Abstract
AIM: To examine the relationships between γ -glutamyl-transferase (GGT), alanine-aminotransferase (ALT), aspartate-aminotransferase (AST) and various metabolic parameters, C-reactive protein (CRP) and an oxidative stress marker (nitrotyrosine, NT) in subjects without any metabolic abnormalities from a population-based sample.
METHODS: Two hundred and five subjects with normal body mass index (BMI), glucose tolerance, and without any metabolic abnormality were studied out of 1 339 subjects, without known liver diseases, alcohol abuse or use of hepatotoxic drugs, who are representative of the 45-64 aged population of Asti (north-western Italy).
RESULTS: In all patients metabolic parameters and hs-CRP levels linearly increase from the lowest to the highest ALT and GGT tertiles, while in subjects without metabolic abnormalities, there is a significant association between fasting glucose, uric acid, waist circumference, hs-CRP, triglyceride values, and GGT levels. In these subjects, male sex, higher hs-CRP and glucose levels are associated with GGT levels in a multiple regression model, after adjustments for multiple confounders. In the same model, median NT levels are significantly associated with the increasing GGT tertile (β = 1.06; 95%CI 0.67-1.45), but not with the AST and ALT tertiles. In a multiple regression model, after adjusting for age, sex, BMI, waist, smoking, and alcohol consumption, both NT (β = 0.05; 95%CI 0.02-0.08) and hs-CRP levels (β = 0.09; 95%CI 0.03-0.15) are significantly associated with fasting glycemia.
CONCLUSION: GGT, an easy, universally standardized and available measurement, could represent an early marker of sub-clinical inflammation and oxidative stress in otherwise healthy individuals. Prospective studies are needed to establish if GGT could predict future diabetes in these subjects.
Keywords: Alanine aminotransferase, Aspartate aminotransferase, Body mass index, C-reactive protein, γ-Glutamyl transferase, Metabolic syndrome, Nitrotyrosine
INTRODUCTION
Increased levels of the liver enzymes, γ-glutamyl transferase (GGT), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) have been found to be associated with diabetes, cardiovascular risk factors and components of the insulin resistance syndrome, even within normal reference intervals[1-12]. In many prospective studies, strong relationships between GGT or ALT concentrations and incident diabetes have also been observed in non-drinkers, in individuals with normal levels of liver enzymes, independently of classical cardiovascular risk factors[2-10]. However, a strong interaction between body mass index (BMI) and GGT has been described, suggesting that this enzyme acts as an intervening factor in the association between obesity and diabetes[1,4,5,7,9]. Some authors have speculated that visceral fat could play a role in the association of GGT with metabolic abnormalities, or that this enzyme could be considered as a reliable marker of visceral fat[1,2,13]. Furthermore, GGT and ALT might be interpreted as markers of hepatic steatosis, a condition well known to be associated with insulin resistance, type 2 diabetes and the metabolic syndrome[14,15]. Thus, the associations recently found between the levels of liver enzymes and insulin resistance might be mediated by a fatty liver, which is accompanied by higher GGT and aminotransferase concentrations.
Increasing data are available about the associations between GGT levels and markers of oxidative stress (directly with F2-isoprostanes, an oxidative damage product of arachidonic acid; inversely with serum and dietary antioxidant vitamins), suggesting that the strong associations between cardiovascular risk factors might be explained by some oxidative mechanism[4,16-18]. Indeed, oxidative processes are key components of chronic inflammation, acting on multiple pathways and amplifying inflammatory reactions[19]. Recent reports have found associations between elevated liver enzymes and several inflammatory parameters[3,4,9,10,20,21].
However, previous studies have always included a group of overweight, or obese subjects, or did not take into account the deposition of body fat; thus adiposity might be a confounding factor. Two recent papers suggested that a non-alcoholic fatty liver could be considered as an early predictor of metabolic disorders also in non-obese non-diabetic Asiatic cohorts; however, in these studies, the control group included individuals with other components of the metabolic syndrome and Asians have a higher proportion of visceral fat and a lower lean body mass than white subjects with the same BMI[22,23]. It seems thus interesting to evaluate the associations between the liver enzyme levels and metabolic and inflammatory parameters in a cohort of Caucasian subjects without any metabolic abnormalities, which could potentially influence this association.
Therefore, the aims of the present study are to examine the relationships between liver enzyme concentrations and various metabolic parameters, a marker of inflammation (C-reactive protein, CRP) and a marker of oxidative stress (nitrotyrosine, NT) in subjects without any metabolic abnormalities from a population-based cohort of middle-aged individuals, recognizing that the cross-sectional design of the study does not definitively establish causal or temporal relationships, and should be considered hypothesis-generating only.
MATERIALS AND METHODS
All the patients aged between 45 and 64 years under the practice of six family physicians from the province of Asti (north-western Italy), whose patients are representative of the local health districts, were enrolled for a metabolic screening. Of these 1 877 subjects, 1 658 (88.3%) accepted to be interviewed on personal habits, and to be tested for several clinical and laboratory measurements, giving written consent, while 219 refused. The resident population of corresponding age, in the same area, and missing patients show the same percentage of males, level of education, known diabetes, and subjects living in a rural area than participating patients.
All procedures were in accordance with the Declaration of Helsinki.
Subjects with >30 g/d alcohol consumption, with known liver or gastrointestinal diseases, as recorded by the family physicians, with liver enzyme concentrations higher than three times the upper limit of the sex-related reference range, or on corticosteroids, methotrexate, amiodarone, tamoxifen or other hepatotoxic drugs were excluded. Estrogen users (both on contraceptive medications or estrogen replacement therapy) were included, all being on low-dose estrogen drugs.
One thousand and three hundred and thirty-nine subjects (80.8%) were evaluated.
In the morning, a fasting venous blood sample was drawn to measure glucose, total and HDL-cholesterol, uric acid, triglyceride, insulin, liver enzymes, and hs-CRP levels.
Weight and height were measured after an overnight fast. Overweight and obese subjects were those with BMI respectively, ≥25, <30, and ≥30 kg/m2. Waist circumference was measured with a plastic tape meter at the level of the umbilicus. Systolic and diastolic blood pressures were measured twice with a standard mercury sphygmomanometer in a sitting position, after at least 10 min of rest. Values reported are the mean of the two determinations. A resting electrocardiogram (ECG) was performed in all the subjects, and interpreted according to the Minnesota Code criteria. If fasting serum glucose value was ≥6.1 mmol/L, a second fasting glucose determination was then performed. Diabetes and impaired fasting glucose (IFG) were diagnosed according to published recommendations[24].
The metabolic syndrome (MS) was defined as the presence of at least three of the following five criteria: fasting serum glucose ≥6.1 mmol/L; arterial blood pressure ≥130/85 mmHg; plasma triglycerides ≥1.69 mmol/L; HDL-cholesterol <1.29 mmol/L (females) and <1.04 mmol/L (males); waist >88 cm (females) and >102 cm (males), in line with the National Cholesterol Education Program (Adult Treatment Panel III) (NCEP-ATP III) criteria[25].
Insulin resistance was calculated from the homeostasis model assessment (HOMA-IR), according to published algorithm[26].
Vascular disease was assessed on the basis of the Rose questionnaire, ECG evidence of ischemic heart disease, and history of documented events, recorded by the family physician (angina, previous myocardial infarction, coronary artery by-pass graft or other invasive procedures to treat coronary artery disease, transient ischemic attacks, strokes, gangrene, amputation, vascular surgery, intermittent claudication, absence of foot pulses, and abnormal brachial and posterior tibial blood pressure using Doppler techniques).
Former or present smokers were defined as ever smokers. Alcohol intake was assessed by multiplying the mean daily consumption for each beverage by the ethanol content, to give grams of alcohol/d (one can/bottle/glass of beer = 13 g, one glass of wine = 12 g, one standard drink of spirit = 14 g).
Later, we identified 205 subjects with normal BMI (<25 kg/m2) and without any component of the MS. To avoid considering as normoglycemic individuals who could be classified as hyperglycemic after the stimulatory test, these subjects were submitted to the 75 g oral glucose tolerance test (OGTT), performed and interpreted in accordance to the guidelines[24]. All of the subjects showed normal glucose tolerance at the OGTT. NT levels were measured in all these subjects.
Serum glucose was measured by the glucose oxidase method (HITACHI 911 Analyzer, Sentinel Ch., Milan, Italy), and serum insulin by immunoradiometric assay (Radim SpA, Pomezia, Italy; intra-assay CV: 1.6-2.2%, inter-assay CV: 6.1-6.5%). Plasma triglycerides and HDL-cholesterol were measured by enzymatic colorimetric assay (HITACHI 911 Analyzer), the latter after precipitation of LDL and VLDL fractions using heparin-MnCl2 solution (Mn2+ concentration: 0.092 mol/L) and centrifugation at 4 °C.
AST was evaluated by a kinetic determination. Malate dehydrogenase catalyzes the reaction of oxalacetic acid with β-NADH2 by forming lactic acid and β-NAD (HITACHI 911 Analyzer). ALT was evaluated by a kinetic determination. Lactate dehydrogenase catalyzes the reaction of pyruvic acid with β-NADH2 by forming lactic acid and β-NAD (HITACHI 911 Analyzer). GGT was evaluated by an enzymatic colorimetric method (HITACHI 911 Analyzer).
Uric acid was evaluated by an enzymatic colorimetric method with uricase (HITACHI 911 Analyzer). Serum hs-CRP levels were determined via a high-sensitivity latex agglutination method on the HITACHI 911 Analyzer. The kit had a minimum detection of less than 0.05 mg/L and a measurable concentration range of up to 160 mg/L. The intra-assay and inter-assay CVs were respectively 0.8-1.3% and 1.0-1.5%. Plasma NT values were determined by an ELISA kit (HyCult Biotechnology b.v., sold in Italy by Pantec, Turin; inter-assay and intra-assay CV respectively: 7±4% and 5±2%). All samples were run in blind.
Statistical analysis
Since the distribution of GGT, hs-CRP, insulin, HOMA, triglyceride, and NT values were highly skewed, the levels of these variables were log-transformed, in order to obtain a normal distribution. In all the analyses, the log-transformed values of these variables were used. For an easy interpretation, median (and range) of not transformed values are reported.
Linear (unadjusted) and multiple regression analyses were performed to evaluate the associations between liver enzyme levels with metabolic, inflammatory, and oxidative parameters, after adjustments for multiple confounders.
RESULTS
BMI, waist, blood pressure, fasting glucose, and insulin, HOMA-IR, triglyceride, low HDL-cholesterol, uric acid, and hs-CRP levels, percentages of males and prevalence of the metabolic syndrome increase from the lowest to the highest tertiles of ALT and GGT, and their values are significantly associated with these enzyme levels (Table 1). This trend is less evident within AST tertiles.
Table 1.
Clinical and laboratory characteristics of all the patients, according to liver enzyme tertiles (left); associations of the variables listed with liver enzyme levels, as a continuous variable, by unadjusted linear regression analyses (right)
| AST | 1st tertile (≤14 U/L) | 2nd tertile (>14≤19 U/L) | 3rd tertile (>19 U/L) | β; 95%CI | ||
| Number | 452 | 440 | 447 | |||
| Age (yr) | 54.0±5.8 | 54.7±5.8 | 55.0±5.5 | 0.08; 0.002 | 0.15 | |
| Male (%) | 31.9 | 34.1 | 47.2 | 1.88; 1.00 | 2.76 | |
| Alcoholics (%) | 41.6 | 45.0 | 51.8 | 1.28; 0.42 | 2.14 | |
| Alcohol (g/day within drinkers) | 14.4±8.7 | 15.8±8.9 | 16.3±8.5 | 0.03; -0.05 | 0.11 | |
| Ever smokers (%) | 48.3 | 40.2 | 41.4 | -0.41; -1.27 | 0.45 | |
| BMI (kg/m2) | 26.3±5.1 | 26.1±4.4 | 27.1±4.9 | 0.13; 0.04 | 0.22 | |
| Waist (cm) | 88.6±13.4 | 89.3±12.4 | 92.5±13.0 | 0.09; 0.06 | 0.12 | |
| Systolic blood pressure (mmHg) | 132.1±15.3 | 132.8±16.2 | 134.8±16.3 | 0.034; 0.007 | 0.06 | |
| Diastolic blood pressure (mmHg) | 82.3±8.7 | 82.9±9.5 | 83.7±9.6 | 0.05;-0.02 | 0.078 | |
| Uric acid (mmol/L) | 178.4±53.5 | 190.3±59.5 | 208.2±59.5 | 0.04;-0.007 | 0.087 | |
| Insulin (pmol/L) | 10.2 (0.6-259.8) | 10.8 (1.2-137.4) | 11.4 (0.6-409.8) | 0.74; 0.26 | 1.22 | |
| HOMA insulin resistance (mU/mL×mmol/L) | 0.40 (0.02-31.8) | 0.44 (0.05-16.6) | 0.47 (0.02-25.9) | 0.74; 0.30 | 1.18 | |
| Fasting glucose (mmol/L) | 5.8±1.9 | 5.6±1.2 | 5.9±1.7 | 0.27; 0.0 | 0.54 | |
| HDL-cholesterol (mmol/L) | 1.6±0.4 | 1.6±0.4 | 1.6±0.4 | -0.46;-1.69 | 0.77 | |
| Triglycerides (mmol/L) | 1.2 (0.5-5.2) | 1.3 (0.6-8.7) | 1.4 (0.4-5.4) | 1.45; 0.5 | 2.5 | |
| Hs-CRP (mg/L) | 1.40 (0.20-50.8) | 1.30 (0.20-49.5) | 1.60 (0.10-130.9) | 0.33;-0.07 | 0.73 | |
| Metabolic syndrome (%) | 20.1 | 19.3 | 26.4 | 1.54; 0.5 | 2.6 | |
| ALT | 1st tertile (≤14 U/L) | 2nd tertile(>14≤22 U/L) | 3rd tertile(>22 U/L) | β; 95%CI | ||
| Number | 444 | 459 | 436 | |||
| Age (yr) | 53.8±5.9 | 55.2±5.7 | 54.6±5.4 | 0.03; -0.09 | 0.16 | |
| Male (%) | 23.4 | 35.1 | 55.0 | 5.1; 3.7 | 6.6 | |
| Alcoholics (%) | 45.5 | 44.2 | 48.7 | 0.97; -0.46 | 2.4 | |
| Alcohol (g/day within drinkers) | 15.2±8.4 | 16.0±9.0 | 15.5±8.8 | 0.03; -0.10 | 0.17 | |
| Ever smokers (%) | 42.9 | 42.3 | 44.9 | -0.07; -1.5 | 1.4 | |
| BMI (kg/m2) | 25.0±4.5 | 26.6±4.6 | 28.0±4.9 | 0.69; 0.55 | 0.83 | |
| Waist (cm) | 85.4±12.9 | 89.7±12.1 | 95.3±12.3 | 0.29; 0.23 | 0.35 | |
| Systolic blood pressure (mmHg) | 131.1±16.0 | 133.0±15.3 | 135.5±16.3 | 0.09; 0.05 | 0.13 | |
| Diastolic blood pressure (mmHg) | 81.7±9.3 | 82.8±8.8 | 84.4±9.5 | 0.15; 0.07 | 0.23 | |
| Uric acid (mmol/L) | 172.5±53.5 | 190.3±53.5 | 214.1±59.5 | 0.05; 0.04 | 0.06 | |
| Insulin (pmol/L) | 10.2 (0.6-151.2) | 10.8 (0.6-409.8) | 15.0 (0.6-244.2) | 3.4; 2.6 | 4.2 | |
| HOMA insulin resistance (mU/mL×mmol/L) | 0.38 (0.02-14.3) | 0.43 (0.02-31.8) | 0.63 (0.02-19.9) | 3.5; 2.8 | 4.2 | |
| Fasting glucose (mmol/L) | 5.5±1.4 | 5.6±1.2 | 6.1±2.1 | 1.5; 1.1 | 1.9 | |
| HDL-cholesterol (mmol/L) | 1.6±0.4 | 1.6±0.3 | 1.5±0.3 | -6.8; -8.8 | -4.8 | |
| Triglycerides (mmol/L) | 1.2 (0.5-8.0) | 1.3 (0.4-8.7) | 1.5 (0.5-5.4) | 5.8; 4.2 | 7.4 | |
| Hs-CRP (mg/L) | 1.10 (0.10-50.8) | 1.40 (0.20-49.5) | 1.60 (0.10-130.9 | 1.4; 0.8 | 2.1 | |
| Metabolic syndrome (%) | 12.8 | 21.1 | 32.1 | 5.5; 3.8 | 7.2 | |
| GGT | 1st tertile (≤12 U/L) | 2nd tertile (>12≤20 U/L) | 3rd tertile(>20 U/L) | β; 95%CI | ||
| Number | 412 | 460 | 467 | |||
| Age (yr) | 53.6±5.5 | 54.8±5.8 | 55.1±5.7 | 0.009; 0.003 | 0.015 | |
| Male (%) | 15.5 | 38.9 | 56.1 | 0.43; 0.37 | 0.50 | |
| Alcoholics (%) | 36.6 | 47.9 | 52.7 | 0.15; 0.08 | 0.22 | |
| Alcohol (g/day within drinkers) | 14.1±7.9 | 15.0±9.0 | 16.9±8.8 | 0.013; 0.007 | 0.019 | |
| Ever smokers (%) | 36.9 | 43.6 | 48.8 | 0.13; 0.06 | 0.20 | |
| BMI (kg/m2) | 24.9±4.2 | 26.7±4.7 | 27.7±5.0 | 0.03; 0.02 | 0.04 | |
| Waist (cm) | 84.4±12.5 | 90.4±12.4 | 94.8±12.2 | 0.016; 0.014 | 0.018 | |
| Systolic blood pressure (mmHg) | 129.7±15.0 | 133.4±15.4 | 136.1±16.8 | 0.006; 0.004 | 0.008 | |
| Diastolic blood pressure (mmHg) | 81.5±8.7 | 82.8±9.3 | 84.4±9.6 | 0.009; 0.005 | 0.013 | |
| Uric acid (mmol/L) | 166.5±47.6 | 190.3±59.5 | 214.1±59.5 | 0.0037;0.003 | 0.004 | |
| Insulin (pmol/L) | 10.2 (0.6-120.0) | 10.8 (1.2-409.8) | 13.8 (0.6-396.0) | 0.13; 0.09 | 0.17 | |
| HOMA insulin resistance (mU/mL×mmol/L) | 0.37 (0.02-5.13) | 0.43 (0.04-25.9) | 0.60 (0.02-31.8) | 0.14; 0.10 | 0.18 | |
| Fasting glucose (mmol/L) | 5.4±1.1 | 5.7±1.4 | 6.1±2.1 | 0.09; 0.07 | 0.11 | |
| HDL-cholesterol (mmol/L) | 1.7±0.3 | 1.6±0.3 | 1.5±0.3 | -0.32;-0.22 | -0.42 | |
| Triglycerides (mmol/L) | 1.1 (0.4-4.4) | 1.3 (0.4-8.7) | 1.5 (0.6-8.0) | 0.44; 0.36 | 0.52 | |
| Hs-CRP (mg/L) | 1.00 (0.10-20.0) | 1.40 (0.20-50.8) | 1.80 (0.20-130.9) | 0.12; 0.09 | 0.15 | |
| Metabolic syndrome (%) | 11.6 | 20.2 | 32.8 | 0.35; 0.27 | 0.43 |
Median (range) is reported for not-normally distributed variables; their log-transformed values and log-GGT values are used in the analyses.
In subjects with normal BMI and without any component of the MS, there is a significant association of percentage of males, waist circumference, glucose, uric acid, hs-CRP, and triglyceride values with increasing GGT levels (Table 2). Waist, uric acid levels and percentages of males are significantly associated with ALT levels, while there is no significant association with AST values.
Table 2.
Clinical and laboratory characteristics of the subjects with normal BMI and without any component of the metabolic syndrome, according to the tertile of liver enzyme concentrations (left); associations of the variables listed with liver enzyme levels, as a continuous variable, by unadjusted linear regression analyses (right)
| AST | 1st tertile (≤13 U/L) | 2nd tertile (>13≤17 U/L) | 3rd tertile (>17 U/L) | β; 95%CI | ||
| Number | 67 | 69 | 69 | |||
| Age (yr) | 52.2±5.5 | 52.5±5.7 | 52.1±5.1 | 0.001;-0.16 | 0.16 | |
| Male (%) | 22.4 | 34.8 | 42.0 | 0.8; -1.0 | 2.6 | |
| Alcoholics (%) | 47.8 | 47.8 | 56.5 | 1.2; -0.5 | 2.9 | |
| Alcohol (g/day within drinkers) | 15.5±9.1 | 13.8±9.0 | 18.5±9.1 | 0.05; -0.07 | 0.18 | |
| Ever smokers (%) | 47.8 | 39.1 | 44.9 | -0.63;-2.35 | 1.09 | |
| BMI (kg/m2) | 21.8±2.0 | 22.1±1.9 | 22.1±2.1 | 0.07;-0.35 | 0.49 | |
| Waist (cm) | 76.3±8.1 | 78.4±7.8 | 75.3±5.6 | 0.08;-0.02 | 0.18 | |
| Systolic blood pressure (mmHg) | 117.4±7.7 | 116.7±7.4 | 116.6±9.1 | -0.05;-0.15 | 0.05 | |
| Diastolic blood pressure (mmHg) | 74.9±6.0 | 75.1±5.8 | 75.3±5.6 | -0.01;-0.16 | 0.14 | |
| Uric acid (mmol/L) | 154.6±47.6 | 160.6±41.6 | 172.5±53.5 | 0.014;-0.004 | 0.03 | |
| Insulin (pmol/L) | 6.0 (0.6-49.2) | 9.6 (1.2-108.6) | 9.0 (3.0-72.0) | 0.41;-0.90 | 1.72 | |
| HOMA insulin resistance (mU/mL´mmol/L) | 0.25 (0.02-2.08) | 0.34 (0.05-5.40) | 0.30 (0.10-2.28) | 0.48;-0.85 | 1.81 | |
| Fasting glucose (mmol/L) | 5.1±0.4 | 5.1±0.5 | 5.2±0.7 | 0.84;-0.68 | 2.36 | |
| HDL-cholesterol (mmol/L) | 1.8±0.3 | 1.7±0.4 | 1.8±0.3 | 1.73;-0.73 | 4.19 | |
| Triglycerides (mmol/L) | 1.0 (0.5-1.7) | 1.1 (0.6-1.7) | 1.0 (0.4-1.7) | -0.48;-3.65 | 2.69 | |
| Hs-CRP (mg/L) | 0.80 (0.10-20.0) | 0.60 (0.20-12.6) | 0.70 (0.20-8.90) | -0.16;-0.96 | 0.64 | |
| ALT | 1st tertile (≤12 U/L) | 2nd tertile (>12≤17 U/L) | 3rd tertile (>17 U/L) | β; 95%CI | ||
| Number | 66 | 67 | 72 | |||
| Age (yr) | 51.9±5.5 | 52.3±5.4 | 52.6±5.5 | -0.04; -0.29 | 0.21 | |
| Male (%) | 15.1 | 35.8 | 47.2 | 4.43; 1.57 | 7.29 | |
| Alcoholics (%) | 45.4 | 50.7 | 55.6 | 2.02; -0.72 | 4.76 | |
| Alcohol (g/day within drinkers) | 14.8±9.3 | 16.7±9.1 | 16.6±9.4 | 0.03; -0.2 | 0.26 | |
| Ever smokers (%) | 47.0 | 46.3 | 38.9 | -1.10; -3.86 | 1.66 | |
| BMI (kg/m2) | 21.8±2.1 | 21.5±2.1 | 22.6±1.7 | 0.55; -0.13 | 1.23 | |
| Waist (cm) | 76.8±7.2 | 75.4±8.8 | 81.2±7.3 | 0.23; 0.07 | 0.39 | |
| Systolic blood pressure (mmHg) | 115.2±9.0 | 117.4±6.3 | 117.9±8.4 | 0.09; -0.08 | 0.26 | |
| Diastolic blood pressure (mmHg) | 75.3±6.3 | 74.0±5.3 | 75.9±5.7 | 0.15; -0.08 | 0.38 | |
| Uric acid (mmol/L) | 154.6±41.6 | 160.6±53.5 | 172.5±47.6 | 0.035; 0.008 | 0.06 | |
| Insulin (pmol/L) | 7.5 (0.6-108.6) | 9.6 (2.4-49.2) | 8.4 (1.2-72.0) | 1.24; -0.88 | 3.36 | |
| HOMA insulin resistance (mU/mL´mmol/L) | 0.29 (0.02-5.40) | 0.34 (0.09-2.08) | 0.30 (0.05-2.56) | 1.41; -0.73 | 3.54 | |
| Fasting glucose (mmol/L) | 5.2±0.6 | 5.0±0.5 | 5.2±0.6 | 1.43; -1.02 | 3.88 | |
| HDL-cholesterol (mmol/L) | 1.8±0.3 | 1.7±0.4 | 1.7±0.4 | -2.07; -6.05 | 1.91 | |
| Triglycerides (mmol/L) | 0.9 (0.5-1.7) | 1.0 (0.4-1.5) | 1.0 (0.4-1.7) | 0.91; -4.22 | 6.04 | |
| Hs-CRP (mg/L) | 0.80 (0.10-20.0) | 0.50 (0.20-17.8) | 0.95 (0.20-12.6) | 1.15; -0.12 | 2.42 | |
| GGT | 1st tertile (≤10 U/L) | 2nd tertile (>10≤16 U/L) | 3rd tertile (>16 U/L) | β; 95%CI | ||
| Number | 68 | 71 | 66 | |||
| Age (yr) | 51.7±5.2 | 52.3±5.6 | 52.8±5.5 | 0.009; -0.008 | 0.026 | |
| Male (%) | 17.6 | 29.6 | 53.0 | 0.48; 0.29 | 0.66 | |
| Alcoholics (%) | 36.8 | 54.9 | 60.6 | 0.24; 0.06 | 0.42 | |
| Alcohol (g/day within drinkers) | 11.2±7.9 | 16.6±8.6 | 18.6±9.6 | 0.012; -0.002 | 0.026 | |
| Ever smokers (%) | 35.3 | 45.1 | 51.5 | 0.14; -0.05 | 0.33 | |
| BMI (kg/m2) | 21.7±1.9 | 22.0±2.0 | 22.3±2.1 | 0.034; -0.012 | 0.08 | |
| Waist (cm) | 76.4±7.2 | 76.4±8.6 | 81.1±7.7 | 0.19; 0.18 | 0.20 | |
| Systolic blood pressure (mmHg) | 116.0±9.0 | 117.3±6.3 | 117.4±8.7 | 0.008;-0.004 | 0.02 | |
| Diastolic blood pressure (mmHg) | 74.6±6.3 | 74.9±5.7 | 75.8±5.4 | 0.012;-0.004 | 0.03 | |
| Uric acid (mmol/L) | 148.7±35.7 | 166.5±41.6 | 178.4±59.5 | 0.004; 0.002 | 0.006 | |
| Insulin (pmol/L) | 9.6 (3.0-72.0) | 9.0 (0.6-63.6) | 8.4 (1.2-108.6) | 0.004;-0.14 | 0.14 | |
| HOMA insulin resistance (mU/mL´mmol/L) | 0.34 (0.10-2.28) | 0.30 (0.02-2.56) | 0.30 (0.04-5.40) | 0.047; -0.09 | 0.18 | |
| Fasting glucose (mmol/L) | 4.8±0.4 | 5.1±0.5 | 5.5±0.5 | 0.35; 0.19 | 0.51 | |
| HDL-cholesterol (mmol/L) | 1.8±0.3 | 1.7±0.3 | 1.7±0.4 | -0.12; -0.39 | 0.15 | |
| Triglycerides (mmol/L) | 0.9 (0.4-1.5) | 1.0 (0.4-1.7) | 1.1 (0.7-1.7) | 0.52; 0.19 | 0.85 | |
| Hs-CRP (mg/L) | 0.50 (0.20-8.30) | 0.80 (0.10-8.90) | 0.95 (0.20-20.0) | 0.21; 0.13 | 0.29 |
Median (range) is reported for not-normally distributed variables; their log-transformed values and log-GGT values are used in the analyses.
Table 3 describes the associations between the levels of each liver enzyme and age, gender, BMI, waist circumference, alcohol consumption, smoking habits, hs-CRP, HOMA-IR, and triglyceride uric acid levels (and hypertension in all the cohort) in a multiple regression model, after introducing all these as independent variables into the model. In all patients: waist levels are significantly associated with AST levels; male sex, waist, and higher levels of HOMA-IR, triglycerides, uric acid with ALT levels; male sex, alcohol and higher levels of hs-CRP, triglycerides, uric acid with GGT values.
Table 3.
Associations of different clinical and laboratory variables with liver enzyme levels in all the patients (left) and in subjects with normal BMI and without any component of the metabolic syndrome (right) in a multiple regression model
|
ALL |
Normal |
BMI and no component of the MS |
||||
|
β |
95%CI |
β |
95%CI |
|||
| AST | ||||||
| Age | 0.02 | -0.06 | 0.10 | -0.015 | -0.17 | 0.14 |
| Male | 0.58 | -0.54 | 1.70 | -0.70 | -3.05 | 1.65 |
| Ever smoking | -1.06 | -2.16 | 0.04 | -0.54 | -2.34 | 1.26 |
| BMI | -0.13 | -0.29 | 0.03 | -0.25 | -0.83 | 0.33 |
| Waist | 0.09 | 0.03 | 0.15 | 0.12 | -0.04 | 0.28 |
| G/day alcohol | 0.04 | 0.0 | 0.08 | 0.07 | -0.02 | 0.16 |
| Hs-CRP | -0.015 | -0.06 | 0.03 | -0.29 | -0.55 | 1.13 |
| HOMA-IR | 0.25 | -0.28 | 0.77 | 0.26 | -1.11 | 1.63 |
| Triglycerides | 0.17 | -0.91 | 1.25 | -1.39 | -4.76 | 1.98 |
| Uric acid | 0.01 | 0.0 | 0.02 | 0.01 | -0.009 | 0.03 |
| ALT | ||||||
| Age | -0.09 | -0.21 | 0.02 | -0.12 | -0.37 | 0.13 |
| Male | 3.32 | 1.58 | 5.06 | 3.36 | -0.32 | 7.04 |
| Ever smoking | 1.13 | -0.30 | 2.56 | -2.27 | -5.11 | 0.57 |
| BMI | 0.22 | -0.01 | 0.45 | 0.07 | -0.83 | 0.97 |
| Waist | 0.10 | 0.01 | 0.19 | 0.11 | -0.14 | 0.36 |
| G/day alcohol | 0.015 | -0.05 | 0.08 | 0.03 | -0.11 | 0.17 |
| Hs-CRP | -0.04 | -0.74 | 0.66 | 1.16 | -0.15 | 2.47 |
| HOMA-IR | 1.85 | 1.03 | 2.67 | 0.91 | -1.25 | 3.07 |
| Triglycerides | 2.07 | 0.37 | 3.77 | -0.88 | -6.15 | 4.39 |
| Uric acid | 0.015 | 0.001 | 0.03 | 0.01 | -0.02 | 0.04 |
| GGT | ||||||
| Age | -0.0004 | -0.006 | 0.006 | -0.003 | -0.019 | 0.013 |
| Male | 0.29 | 0.21 | 0.37 | 0.31 | 0.10 | 0.52 |
| Ever smoking | 0.02 | -0.04 | 0.07 | 0.025 | -0.16 | 0.20 |
| BMI | -0.0003 | -0.012 | 0.012 | -0.02 | -0.07 | 0.04 |
| Waist | 0.004 | 0.0 | 0.008 | 0.0035 | -0.012 | 0.019 |
| G/day alcohol | 0.006 | 0.002 | 0.010 | 0.005 | -0.003 | 0.014 |
| Hs-CRP | 0.09 | 0.05 | 0.12 | 0.19 | 0.11 | 0.27 |
| HOMA-IR | 0.03 | -0.01 | 0.07 | -0.05 | -0.19 | 0.09 |
| Triglycerides | 0.21 | 0.13 | 0.29 | 0.26 | -0.05 | 0.57 |
| Uric acid | 0.0012 | 0.0006 | 0.0018 | 0.002 | 0.0 | 0.004 |
Multiple regression analyses with adjustments for all the variables listed.
In the subgroup of subjects with normal BMI, without any component of the MS, male sex and higher hs-CRP levels are associated with GGT levels, while no significant association is evident for the other liver enzymes. Fasting glucose is significantly associated with GGT levels in the same model, after adjusting for all previous variables, but insulin instead of HOMA-IR levels (β = 0.23; 95%CI 0.07-0.39); fasting glucose is not significantly associated with AST or ALT values.
In the subgroup of normal BMI subjects, the median NT levels are significantly associated with the increasing GGT tertile (β = 1.07; 95%CI 0.70-1.44), but not with the AST and ALT tertiles in a simple regression (Figure 1). After adjustments for age, sex, BMI, waist, smoking, and alcohol consumption, this association remains significant (β = 1.06; 95%CI 0.67-1.45).
Figure 1.
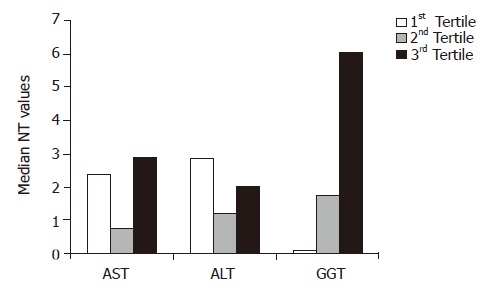
Median NT values for tertile of liver enzyme levels in subjects with normal BMI and without any component of the metabolic syndrome. At simple and multiple regression analyses NT values are associated with increasing GGT tertiles (P<0.001), but not with ALT or AST tertiles.
Median NT levels are significantly correlated with: fasting glucose (β = 1.00; 95%CI 0.45-1.55) and waist circumference (β = 0.04; 95%CI 0.002-0.08). In a multiple regression model, after adjustments for age, sex, BMI, waist, smoking, and alcohol consumption, both NT (β = 0.05; 95%CI 0.02-0.08) and hs-CRP levels (β = 0.09; 95%CI 0.03-0.15) are significantly associated with fasting glycemia.
Correlations are similar also in non-drinkers, and after adjusting for AST or ALT levels (data not shown). Data do not change after excluding the subjects with CRP>10 (respectively n = 48 in the entire cohort and n = 2 in the subgroup of normal BMI), those with cardiovascular diseases (respectively n = 50 and n = 3) or those subjects on estrogen therapy (respectively n = 73 and n = 12).
DISCUSSION
Prospective studies have described that high levels of ALT and GGT[2-10] are associated with subsequent development of diabetes, while the association between AST levels and metabolic abnormalities is weaker and often attenuated or abolished after adjustment for adiposity[2-6,8-10].
Accordingly, we have found in all our patients a worse metabolic pattern in subjects within the highest ALT and GGT tertiles than those within the highest AST tertile. Increased ALT and GGT levels are associated with hepatic insulin resistance and a subsequent decline in hepatic insulin sensitivity[1-5] and GGT seems implicated in oxidative stress[4,16-18].
Excess deposition of fat in the liver, the non-alcoholic fatty liver disease, shows strong cross-sectional associations with obesity, insulin resistance and type 2 diabetes[14,15] and is associated with liver enzyme elevation. Thus, the relationships previously found might reflect associations between ALT or GGT levels and obesity or insulin resistance. Accordingly, authors have found that the associations between GGT levels and diabetes or blood pressure have been attenuated on adjustment for known risk factors for diabetes or for plasma insulin levels and have speculated that visceral fat could play a role in the association of GGT with type 2 diabetes[2,4,27].
The subgroup of subjects free from abnormal glucose, lipid, pressure values and with normal BMI and waist circumference avoids these possible interference from confounding factors. Within this group, increased GGT levels are significantly associated with hs-CRP and fasting glucose values, after multiple adjustments.
There is a progressive and significant increment of NT levels with increasing GGT tertile, not evident within the tertiles of the other liver enzymes that were tested (Figure 1). Furthermore, NT levels are significantly associated to fasting glucose values.
NT, generated from the oxidation of tyrosine, has been considered as a measure of oxidative injury from peroxynitrite (deriving from the reaction of nitric oxide with superoxide anion radicals), and reported to be elevated in diabetes, a condition associated with oxidative stress[28,29].
It could be speculated that the association between GGT levels and fasting glucose, not confounded by other metabolic abnormalities in the subgroup of “metabolically” healthy individuals, could be due to the adverse oxidative pattern of these subjects, suggested by the sharp increase of NT levels in the individuals within the highest GGT tertile.
In line with this, previous studies have reported a primary role for GGT in metabolizing extracellular reduced glutathione, a cellular antioxidant, allowing for precursor amino acids to be reutilized for the intracellular synthesis of glutathione[4,5,18]. Furthermore, dietary and serum antioxidants inversely predicted future GGT levels, while these latter are associated to F2-isoprostanes, a marker of oxidative damage[4,16-18]. It has been speculated that elevated GGT levels might be a defensive response to oxidative stress or, otherwise a marker of oxidative stress, being involved directly in the generation of reactive oxygen species, especially in the presence of iron or other transition metals, inducing lipid peroxidation in human biological membranes[17,18,30,31]. Whether GGT is a causative factor for oxidative stress or a marker is currently unknown.
Aminotransferases were not associated with NT levels (Figure 1), in agreement with studies that showed lower associations between ALT levels and antioxidant vitamins and micronutrients, at least in individuals with normal BMI or fat distribution[18,32].
Thus, in subjects without other metabolic abnormalities, serum GGT might represent a marker of oxidative stress, and justify the associations found with higher fasting glucose values in these healthy individuals.
We cannot exclude the possibility that abnormal GGT values reflect fatty liver deposition, predating the development of subsequent diabetes; furthermore, hepatic ultrasonography or more invasive instrumental methods have not been performed in our subjects. Otherwise, they could be considered as “metabolically obese, normal weight” individuals, in consideration of their higher, though within the normal range, waist values[33]. Indeed our subjects with elevated GGT levels do not show higher insulin levels or insulin resistance (Table 2) and fatty liver has been associated with hyperinsulinemia and insulin resistance[14,15], and associations remain significant, after adjustments for waist circumference and BMI. Accordingly, abnormal liver enzymes have been found in a proportion of normal BMI individuals without fatty liver by ultrasound[34].
Furthermore, the correlations found are independent by adjustment for aminotransferase levels, which are better indicators of hepatocellular statuses and these latter are not associated with glycemia in the subgroup of normal BMI individuals.
Finally, increased GGT levels are conventionally considered as a marker of alcoholic abuse; however, only moderate drinkers have been included in the study, adjustments for alcohol intake have been performed, and correlations do not change after including only non-drinkers.
Another possible mechanism implicated is chronic inflammation: hs-CRP, an acute-phase reactant of hepatic origin and a sensitive marker for systemic inflammation, predicts the occurrence of diabetes, the metabolic syndrome and atherosclerotic diseases in healthy subjects[35]. Previous studies have found associations between GGT and CRP or other inflammatory parameters, suggesting that this enzyme represents the expression of sub-clinical inflammation, and has a role in cellular stress[3,4,9,10,20,21]. In our healthy cohort, hs-CRP levels are significantly associated either with highest GGT values or with fasting glucose. Oxidative processes might have an implication in chronic inflammation[19]; it has been hypothesized that elevation in GGT might occur before an elevation in CRP, and the related oxidative stress would give rise to a subsequent inflammatory response[21].
Again, aminotransferases are not associated with hs-CRP in normal BMI individuals, in line with previous reports, which did not find such an association[6,8,21].
Another explanation might be related to the liver response to pro-inflammatory cytokine tumor necrosis factor-α, giving fatty hepatic changes, or to the inflammatory processes accompanying non-alcoholic fatty liver and contributing to the systemic inflammation observed in these subjects, frequently affected by the MS[20,36]. However, the lack of a significant association between hs-CRP and ALT (a better marker for liver fat accumulation) levels in our normal BMI individuals is against this pathogenetic hypothesis at least in this subgroup. Accordingly, other authors demonstrated that CRP and aminotransferases predicted diabetes independent of each other, with similar magnitude associations[8].
Since the majority of healthy subjects within the highest GGT tertile show enzyme levels within targets of normality (89%), it could be suggested that variations within the normal ranges of GGT are associated with a worse oxidative or inflammatory pattern.
A single measurement of glucose, insulin, hs-CRP or liver enzyme levels represents a limitation of the present study, although common to most epidemiological studies. However, random errors due to the fluctuations of laboratory measurements usually lead to a reduced estimate of the associated strength. Serum oxidative markers could not directly reflect hepatocellular levels, and the existence of oxidative stress depends on the relative balance of reactive oxygen species and all the microenvironment antioxidant defenses, that could not be evaluated in such an observational study.
Whether elevated GGT levels should be added to the cluster of cardiovascular risk factors that form the metabolic syndrome and link insulin resistance to cardiovascular disease, as supported by literature[37,38] or if elevated GGT levels represent a marker of oxidative stress or inflammation could not be established by a cross-sectional study. Nevertheless, the hypothesis that elevated GGT levels may represent an early, easy, and inexpensive marker for a higher subsequent metabolic risk in apparently healthy subjects seems intriguing and worthy of future investigation.
In conclusion, in adult healthy subjects without any measurable metabolic abnormality, those with the highest GGT levels present with either higher fasting glucose values (even within the range of normality) and evidence of some oxidative stress or inflammation; aminotransferase levels do not show these correlations. The follow-up of these individuals would determine if GGT values might be considered as an early predictor of subsequent diabetes occurrence.
ACKNOWLEDGMENTS
We are indebted to Dr Carla Baldi, Dr Lorenzo Benini, Dr Ferruccio Dusio, Dr Giuseppe Forastiere, Dr Claudio Lucia, Dr Claudio Nuti, for their precious assistance in performing the study.
Footnotes
Supported by a grant: “Progetto di Ricerca Sanitaria Finalizzata, Regione Piemonte, 2003”
Science Editor Guo SY Language Editor ELsevier HK
References
- 1.Nilssen O, Førde OH. Seven-year longitudinal population study of change in gamma-glutamyltransferase: the Tromsø Study. Am J Epidemiol. 1994;139:787–792. doi: 10.1093/oxfordjournals.aje.a117075. [DOI] [PubMed] [Google Scholar]
- 2.Perry IJ, Wannamethee SG, Shaper AG. Prospective study of serum gamma-glutamyltransferase and risk of NIDDM. Diabetes Care. 1998;21:732–737. doi: 10.2337/diacare.21.5.732. [DOI] [PubMed] [Google Scholar]
- 3.Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, Tataranni PA. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:1889–1895. doi: 10.2337/diabetes.51.6.1889. [DOI] [PubMed] [Google Scholar]
- 4.Lee DH, Jacobs DR, Gross M, Kiefe CI, Roseman J, Lewis CE, Steffes M. Gamma-glutamyltransferase is a predictor of incident diabetes and hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin Chem. 2003;49:1358–1366. doi: 10.1373/49.8.1358. [DOI] [PubMed] [Google Scholar]
- 5.Lee DH, Ha MH, Kim JH, Christiani DC, Gross MD, Steffes M, Blomhoff R, Jacobs DR. Gamma-glutamyltransferase and diabetes--a 4 year follow-up study. Diabetologia. 2003;46:359–364. doi: 10.1007/s00125-003-1036-5. [DOI] [PubMed] [Google Scholar]
- 6.Sattar N, Scherbakova O, Ford I, O'Reilly DS, Stanley A, Forrest E, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J. Elevated alanine aminotransferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the west of Scotland coronary prevention study. Diabetes. 2004;53:2855–2860. doi: 10.2337/diabetes.53.11.2855. [DOI] [PubMed] [Google Scholar]
- 7.Lee DH, Silventoinen K, Jacobs DR, Jousilahti P, Tuomileto J. gamma-Glutamyltransferase, obesity, and the risk of type 2 diabetes: observational cohort study among 20,158 middle-aged men and women. J Clin Endocrinol Metab. 2004;89:5410–5414. doi: 10.1210/jc.2004-0505. [DOI] [PubMed] [Google Scholar]
- 8.Hanley AJ, Williams K, Festa A, Wagenknecht LE, D'Agostino RB, Kempf J, Zinman B, Haffner SM. Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2004;53:2623–2632. doi: 10.2337/diabetes.53.10.2623. [DOI] [PubMed] [Google Scholar]
- 9.Nakanishi N, Nishina K, Li W, Sato M, Suzuki K, Tatara K. Serum gamma-glutamyltransferase and development of impaired fasting glucose or type 2 diabetes in middle-aged Japanese men. J Intern Med. 2003;254:287–295. doi: 10.1046/j.1365-2796.2003.01198.x. [DOI] [PubMed] [Google Scholar]
- 10.Nakanishi N, Suzuki K, Tatara K. Serum gamma-glutamyltransferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2004;27:1427–1432. doi: 10.2337/diacare.27.6.1427. [DOI] [PubMed] [Google Scholar]
- 11.Sakugawa H, Nakayoshi T, Kobashigawa K, Nakasone H, Kawakami Y, Yamashiro T, Maeshiro T, Tomimori K, Miyagi S, Kinjo F, et al. Metabolic syndrome is directly associated with gamma glutamyl transpeptidase elevation in Japanese women. World J Gastroenterol. 2004;10:1052–1055. doi: 10.3748/wjg.v10.i7.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu CM, Tung TH, Liu JH, Chen VT, Lin CH, Hsu CT, Chou P. A community-based epidemiological study of elevated serum alanine aminotransferase levels in Kinmen, Taiwan. World J Gastroenterol. 2005;11:1616–1622. doi: 10.3748/wjg.v11.i11.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Barneveld T, Seidell JC, Traag N, Hautvast JG. Fat distribution and gamma-glutamyl transferase in relation to serum lipids and blood pressure in 38-year old Dutch males. Eur J Clin Nutr. 1989;43:809–818. [PubMed] [Google Scholar]
- 14.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 15.Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, Cassader M, David E, Cavallo-Perin P, Rizzetto M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367–372. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 16.Lee DH, Gross MD, Jacobs DR. Association of serum carotenoids and tocopherols with gamma-glutamyltransferase: the Cardiovascular Risk Development in Young Adults (CARDIA) Study. Clin Chem. 2004;50:582–588. doi: 10.1373/clinchem.2003.028852. [DOI] [PubMed] [Google Scholar]
- 17.Lee DH, Steffen LM, Jacobs DR. Association between serum gamma-glutamyltransferase and dietary factors: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2004;79:600–605. doi: 10.1093/ajcn/79.4.600. [DOI] [PubMed] [Google Scholar]
- 18.Lim JS, Yang JH, Chun BY, Kam S, Jacobs DR, Lee DH. Is serum gamma-glutamyltransferase inversely associated with serum antioxidants as a marker of oxidative stress? Free Radic Biol Med. 2004;37:1018–1023. doi: 10.1016/j.freeradbiomed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 19.Pleiner J, Mittermayer F, Schaller G, Marsik C, MacAllister RJ, Wolzt M. Inflammation-induced vasoconstrictor hyporeactivity is caused by oxidative stress. J Am Coll Cardiol. 2003;42:1656–1662. doi: 10.1016/j.jacc.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Kerner A, Avizohar O, Sella R, Bartha P, Zinder O, Markiewicz W, Levy Y, Brook GJ, Aronson D. Association between elevated liver enzymes and C-reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2005;25:193–197. doi: 10.1161/01.ATV.0000148324.63685.6a. [DOI] [PubMed] [Google Scholar]
- 21.Lee DH, Jacobs DR. Association between serum gamma-glutamyltransferase and C-reactive protein. Atherosclerosis. 2005;178:327–330. doi: 10.1016/j.atherosclerosis.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169–2175. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki A, Angulo P, Lymp J, St Sauver J, Muto A, Okada T, Lindor K. Chronological development of elevated aminotransferases in a nonalcoholic population. Hepatology. 2005;41:64–71. doi: 10.1002/hep.20543. [DOI] [PubMed] [Google Scholar]
- 24.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 25.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Ikai E, Ishizaki M, Suzuki Y, Ishida M, Noborizaka Y, Yamada Y. Association between hepatic steatosis, insulin resistance and hyperinsulinaemia as related to hypertension in alcohol consumers and obese people. J Hum Hypertens. 1995;9:101–105. [PubMed] [Google Scholar]
- 28.Ceriello A, Mercuri F, Quagliaro L, Assaloni R, Motz E, Tonutti L, Taboga C. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia. 2001;44:834–838. doi: 10.1007/s001250100529. [DOI] [PubMed] [Google Scholar]
- 29.Ceriello A, Quagliaro L, Catone B, Pascon R, Piazzola M, Bais B, Marra G, Tonutti L, Taboga C, Motz E. Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care. 2002;25:1439–1443. doi: 10.2337/diacare.25.8.1439. [DOI] [PubMed] [Google Scholar]
- 30.Drozdz R, Parmentier C, Hachad H, Leroy P, Siest G, Wellman M. gamma-Glutamyltransferase dependent generation of reactive oxygen species from a glutathione/transferrin system. Free Radic Biol Med. 1998;25:786–792. doi: 10.1016/s0891-5849(98)00127-0. [DOI] [PubMed] [Google Scholar]
- 31.Paolicchi A, Minotti G, Tonarelli P, Tongiani R, De Cesare D, Mezzetti A, Dominici S, Comporti M, Pompella A. Gamma-glutamyl transpeptidase-dependent iron reduction and LDL oxidation--a potential mechanism in atherosclerosis. J Investig Med. 1999;47:151–160. [PubMed] [Google Scholar]
- 32.Ruhl CE, Everhart JE. Relation of elevated serum alanine aminotransferase activity with iron and antioxidant levels in the United States. Gastroenterology. 2003;124:1821–1829. doi: 10.1016/s0016-5085(03)00395-0. [DOI] [PubMed] [Google Scholar]
- 33.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 34.Sakugawa H, Nakayoshi T, Kobashigawa K, Nakasone H, Kawakami Y, Yamashiro T, Maeshiro T, Tomimori K, Miyagi S, Kinjo F, et al. Alanine aminotransferase elevation not associated with fatty liver is frequently seen in obese Japanese women. Eur J Clin Nutr. 2004;58:1248–1252. doi: 10.1038/sj.ejcn.1601956. [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–2825. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 36.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 37.Wannamethee G, Ebrahim S, Shaper AG. Gamma-glutamyltransferase: determinants and association with mortality from ischemic heart disease and all causes. Am J Epidemiol. 1995;142:699–708. doi: 10.1093/oxfordjournals.aje.a117699. [DOI] [PubMed] [Google Scholar]
- 38.Jousilahti P, Rastenyte D, Tuomilehto J. Serum gamma-glutamyl transferase, self-reported alcohol drinking, and the risk of stroke. Stroke. 2000;31:1851–1855. doi: 10.1161/01.str.31.8.1851. [DOI] [PubMed] [Google Scholar]


