biopsies. Only 234 patients of this series had gastric biopsies in addition to their esophageal specimens. Among this subgroup who had biopsy demonstrated CLE and from whom concomitant gastric biopsies were taken, 77 patients (32.9%) had H pylori in their gastric biopsies, 20 of them had H pylori in both the esophageal and gastric specimens. The finding of H pylori in CLE biopsies was not associated with increase in the prevalence of esophagitis (52% vs 58%), esophageal strictures (11% vs 16%), esophageal ulcers (23% vs 21%) or esophageal adenocarcinoma (4.5% vs 7.6%).
DISCUSSION
The present series confirms the findings of other studies[10,11] that the prevalence of CLE increases with age and reaches a peak in late middle age. Eighty-two percent of our patients (487/597) were diagnosed when they were over 50 years of age. The high prevalence of CLE occurs mainly in later middle age and the elderly as shown by our population with a mean age of 59 in males and 68 in females, although it can be seen in younger patients also. However, only 2.5% of patients (15/597) were under 30 years of age at the time of diagnosis. One patient had a diagnosis of CLE below the age of 10 and this was a 3-year old girl who presented with repeated vomiting. Endoscopic and histological examination confirmed the diagnosis of CLE and anti-reflux surgery was performed on this patient at the age of four. This case could be explained on the basis of the few cases of congenital Barrett’s esophagus reported previously[12].
Although there were slightly more males than females in the whole of our series (56% vs 44%), we have found that below the age of 60, there is a clear male predominance of 3:1. In contradiction to other published data, which showed either a male predominance[13] or an equal sex distribution[10,14], we observed a female predominance of 1.5:1 in patients above the age of 70.
A large proportion of patients with CLE in the general population will remain undiagnosed unless complications or carcinoma develop. CLE itself causes no symptoms[15] whereas the main symptoms, which bring patients to medical attention, are related to the reflux symptoms or complications of CLE such as ulceration, stricture, bleeding or adenocarcinoma. There is no correlation between severity of symptoms and histological findings, as about 17% of our patients with CLE had no esophageal symptoms. It is clear therefore that the selection of patients for a surveillance program cannot be based on symptomatology. This conclusion was also made in earlier patient series[10,16,17].
Our study confirms that patients with CLE have an increased incidence of esophageal adenocarcinoma. There were 7 patients who developed adenocarcinoma within 6 mo of initial diagnosis, before their first annual review endoscopy. This is likely to reflect the presence of undetected malignancy at the time of initial endoscopy. This probably represents a sampling error in the biopsies taken and therefore these seven patients were included in the prevalence rather than the incidence data[6].
This study identified male sex, and benign esophageal stricture to be independent risk factors (at least four-fold increased risk) for the development of esophageal adenocarcinoma in patients with CLE. Male patients with benign esophageal stricture constituted 86% of all patients who developed esophageal malignancy. The risk of malignancy was not related to the age of the patients, smoking, presence of SCE, length of columnar-lined segment, or the presence of esophageal ulceration or hiatul hernia on endoscopy or H pylori in esophageal or gastric biopsies. Previous reports identified white ethnicity[18], older age[19], male sex[13,14,20], SCE[21,22], long columnar segment[10,19,23-28], large hiatal hernia size[26,27], esophageal ulcer or stricture[10,29-31], severe acid reflux[26,32], obesity[33,34] and smoking and alcohol[20,35] to be associated with the progression from columnar epithelium to adenocarcinoma. However, some of these reports were based on observations or univariate analyses, and did not control for other variables as in the current study.
There was no significant association between age >60 years and the risk of malignant progression. Most patients are diagnosed with CLE in their sixth or seventh decade of life. Patients with Barrett’s are often reported to be of the same age as those who develop cancer[26]. On the other hand, other studies have reported that the risk of esophageal adenocarcinoma increases with age. Gopal et al[19] reported that the risk of dysplasia increased by 3.3% per year of age. A potential problem is that the mean age of patients with dysplasia is quite high, increasing the expected operative mortality and tending to preclude operative intervention. Clearly, surveillance should be offered only for those patients in whom esophagectomy is considered as a therapeutic option, if early carcinoma is detected. On the other hand, with the availability of ablation therapy, one can argue that even patients who are not fit for esophagectomy will benefit from the treatment of their high-grade dysplasia or early adenocarcinoma using photoablation irrespective of their age.
The increased susceptibility of male subjects with CLE to the development of esophageal malignancy has been reported previously[20,36,37]. In these series, men constituted 67-100% of all patients who developed esophageal adenocarcinoma (77% in this report). Our finding that benign stricture formation, which complicated 16% of all patients with CLE, was a risk factor for malignant transformation that supports previous reports[10,30,31,38,39]. Careful endoscopic surveillance of patients with benign esophageal strictures is therefore required, despite the fact that malignant progression occurred in only one in eight patients.
We found no evidence to support the suggestion that a longer CLE segment is associated with a greater risk of carcinoma. Although the length of columnar segment was significantly greater in patients who developed esophageal adenocarcinoma compared with those who did not, this was not an independent risk factor for malignant transformation. This finding is in concordance with that of Robertson et al[16] who showed that the progression of the metaplastic epithelium up the esophagus, which occurred in 20% of their patients, was not associated with an increased risk of malignancy, as out of the 11 patients who had progression up to 6 cm, only 1 developed carcinoma. Additionally, the marked association between adenocarcinoma of the gastroesophageal junction and ‘short-segment’ Barrett’s mucosa[21,22,25,40] underscores the importance of the ‘short-segment’, and calls for follow-up program similar to that of longer segments of CLE. Rudolph et al[28] reported that segment length was not related to cancer risk in the full cohort of patients with CLE, and when patients with high-grade dysplasia at baseline were excluded; however, a non-significant trend was observed; a 5 cm difference in segment length was associated with a 1.7-fold increase in cancer risk. These authors concluded that the risk for esophageal adenocarcinoma in patients with short-segment Barrett esophagus was not substantially lower than that in patients with longer segments. They suggested that until more data are available, the frequency of endoscopic surveillance should be selected without regard to segment length.
There are some indications that the risk of cancer is proportionate to the anatomic extent of CLE[19,26,27]. A few studies have shown that the risk of adenocarcinoma increases with the length of the columnar segment and they suggested considering patients with CLE of more than 8 or 10 cm in length for surveillance. For example, Iftikhar et al[24] reported that among 102 patients with CLE, 12 were found to have dysplasia; all of them had a columnar segment of 8 cm or more at the time of diagnosis and no patient with a columnar segment of less than 8 cm was found to have dysplasia or adenocarcinoma. In two retrospective studies conducted by Harle et al[41] and Rosenberg et al[42], 94% and 88% of patients with adenocarcinoma had long segments (>10 cm) of columnar epithelium. Both series suggested that an extended length of Barrett’s esophagus is associated with a higher risk of malignant progression. Schnell et al[23] reported a series of 238 patients with CLE. Adenocarcinoma was found in 7% of the 129 patients with segments less than 2 cm in length, in 12% of 50 patients with 3-5 cm segments, in 31% of 45 patients with 6-10 cm Barrett’s segments and in 43% of the 14 patients who had segments of more than 10 cm in length. They concluded that there is a strong association between the length of CLE and risk of adenocarcinoma. Gopal et al[19] reported that the risk of dysplasia increased by 14%/cm of increased length, and similarly Avidan et al[26] reported that each 1-cm elongation of Barrett’s mucosa carried with it a 17% increase in the risk of developing high-grade dysplasia or carcinoma. On the other hand, other studies have suggested that shorter lengths of CLE may have been obscured by the tumor and therefore missed at resection. For example, Hamilton et al[40] found that 64% of the resected specimens of adenocarcinoma of the esophagus and GEJ were associated with Barrett’s esophagus although the Barrett’s mucosa was identified by endoscopic biopsies in only 38% of cases. When adenocarcinoma develops in a short segment of specialized epithelium, the tumor may destroy the area of specialized epithelium leaving no trace of such epithelium. Schnell et al[21] did indeed report four patients with adenocarcinoma in short segment of specialized epithelium. Cameron et al[22] reported that SCE was found in 9 of 9 (100%) cases of esophageal adenocarcinomas and in 10 of 24 (42%) of cases of junctional adenocarcinomas. SCE was found in 8 of 12 (67%) of junctional adenocarcinoma of 6 cm or less in length but in only 2 of 12 (17%) of larger tumors. Again they concluded that junctional adenocarcinomas are associated with both short and long segments of Barrett’s esophagus and larger tumors probably overgrow and conceal the underlying SCE from which they arise. In 38% of the patients who developed cancer, the metaplastic segment was less than 3 cm[43] Schnell et al[21] emphasizes that patients with short segments should be considered at risk and should be followed in the same way as their counterparts with longer segments of CLE. The above studies clearly demonstrate that carcinoma can develop in short as well as long segment of CLE. It is now established that short segment CLE does carry a risk of malignant progression, albeit currently this is difficult to quantify[44].
Specialized epithelium is the most common and distinctive type of columnar epithelium found in CLE. Although dysplasia and carcinoma develop mainly in the presence of SCE, we cannot regard this type of epithelium as the sole indication for surveillance because histology has shown that it is present in most patients with CLE. Indeed nowadays its presence is rather a criterion to establish the diagnosis of CLE. There are, however, a few cases of adenocarcinoma, which can develop in columnar epithelium of other histological types (junctional or fundic type).
The reported data concerning the influence of alcohol consumption on the malignant progression of CLE is controversial. Most published reports including the current study suggest that alcohol ingestion has no or only little effect[26]. The reported decline in the incidence of oral cavity and pharynx cancers, which are traditionally, related to alcohol consumption contrasts with the increase in the incidence of esophageal adenocarcinoma[45]. Gammon et al[46] reported a decrease in the risk associated with wine drinking and no increase in the risk by the use of other alcoholic beverages. Similarly, Garridou et al[47] reported that wine might have a protective effect. On the other hand, other investigators have reported an increased risk of esophageal adenocarcinoma with high alcohol intake[20,35,48-50]. Zhang et al[48] reported a statistically not significant twofold increase in the risk of esophageal adenocarcinoma in those who consume alcohol when compared with nondrinkers. Kabat et al[49] reported that only hard liquor intake was associated with esophageal adenocarcinoma in males, and only daily beer intake was associated with adenocarcinoma in females. Barrett’s esophagus was reported to occur more frequently among subjects who consume large amounts of alcohol and alcohol consumption was also a risk factor for an increased length of Barrett’s mucosa[51,52]. There is a well-known association between alcohol intake and risk of upper digestive tract cancers from epidemiological studies[53]. One possible mechanism is that this increased risk is due to a direct exposure of the esophageal mucosa to high alcohol concentrations but systemic effects could also be important.
There were no differences between smokers and non-smokers with regard to the length of the columnar segment and the presence or absence of Barrett’s complications such as ulcer, stricture or adenocarcinoma. Several reports[26,54,55], as well as the current one, did not support the suggested increased risk of malignant transformation with smoking. Cooper and Barbezat[54] reported a series of 52 patients with CLE and 25 of them were smokers or ex-smokers. They also found no difference in the clinical characteristics between the smokers and non-smokers. Other investigators have shown a higher proportion of smokers in patients with CLE[56] and some suggested that the malignant progression of Barrett’s metaplasia was higher in patients who smoked[20,35,50]. On the other hand, Levi et al[55] evaluated the relationship between tobacco, alcohol and the risk of esophageal adenocarcinoma in CLE in an endoscopy-clinic-based case-control study of 30 cases of adenocarcinoma and 140 controls with non-malignant CLE. Among the cases, 18 (60%) were non-smokers and 14 (47%) non-drinkers, the corresponding proportions in the controls being 52% and 44%. Thus, there was no apparent relation between tobacco, alcohol and the risk of esophageal adenocarcinoma. They suggested that the findings of their study, although based on a limited number of cases, indicate that alcohol and tobacco are unlikely to play a major role in the etiology of adenocarcinoma in CLE. Overall, alcohol and tobacco may be risk factors for esophageal adenocarcinoma, but are not as important as they are in the etiology of esophageal squamous cell carcinoma[57].
Esophagitis and hiatal hernia were more common in the non-malignant group than the malignant group. But these differences have no clinical value, as it will not differentiate between the two groups. The apparent protective effect of esophagitis against malignant progression reflects the natural history of the disease. Esophagitis precedes the replacement of squamous with columnar epithelium, which in turn may become progressively dysplastic with final transformation into adenocarcinoma[12,58,59]. Additionally, columnar epithelium, unlike squamous, is less sensitive to injury secondary to GER and thus more resistant to inflammation[60] .
There was an association between the prevalence of esophagitis and esophageal ulcerations with the history of NSAIDs ingestion within the non-cancer patients; however, there was no significant difference between the non-malignant group and malignant group regarding the prevalence of NSAIDs ingestion. It is likely that NSAIDs are prescribed or self-prescribed for esophagitis. Fifty-five percent of patients in the non-malignant group were taking these drugs at the time of diagnosis and 62% of them were found to have esophagitis or esophageal ulcerations. Cooper and Barbezat[54] reported similar findings and suggested that patients with CLE should avoid taking NSAIDs. However, NSAIDs are now proposed in intervention trials for patients with CLE. Increased expression of the cyclooxygenase 2 enzyme is proposed to be central to the development of esophageal cancer. Since this enzyme is inhibited by NSAIDs, these drugs hold promise as cancer chemopreventive agents in Barrett’s esophagus patients[61]. Several preventive strategies against esophageal adenocarcinoma are under investigation using NSAIDs[62].
We found that esophageal ulcerations were present in 25% of patients in the carcinoma group (11/44) and in 21% of the patients who had no carcinoma (117/553); a difference that was not statistically significant. No case of esophageal perforation due to these ulcers was found in our series. These findings disagree with previous series, which suggested that the risk of developing carcinoma is increased in the presence of esophageal ulcers[10,29-31].
In concordance with previous work suggesting that stricture formation in CLE is a risk factor of malignant progression[10,30,31,39], we observed that esophageal strictures were present in 36% of patients in the carcinoma group (16/44) and in only 14% of the patients without carcinoma (77/553); a difference that was statistically significant. Theoretically it is possible that some of the strictures of the patients who presented or developed adenocarcinoma within 6 mo of CLE diagnoses could be malignant strictures (missed cancers) which were not detected histologically due to sampling error.
We found that H pylori colonization of the esophagus was not a risk factor for malignant progression. Our data indicate that H pylori can colonize the CLE, but its prevalence rate in the esophageal biopsies of these patients or in the subgroups that had complications or carcinoma is low and it is unlikely that H pylori has a significant role in the pathogenesis of CLE or its complications. Similar conclusion was reported before[63,64]. The prevalence rate of H pylori in the gastric biopsies of the same patients is similar or less than that in the normal population. Weston et al[65] found that Barrett’s high-grade dysplasia and adenocarcinoma were significantly more prevalent in patients who are not infected with H pylori. They suggested that H pylori appear to have a protective effect against the development of Barrett’s adenocarcinoma.
While the role of endoscopic surveillance in low-risk patients with CLE is controversial, efficient and effective screening would target high-risk patients. This study suggests that such programs may be largely directed to male subjects and those who develop benign esophageal strictures. We have previously shown that immunohistochemical detection of cyclin D1 in esophageal biopsies of patients with CLE is a sensitive tool for identifying subgroup of patients who may be at a higher risk[66]. But given that multiple genetic alterations, which are implicated in the natural history of esophageal adenocarcinoma, a combination of clinical risk factors and carefully validated biomarkers including cyclin D1, might improve still further the predictive value of the molecular approach[66,67].
In conclusion, we have found that 44 patients presented or developed esophageal adenocarcinoma of which 34 (77%) were males. Independent risk factors for progression from columnar metaplasia to esophageal adenocarcinoma were male sex, and the development of benign esophageal stricture. No other clinical characteristics differentiate between the non-malignant and malignant group. Most esophageal adenocarcinoma occurs in men with specialized epithelium. This subgroup may constitute a clinically recognized group at a high risk of cancer and particularly suitable for endoscopic surveillance. In patients with CLE, endoscopic surveillance for the early detection of adenocarcinoma may be restricted to male subjects, as well as patients who develop benign esophageal strictures. A large proportion of patients with CLE have no esophageal symptoms making recruitment into endoscopic surveillance programs problematic.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Stein HJ, Siewert JR. Barrett's esophagus: pathogenesis, epidemiology, functional abnormalities, malignant degeneration, and surgical management. Dysphagia. 1993;8:276–288. doi: 10.1007/BF01354551. [DOI] [PubMed] [Google Scholar]
- 2.Naef AP, Savary M, Ozzello L. Columnar-lined lower esophagus: an acquired lesion with malignant predisposition. Report on 140 cases of Barrett's esophagus with 12 adenocarcinomas. J Thorac Cardiovasc Surg. 1975;70:826–835. [PubMed] [Google Scholar]
- 3.Bartlesman JF, Hameeteman W, Tÿtgat GN. Barrett's oesophagus. Eur J Cancer Prev. 1992;1:323–325. doi: 10.1097/00008469-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Cameron AJ, Zinsmeister AR, Ballard DJ, Carney JA. Prevalence of columnar-lined (Barrett's) esophagus. Comparison of population-based clinical and autopsy findings. Gastroenterology. 1990;99:918–922. doi: 10.1016/0016-5085(90)90607-3. [DOI] [PubMed] [Google Scholar]
- 5.Spechler SJ. Endoscopic surveillance for patients with Barrett esophagus: does the cancer risk justify the practice? Ann Intern Med. 1987;106:902–904. doi: 10.7326/0003-4819-106-6-902. [DOI] [PubMed] [Google Scholar]
- 6.Bani-Hani K, Sue-Ling H, Johnston D, Axon AT, Martin IG. Barrett's oesophagus: results from a 13-year surveillance programme. Eur J Gastroenterol Hepatol. 2000;12:649–654. [PubMed] [Google Scholar]
- 7.Cameron AJ, Ott BJ, Payne WS. The incidence of adenocarcinoma in columnar-lined (Barrett's) esophagus. N Engl J Med. 1985;313:857–859. doi: 10.1056/NEJM198510033131404. [DOI] [PubMed] [Google Scholar]
- 8.Spechler SJ. The frequency of cancer in patients with Barrett's esophagus. Acta Endoscopica. 1992;22:541–544. [Google Scholar]
- 9.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 10.Macdonald CE, Wicks AC, Playford RJ. Ten years' experience of screening patients with Barrett's oesophagus in a university teaching hospital. Gut. 1997;41:303–307. doi: 10.1136/gut.41.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron AJ, Lomboy CT. Barrett's esophagus: age, prevalence, and extent of columnar epithelium. Gastroenterology. 1992;103:1241–1245. doi: 10.1016/0016-5085(92)91510-b. [DOI] [PubMed] [Google Scholar]
- 12.Borrie J, Goldwater L. Columnar cell-lined esophagus: assessment of etiology and treatment. A 22 year experience. J Thorac Cardiovasc Surg. 1976;71:825–834. [PubMed] [Google Scholar]
- 13.Wright TA, Gray MR, Morris AI, Gilmore IT, Ellis A, Smart HL, Myskow M, Nash J, Donnelly RJ, Kingsnorth AN. Cost effectiveness of detecting Barrett's cancer. Gut. 1996;39:574–579. doi: 10.1136/gut.39.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menke-Pluymers MB, Hop WC, Dees J, van Blankenstein M, Tilanus HW. Risk factors for the development of an adenocarcinoma in columnar-lined (Barrett) esophagus. The Rotterdam Esophageal Tumor Study Group. Cancer. 1993;72:1155–1158. doi: 10.1002/1097-0142(19930815)72:4<1155::aid-cncr2820720404>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Sjogren RW, Johnson LF. Barrett's esophagus: a review. Am J Med. 1983;74:313–321. doi: 10.1016/0002-9343(83)90635-6. [DOI] [PubMed] [Google Scholar]
- 16.Robertson CS, Mayberry JF, Nicholson DA, James PD, Atkinson M. Value of endoscopic surveillance in the detection of neoplastic change in Barrett's oesophagus. Br J Surg. 1988;75:760–763. doi: 10.1002/bjs.1800750813. [DOI] [PubMed] [Google Scholar]
- 17.Achkar E, Carey W. The cost of surveillance for adenocarcinoma complicating Barrett's esophagus. Am J Gastroenterol. 1988;83:291–294. [PubMed] [Google Scholar]
- 18.Rogers EL, Goldkind SF, Iseri OA, Bustin M, Goldkind L, Hamilton SR, Smith RL. Adenocarcinoma of the lower esophagus. A disease primarily of white men with Barrett's esophagus. J Clin Gastroenterol. 1986;8:613–618. doi: 10.1097/00004836-198612000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Gopal DV, Lieberman DA, Magaret N, Fennerty MB, Sampliner RE, Garewal HS, Falk GW, Faigel DO. Risk factors for dysplasia in patients with Barrett's esophagus (BE): results from a multicenter consortium. Dig Dis Sci. 2003;48:1537–1541. doi: 10.1023/a:1024715824149. [DOI] [PubMed] [Google Scholar]
- 20.Skinner DB, Walther BC, Riddell RH, Schmidt H, Iascone C, DeMeester TR. Barrett's esophagus. Comparison of benign and malignant cases. Ann Surg. 1983;198:554–565. doi: 10.1097/00000658-198310000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnell TG, Sontag SJ, Chejfec G. Adenocarcinomas arising in tongues or short segments of Barrett's esophagus. Dig Dis Sci. 1992;37:137–143. doi: 10.1007/BF01308357. [DOI] [PubMed] [Google Scholar]
- 22.Cameron AJ, Lomboy CT, Pera M, Carpenter HA. Adenocarcinoma of the esophagogastric junction and Barrett's esophagus. Gastroenterology. 1995;109:1541–1546. doi: 10.1016/0016-5085(95)90642-8. [DOI] [PubMed] [Google Scholar]
- 23.Schnell T, Sontag S, Chejfec G, Miller T, Kurucar C, O'Connell S, Brand L. Does length (L) of Barrett's esophagus (BE) correlate with age, cigarette or alcohol consumption or risk of adenocarcinoma (adCa) (abstract)? Gastroenterology. 1990;98:A120. [Google Scholar]
- 24.Iftikhar SY, James PD, Steele RJ, Hardcastle JD, Atkinson M. Length of Barrett's oesophagus: an important factor in the development of dysplasia and adenocarcinoma. Gut. 1992;33:1155–1158. doi: 10.1136/gut.33.9.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark GW, Smyrk TC, Burdiles P, Hoeft SF, Peters JH, Kiyabu M, Hinder RA, Bremner CG, DeMeester TR. Is Barrett's metaplasia the source of adenocarcinomas of the cardia? Arch Surg. 1994;129:609–614. doi: 10.1001/archsurg.1994.01420300051007. [DOI] [PubMed] [Google Scholar]
- 26.Avidan B, Sonnenberg A, Schnell TG, Chejfec G, Metz A, Sontag SJ. Hiatal hernia size, Barrett's length, and severity of acid reflux are all risk factors for esophageal adenocarcinoma. Am J Gastroenterol. 2002;97:1930–1936. doi: 10.1111/j.1572-0241.2002.05902.x. [DOI] [PubMed] [Google Scholar]
- 27.Weston AP, Badr AS, Hassanein RS. Prospective multivariate analysis of clinical, endoscopic, and histological factors predictive of the development of Barrett's multifocal high-grade dysplasia or adenocarcinoma. Am J Gastroenterol. 1999;94:3413–3419. doi: 10.1111/j.1572-0241.1999.01602.x. [DOI] [PubMed] [Google Scholar]
- 28.Rudolph RE, Vaughan TL, Storer BE, Haggitt RC, Rabinovitch PS, Levine DS, Reid BJ. Effect of segment length on risk for neoplastic progression in patients with Barrett esophagus. Ann Intern Med. 2000;132:612–620. doi: 10.7326/0003-4819-132-8-200004180-00003. [DOI] [PubMed] [Google Scholar]
- 29.Van der Veen AH, Dees J, Blankensteijn JD, Van Blankenstein M. Adenocarcinoma in Barrett's oesophagus: an overrated risk. Gut. 1989;30:14–18. doi: 10.1136/gut.30.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moghissi K, Sharpe DA, Pender D. Adenocarcinoma and Barrett's oesophagus. A clinico-pathological study. Eur J Cardiothorac Surg. 1993;7:126–131. doi: 10.1016/1010-7940(93)90034-9. [DOI] [PubMed] [Google Scholar]
- 31.Lerut T, Coosemans W, Van Raemdonck D, Dillemans B, De Leyn P, Marnette JM, Geboes K. Surgical treatment of Barrett's carcinoma. Correlations between morphologic findings and prognosis. J Thorac Cardiovasc Surg. 1994;107:1059–1065; discussion 1059-1065;. doi: 10.1007/978-4-431-68246-2_25. [DOI] [PubMed] [Google Scholar]
- 32.Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 33.Brown LM, Swanson CA, Gridley G, Swanson GM, Schoenberg JB, Greenberg RS, Silverman DT, Pottern LM, Hayes RB, Schwartz AG. Adenocarcinoma of the esophagus: role of obesity and diet. J Natl Cancer Inst. 1995;87:104–109. doi: 10.1093/jnci/87.2.104. [DOI] [PubMed] [Google Scholar]
- 34.Lagergren J, Bergström R, Nyrén O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999;130:883–890. doi: 10.7326/0003-4819-130-11-199906010-00003. [DOI] [PubMed] [Google Scholar]
- 35.Gray MR, Donnelly RJ, Kingsnorth AN. The role of smoking and alcohol in metaplasia and cancer risk in Barrett's columnar lined oesophagus. Gut. 1993;34:727–731. doi: 10.1136/gut.34.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allison PR, Johnstone AS. The oesophagus lined with gastric mucous membrane. Thorax. 1953;8:87–101. doi: 10.1136/thx.8.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haggitt RC, Tryzelaar J, Ellis FH, Colcher H. Adenocarcinoma complicating columnar epithelium-lined (Barrett's) esophagus. Am J Clin Pathol. 1978;70:1–5. doi: 10.1093/ajcp/70.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Chow WH, Finkle WD, McLaughlin JK, Frankl H, Ziel HK, Fraumeni JF. The relation of gastroesophageal reflux disease and its treatment to adenocarcinomas of the esophagus and gastric cardia. JAMA. 1995;274:474–477. [PubMed] [Google Scholar]
- 39.van der Burgh A, Dees J, Hop WC, van Blankenstein M. Oesophageal cancer is an uncommon cause of death in patients with Barrett's oesophagus. Gut. 1996;39:5–8. doi: 10.1136/gut.39.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton SR, Smith RR, Cameron JL. Prevalence and characteristics of Barrett esophagus in patients with adenocarcinoma of the esophagus or esophagogastric junction. Hum Pathol. 1988;19:942–948. doi: 10.1016/s0046-8177(88)80010-8. [DOI] [PubMed] [Google Scholar]
- 41.Harle IA, Finley RJ, Belsheim M, Bondy DC, Booth M, Lloyd D, McDonald JW, Sullivan S, Valberg LS, Watson WC. Management of adenocarcinoma in a columnar-lined esophagus. Ann Thorac Surg. 1985;40:330–336. doi: 10.1016/s0003-4975(10)60062-8. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg JC, Budev H, Edwards RC, Singal S, Steiger Z, Sundareson AS. Analysis of adenocarcinoma in Barrett's esophagus utilizing a staging system. Cancer. 1985;55:1353–1360. doi: 10.1002/1097-0142(19850315)55:6<1353::aid-cncr2820550632>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 43.Levine DS, Haggitt RC, Blount PL, Rabinovitch PS, Rusch VW, Reid BJ. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett's esophagus. Gastroenterology. 1993;105:40–50. doi: 10.1016/0016-5085(93)90008-z. [DOI] [PubMed] [Google Scholar]
- 44.Sharma P. Short segment Barrett esophagus and specialized columnar mucosa at the gastroesophageal junction. Mayo Clin Proc. 2001;76:331–334. doi: 10.4065/76.3.331. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds JC, Rahimi P, Hirschl D. Barrett's esophagus: clinical characteristics. Gastroenterol Clin North Am. 2002;31:441–460. doi: 10.1016/s0889-8553(02)00019-5. [DOI] [PubMed] [Google Scholar]
- 46.Gammon MD, Schoenberg JB, Ahsan H, Risch HA, Vaughan TL, Chow WH, Rotterdam H, West AB, Dubrow R, Stanford JL, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277–1284. doi: 10.1093/jnci/89.17.1277. [DOI] [PubMed] [Google Scholar]
- 47.Garidou A, Tzonou A, Lipworth L, Signorello LB, Kalapothaki V, Trichopoulos D. Life-style factors and medical conditions in relation to esophageal cancer by histologic type in a low-risk population. Int J Cancer. 1996;68:295–299. doi: 10.1002/(SICI)1097-0215(19961104)68:3<295::AID-IJC5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 48.Zhang ZF, Kurtz RC, Sun M, Karpeh M, Yu GP, Gargon N, Fein JS, Georgopoulos SK, Harlap S. Adenocarcinomas of the esophagus and gastric cardia: medical conditions, tobacco, alcohol, and socioeconomic factors. Cancer Epidemiol Biomarkers Prev. 1996;5:761–768. [PubMed] [Google Scholar]
- 49.Kabat GC, Ng SK, Wynder EL. Tobacco, alcohol intake, and diet in relation to adenocarcinoma of the esophagus and gastric cardia. Cancer Causes Control. 1993;4:123–132. doi: 10.1007/BF00053153. [DOI] [PubMed] [Google Scholar]
- 50.Spechler SJ, Robbins AH, Rubins HB, Vincent ME, Heeren T, Doos WG, Colton T, Schimmel EM. Adenocarcinoma and Barrett's esophagus. An overrated risk? Gastroenterology. 1984;87:927–933. [PubMed] [Google Scholar]
- 51.Avidan B, Sonnenberg A, Schnell TG, Sontag SJ. Hiatal hernia and acid reflux frequency predict presence and length of Barrett's esophagus. Dig Dis Sci. 2002;47:256–264. doi: 10.1023/a:1013797417170. [DOI] [PubMed] [Google Scholar]
- 52.Conio M, Filiberti R, Blanchi S, Ferraris R, Marchi S, Ravelli P, Lapertosa G, Iaquinto G, Sablich R, Gusmaroli R, et al. Risk factors for Barrett's esophagus: a case-control study. Int J Cancer. 2002;97:225–229. doi: 10.1002/ijc.1583. [DOI] [PubMed] [Google Scholar]
- 53.Grønbaek M, Becker U, Johansen D, Tønnesen H, Jensen G, Sørensen TI. Population based cohort study of the association between alcohol intake and cancer of the upper digestive tract. BMJ. 1998;317:844–847. doi: 10.1136/bmj.317.7162.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper BT, Barbezat GO. Barrett's oesophagus: a clinical study of 52 patients. Q J Med. 1987;62:97–108. [PubMed] [Google Scholar]
- 55.Levi F, Ollyo JB, La Vecchia C, Boyle P, Monnier P, Savary M. The consumption of tobacco, alcohol and the risk of adenocarcinoma in Barrett's oesophagus. Int J Cancer. 1990;45:852–854. doi: 10.1002/ijc.2910450511. [DOI] [PubMed] [Google Scholar]
- 56.Herlihy KJ, Orlando RC, Bryson JC, Bozymski EM, Carney CN, Powell DW. Barrett's esophagus: clinical, endoscopic, histologic, manometric, and electrical potential difference characteristics. Gastroenterology. 1984;86:436–443. [PubMed] [Google Scholar]
- 57.Day NE, Munoz N. Esophagus. In: Schottenfeld D, Fraumeni JF Jr, eds , editors. Cancer epidemiology and prevention Philadelphia: WB Saunders Co; 1982. pp. 596–623. [Google Scholar]
- 58.Goldman MC, Beckman RC. Barrett syndrome. Case report with discussion about concepts of pathogenesis. Gastroenterology. 1960;39:104–110. [PubMed] [Google Scholar]
- 59.Halvorsen JF, Semb BK. The "Barrett syndrome" (the columnar-lined lower oesophagus): an acquired condition secondary to reflux oesophagitis. A case report with discussion of pathogenesis. Acta Chir Scand. 1975;141:683–687. [PubMed] [Google Scholar]
- 60.Jankowski J. Gene expression in Barrett's mucosa: acute and chronic adaptive responses in the oesophagus. Gut. 1993;34:1649–1650. doi: 10.1136/gut.34.12.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morgan G, Vainio H. Barrett's oesophagus, oesophageal cancer and colon cancer: an explanation of the association and cancer chemopreventive potential of non-steroidal anti-inflammatory drugs. Eur J Cancer Prev. 1998;7:195–199. doi: 10.1097/00008469-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Heath EI, Limburg PJ, Hawk ET, Forastiere AA. Adenocarcinoma of the esophagus: risk factors and prevention. Oncology (Williston Park) 2000;14:507–514; discussion 518-520, 522-523. [PubMed] [Google Scholar]
- 63.Paull G, Yardley JH. Gastric and esophageal Campylobacter pylori in patients with Barrett's esophagus. Gastroenterology. 1988;95:216–218. doi: 10.1016/0016-5085(88)90316-2. [DOI] [PubMed] [Google Scholar]
- 64.Talley NJ, Cameron AJ, Shorter RG, Zinsmeister AR, Phillips SF. Campylobacter pylori and Barrett's esophagus. Mayo Clin Proc. 1988;63:1176–1180. doi: 10.1016/s0025-6196(12)65402-0. [DOI] [PubMed] [Google Scholar]
- 65.Weston AP, Badr AS, Topalovski M, Cherian R, Dixon A, Hassanein RS. Prospective evaluation of the prevalence of gastric Helicobacter pylori infection in patients with GERD, Barrett's esophagus, Barrett's dysplasia, and Barrett's adenocarcinoma. Am J Gastroenterol. 2000;95:387–394. doi: 10.1111/j.1572-0241.2000.01758.x. [DOI] [PubMed] [Google Scholar]
- 66.Bani-Hani K, Martin IG, Hardie LJ, Mapstone N, Briggs JA, Forman D, Wild CP. Prospective study of cyclin D1 overexpression in Barrett's esophagus: association with increased risk of adenocarcinoma. J Natl Cancer Inst. 2000;92:1316–1321. doi: 10.1093/jnci/92.16.1316. [DOI] [PubMed] [Google Scholar]
- 67.Wild CP, Forman D. Surveillance for Barrett's oesophagus. It is too early to dismiss surveillance programmes. BMJ. 2001;322:1125; author reply 1126. [PubMed] [Google Scholar]


