Abstract
AIM: To elucidate the effect of p27KIP1 on cell cycle and apoptosis regulation in gastric carcinoma cells.
METHODS: The whole length of p27KIP1 cDNA was transfected into human gastric cancer cell line SCG7901 by lipofectamine. Expression of p27KIP1 protein or mRNA was analyzed by Western blot and RNA dot blotting, respectively. Effect of p27KIP1 on cell growth was observed by MTT assay and anchorage-independent growth in soft agar. Tumorigenicity in nude mice was used to assess the in vivo biological effect of p27KIP1. Flow cytometry, TUNEL, and electron microscopy were used to assess the effect of p27KIP1 on cell cycle and apoptosis.
RESULTS: Expression of p27KIP1 protein or mRNA increased evidently in SCG7901 cells transfected with p27KIP1. The cell growth was reduced by 31% at 48 h after induction with zinc determined by cell viability assay. The alteration of cell malignant phenotype was evidently indicated by the loss of anchorage-independent growth ability in soft agar. The tumorigenicity in nude mice was reduced evidently (0.55±0.14 cm vs 1.36±0.13 cm, P<0.01). p27KIP1 overexpression caused cell arrest with 36% increase (from 33.7% to 69.3%, P<0.01) in G1 population. Prolonged p27KIP1 expression induced apoptotic cell death reflected by pre-G1 peak in the histogram of FACS, which was also confirmed by TUNEL assay and electron microscopy.
CONCLUSION: p27KIP1 can prolong cell cycle in G1 phase and lead to apoptosis. p27KIP1 may be a good candidate for cancer gene therapy.
Keywords: Cell cycle, Apoptosis, Gastric neoplasm, p27KIP1
INTRODUCTION
The number of cells is regulated by a balance between proliferation, growth arrest and programmed cell death (apoptosis, PCD). Disorders of the cell cycle and apoptosis are closely related to the progression and aggressiveness of cancers[1]. p27KIP1, a member of the Cip/Kip family of cyclin-dependent kinase inhibitors (CDKI), was first identified as a negative cell cycle regulator in transforming growth factor β-treated cells and in G1 phase quiescent cells by cell contact inhibition[2,3]. p27KIP1 binds to a wide variety of cyclin/CDK complexes including CDK2 and CDK4[4], inhibits kinase activity[5] and blocks cell cycle[6-8]. The overexpression of p27KIP1 protein in mammalian cells induces G1 arrest of the cell cycle and apoptosis[9,10]. Decreased p27KIP1, which is positively correlated with a deceased rate of apoptosis, may be not only an indicator of tumor aggressiveness, but also an important prognostic marker in gastric carcinoma[11].
In this study, an inducible expression system was used to induce overexpression of p27KIP1 in human gastric cancer cells. Then whether transfection of SCG7901 with p27KIP1 gene could alter the cell and molecular biology as well as the spontaneous rate of apoptosis was studied.
MATERIALS AND METHODS
Cell culture
Human gastric cancer cell line SCG7901 was provided by the Department of Pathology, Fourth Military Medical University (Xi'an, China). SCG7901 cells were cultured in Eagle’s medium containing phenol red supplemented with 50 mL/L fetal bovine serum (FBS) in a humidified atmosphere of 950 mL/L air and 50 mL/L CO2 at 37 °C. All the media were supplemented with 2 mmol/L L-glutamine, 100 mg/L penicillin and 100 kU/L streptomycin. Culture medium and supplements were obtained from Gibco BRL.
Plasmid construction and DNA transfection
pGEM T-Easy-KIP1 vector containing human full-length cDNA of p27KIP1 was used. Complementary DNA of p27KIP1 cleaved from the pGEM T-Easy-KIP1 plasmid by EcoRI was subcloned into the inducible vector neo-control pMD-neo vector to generate pMD-KIP1 containing 0.6 kb p27KIP1 cDNA and controlled expression of protein upon addition of 100 mmol/L zinc as an external inducer. Clonfectin (Clontech, USA) was used to transfect p27 KIP1 inducible expression vectors into SGC7901 cells. Logarithmically growing cells were transfected with 1 μg of plasmids and 2 μL of clonfectin reagent according to the manufacturer’s instructions. For stable transfection, the transfected SGC7901 cells were selected in medium plus 0.5 g/L G418 (Life Technologies, USA) for 2 wk, then the G418 concentration was reduced to 0.2 g/L. Stable SGC7901 cell line transfected with pMD-KIP1 vector was treated by continuous exposure to 100 mmol/L ZnSO4.
Western blot analysis of transgene expression
Monolayers of the cells were rinsed with PBS and lysed with SDS-PAGE loading buffer (50 mmol/L Tris-HCl pH 6.8, 100 mmol/L dithiothreitol, 2 g/L SDS). Mock and pMD-neo transfected cells served as controls. Samples were analyzed by SDS-PAGE and transferred into Hybond-C super membranes. The membranes were blocked with 50 mL/L skim-milk and 1 g/L Tween-20, then probed with primary antibody overnight according to the manufacturer’s instructions, washed in PBS, 2 g/L Tween-20 and then incubated with appropriate horseradish-peroxidase (HRP) conjugated second antibody. After being washed, the membranes were developed by DAB reagents according to the manufacturer’s guide (Dako Co., USA). p27KIP1 (Santa Cruz Biotechnology, USA) was detected after HRP-conjugated anti-rabbit IgG (Fc) was used. The level of β-actin was used as a control for equal loading of protein.
Total RNA extraction and RNA dot blotting
Total RNA was extracted from SGC7901 cells using TRIzol (Life Technologies, USA) following a standard acid-guanidium-phenolchloroform method. The digoxingenin-labeled specific DNA probe was obtained as follows. The plasmid containing p27KIP1 cDNA was used as template, a pair of primers (-5 °C-ggggtaccatgtcaaacgtgcgagt-3 °Cand -5 °C-gctcaacgtttgacgtcttc-3 °C) was used to amplify the sequence. The PCR system containing Dig-11-dUTP and four normal dNTPs and Taq polymerase were used. After a standard PCR procedure (94 °C to 30 s, 60 °C for 30 s, 72 °C for 1 min, 30 cycles), the PCR product was purified by 1% agarose electrophoresis and DNA extraction. The labeling efficiency was further determined by Dot blot of serially diluted plasmid. Finally, the digoxingenin-labeled DNA probe was used in the experiment. RNA Dot blot was performed according to the Dig system user’s guide for filter hybridization (Boehringer Mannheim, Germany). Briefly, RNA sample was diluted in RNA dilution buffer (a mixture of DEPC-treated H2O, 20×SSC, and formaldehyde at 5:3:2). The membrane was marked slightly with a pencil to identify each dilution before spotting. One microliter of the RNA sample was spotted onto a dry nylon membrane using a micropipettor. Then the RNA was fixed to the membrane by baking in an oven at 120 °Cfor 30 min. Hybridization and the following procedures were similar to in situ hybridization. Hybridization mixture contained 5×SSC, 500 g/L deionized formamide, 1 g/L sodium-lauroyl sarcosine, 0.2 g/L SDS, 20 g/L blocking reagent and a 25 ng/mL DNA probe. The level of GADPH mRNA was used as a control for equal loading of RNA.
MTT and colony assay
SGC7901/KIP1 and SGC7901/pMD cells (1×104) were seeded in 96-well plates and continuously exposed to 100 mmoL/L ZnSO4. After treatment, 50 μL of 5 g/L 3-(4,4-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide (MTT) in PBS was added to each well, incubated for 4 h at 37 °C and the formed formazan crystals were dissolved in 50 μL of dimethyl sulfoxide. The absorbance was recorded at 490 nm on a microplate reader (BioRad). Cell proliferation was expressed as IC50 for cells. Soft agar was prepared with the 6 g/L bottom agar layer and the 4 g/L top agar layer. The number of cells plated for each clone was 2 000. Plates were incubated at 37 °C with 50 mL/L CO2 for 2 wk and the colonies were counted and photographed under a phase contrast microscope (Nikon, Tokyo, Japan).
Tumorigenicity tests in nude mice
p27KIP1-transfected SGC7901/KIP1 cells were injected into the right flank of a nude mouse at 2.5×106. pMD-neo-transfected SGC7901/pMD cells served as control. Each group had five nude mice. From the second day, each nude mouse received 100 mmol/L ZnSO4 at a dose of 0.2 mL daily by subcutaneous injection for over 4 wk. The time of tumorigenicity and the tumor volume were measured.
Cell cycle analysis
SGC7901 cells including adherent cells (with trypsin-EDTA) and nonadherent cells were harvested. All cells were washed with PBS, resuspended and incubated in 700 mL/L ethanol for at least 12 h at 4 °C to permeabilize the plasma membrane. Cells were centrifuged at 1 000 r/min, resuspended in 100 mg/L RNase and 10 mg/L propidium iodide, incubated for 15 min at 25 °Cin the dark. Single color fluorescent flow cytometry was performed with a FACScalibur flow cytometer (Becton Dickinson, USA). The histograms were analyzed with Multiplus Software П.
Electron microscopy
SGC7901/pMD cells were gently washed with serum-free medium and fixed with 25 g/L glutaraldehyde in 0.1 mol/L sodium cacodylate buffer. These cells were scraped from the surface of the dishes and pelleted by spinning for 5 min at 10 000 g. The cells were osmicated with 10 g/L osmium tetroxide. The block was stained, dehydrated in graded ethanol, infiltrated with propylene oxide, embedded with EMBED overnight and cured in a 60 °C oven for 48 h. Silver sections were cut with an Ultracut E microtome, collected on a formvar and carbon-coated grid, stained with uranyl acetate and Reynold’s lead citrate, and viewed under a JEOL 100 CX electron microscope.
Apoptosis analyzed by TUNEL assay
SGC7901/KIP1 and SGC7901/pMD cells were harvested for TUNEL staining. The proportion of cells showing DNA fragmentation was measured by incorporation of fluorescein (FITC)-12-dUTP into DNA using terminal deoxynucleotidyltransferase (TdT). The kit was bought from Boehringer Mannheim (In Situ Cell Death Detection). Cells were fixed by 40 g/L paraformaldehyde in PBS overnight at 4 °C. The samples were washed thrice with PBS and permeabilized by 2 g/L Triton X-100 in PBS for 15 min on ice. After being washed twice, cells were equilibrated at room temperature for 15-30 min in a equilibration buffer with 30 g/L BSA and 200 mL/L normal bovine serum in PBS at pH 7.4, then the slides were covered with the TUNEL mixture (calf thymus TdT, FITC-12-dUTP, and cobalt chloride in 1×reaction buffer) for 1-2 h at 37 °Cin the dark. The tailing reaction was terminated by 2×standard saline citrate (SSC). The samples were washed thrice with PBS and analyzed by fluorescence microscopy. Routine HE staining was also conducted. Negative control was performed by omitting TdT. Quantitative analysis of apoptosis was represented by apoptotic index (AI) as described previously. AI referred to percentages from at least 1 000 counts apoptotic and non-apoptotic cells.
Statistical analysis
Fisher’s exact test was performed. P<0.05 was considered statistically significant.
RESULTS
Inducible expression of p27KIP1 in SGC7901 cells
Western blot analysis demonstrated p27KIP1 expression 0, 4, 24, 48, and 72 h after the addition of ZnSO4. Mock and pMD-neo vector-transfected cells as a control contained no detectable level of p27KIP1 in the presence of 100 mmol/L ZnSO4. However, p27KIP1 expression was increased at 4 h and further increased to a steady level at 24 h after the addition of ZnSO4 as determined by the Western blot analysis (Figure 1). In RNA dot blotting survey of the expression, p27KIP1 mRNA was very low in untransfected or noninduced SGC7901 cells. In contrast, mRNA for p27KIP1 was expressed at a high level 4 and 24 h after the addition of ZnSO4 (Figure 2). This expression pattern was rather consistent with the expression of protein.
Figure 1.
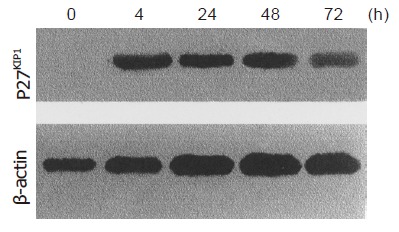
Induction of p27KIP1 expression by Western blotting analysis.
Figure 2.
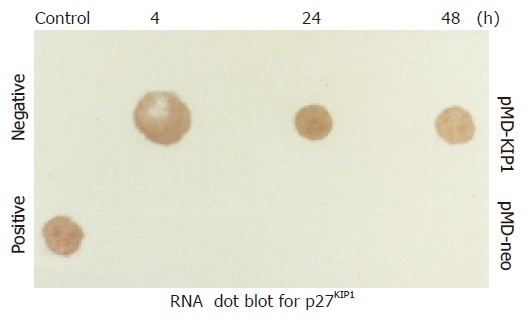
Expression of p27KIP1 in mRNA level by RNA dot blot analysis.
Inhibition of tumor cell growth by exogenous p27KIP1
Exogenous p27KIP1 inhibited cell growth significantly. SGC7901 cell viability was determined by MTT assay (Figure 3). The cell viability rate decreased from 88% to 57% 48 h after p27KIP1 induction with zinc treatment (P<0.01). Another biological feature of malignant cells was anchorage-independent growth capability. The neo-control SGC7901/pMD and SGC7901 cells formed large colonies in each well after 2 wk, and the rate of colony formation was increased by 80% and 60%, respectively. SGC7901/KIP1 cells showed markedly lower ability to form colony with a rate of 30% (P<0.01). Moreover, tumors were generated in nude mice on the 7th d from SGC7901/pMD cells, but SGC7901/KIP1 cells generated tumor on the 12th d and grew slowly. The tumor diameter generated from SGC7901/KIP1 cells was smaller than that from SGC7901/pMD cells (0.55±0.14 vs 1.36±0.13 cm, P<0.01, Figure 4). These data indicated that the exogenous p27KIP1 had a cytostatic effect on cell growth.
Figure 3.
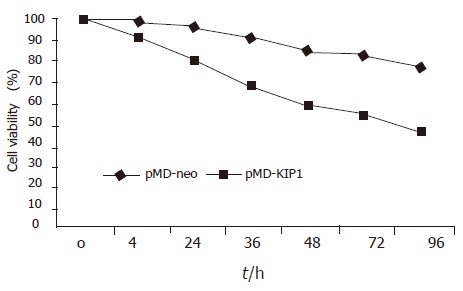
Growth course of SGC7901 cells.
Figure 4.
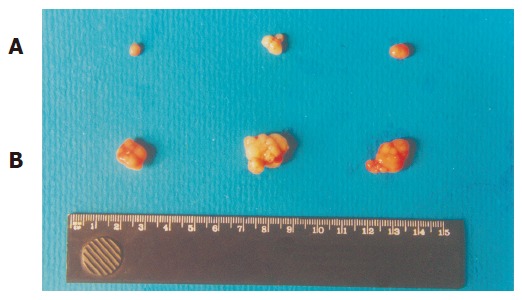
Tumorigenicity in nude mice. A: SGC7901/KIP1 group; B: SGC7901 group.
Overexpression of p27KIP1 prolonged cell cycle in G1 phase
p27KIP1 was identified as a negative cell cycle regulator in transforming growth factor β-treated cells and in G1 phase quiescent cells by cell contact inhibition. The overexpression of p27KIP1 protein in mammalian cells induced G1 arrest of the cell cycle. In order to investigate whether p27KIP1 had the ability to induce G1 arrest in SGC7901 cells, cell cycle analysis was performed on transfected SGC7901 cells. Flow cytometry analysis showed that overexpression of p27KIP1 increased remarkably the proportion of SGC7901 cells with G1 phase DNA content increased from 33.7% to 69.3% (P = 0.000) and decreased with S phase DNA content at 48 h after induction (Figure 5). There was no significant change in the proportion of cells with G2/M phase DNA content.
Figure 5.
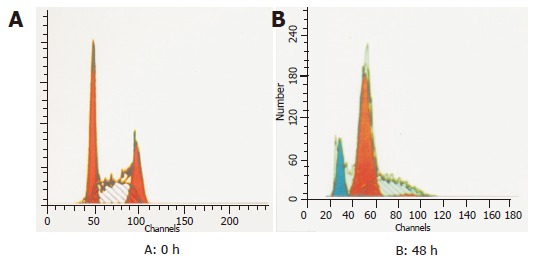
Flow cytometric analysis of SGC7901 cells at 0 h (A) and 48 h (B).
Overexpression of p27KIP1-induced apoptosis in SGC7901 cells
Morphological changes SGC7901/KIP1 cells-transfected p27KIP1 altered their morphology and induced DNA strand breaks in a manner consistent with apoptosis. The changes were indeed induced by apoptosis rather than necrosis, which was confirmed by electron microscopy. SGC7901/KIP1 cells showed compacted nuclear chromatin with fine granular masses marginated against the nuclear-enveloped and condensed cytoplasm, the nuclear outline was convoluted and the organelles were preserved (Figure 6).
Figure 6.
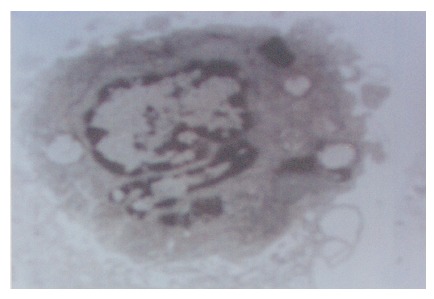
Ultrastructural changes associated with apoptosis in SGC7901/KIP1 cells (TEM×7 500).
TUNEL assay To determine whether overexpression of p27KIP1 was able to induce apoptosis in SGC7901 cells, TUNEL assay was performed. Compared to the pMD-neo-transfected SGC7901/pMD cells, p27KIP1-overexpressed SGC7901/KIP1 cells showed obvious morphological changes. Some apoptotic cells appeared round and condensed cytoplasm as well as highly fragmented chromatin. TUNEL-positive cells were found with Kelly or green fluorescence in nucleoli. Cells without apoptosis observed by fluorescence microscopy had no fluorescence. Apoptotic index significantly increased in p27KIP1-transfected SGC7901/KIP1 cells 24 and 48 h after being exposed to 100 mmol/L ZnSO4 (3.0 vs 21.2, 3.6 vs 26.3, P<0.05).
Flow cytometry In order to determine the effect of p27KIP1 overexpression on apoptosis in SGC7901 cells, cells were exposed to 100 mmol/L ZnSO4 for 48 h and apoptotic damage of DNA was detected according to the sub-G1 peak on a flow cytometer. Overexpression of p27KIP1 showed significantly higher apoptotic peak than normal control as the sub-G1 peak indicated. The apoptotic cells accounted for about 14.7% (Figure 5).
Taken together, p27KIP1 overexpression could inhibit proliferation of SGC7901 cells and subsequently induce apoptosis.
DISCUSSION
Gastric cancer is the second most common fatal malignancy in the world[12-14] and is still the leading cause of cancer-related deaths in China. The development of gastric cancer is a multi-factorial process[15]. A balance between proliferation and apoptosis of tumor cells is important for tumor growth. In gastric cancer, proliferative activity and apoptosis of cancer cells are associated with tumor growth[16]. Cyclins, cyclin-dependent kinase (CDK) and their inhibitors regulate cell growth, differentiation, survival and death. Cell cycle which progresses sequentially through G1, DNA synthesis (S), G2, and mitosis (M) phases, regulates DNA replication and chromosomal segregation into the daughter cells. Gene abnormalities and aberrant expression of cell cycle regulators play a pivotal role in the pathogenesis of gastrointestinal tumors[17], of which CDKIs are major regulators.
p27KIP1 is a member of the Cip/Kip family of CDKIs that regulate cell cycle progression, thus inhibiting various cycle-CDK complexes. Physiologically, p27KIP1 is believed to primarily regulate progression of cells from late G1 into S phase by interacting with cyclin E-CDK2 complexes[9]. p27KIP1 has been implicated as a mediator of growth arrest due to TGF-β, cAMP and other extracellular factors. Moreover, elevated expression of p27KIP1 protein leads to G1 arrest in many cell types and promotes neuronal differentiation in mouse neuroblastoma cells, while inhibition of p27KIP1 expression through the use of antisense technology prevents G1 arrest and/or suppresses entry of fibroblasts into a state of quiescence in response to mitogen depletion.
Interestingly, in many types of tumors such as gastric[18,19], prostate[20,21] and breast carcinoma, the expression of p27KIP1 gene is downregulated[22,23]. Loss of p27KIP1 expression may result in tumor development and/or progression. However, this loss of expression does not appear from the result of gene mutations. It is believed that the aberrant expression of p27KIP1 plays a pivotal role in the pathogenesis of carcinoma. Malignant human renal tumor cell transfection with p27KIP1 gene leads to inhibition of proliferation and cell cycle arrest in G1[24]. Ectopic overexpression of p27KIP1 is associated with a striking decrease in aneuploid cells, loss of anchorage-independent growth in soft agar and failure to induce tumor development in a xenograft model[25]. In this report, we have also demonstrated that p27KIP1 overexpression could inhibit proliferation of SGC7901 cells. The cell growth was reduced by 31% at 48 h, after the induction with zinc as determined by cell viability assay. The alteration of cell malignant phenotype was evidently indicated by the loss of anchorage-independent growth ability in soft agar. The tumorigenicity in nude mice was reduced evidently (P<0.01). p27KIP1 overexpression increased cell arrest from 33.7% to 69.3% (P<0.01) in G1 population.
Recent evidence demonstrated that high levels of p27KIP1 protein induced by adenovirus vector leads to growth arrest as well as enhancement of apoptosis in several cell lines from different species and tissues of various origins[11,25]. Decreased p27KIP1, which is positively correlated with a decreased rate of apoptosis, may be not only an indicator of tumor aggressiveness, but also an important prognostic marker in gastric carcinoma[16]. In this study, we have demonstrated that overexpression of p27KIP1 not only prolonged cell cycle in G1 phase of SGC7901 cells but also subsequently induced apoptosis as reflected by pre-G1 peak in the histogram of FACS, TUNEL assay and electron microscopy. This is the first report in which a significant correlation between p27KIP1 expression and cell cycle arrest and apoptosis has been demonstrated in SGC7901 cell line.
Mice lacking p27KIP1 are abnormally large and display multiple organ hyperplasia indicative of tissue overgrowth[26,27]. Thus, it is possible that p27KIP1 plays an important role in maintaining organ size through the induction of apoptosis. However, the mechanism of tumor growth suppression appears to include p27KIP1 but also various other properties such as p53. p53 serves as a major G1 checkpoint regulator through the induction of p21 but not of p27KIP1 and p57, while it induces apoptosis. The p53-mediated CDK inhibitor p21 does not lead to significant apoptosis though both p21 and p27KIP1 induce G1 arrest through their potential abilities to inhibit CDK activity. These findings suggest that the inhibition of CDK activity may not be sufficient to induce apoptotic cell death. A possible explanation of these differences between p27KIP1 and p53-mediated p21 function is that p27KIP1 may have other functions in addition to its notable function as a CDKI. It can also be speculated that p27KIP1, which has the potential to induce growth arrest and apoptosis, may play a more important role in tumor growth suppression than p53-mediated p21. Further understanding of the mechanism involved in this process can improve chemotherapeutic strategies aimed at achieving high level p27KIP1 expression and gene therapy.
Footnotes
Science Editor Wang XL and Li WZ Language Editor Elsevier HK
References
- 1.Krug U, Ganser A, Koeffler HP. Tumor suppressor genes in normal and malignant hematopoiesis. Oncogene. 2002;21:3475–3495. doi: 10.1038/sj.onc.1205322. [DOI] [PubMed] [Google Scholar]
- 2.Nho RS, Sheaff RJ. p27kip1 contributions to cancer. Prog Cell Cycle Res. 2003;5:249–259. [PubMed] [Google Scholar]
- 3.Hengst L, Dulic V, Slingerland JM, Lees E, Reed SI. A cell cycle-regulated inhibitor of cyclin-dependent kinases. Proc Natl Acad Sci USA. 1994;91:5291–5295. doi: 10.1073/pnas.91.12.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blain SW, Scher HI, Cordon-Cardo C, Koff A. p27 as a target for cancer therapeutics. Cancer Cell. 2003;3:111–115. doi: 10.1016/s1535-6108(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 5.Alkarain A, Slingerland J. Deregulation of p27 by oncogenic signaling and its prognostic significance in breast cancer. Breast Cancer Res. 2004;6:13–21. doi: 10.1186/bcr722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chetty R. p27 Protein and cancers of the gastrointestinal tract and liver: an overview. J Clin Gastroenterol. 2003;37:23–27. doi: 10.1097/00004836-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Russo AA, Jeffrey PD, Patten AK, Massagué J, Pavletich NP. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, Scheithauer BW. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154:313–323. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davison EA, Lee CS, Naylor MJ, Oakes SR, Sutherland RL, Hennighausen L, Ormandy CJ, Musgrove EA. The cyclin-dependent kinase inhibitor p27 (Kip1) regulates both DNA synthesis and apoptosis in mammary epithelium but is not required for its functional development during pregnancy. Mol Endocrinol. 2003;17:2436–2447. doi: 10.1210/me.2003-0199. [DOI] [PubMed] [Google Scholar]
- 11.Katayose Y, Kim M, Rakkar AN, Li Z, Cowan KH, Seth P. Promoting apoptosis: a novel activity associated with the cyclin-dependent kinase inhibitor p27. Cancer Res. 1997;57:5441–5445. [PubMed] [Google Scholar]
- 12.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 13.Bani-Hani KE, Yaghan RJ, Heis HA, Shatnawi NJ, Matalka II, Bani-Hani AM, Gharaibeh KA. Gastric malignancies in Northern Jordan with special emphasis on descriptive epidemiology. World J Gastroenterol. 2004;10:2174–2178. doi: 10.3748/wjg.v10.i15.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong JE, Ito Y, Correa P, Peeters KC, van de Velde CJ, Sasako M, Macdonald J. Therapeutic strategies in gastric cancer. J Clin Oncol. 2003;21:267s–269s. doi: 10.1200/JCO.2003.08.536. [DOI] [PubMed] [Google Scholar]
- 15.Oluwasola AO, Ogunbiyi JO. Gastric cancer: aetiological, clinicopathological and management patterns in Nigeria. Niger J Med. 2003;12:177–186. [PubMed] [Google Scholar]
- 16.Ohtani M, Isozaki H, Fujii K, Nomura E, Niki M, Mabuchi H, Nishiguchi K, Toyoda M, Ishibashi T, Tanigawa N. Impact of the expression of cyclin-dependent kinase inhibitor p27Kip1 and apoptosis in tumor cells on the overall survival of patients with non-early stage gastric carcinoma. Cancer. 1999;85:1711–1718. [PubMed] [Google Scholar]
- 17.Koide N, Nishio A, Igarashi J, Kajikawa S, Adachi W, Amano J. Alpha-fetoprotein-producing gastric cancer: histochemical analysis of cell proliferation, apoptosis, and angiogenesis. Am J Gastroenterol. 1999;94:1658–1663. doi: 10.1111/j.1572-0241.1999.01158.x. [DOI] [PubMed] [Google Scholar]
- 18.Nitti D, Belluco C, Mammano E, Marchet A, Ambrosi A, Mencarelli R, Segato P, Lise M. Low level of p27(Kip1) protein expression in gastric adenocarcinoma is associated with disease progression and poor outcome. J Surg Oncol. 2002;81:167–175; discussion 175-176. doi: 10.1002/jso.10172. [DOI] [PubMed] [Google Scholar]
- 19.Philipp-Staheli J, Kim KH. Payne SR., Gurley KE, Liggitt D, Longto G, Kemp CJ. Pathway-specific tumor suppression. Reduction of p27 accelerates gastrointestinal tumorigenesis in Apc mutant mice, but not in Smad3 mutant mice. Cancer Cell. 2002;1:355–368 DOI : 10.1016/S1535-6108(02)00054-5. doi: 10.1016/s1535-6108(02)00054-5. [DOI] [PubMed] [Google Scholar]
- 20.Fernández PL, Arce Y, Farré X, Martínez A, Nadal A, Rey MJ, Peiró N, Campo E, Cardesa A. Expression of p27/Kip1 is down-regulated in human prostate carcinoma progression. J Pathol. 1999;187:563–566. doi: 10.1002/(SICI)1096-9896(199904)187:5<563::AID-PATH292>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Tsihlias J, Kapusta LR, DeBoer G, Morava-Protzner I, Zbieranowski I, Bhattacharya N, Catzavelos GC, Klotz LH, Slingerland JM. Loss of cyclin-dependent kinase inhibitor p27Kip1 is a novel prognostic factor in localized human prostate adenocarcinoma. Cancer Res. 1998;58:542–548. [PubMed] [Google Scholar]
- 22.Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 23.Tsihlias J, Kapusta L, Slingerland J. The prognostic significance of altered cyclin-dependent kinase inhibitors in human cancer. Annu Rev Med. 1999;50:401–423. doi: 10.1146/annurev.med.50.1.401. [DOI] [PubMed] [Google Scholar]
- 24.Katner AL, Gootam P, Hoang QB, Gnarra JR, Rayford W. A recombinant adenovirus expressing p7(Kip1) induces cell cycle arrest and apoptosis in human 786-0 renal carcinoma cells. J Urol. 2002;168:766–773. [PubMed] [Google Scholar]
- 25.Katner AL, Hoang QB, Gootam P, Jaruga E, Ma Q, Gnarra J, Rayford W. Induction of cell cycle arrest and apoptosis in human prostate carcinoma cells by a recombinant adenovirus expressing p27(Kip1) Prostate. 2002;53:77–87. doi: 10.1002/pros.10124. [DOI] [PubMed] [Google Scholar]
- 26.Cipriano SC, Chen L, Burns KH, Koff A, Matzuk MM. Inhibin and p27 interact to regulate gonadal tumorigenesis. Mol Endocrinol. 2001;15:985–996. doi: 10.1210/mend.15.6.0650. [DOI] [PubMed] [Google Scholar]
- 27.Sotillo R, Dubus P, Martín J, de la Cueva E, Ortega S, Malumbres M, Barbacid M. Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors. EMBO J. 2001;20:6637–6647. doi: 10.1093/emboj/20.23.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]


