Abstract
Although various animal models have been developed to clarify gastric carcinogenesis, apparent mechanism of gastric cancer was not clarified in recent years. Since the recognition of the pathogenicity of Helicobacter pylori (H pylori), several animal models with H pylori infection have been developed to confirm the association between H pylori and gastric cancer. Nonhuman primate and rodent models were suitable for this study. Japanese monkey model revealed atrophic gastritis and p53 mutation after long-term infection of H pylori. Mongolian gerbil model showed the development of gastric carcinoma with H pylori infection alone, as well as with combination of chemical carcinogens, such as N-methyl-N-nitrosourea and N-methyl-N-nitro-N'-nitrosoguanidine. The histopathological changes of these animal models after H pylori inoculation are closely similar to those in human beings with H pylori infection. Eradication therapy attenuated the development of gastric cancer in H pylori-infected Mongolian gerbil. Although several features of animal models differ from those seen in human beings, these experimental models provide a starting point for further studies to clarify the mechanism of gastric carcinogenesis as a result of H pylori infection and assist the planning of eradication therapy to prevent gastric carcinoma.
Keywords: Helicobacter pylori, Gastric carcinoma, Animal model, Japanese monkey, Mongolian gerbil
INTRODUCTION
Gastric cancer is one of the main causes of cancer-related mortality, especially, in East Asia. To clarify the mechanism of gastric cancer development, many experimental models have been used. However, almost all experimental animals, that showed spontaneous gastric cancer were very rare[1]; therefore several animal models were established using chemical carcinogens, such as N-methyl-N-nitrosourea (MNU)[2,3] and N-methyl-N-nitro-N’-nitrosoguanidine (MNNG)[4,5], which showed a high rate of gastric cancer development, especially in the antrum.
Since Warren and Marshall[6] revealed the microorganism which inhabits the stomach, Helicobacter pylori (H pylori) was considered as the major factor of many kind of gastroduodenal diseases, such as acute gastritis[7-9], chronic atrophic gastritis[9,10], intestinal metaplasia[11], peptic ulcer[12,13], mucosal associated lymphoid tissue lymphoma[14], gastric cancer[15-18], and others[19,20].
Previously, a large number of epidemiological studies indicated that H pylori infection has a close relation with gastric cancer[15-18]. Therefore, the International Agency for Research on Cancer (IARC) conference of the World Health Organization (WHO) defined H pylori as a definite carcinogen (Group I) to the human stomach based on three prospective case-control studies[15-17] reported in 1991[21].
However, the mechanisms by which H pylori infection develop gastric cancer are not defined in detail. In further studies, attempts have been made to reveal the possible mechanisms by which H pylori contributes to the development of gastric carcinoma and many researchers have developed animal models of infection using Helicobacter species.
Previously, a large number of animal experimental models have been developed to define the association between H pylori infection and gastroduodenal disease, such as piglet[22], beagle dog[23], mice[24], rhesus monkey[25], Japanese monkey (Macaca fuscata)[9,26,27], Mongolian gerbil[28], and others. In the beginning of the development of experimental models, only a few models had long periods of infection.
We have reported the results of a 5-year study on H pylori infection using Japanese monkeys (Macaca fuscata)[27] and have obtained the findings that advance gastric mucosal atrophy, increase proliferation and mutation of p53 in gastric epithelial cells[29,30].
Several experiments, which demonstrated that chronic H pylori infection models of Mongolian gerbils developed gastric carcinoma, were conducted[31-33]. In these experiments, the animals were mainly divided into two groups: one group was infected with H pylori alone and the group was given a known carcinogen such as MNU and MNNG in addition to persistent H pylori infection. The results of these experiments revealed that animals in different groups developed different histopathological types of gastric carcinoma. These results will be very useful to elucidate the mechanism of gastric carcinogenesis due to H pylori infection.
GASTRIC CANCER AND JAPANESE MONKEY
The nonhuman primate animals are useful to clarify the relationship between H pylori and gastric diseases. Their stomachs are similar to those of human beings anatomically, physiologically, and dietary, compared with rodent animals. They have 10-20 years of long life span, which enables long-term follow-up with endoscopy and repeated histological examinations of the stomach using biopsy or endoscopic resected specimens. Several primate animals have been reported to be successful in experimental transmission of H pylori in chimpanzees (Pan troglodytes)[34], and species of macaques: rhesus monkey (M. mulatta)[25], cynomolgus monkey (M. fascicularis)[25], and Japanese monkey (M. fuscata)[9,26,27]. In these animals, some kinds of Macaque species are available for a wide variety of research field. We have established the Japanese monkey model with H pylori infection. This experimental model is very useful and a promising nonhuman primate model[9,26,27].
The methods of development of this monkey model are described briefly. The bacterial strains used were H pylori MCO 88155, MCO 88099, MCO 88142, and MCO 88156, isolated from two patients with duodenal ulcers and two with gastric ulcers. The colonies were suspended in 5 mL of sterile saline, and the bacterial concentration was adjusted to 109 CFU/mL. These were resuspended in 8 mL of sterile saline, and 5 mL of the final resuspension was used in each monkey. The animals were given ampicillin orally to eradicate spiral bacteria other than H pylori. After treatment with ampicillin, spiral bacteria were not found in any of the stomachs. The monkeys were sprayed with 5 mL of a mixed suspension of four bacterial strains endoscopically around their antrum. The gastric mucosa was examined endoscopically, and endoscopic mucosal resection was performed repeatedly during 6 years of observation.
One week after inoculation, all infected monkeys showed endoscopic acute gastritis accompanied by marked erythema and edema. These findings were consistent with the acute gastric mucosal lesion observed in the human stomach. Infection of H pylori was recognized by culture, the rapid urease test, histology, and the elevation of H pylori-specific IgG in plasma. In the early phase of infection, infiltration of monocytes and polymorphonuclear leukocytes were marked in the edematous lamina propria and superficial erosions were evident. After 3 mo of inoculation, infiltration of mononuclear cells and plasma cells were predominant in the lamina propria layer. However, no superficial erosions and atrophic changes were observed.
In the infected group, the gastritis score which was evaluated by a scoring system based on the method of Rauws et al[35]. were markedly increased in the antral mucosa 1 wk after inoculation (P<0.001). The score then gradually decreased throughout the whole investigation period, but remained significantly higher (P<0.01) than that of the control group.
Six months after inoculation, the pyloric glandular height was apparently lower in the infected animals than in controls. Furthermore, the atrophic change advanced gradually throughout the 5-year observation period[27]. Endoscopically, according to the endoscopic-atrophic-border scale described by Kimura and Takemoto[36], gastric atrophy also gradually advanced for more than 3 years. These findings indicated evidently that H pylori infection caused atrophic gastritis in the Japanese monkey model. Cell proliferation activity, which was revealed with immunohistochemical detection of Ki-67 in the antral mucosa of infected animals, was significantly accelerated throughout the entire observation period (Figure 1). Immunohistochemical detection of p53 and point mutation of p53 was exhibited in the gastric mucosa[29,30] of this model. Genetic alterations in exons 5-8 of the p53 gene were uncommon in the H pylori-uninfected monkeys, whereas a higher prevalence of missense mutations in the p53 gene appeared in association with H pylori infection (Table 1). The number of mutations in the p53 gene increased as the gastric atrophy score increased, which depends on the duration of H pylori infection[30]. These findings of Japanese monkey model may explain the potential mechanism for the causal role of H pylori in the chain of events leading to gastric carcinoma. This monkey model facilitates investigation of the correlation between the long-term sequence of H pylori infection and gradual gastric mucosal change. Although many pathophysiological changes were seen in H pylori-infected gastric mucosa, this Japanese monkey model did not show the development of gastric carcinoma. In their long life span, which is similar to human beings, further continuous infection may be needed to the more dramatic histological change.
Figure 1.
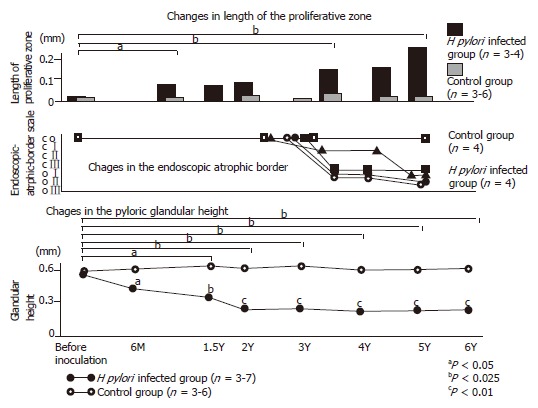
Gastric mucosal alteration of Japanese monkey model with H pylori infection. Upper graph showed the gradual increase of the proliferative zone of H pylori-infected Japanese monkey model. Middle graph showed the alteration of endoscopic-atrophic-border scale of this model. Macroscopically, gastric atrophy advanced for more than 3 yr. Lower graph showed the alteration of the pyloric glandular height. Six months after inoculation, the pyloric glandular height was apparently lower in the infected animals than in controls. Furthermore, the atrophic change advanced gradually throughout the 6-yr observation period.
Table 1.
Duration of H pylori infection and number of point mutations in exon 5-8 of the p53 gene
| Monkey | Duration of H pylori infection (yr) | Number of nucleotide (amino acid) substitutions in p53 | Atrophy score1 | Intensity of p53 immunostaining2 | |||
| Ex 5 | Ex 6 | Ex 7 | Ex 8 | - | |||
| A | 1.5 | 0(0) | 2 (1) | 0 (0) | 1 (1) | 2 | - |
| B | 2 | 4 (0) | 2 (2) | 5 (4) | 4 (3) | 3 | + |
| C | 3 | 4 (2) | 1 (0) | 2 (2) | 2 (2) | 4 | + |
| D | 3 | 2 (1) | 1 (0) | 1 (1) | 4 (2) | 5 | + |
| E | 3.5 | 5 (2) | 2 (1) | 5 (4) | 6 (4) | 4 | |
| F | 4.5 | 5 (1) | 3 (1) | 4 (3) | 5 (4) | 6 | - |
| G | 5 | 4 (1) | 3 (1) | 2 (1) | 8 (6) | 8 | + |
| H | 7.5 | 8 (5) | 2 (1) | 5 (4) | 8 (4) | 12 | ++ |
, The atrophy score was calculated as the sum of the histological evaluations of five gastric specimens according to Updated Sydney System.
, The intensity of p53 immunostaining was classified into four grades: -, no staining; +, mild staining; ++, moderate staining; +++, intense staining.
DEVELOPMENT OF THE RODENT MODEL
Several rodent models were established for examining the etiological feature of Helicobacter species infection, such as mice[24,37], rat[38], and Mongolian gerbil[28]. Compared with nonhuman primate models, rodent models are treated easily, and are economical.
Marchetti et al[39]. reported the several clinical isolates colonized the stomach of SPF conditioned mice (CD1 mice) and Balb/c mice; however, colonization was very low. Lee et al[40]. reported the quite good colonization by using the Sydney strain of H pylori (strain SS1), which is cagA and vacA positive.
These rodent models showed meager development of spontaneous gastric cancer. Cui et al[41]. reported the development of spontaneous gastric carcinoma, classified as malignant enterochromaffin-like (ECL) carcinomas in female cotton rats (Sigmodon hispidus). Previously, chemical carcinogens such as MNNG and MNU have been often used in the rodent species for the investigation of experimental gastric cancer[2-5].
From the recognition of Helicobacter species’ pathogenicity, Fox et al[42]. reported a possible carcinogenic role for Helicobacter species in the gastric mucosa after oral administration of MNNG in ferrets infected with Helicobacter mustelae. Nine out of the ten ferrets, which were given a dose of 50 mg/kg MNNG orally, developed adenocarcinoma. This was the first experimental study using carcinogens combined with infection with a Helicobacter species. Although spontaneous gastric adenocarcinomas have been reported[43] in aged ferrets with H mustelae even in the absence of carcinogen exposure, an important additional problem is that the bacterium used was not H pylori but H mustelae. Fox et al[44]. also described the development of gastric adenocarcinoma, which was led from severe gastritis in C57BL/6 mice with Helicobacter felis inoculation. In their report, p53+/- mice showed significant low prevalence of fundic lymphoplasmacytic infiltration and submucosal lymphoid follicle formation than those in C57BL/6 mice. They indicated two distinct roles of p53, one of them displayed the gastric cancer risk. However, deletion of one p53 allele results in a down-regulated Th1 response to Helicobacter infection, which may indirectly protect against the development of gastric cancer associated with chronic inflammation.
The differences between H felis in mice and H pylori in human beings are the lack of induction of neutrophil and cag pathogenicity island, which are recognized as main pathogens of H pylori. Kim et al[45]. reported that C57BL/6 mice infected with H pylori (SS1 strain) showed no evidence of gastric adenoma, dysplasia, and carcinoma during 80 wk of infection. They explained this result by the balance that exists between cell proliferation and apoptosis.
Transgenic hypergastrinemic (INS-GAS) mouse model have also been useful for the investigation of gastric carcinogenesis. Fox et al[46]. reported that male INS-GAS mice infected with H pylori developed atrophy, intestinal metaplasia, and dysplasia and adenocarcinoma. This murine model with H pylori cagE mutant showed the deletion of development of cancer. In contrast, none of the female mice with H pylori infection developed adenocarcinoma. However, IL-1 levels showed no significant difference between males and females. Fox et al. concluded that the INS-GAS model is effective for investigating discrete host-microbial interactions that culminate in gastric cancer within the context of biologic conditions induced by H pylori.
DEVELOPMENT OF MONGOLIAN GERBIL MODEL
Yokota et al[28]. developed the experimental Mongolian gerbil model with H pylori infection, in which only a mild inflammatory infiltration in the gastric mucosa was seen during two months of their observation. Hirayama et al[47]. described that ulcers and intestinal metaplasia were produced 6 mo after inoculation with H pylori in Mongolian gerbils.
In our laboratory, 5-wk-old male Mongolian gerbils weighing 30-40 g (Seiwa Experimental Animals Co. Ltd., Fukuoka, Japan) were used[48]. H pylori ATCC-43504 possessing the cagA gene and expressing vacuolating cytotoxin was used. A 4-d culture on blood agar at 37 °C under microaerophilic conditions was harvested and incubated in brucella broth (DIFCO Laboratories, Detroit, MI, USA) with 10% horse serum for 24 h. Inoculum size was adjusted with sterile saline to produce the optical density of McFarland 4 at 540 nm. Mongolian gerbils were housed five per cage, starved for 24 h, and then fed with chow (Oriental Yeast Co., Tokyo, Japan) and water ad libitum beginning 12 h after H pylori inoculation. On the day of infection, the Mongolian gerbils were challenged orally with vehicle or 109 CFU H pylori in 1.0 mL of brucella broth with 10% horse serum. The spiral bacteria were observed in the mucus and gastric pits of all inoculated animals from 1 mo after inoculation throughout the whole observation period. However, nearly half of the animals had barely detectable H pylori in the stomach by bacterial culture. The bacterial counts from the stomachs of gerbils 1 and 6 mo after H pylori inoculation were 25 and 410 CFU/10 mg of gastric tissue, respectively[48]. These levels of colonized bacteria were nearly 1/10 to 1/100 than those of human being and monkey.
Mongolian gerbils with H pylori infection showed irregularly thickened gastric walls and spotty hemorrhages and erosions macroscopically, 1 year after inoculation. A severe infiltration of polymorphonuclear and mononuclear cells was seen in the lamina propria and mononuclear cells infiltration with lymphoid follicle in the submucosa, 1 mo after H pylori inoculation (Figure 2A). Erosion of the gastric mucosa appeared soon after inoculation, whereas gastric ulcers, gastritis cystica profunda (Figure 2B), and atrophy with goblet cell metaplasia (Figures 2C and D) occurred between 3 and 6 mo after inoculation[48,49]. Moreover, Suzuki et al[50]. reported that H pylori inoculation induced neutrophil followed by an increase in the level of lipid peroxidation and activated glutathione (antioxidant) turnover. These sequential changes of histological changes in gastric mucosa were quite similar to those observed in human beings. Therefore, Mongolian gerbil model may be useful to study the relationship between H pylori infection and gastric lesions, which include gastric malignancy.
Figure 2.
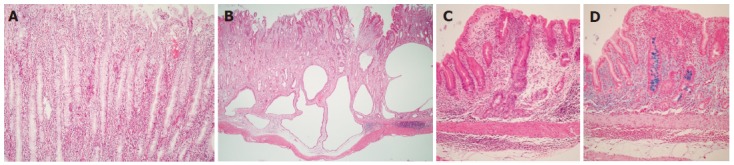
Microscopic views of the gastric body of Mongolian gerbils at 18 mo after H pylori inoculation. A: Severe infiltration of polymorphonuclear and mononuclear cells were seen in the lamina propria. (HE stain, x100); B: Some glands have extended into the submucosa but not into the proper muscularis layer. Severe infiltration of mononuclear cells in the submucosa (HE stain, x10); C: Intestinal metaplasia is seen scattering in gastric mucosa (HE stain, x10); D: Intestinal metaplasia (Alcian blue stain (pH 2.5); original magnification, x10).
GASTRIC CANCER AND MONGOLIAN GERBIL MODEL
Mongolian gerbils have also been induced by the development of gastric carcinoma with chemical carcinogen alone[51]. In addition, the results of several experimental studies have confirmed that administration of MNNG or MNU to Mongolian gerbils with chronic H pylori infection enhanced the development of different histopathological types of gastric carcinoma (Table 2)[31-33].
Table 2.
Gastric carcinogenesis in Helicobacter pylori-infected Mongolian gerbils
| Author | Year | Strain | Study design (ppm) | Incidence of cancer (%) | Duration of experiment (wk) |
| Sugiyama et al. | 1998 | ATCC43504 | HP→MNU (10) HP alone MNU (30) → HP MNU alone | 7/19 (36.8) 0/20 (0) 6/18 (33.3) 0/74 (0) | 40 40 40 40 |
| Tokieda et al. | 1999 | ATCC43504 | HP → MNNG (50) persistent HP positive HP eradicated Br → MNNG (50) | 5/17 (29.4) 5/8 (62.5) 0/9 (0) 3/22 (13.6) | 52 52 52 50 |
| Shimizu et al. | 1999 | ATCC43504 | MNNG (300) → HP MNNG (300) → Br MNNG (60) → HP MNNG (60) → Br HP → MNNG (100) Br → MNNG (100) HP → MNNG (20) Br → MNNG (20) HP alone | 12/27 (44.4) 1/19 (5.3) 6/25 (24.0) 0/20 (0) 4/27 (14.8) 3/18 (16.7) 15/25 (60) 1/20 (5) 0/20 (0) | 50 50 50 50 50 50 50 50 50 |
HP, Helicobacter pylori; MNU, N-methyl-N-nitrosourea; MNNG, N-methyl-N’-nitroso-N-nitrosoguanidine; Br, Brucella broth.
Sugiyama et al[31]. reported the development of carcinoma in the Mongolian gerbils evaluated at 40 wk after an experiment in which 7-wk-old animals were inoculated with H pylori (ATCC43504) and given 10 or 30 ppm MNU before or after inoculation. In this report, only the groups of the animals, which were administered with both H pylori and MNU, developed gastric cancers; more specifically, they developed different types of adenocarcinoma, such as well-differentiated, poorly differentiated, and signet ring cell carcinoma. These interesting experimental results support the results so far obtained in largescale epidemiological investigations[52].
The group inoculated with H pylori after being given MNU showed a distinctive initiation-promotion effect, whereas the group to which MNU was given after inoculation with H pylori appeared to demonstrate the simultaneous action of these two factors, with H pylori acting as a coinitiator. No gastric carcinoma was found within 40 wk of H pylori infection alone.
Tokieda et al[32]. conducted a study in which 5-wk-old Mongolian gerbils were inoculated with H pylori (ATCC43504) and orally given MNNG at 50 g/mL for 20 wk for comparison against animals administered with MNNG alone. At the 52nd wk after initiation, the group treated with MNNG and H pylori developed gastric carcinoma at a significantly higher frequency than the group treated with MNNG alone. In addition, cell proliferation was revealed to be markedly accelerated in those animals infected with H pylori with evaluation using a labeling index of 5-bromo-2’-deoxyuridine. This result suggests the possibility of explaining the link between H pylori infection and early events in gastric carcinogenesis. One of the interests in this study is that administration of MNNG reduced the infection rate of H pylori with the lapse of time, due to the likelihood of MNNG showing low-level (200 μg/mL) antibacterial activity against H pylori[32]. It is also of interest that H pylori-free animals did not develop gastric carcinoma even with MNNG administration. This result indicated a stronger carcinogenic role of H pylori infection. Although it has been reported by Sugiyama[31] that H pylori can persistently colonize the stomach of MNUtreated Mongolian gerbils, it is interesting that the two studies[32,33] report that MNNG administration eradicates H pylori infection, resulting in a reduction of its carcinogenic effects in the stomach. In H pylori-infected Mongolian gerbils with MNNG administration, duodenogastric reflux due to surgical procedure might attenuate the effect of H pylori on gastric tumorigenesis[53]. Because of our study, which indicated that bile reflux might lead to H pylori eradication[54], their results may probably depend on the H pylori eradication.
CARCINOGENICITY OF H PYLORI INFECTION ALONE
Although these studies showed marked increase of the chemical carcinogenic risk in the Mongolian gerbils, direct relationship between H pylori and gastric carcinogenesis was not indicated.
Two experimental studies attempted to confirm prior epidemiological studies that have demonstrated an association between H pylori infection and gastric carcinogenesis in human beings using Mongolian gerbils chronically infected with this bacterium (Table 3)[55,56]. Both studies confirmed gastric carcinogenesis resulting from H pylori infection alone, and were the first papers to fulfill Koch’s postulates concerning H pylori infection and gastric carcinoma.
Table 3.
Gastric carcinogenesis in Helicobacter pylori-infected Mongolian gerbils
| Author | Year | Strain | cagA gene | Vacuolating | Incidence of | Duration of | Histological type of |
| cytotoxin | cancer (%) | experiment (wk) | carcinoma | ||||
| Watanabe et al. | 1998 | TN2GF41 | + | + | 10/27 (37) | 62 | Well differentiated |
| adenocarinoma | |||||||
| Honda et al. | 1998 | ATCC435042 | + | + | 2/5 (40) | 72 | Well differentiated |
| adenocarinoma | |||||||
| Hirayama et al. | 1999 | ATCC435042 | + | + | 1/56 (1.8) | 64 | Well differentiated |
| adenocarinoma | |||||||
| Ogura et al. | 2000 | TN22 | + | + | 1/23 (4) | 62 | Well differentiated |
| adenocarinoma | |||||||
| Zheng et al. | 2004 | ATCC435042 | + | + | 3/17 (18) | 84 | Well differentiated |
| H pylori 1613 | + | + | adenocarinoma |
H pylori isolated from patient with gastric ulcer;
Type of strains;
H pylori isolated from patient with gastric adenocarcinoma.
Watanabe et al[55]. used H pylori isolated from patients with gastric ulcer (TN2GF4), and Honda et al[56]. used ATCC43504 type strain, both of which were inoculated into 5-wk-old SPF Mongolian gerbils. The results showed that 37% (10 out of 27) of the animals in the former study developed well-differentiated adenocarcinoma at 62 wk after inoculation, whereas 40% (2 out of 5) of the animals in the latter study developed well-differentiated adenocarcinoma at 72 wk after inoculation (Figure 3). Both of these strains contained cagA and produced vacuolating cytotoxins. Sequential histopathological changes leading to carcinogenesis of the gastric mucosa were found to be common to the two studies, and very closely resembled the histopathological changes in human gastric mucosa caused by H pylori infection.
Figure 3.
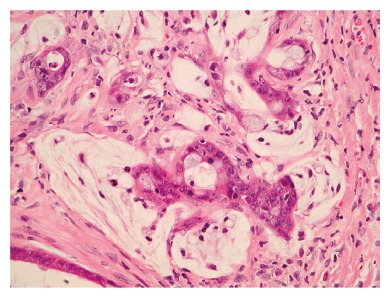
Microscopic views of the gastric mucosa of Mongolian gerbils at 18 mo after H pylori inoculation. Well-differentiated adenocarcinoma has extended into the muscular layer. Atypical glands and nuclei and abnormal mitosis are evident (HE stain, x40).
Hirayama et al[57]. reported that poorly differentiated adenocarcinoma and carcinoid were developed in Mongolian gerbils model with H pylori (ATCC43504 type strain) infection alone. Zheng et al[58]. reported that Mongolian gerbils models, which were infected with H pylori (ATCC43504) and H pylori 161 (isolated from a Chinese patient with gastric adenocarcinoma) showed the development of well-differentiated adenocarcinoma (Table 3). Ogura et al[59]. reported the development of well-differentiated gastric cancer in wild type (TN2) and isogenic mutant of vacA (TN2ΔvacA) of Mongolian gerbil.
Mongolian gerbils’ model also showed the development of gastric carcinoid[57,59,60]. In our laboratory, ECL cell tumors with marked atrophic gastritis and with hypergastrinemia were observed in the fundic gland area of infected Mongolian gerbils, 24 mo after inoculation (Figure 4); in contrast, adenocarcinoma developed in pyloric gland area. Histopathological findings of the entire observation period in Mongolian gerbils after H pylori inoculation are summarized in Table 4.
Figure 4.
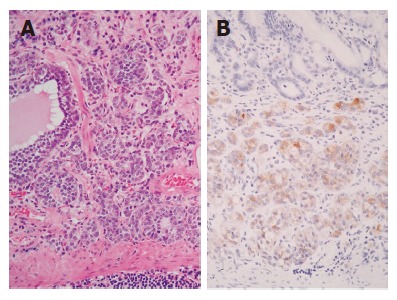
Gastric carcinoid in the stomach of a Mongolian gerbils colonized for 24 mo by H pylori. Microscopic view showing intramucosal carcinoid tumor (A: HE stain, x20; B: immunohistochemistry of chromogranin A, x20’).
Table 4.
Gastric carcinogenesis in Helicobacter pylori-infected Mongolian gerbils
| Histropathological findings | Mo | |||
| 6 | 12 | 18 | 24 | |
| Gastritis | 5/5 | 4/4 | 5/5 | 10/10 |
| Gastric ulcer | 4/5 | 3/4 | 5/5 | 5/10 |
| Atrophy | 4/5 | 4/4 | 5/5 | 10/10 |
| Intestinal metaplasia | 2/5 | 3/4 | 5/5 | 10/10 |
| Dysplasia | 0/5 | 2/4 | 4/5 | 10/10 |
| Gastric cancer | 0/5 | 0/4 | 2/5 | 5/10 |
| Gastric carcinoid | 0/5 | 0/4 | 0/5 | 5/10 |
Data represent positive case/control Uninfected control. animals (n = 5 each) showed no abnormal findings.
Ogura et al[59]. discussed the virulence factors of H pylori in Mongolian gerbils. Experimental gastric cancer derived in Mongolian gerbils with wild type of H pylori and vacA mutant infection, whereas cagE mutant induced far milder change of gastritis and induced no gastric cancer, which indicates the essential role of cagPAI in the gastric diseases with H pylori infection.
PREVENTION OF GASTRIC CARCINOMA BY ERADICATION OF H PYLORI
Shimizu et al[61]. reported that the incidence of adenocarcinomas in MNU-administered Mongolian gerbils with H pylori infection (15 out of 23) was significantly higher than in MNU-administered Mongolian gerbils that underwent H pylori eradication (5 out of 24). Their results suggest that H pylori eradication may prevent gastric carcinogenesis, and Mongolian gerbil’s models have also been useful to study the prevention of gastric carcinogenesis in human beings.
DIFFERENCES BETWEEN ANIMAL MODELS AND HUMAN BEINGS
Although the Japanese monkey model and Mongolian gerbil model showed the similar change of human stomach that was infected with H pylori, several features of animal models differ from those seen in human beings. In Japanese monkey model intestinal metaplasia was not seen during the whole observation period. Severe gastritis and lymphoid follicular hyperplasia in the submucosal layer and gastritis cystica profunda, which are seen in Mongolian gerbils, are not observed in human gastric mucosa.
Table 5 shows our tentative opinion on p53 and H pylori infection in animal model and human beings. Although no gastric carcinoma developed in Japanese monkey model, Mongolian gerbil model showed gastric carcinoma resulting from H pylori infection alone. In human and Japanese monkey, both p53 immunostaining[29,62-64] and point mutations[30,65] were observed in H pylori infection. In Mongolian gerbil, the p53 immunostaining was detected in gastric cancer but not in atrophic gastritis; moreover, there were no p53 mutations in exons 5 to 8 in infected gastric mucosa[66].
Table 5.
Relation between p53 and H pylori and gastric mucosal change (tentative opinion)
| Condition | Human | Japanese monkey | Mongolian gerbil |
| H pylori and p53 | ++ | ++ | +1 |
| overexpression (histology) | |||
| H pylori and p53 point mutation | ++ | ++ | - |
| H pylori and atrophic gastritis | ++ | ++ | ++ |
| H pylori and intestinal metaplasia | ++ | - | ++ |
| H pylori and gastric cancer | +2 | - | ++ |
The p53 overexpression was observed only in gastric cancer;
Not proven by interventional study; ++, strong evidence; +, weak evidence; -, no evidence
Suzuki et al[67]. reported that Mongolian gerbil model showed significant attenuation of apoptosis and promotion of cell proliferation than those seen in mice model with H pylori inoculation. Crabtree et al[68]. also described the differences of mucosal cytokine response between Mongolian gerbils and mice, and gender differences in the magnitude of cytokine response to H pylori. The differences of features between the species suggested that the pathogens of gastric diseases does not associate only with H pylori and may reflect in part other host factors.
Sonic hedgehog (Shh) is an important endometrial morphogenetic signal during the development of the vertebrate gut. Shh controls gastrointestinal patterning in general and gastric gland formation in particular. Suzuki et al[69]. reported that the long-term colonization of H pylori led to attenuation of Shh expression. Loss of Shh expression correlated with the loss of parietal cells, disturbed maturation of the mucous neck cell-zymogenic cell lineage. van den Brink et al[70]. described the loss of Shh expression in the intestinal metaplasia of the human stomach. Loss of Shh expression not only in intestinal metaplasia, but also in the tissue of H pylori-induced fundic gland atrophy is important for considering the possible link to preneoplastic lesion formation[69].
QUITE A NEW CONCEPT OF GASTRIC CANCER ORIGIN
In 2004, Houghton et al[71]. reported the innovative idea of gastric cancer origin with the usage of H. felis/C57BL/6 mouse model. Previously, tissue stem cells have been recognized as the origin of carcinoma. However, their study showed that bone marrow-derived cells (BMDCs) might also represent a potential source of malignancy. Female C57BL/6 mice after undergoing lethal irradiation were transplanted with bone marrow from male C57BL/6JGtrosa26 (ROSA26), which was labeled with X-galactosidase or green fluorescent protein. In this model, gastric mucosal apoptosis increased at 6- 8 wk after H. felis inoculation. After 52 wk of inoculation, beta-galactosidase (gal) and trefoil factor2 (TFF2) positive cells increased gradually, then 90% of the gastric mucosa at the squamocolumnar junction was replaced with cells derived from the donor marrow. One year after infection, intramucosal carcinoma or high-grade gastrointestinal intraepithelial neoplasia were seen in these mice. No evidence of BMDC engraftment was seen in H. felis uninfected mice. Authors indicated that BMDC originated the epithelial cancer and the necessity of Helicobacter infection in this process.
CONCLUSION
In various experimental models, nonhuman primate and rodent models showed the variable evidences, which clarified the association between H pylori infection and gastric cancer.
Experiments developed using Mongolian gerbils have demonstrated that H pylori infection is clearly responsible for gastric carcinogenesis, and provide important confirmation of the statements issued by IARC/WHO. It will be of critical importance to extrapolate the sequential histopathological changes found in the Mongolian gerbil to lesions in the human gastric mucosa, since this model is proven to provide important pointers for the study of the mechanism of gastric carcinogenesis as a result of H pylori infection. While Koch’s postulates for H pylori and gastric carcinoma have now been fulfilled, an important question to be addressed is why the Mongolian gerbil is the only species in which carcinogenesis has been experimentally induced by infection with H pylori.
Footnotes
Science Editor Xia HHX and Guo SY Language Editor ELsevier HK
References
- 1.Cui G, Qvigstad G, Falkmer S, Sandvik AK, Kawase S, Waldum HL. Spontaneous ECLomas in cotton rats (Sigmodon hispidus): tumours occurring in hypoacidic/hypergastrinaemic animals with normal parietal cells. Carcinogenesis. 2000;21:23–27. doi: 10.1093/carcin/21.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Fort L, Taper HS, Brucher JM. Gastric carcinogenesis in rat induced by methylnitrosourea (MNU). Morphology, and histochemistry of nucleases. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1974;81:51–62. doi: 10.1007/BF00303600. [DOI] [PubMed] [Google Scholar]
- 3.Fujita M, Taguchi T, Takami M, Usugane M, Takahashi A. Lung metastasis of canine gastric adenocarcinoma induced by N-methyl-N'-nitro-N-nitroguanidine. Gan. 1975;66:107–108. [PubMed] [Google Scholar]
- 4.Kartasheva LA, Bykorez AI. Induction of stomach tumors in rats by N-methyl-N-nitroso-N1-nitroguanidine. Vopr Onkol. 1975;21:50–55. [PubMed] [Google Scholar]
- 5.Koestner AW, Ruecker FA, Koestner A. Morphology and pathogenesis of tumors of the thymus and stomach in Sprague-Dawley rats following intragastric administration of methyl nitrosourea (MNU) Int J Cancer. 1977;20:418–426. doi: 10.1002/ijc.2910200314. [DOI] [PubMed] [Google Scholar]
- 6.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 7.Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch's postulates for pyloric Campylobacter. Med J Aust. 1985;142:436–439. doi: 10.5694/j.1326-5377.1985.tb113443.x. [DOI] [PubMed] [Google Scholar]
- 8.Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987;82:192–199. [PubMed] [Google Scholar]
- 9.Fujioka T, Shuto R, Kodama R, Fujiyama K, Kubota T, Murakami K, Perparim K, Nasu M. Experimental model for chronic gastritis with Helicobacter pylori: long term follow-up study in H pylori-infected Japanese macaques. Eur J Gastroenterol Hepatol. 1993;5(supple 1):S73–S78. [PubMed] [Google Scholar]
- 10.Kuipers EJ, Uyterlinde AM, Peña AS, Roosendaal R, Pals G, Nelis GF, Festen HP, Meuwissen SG. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525–1528. doi: 10.1016/s0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- 11.Sakaki N, Momma K, Egawa N, Yamada Y, Kan T, Ishiwata J. The influence of Helicobacter pylori infection on the progression of gastric mucosal atrophy and occurrence of gastric cancer. Eur J Gastroenterol Hepatol. 1995;7 Suppl 1:S59–S62. [PubMed] [Google Scholar]
- 12.Schubert TT, Bologna SD, Nensey Y, Schubert AB, Mascha EJ, Ma CK. Ulcer risk factors: interactions between Helicobacter pylori infection, nonsteroidal use, and age. Am J Med. 1993;94:413–418. doi: 10.1016/0002-9343(93)90153-g. [DOI] [PubMed] [Google Scholar]
- 13.Maaroos HI, Kekki M, Vorobjova T, Salupere V, Sipponen P. Risk of recurrence of gastric ulcer, chronic gastritis, and grade of Helicobacter pylori colonization. A long-term follow-up study of 25 patients. Scand J Gastroenterol. 1994;29:532–536. doi: 10.3109/00365529409092468. [DOI] [PubMed] [Google Scholar]
- 14.Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 15.Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 17.Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 18.An international association between Helicobacter pylori infection and gastric cancer. The EUROGAST Study Group. Lancet. 1993;341:1359–1362. [PubMed] [Google Scholar]
- 19.Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet. 1998;352:878. doi: 10.1016/S0140-6736(05)60004-9. [DOI] [PubMed] [Google Scholar]
- 20.Sato R, Murakami K, Watanabe K, Okimoto T, Miyajima H, Ogata M, Ohtsuka E, Kodama M, Saburi Y, Fujioka T, et al. Effect of Helicobacter pylori eradication on platelet recovery in patients with chronic idiopathic thrombocytopenic purpura. Arch Intern Med. 2004;164:1904–1907. doi: 10.1001/archinte.164.17.1904. [DOI] [PubMed] [Google Scholar]
- 21.Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [Google Scholar]
- 22.Krakowka S, Morgan DR, Kraft WG, Leunk RD. Establishment of gastric Campylobacter pylori infection in the neonatal gnotobiotic piglet. Infect Immun. 1987;55:2789–2796. doi: 10.1128/iai.55.11.2789-2796.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radin MJ, Eaton KA, Krakowka S, Morgan DR, Lee A, Otto G, Fox J. Helicobacter pylori gastric infection in gnotobiotic beagle dogs. Infect Immun. 1990;58:2606–2612. doi: 10.1128/iai.58.8.2606-2612.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karita M, Kouchiyama T, Okita K, Nakazawa T. New small animal model for human gastric Helicobacter pylori infection: success in both nude and euthymic mice. Am J Gastroenterol. 1991;86:1596–1603. [PubMed] [Google Scholar]
- 25.Euler AR, Zurenko GE, Moe JB, Ulrich RG, Yagi Y. Evaluation of two monkey species (Macaca mulatta and Macaca fascicularis) as possible models for human Helicobacter pylori disease. J Clin Microbiol. 1990;28:2285–2290. doi: 10.1128/jcm.28.10.2285-2290.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shuto R, Fujioka T, Kubota T, Nasu M. Experimental gastritis induced by Helicobacter pylori in Japanese monkeys. Infect Immun. 1993;61:933–939. doi: 10.1128/iai.61.3.933-939.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujioka T, Kodama R, Honda S, Guei-Hua G, Nishizono A, Nasu M. Long-term sequelae of experimental gastritis with Helicobacter pylori: a 5-year follow-up study. J Clin Gastroenterol. 1997;25 Suppl 1:S8–S12. doi: 10.1097/00004836-199700001-00004. [DOI] [PubMed] [Google Scholar]
- 28.Yokota K, Kurebayashi Y, Takayama Y, Hayashi S, Isogai H, Isogai E, Imai K, Yabana T, Yachi A, Oguma K. Colonization of Helicobacter pylori in the gastric mucosa of Mongolian gerbils. Microbiol Immunol. 1991;35:475–480. doi: 10.1111/j.1348-0421.1991.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 29.Kodama M, Fujioka T, Kodama R, Takahashi K, Kubota T, Murakami K, Nasu M. p53 expression in gastric mucosa with Helicobacter pylori infection. J Gastroenterol Hepatol. 1998;13:215–219. doi: 10.1111/j.1440-1746.1998.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 30.Oda T, Murakami K, Nishizono A, Kodama M, Nasu M, Fujioka T. Long-term Helicobacter pylori infection in Japanese monkeys induces atrophic gastritis and accumulation of mutations in the p53 tumor suppressor gene. Helicobacter. 2002;7:143–151. doi: 10.1046/j.1523-5378.2002.00074.x. [DOI] [PubMed] [Google Scholar]
- 31.Sugiyama A, Maruta F, Ikeno T, Ishida K, Kawasaki S, Katsuyama T, Shimizu N, Tatematsu M. Helicobacter pylori infection enhances N-methyl-N-nitrosourea-induced stomach carcinogenesis in the Mongolian gerbil. Cancer Res. 1998;58:2067–2069. [PubMed] [Google Scholar]
- 32.Tokieda M, Honda S, Fujioka T, Nasu M. Effect of Helicobacter pylori infection on the N-methyl-N'-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in mongolian gerbils. Carcinogenesis. 1999;20:1261–1266. doi: 10.1093/carcin/20.7.1261. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu N, Inada K, Nakanishi H, Tsukamoto T, Ikehara Y, Kaminishi M, Kuramoto S, Sugiyama A, Katsuyama T, Tatematsu M. Helicobacter pylori infection enhances glandular stomach carcinogenesis in Mongolian gerbils treated with chemical carcinogens. Carcinogenesis. 1999;20:669–676. doi: 10.1093/carcin/20.4.669. [DOI] [PubMed] [Google Scholar]
- 34.Hazell SL, Eichberg JW, Lee DR, Alpert L, Evans DG, Evans DJ, Graham DY. Selection of the chimpanzee over the baboon as a model for Helicobacter pylori infection. Gastroenterology. 1992;103:848–854. doi: 10.1016/0016-5085(92)90016-r. [DOI] [PubMed] [Google Scholar]
- 35.Rauws EA, Langenberg W, Houthoff HJ, Zanen HC, Tytgat GN. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988;94:33–40. [PubMed] [Google Scholar]
- 36.KimuraK , Takemoto T. Endoscopic atrophy border. Endoscopy. 1969;1:1–3. [Google Scholar]
- 37.Lee A, Fox JG, Otto G, Murphy J. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology. 1990;99:1315–1323. doi: 10.1016/0016-5085(90)91156-z. [DOI] [PubMed] [Google Scholar]
- 38.Danon SJ, Moss ND, Larsson H, Arvidsson S, Ottosson S, Dixon MF, Lee A. Gastrin release and gastric acid secretion in the rat infected with either Helicobacter felis or Helicobacter heilmannii. J Gastroenterol Hepatol. 1998;13:95–103. doi: 10.1111/j.1440-1746.1998.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 39.Marchetti M, Aricò B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 40.Lee A, O'Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 41.Cui G, Qvigstad G, Falkmer S, Sandvik AK, Kawase S, Waldum HL. Spontaneous ECLomas in cotton rats (Sigmodon hispidus): tumours occurring in hypoacidic/hypergastrinaemic animals with normal parietal cells. Carcinogenesis. 2000;21:23–27. doi: 10.1093/carcin/21.1.23. [DOI] [PubMed] [Google Scholar]
- 42.Fox JG, Wishnok JS, Murphy JC, Tannenbaum SR, Correa P. MNNG-induced gastric carcinoma in ferrets infected with Helicobacter mustelae. Carcinogenesis. 1993;14:1957–1961. doi: 10.1093/carcin/14.9.1957. [DOI] [PubMed] [Google Scholar]
- 43.Fox JG, Dangler CA, Sager W, Borkowski R, Gliatto JM. Helicobacter mustelae-associated gastric adenocarcinoma in ferrets (Mustela putorius furo) Vet Pathol. 1997;34:225–229. doi: 10.1177/030098589703400308. [DOI] [PubMed] [Google Scholar]
- 44.Fox JG, Sheppard BJ, Dangler CA, Whary MT, Ihrig M, Wang TC. Germ-line p53-targeted disruption inhibits helicobacter-induced premalignant lesions and invasive gastric carcinoma through down-regulation of Th1 proinflammatory responses. Cancer Res. 2002;62:696–702. [PubMed] [Google Scholar]
- 45.Kim DH, Kim SW, Song YJ, Oh TY, Han SU, Kim YB, Joo HJ, Cho YK, Kim DY, Cho SW, et al. Long-term evaluation of mice model infected with Helicobacter pylori: focus on gastric pathology including gastric cancer. Aliment Pharmacol Ther. 2003;18 Suppl 1:14–23. doi: 10.1046/j.1365-2036.18.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 46.Fox JG, Wang TC, Rogers AB, Poutahidis T, Ge Z, Taylor N, Dangler CA, Israel DA, Krishna U, Gaus K, et al. Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003;124:1879–1890. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 47.Hirayama F, Takagi S, Kusuhara H, Iwao E, Yokoyama Y, Ikeda Y. Induction of gastric ulcer and intestinal metaplasia in mongolian gerbils infected with Helicobacter pylori. J Gastroenterol. 1996;31:755–757. doi: 10.1007/BF02347631. [DOI] [PubMed] [Google Scholar]
- 48.Honda S, Fujioka T, Tokieda T, Gotoh T, Nishizono A, Nasu M. Gastric ulcer, atrophic gastritis, and intestinal metaplasia caused by Helicobacter pylori infection in Mongolian gerbils. Scand J Gastroenterol. 1998;33:454–60 DOI : 10.1080/00365529850171990. doi: 10.1080/00365529850171990. [DOI] [PubMed] [Google Scholar]
- 49.Ikeno T, Ota H, Sugiyama A, Ishida K, Katsuyama T, Genta RM, Kawasaki S. Helicobacter pylori-induced chronic active gastritis, intestinal metaplasia, and gastric ulcer in Mongolian gerbils. Am J Pathol. 1999;154:951–960. doi: 10.1016/S0002-9440(10)65343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki H, Mori M, Seto K, Kai A, Kawaguchi C, Suzuki M, Suematsu M, Yoneta T, Miura S, Ishii H. Helicobacter pylori-associated gastric pro- and antioxidant formation in Mongolian gerbils. Free Radic Biol Med. 1999;26:679–684. doi: 10.1016/s0891-5849(98)00248-2. [DOI] [PubMed] [Google Scholar]
- 51.Tatematsu M, Yamamoto M, Shimizu N, Yoshikawa A, Fukami H, Kaminishi M, Oohara T, Sugiyama A, Ikeno T. Induction of glandular stomach cancers in Helicobacter pylori-sensitive Mongolian gerbils treated with N-methyl-N-nitrosourea and N-methyl-N'-nitro-N-nitrosoguanidine in drinking water. Jpn J Cancer Res. 1998;89:97–104. doi: 10.1111/j.1349-7006.1998.tb00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 53.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 54.Abe H, Murakami K, Satoh S, Sato R, Kodama M, Arita T, Fujioka T. Influence of bile reflux and Helicobacter pylori infection on gastritis in the remnant gastric mucosa after distal gastrectomy. J Gastroenterol. 2005;40:563–569. doi: 10.1007/s00535-005-1589-9. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 56.Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255–4259. [PubMed] [Google Scholar]
- 57.Hirayama F, Takagi S, Iwao E, Yokoyama Y, Haga K, Hanada S. Development of poorly differentiated adenocarcinoma and carcinoid due to long-term Helicobacter pylori colonization in Mongolian gerbils. J Gastroenterol. 1999;34:450–454. doi: 10.1007/s005350050295. [DOI] [PubMed] [Google Scholar]
- 58.Zheng Q, Chen XY, Shi Y, Xiao SD. Development of gastric adenocarcinoma in Mongolian gerbils after long-term infection with Helicobacter pylori. J Gastroenterol Hepatol. 2004;19:1192–1198. doi: 10.1111/j.1440-1746.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- 59.Ogura K, Maeda S, Nakao M, Watanabe T, Tada M, Kyutoku T, Yoshida H, Shiratori Y, Omata M. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J Exp Med. 2000;192:1601–1610. doi: 10.1084/jem.192.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kagawa J, Honda S, Kodama M, Sato R, Murakami K, Fujioka T. Enterocromaffin-like cell tumor induced by Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2002;7:390–397. doi: 10.1046/j.1523-5378.2002.00115.x. [DOI] [PubMed] [Google Scholar]
- 61.Shimizu N, Ikehara Y, Inada K, Nakanishi H, Tsukamoto T, Nozaki K, Kaminishi M, Kuramoto S, Sugiyama A, Katsuyama T, et al. Eradication diminishes enhancing effects of Helicobacter pylori infection on glandular stomach carcinogenesis in Mongolian gerbils. Cancer Res. 2000;60:1512–1514. [PubMed] [Google Scholar]
- 62.Hibi K, Mitomi H, Koizumi W, Tanabe S, Saigenji K, Okayasu I. Enhanced cellular proliferation and p53 accumulation in gastric mucosa chronically infected with Helicobacter pylori. Am J Clin Pathol. 1997;108:26–34. [PubMed] [Google Scholar]
- 63.Satoh K, Kihira K, Kawata H, Tokumaru K, Kumakura Y, Ishino Y, Kawakami S, Inoue K, Kojima T, Satoh Y, et al. p53 expression in the gastric mucosa before and after eradication of Helicobacter pylori. Helicobacter. 2001;6:31–36. doi: 10.1046/j.1523-5378.2001.00003.x. [DOI] [PubMed] [Google Scholar]
- 64.Kodama M, Fujioka T, Murakami K, Okimoto T, Sato R, Watanabe K, Nasu M. Eradication of Helicobacter pylori reduced the immunohistochemical detection of p53 and MDM2 in gastric mucosa. J Gastroenterol Hepatol. 2005;20:941–946. doi: 10.1111/j.1440-1746.2005.03880.x. [DOI] [PubMed] [Google Scholar]
- 65.Murakami K, Fujioka T, Okimoto T, Mitsuishi Y, Oda T, Nishizono A, Nasu M. Analysis of p53 gene mutations in Helicobacter pylori-associated gastritis mucosa in endoscopic biopsy specimens. Scand J Gastroenterol. 1999;34:474–477. doi: 10.1080/003655299750026191. [DOI] [PubMed] [Google Scholar]
- 66.Murakami K, Fujioka T, Kodama M, Honda S, Okimoto T, Oda T, Nishizono A, Sato R, Kubota T, Kagawa J, et al. Analysis of p53 mutations and Helicobacter pylori infection in human and animal models. J Gastroenterol. 2002;37 Suppl 13:1–5. doi: 10.1007/BF02990091. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki H, Miyazawa M, Nagahashi S, Mori M, Seto K, Kai A, Suzuki M, Miura S, Ishii H. Attenuated apoptosis in H. pylori-colonized gastric mucosa of Mongolian gerbils in comparison with mice. Dig Dis Sci. 2002;47:90–99. doi: 10.1023/a:1013219621422. [DOI] [PubMed] [Google Scholar]
- 68.Crabtree JE, Court M, Aboshkiwa MA, Jeremy AH, Dixon MF, Robinson PA. Gastric mucosal cytokine and epithelial cell responses to Helicobacter pylori infection in Mongolian gerbils. J Pathol. 2004;202:197–207. doi: 10.1002/path.1498. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki H, Minegishi Y, Nomoto Y, Ota T, Masaoka T, van den Brink GR, Hibi T. Down-regulation of a morphogen (sonic hedgehog) gradient in the gastric epithelium of Helicobacter pylori-infected Mongolian gerbils. J Pathol. 2005;206:186–197. doi: 10.1002/path.1763. [DOI] [PubMed] [Google Scholar]
- 70.van den Brink GR, Hardwick JC, Nielsen C, Xu C, ten Kate FJ, Glickman J, van Deventer SJ, Roberts DJ, Peppelenbosch MP. Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut. 2002;51:628–633. doi: 10.1136/gut.51.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]


