Abstract
The superior colliculus (SC) plays a critical role in orienting movements, in part by integrating modulatory influences on the sensorimotor transformations it performs. Many species exhibit a robust brain stem cholinergic projection to the intermediate and deep layers of the SC arising mainly from the pedunculopontine tegmental nucleus (PPTg), which may serve to modulate SC function. However, the physiological effects of this input have not been examined in vivo, preventing an understanding of its functional role. Given the data from slice experiments, cholinergic input may have a net excitatory effect on the SC. Alternatively, the input could have mixed effects, via activation of inhibitory neurons within or upstream of the SC. Distinguishing between these possibilities requires in vivo experiments in which endogenous cholinergic input is directly manipulated. Here we used anatomical and optogenetic techniques to identify and selectively activate brain stem cholinergic terminals entering the intermediate and deep layers of the awake mouse SC and recorded SC neuronal responses. We first quantified the pattern of the cholinergic input to the mouse SC, finding that it was predominantly localized to the intermediate and deep layers. We then found that optogenetic stimulation of cholinergic terminals in the SC significantly increased the activity of a subpopulation of SC neurons. Interestingly, cholinergic input had a broad range of effects on the magnitude and timing of SC responses, perhaps reflecting both monosynaptic and polysynaptic innervation. These findings begin to elucidate the functional role of this cholinergic projection in modulating the processing underlying sensorimotor transformations in the SC.
Keywords: in vivo electrophysiology, brain stem cholinergic system, freely moving animals, sensorimotor transformations, oculomotor
the superior colliculus (SC) is a critical node in the network of brain regions underlying sensorimotor transformations. Given that numerous brain areas and subsystems send direct input to the SC, a key aspect of its role involves the integration of modulatory influences on these transformations (Gandhi and Katnani 2011; Kobayashi and Isa 2002). For example, the role of tonic GABAergic inhibition from the substantia nigra pars reticulata (SNr) in preventing unwanted SC-dependent movements has been well studied (Deniau et al. 1978; Hikosaka and Wurtz 1985; Liu and Basso 2008). However, much less is known about the role of cholinergic input in SC processing, despite the conservation of a robust cholinergic projection from the pedunculopontine tegmental nucleus (PPTg) across several mammalian species (Beninato and Spencer 1986; Hall et al. 1989; Harting et al. 1988; Harting and Van Lieshout 1991; Jones and Webster 1988; Wallace and Fredens 1988; Woolf and Butcher 1986).
Studies in slices have shown that cholinergic agonists depolarize SC neurons directly, primarily via nicotinic signaling, and indirectly, by hyperpolarizing the terminals of inhibitory SNr neurons via presynaptic muscarinic receptors (Isa and Hall 2009; Li et al. 2004; Sooksawate et al. 2008, 2011; Sooksawate and Isa 2006). Results of the few in vivo studies performed have been consistent with the slice studies, finding that cholinergic agonists delivered to the SC facilitate whisking-related neural activity in rats (Bezdudnaya and Castro-Alamancos 2014) as well as SC-dependent saccade generation in primates (Aizawa et al. 1999; Watanabe et al. 2005). While these pharmacological studies suggest that cholinergic signaling promotes SC motor output, the effects of endogenous cholinergic input on SC activity are not known.
To address this question, we utilized a mouse model well-suited to awake recordings in response to optogenetic cholinergic-specific stimulation (Ma and Luo 2012; Witten et al. 2010; Zhang et al. 2010). This model system allowed us to determine how intrinsic cholinergic input modulates SC activity in vivo. To validate this model for our physiological studies, we first examined how the anatomy of cholinergic projections from the PPTg to the SC compared between mice and other species in which this projection has been more extensively studied with traditional tracing and immunostaining tools. We found greater innervation of the intermediate and deep layers of the SC than of the superficial layer, consistent with the data from other species (Beninato and Spencer 1986; Hall et al. 1989; Harting and Van Lieshout 1991). We then performed in vivo recordings in the intermediate and deep layers of the SC in response to optogenetic stimulation of cholinergic terminals. We observed robust, often polysynaptic, responses to cholinergic input. Given the critical role of the rodent SC in stimulus-cued orienting movements (Felsen and Mainen 2008, 2012; Hirokawa et al. 2011; Stubblefield et al. 2013), our results suggest that brain stem cholinergic input may have a broad modulatory influence on sensorimotor transformations in the SC.
MATERIALS AND METHODS
Animal subjects.
Procedures using animals were approved by the University of Colorado Animal Care and Use Committee and were in accordance with standards implemented by the National Institutes of Health.
Surgeries for fluorescence reconstruction to examine cholinergic PPTg input to the SC.
Four transgenic [ChAT-Cre; Jackson Labs, strain B6;129S6-Chattm1(cre)Lowl/J] adult (aged 150–200 days) male mice were used for histological injection experiments. Each mouse received two tracer injections, one during each of two surgeries separated by 6 wk. First, we injected a Cre-dependent adeno-associated virus [AAV5-Ef1a-DIO-EYFP; all viruses were obtained from the University of North Carolina Vector Core with permission from Dr. Karl Deisseroth (Stanford University)] into the left PPTg that would be expressed by Cre-expressing ChAT neurons. Mice then underwent a second surgery and were injected with cholera toxin subunit B (CTB; a retrograde tracer) conjugated to Alexa 555 (Life Technologies) into the contralateral (right) medial pontine reticular formation (mPRF) to obtain retrograde labeling in the left SC (Grantyn and Berthoz 1987; Isa et al. 2013; Isa and Sasaki 2002; Sooksawate et al. 2005, 2008) (Fig. 1A). With the exception of injectate and anatomical target, both tracer injection surgeries followed the same method. CTB was injected 6 wk after the AAV injection; total incubation time for AAV and CTB was 8 and 2 wk, respectively. The tracer injection method adheres closely to that described in Thompson et al. (2012). Mice were placed in an enclosed chamber and briefly exposed to anesthesia (isoflurane, 2%). Directly after deep anesthesia, mice were placed in a stereotactic device with a nose cone that delivered 1–1.5% isoflurane to maintain an anesthetized state (verified frequently throughout the surgery via response to foot pinch). The scalp was then shaved, topical antiseptic (Betadine) was applied along with ophthalmic ointment to the eyes, and 250 μl of 2% lidocaine was injected under the surface of the scalp. The skull was exposed by midline incision and scalp retraction, and bregma and lambda were aligned along the horizontal plane (z coordinate). We drilled a small cranial fenestration (1.5 × 1.5 mm) over the approximate location of either the left PPTg (4.5 mm posterior from bregma and 1.1 mm lateral to midline) or the right mPRF (5.5 mm posterior from bregma and 0.4 mm lateral to midline), which is designated as the caudal part of the pontine reticular nucleus by Paxinos and Franklin (2012). Injection pipettes were pulled from borosilicate glass micropipettes (OD: 1.0 mm, ID: 0.5 mm; Sutter Instruments) with a model P97 Sutter Instrument micropipette puller, and the tips were clipped under microscopic inspection to 10- to 15-µm inner diameter with dissection scissors. The volume of the injection was calibrated by making 1-mm demarcations along the shaft of the glass pipette with a fine-tipped black marker (1 mm = 125 nl). Two microliters of the injectate was pipetted onto a small square (0.3 × 0.3 cm) of paraffin film and placed on the surface of the skull at the approximate posterior and lateral coordinates of the first craniotomy. The injection pipette was then lowered into the injectate, and under microscopic inspection ∼500 nl was slowly aspirated. Taking up of the tracer was achieved by having the pipette connected with 35 cm of PE-160 polyethylene tubing (OD: 1.57 mm, ID: 1.14 mm) to a blunted 23-gauge needle connected to a 20-ml Luer-Lok syringe. The injectate-filled micropipette was adjusted to the appropriate injection coordinates and slowly lowered to depth (PPTg, 2.4 mm ventral to the dural surface; mPRF, 4.25 mm ventral to the dural surface). In total, ∼175–200 nl of AAV was injected into the PPTg and 300–400 nl of CTB (1.0 mg/ml) was injected into the mPRF. After the final injection, the surface of the skull was lightly debrided with 0.1 M PBS, the incision was closed with veterinary adhesive, and topical anesthetic was reapplied along with a topical antibiotic (gentamicin).
Fig. 1.
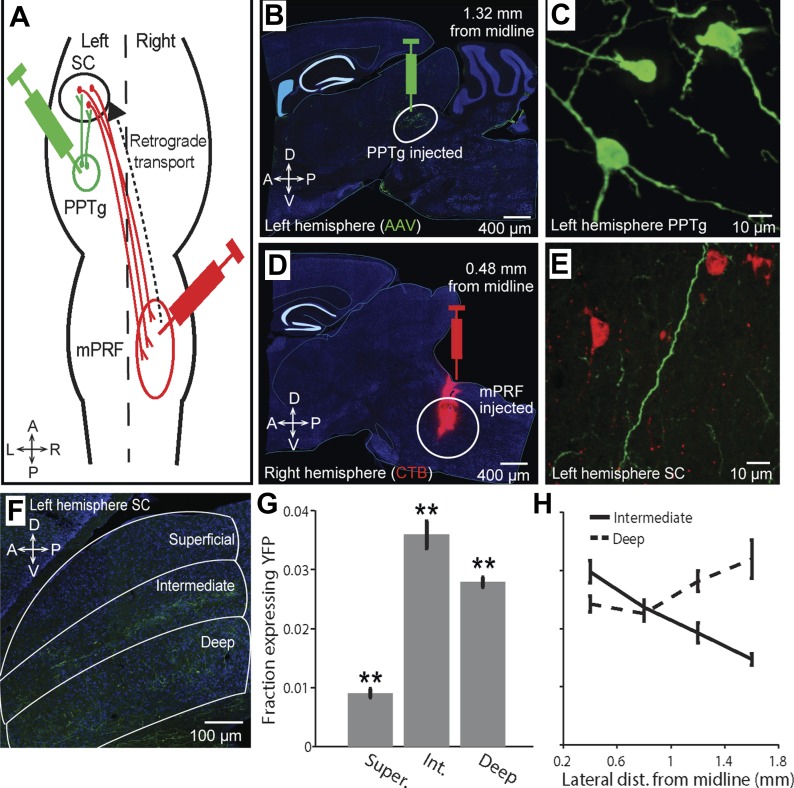
Investigation of the cholinergic projection from the pedunculopontine tegmental nucleus (PPTg) to the superior colliculus (SC). A: schematic overview of the experiments. ChAT-Cre mice were injected with an adeno-associated virus (AAV) containing a Cre-dependent enhanced yellow fluorescent protein (EYFP) construct into the left PPTg to label cholinergic terminals in the left SC and cholera toxin B (CTB) conjugated to Alexa 555 into the right medial pontine reticular formation (mPRF) to retrogradely label principal neurons in the left SC. B: an example sagittal section demonstrating AAV-EYFP expression (green) at the injection site in the PPTg. C: image (×40) of EYFP expression in PPTg neurons. D: an example sagittal section demonstrating CTB-Alexa 555 expression (red) at the injection site in the mPRF. E: image (×40) of retrogradely labeled intermediate-layer SC projection neuron (red) colocalized with cholinergic PPTg axon terminals (green). F: representative sagittal slice used for the fluorescence reconstruction analyses to quantify the fraction of each SC layer that receives PPTg axon terminals (green). G: fraction area of PPTg input to each SC layer was quantified across all mice (3 sliced sagittally and 1 coronally). **P < 0.001, paired t-test. Error bars, means ± SE. H: fraction area of PPTg input to the intermediate and deep SC layers is shown as a function of known lateral distance from the midline of sagittal sections (3 mice). A, anterior; P, posterior; L, left; R, right; D, dorsal; V, ventral; Super., superficial layer; Int., intermediate layer.
Immunocytochemistry for fluorescence reconstruction.
At the end of a 14- to 19-day postoperative period after the second injection (CTB), mice were overdosed with an intraperitoneal injection of pentobarbital sodium (100 mg/kg) and transcardially perfused with saline followed by ice-cold 4% paraformaldehyde (PFA) in 0.1 M PB. After perfusion, brains were postfixed for 3.5 h in 4% PFA in 0.1 M PB and then cryoprotected overnight by immersion in 20% sucrose in 0.1 M PB. Brains were embedded in optimal cutting temperature compound (OCT; Fisher Scientific) and frozen rapidly on dry ice. Serial coronal (1 mouse) and sagittal (3 mice) sections (40 μm) were cut on a cryostat.
Strong fluorescent signals were obtained for yellow fluorescent protein (YFP) expression by immunohistochemical detection with a chicken polyclonal anti-GFP antibody (1:3,000; Aves Labs). Detection of Alexa 555-conjugated CTB was enhanced by application of rabbit anti-CTB antiserum. CTB and the anti-CTB antibody (1:2,000) were purchased as a kit (Life Technologies). After three 10-min washes in 0.1 M PBS, sections were incubated in blocking solution containing 1% BSA, 2% normal goat serum, and 0.3% Triton X-100 in 0.1 M PBS for 1 h and then incubated in a cocktail mixture of rabbit anti-CTB and chicken anti-GFP for 24–48 h. After incubation in antisera, sections were washed three times for 10 min each in 0.1 M PBS. Sections were then incubated in secondary antibodies (1:500 goat anti-rabbit Alexa 568 for CTB; 1:500 goat anti-chicken Alexa 488 for GFP) and 660 fluorescent Nissl stain solution (1:100, NeuroTrace; Life Technologies) for 2 h. After secondary antibody incubation, sections were washed three times in 0.1 M PBS and once in 0.1 M PB, mounted in serial order onto slides, and coverslipped with Fluoromount-G (Southern Biotechnology). As a negative control, sections of a wild-type mouse (no endogenous YFP) were reacted with the anti-GFP antibody and revealed no fluorescence (data not shown). In addition to examining fluorescence, we used these slices to verify successful targeting of the PPTg and mPRF with our AAV and CTB injections, respectively.
Image processing for fluorescence reconstruction.
Image acquisition exposure times for each fluorophore (Alexa 555, Alexa 448, and Alexa 660) were held constant across all sections within each brain specimen. Whole-slide fluorescent images were photographed with Surveyor by Objective Imaging software that controlled the microscope stage as well as enabling image acquisition, with a black-and-white Leica DFC 365FX camera on a Leica DM6000B microscope. To capture whole-slide scans, the multiscan option in the imaging software was used. For each fluorophore, an overlapping grid of images was captured with a ×10 objective. Within each grid, all three channels (FITC, Texas red, Cy5) were obtained sequentially and merged together to prevent side-band excitation of the fluorophores. Images were then stitched together in real time with the best focus algorithm in the Surveyor software, which yielded a mosaic image of the whole microscope slide. Images of individual fluorescent sections were then obtained with the Region of Interest Tool in the Surveyor Viewer Software.
Fluorescent mapping for reconstruction analyses.
To quantify the distribution of ChAT-positive expression across different layers of the SC we wrote an interactive program, “SCLayerGUI,” running in MATLAB 2013a with the Image Processing Toolbox (The MathWorks; program available at https://github.com/neuropil/SCLayerGUI). This function allows us to examine expression in each layer of the SC (superficial, intermediate, and deep), throughout its entire extent in the mediolateral plane (coronally) and the anteroposterior plane (sagittally). To objectively delimit the SC layers, we used the Nissl stain (and not the ChAT labeling) as a guide to trace a polygon bounding each layer. SC layers included both the gray and white sublayers. For each section, we then calculated the mean and standard deviation of the green RGB channel, corresponding to the ChAT-positive label, for the set of pixels within any of the polygons (i.e., across all 3 user-defined SC layers). We defined the threshold for ChAT expression, for each section, as the mean + 2SD of the green RGB channel for this set of pixels. We then quantified positive ChAT expression for each layer by calculating the number of pixels per polygon that exceeded this threshold. Finally, we derived the fraction expressing YFP per layer by dividing the area of expression (corresponding to the number of pixels above threshold) by the total area of the layer. These data are shown in Fig. 1, G and H. This quantification method allows for relatively high-throughput analyses that are unbiased by expression pattern or anatomical distribution—problems commonly associated with manual methods of expression level estimation—and has been validated in other brain regions (Thompson et al. 2012).
Immunocytochemistry for PPTg cholinergic neurons.
To obtain expression of ChR2-EYFP restricted to cholinergic neurons, we generated “ChAT-Cre/ChR2” mice by crossing the transgenic ChAT-Cre mice described above [Jackson Labs, strain B6;129S6-Chattm1(cre)Lowl/J] with Ai32 floxed ChR2-EYFP mice [Jackson Labs, strain B6;129S-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J]. To verify the intended expression pattern, we assessed the colocalization of ChAT and EYFP in several mice from this line. Adult male ChAT-Cre/ChR2 mice were transcardially perfused in 1.6% PFA, and whole brains remained in fixative no longer than 2 h at room temperature. Brains were then transferred into 30% sucrose and 0.1 M PB and refrigerated for 24 h. Sagittal sections were cut on a freezing microtome to 60 μm and were washed 3 × 10 min in 0.1 M PBS before application of blocking solution (7.5% normal donkey serum, 5% BSA, 87.5% PBS + 0.5% Triton X) for 1 h at room temperature. Slices were incubated in primary antibody (goat anti-ChAT; EMD/Millipore) and diluted to 1:200 in blocking solution with a second primary antibody (1:3,000 chicken anti-GFP; Life Technologies) for 48 h at room temperature. Slices were then washed 7 × 10 min in 0.1 M PBS + 0.5% Triton X. Secondary antibody for ChAT identification (1:1,000 donkey anti-goat Alexa 568; Life Technologies) and secondary antibody to GFP (1:500 Alexa 488; Life Technologies) were diluted in blocking solution and applied to sections for 2 h, and blue Nissl stain (1:1,000 NeuroTrace; Life Technologies) was added after 1.5-h incubation time at this step for a duration of 0.5 h. Slices were again washed in 0.1 M PBS 5 × 10 min and mounted onto slides. Restricted YFP expression in ChAT neurons was confirmed with a confocal microscope (Zeiss) using lasers at 488 and 561 nm sequentially for the two fluorophores and then merging them to prevent side-band excitation (3I, Slidebook 5.0).
Optetrode recording drive assembly and implantation.
To stimulate cholinergic terminals while recording nearby SC activity, we assembled and chronically implanted “optetrode” drives. Each drive (modified from Anikeeva et al. 2012) contained an optic fiber (ThorLabs; 200-μm-diameter core, 250-μm outer diameter, 21.5 mm long) from which the cladding was stripped to 1 mm from the light-emitting tip. A scribe was used to precisely cut and align the fiber core and cladding for efficient light output. The opposite end of the fiber was also stripped to fit and securely attach to a ceramic ferrule (Precision Fiber Products) and polished for efficient laser coupling. The assembled fiber was tested with a 473-nm solid-state laser (Shanghai Laser & Optics Century) to determine the coupling efficiency for 80–160 mW/mm2 light power delivery once implanted.
The recording drive consisted of a 16-channel electrode interface board (EIB; Neuralynx) to which thin-walled plastic PEEK tubing (Small Parts) was attached and extended 0.5 mm above the surface of the EIB and 18.25 mm below. Tetrodes, consisting of four twisted polyimide-coated nichrome wires (Kanthal; single-wire diameter, 12.5 μm) gold plated to 0.2- to 0.4-MΩ impedance, were pinned to the EIB and threaded top-down through the plastic tubing. Delrin protective housing (University of Colorado Machine Shop), consisting of a movable thumb nut and vented screw assembly, was then threaded up and around the bottom extent of the tubing and protected tetrodes so that the screw head was securely attached to the EIB. The optic fiber was finally inserted through the plastic tubing from the top surface of the EIB and firmly attached so that 1 mm of the fiber tip extended below the bottom of the tubing. Tetrodes were cut to 200–500 μm below the optic fiber tip and securely glued to all sides of the 1-mm exposed fiber, clear of the fiber tip (see Fig. 2A).
Fig. 2.
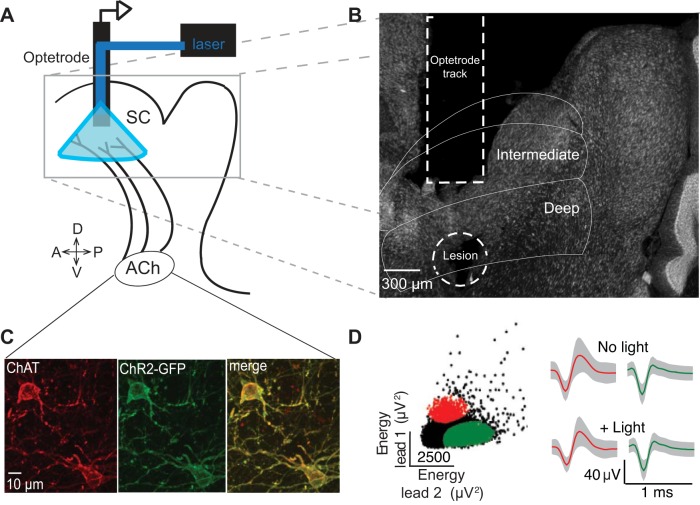
Experimental design and histological confirmation for the stimulation/recording experiments. A: schematic sagittal representation of the experimental design. The optetrode was targeted to the intermediate and deep SC; 473-nm light was delivered to cholinergic terminals expressing ChR2 while extracellular recordings of SC neurons were performed. B: verification of optetrode trajectory through SC and final recording depth. Electrolytic lesion is circled. C: example image from 1 ChAT-Cre/ChR2 mouse consistent with ChR2 expression (green) being limited to ChAT-positive neurons (red) in our ChAT-Cre/ChR2 line. D, left: isolation of the activity of 2 neurons, based on features of the waveforms recorded on each lead, from a representative recording session. Right: for each cluster, the average waveforms on the dominant lead (red, lead 1; green, lead 2) were similar for light-driven spikes (+ Light) and spontaneous spikes (No light). Means ± SD of waveforms are shown.
To chronically implant adult ChAT-Cre/ChR2 mice with optetrode drives (we recorded from these mice because they exhibited, in preliminary experiments, more robust ChR2 expression than virally injected ChAT-Cre mice), anesthesia was induced with 2% isoflurane and maintained between 1% and 1.5% throughout the procedure. Body temperature was maintained with a heating pad. The mouse was placed in a stereotaxic frame (Kopf Instruments), a small incision was made in the scalp, and a craniotomy was performed with a dental drill. The drive was targeted to the intermediate and deep layers of the left SC between 3.75 and 4.04 mm posterior to bregma, 0.9–1.0 mm lateral to the midline, and 1.3 mm ventral to the SC surface. The Delrin housing of the drive was firmly affixed to the skull with two small screws, luting (3M), and dental acrylic (A-M Systems). After surgery, mice were immediately rehydrated with sterile 0.9% saline (1 ml/kg) and administered the analgesics Ketofen (Pfizer; 5 mg/kg) for 3 days and children's ibuprofen (Safeway; 50 mg·kg−1·day−1) for 7 days after surgery. The incision site was treated with a topical antibiotic. Mice were allowed to recover for at least 5 days before recordings began.
Optical stimulation and chronic extracellular recordings.
All recordings were conducted in fully awake mice allowed to move freely within an open box that was 24.5 cm long × 20 cm wide × 19 cm tall. Typically, mice alternated between periods of stillness and exploratory movement; these epoch transitions were not correlated with optical stimulation parameters. We optically stimulated cholinergic terminals in the SC and recorded the responses of SC neurons. For optical stimulation, the solid-state diode laser delivered 473-nm light at a daily tested power output between 80 and 160 mW/mm2. At this power range, we assessed that light penetrated up to 300 μm of neural tissue with no thermal damage (Al-Juboori et al. 2013). The laser was controlled with purpose-written MATLAB software to deliver light stimulation pulses at four different frequencies for each recording session. Frequencies of 2, 4, 8, and 16 Hz were presented in blocks of 90–150 trials, with order randomized across recording sessions. Pulse-on duration was fixed at 25 ms, and pulse-off duration was 475 ms for 2-Hz, 225 ms for 4-Hz, 100 ms for 8-Hz, and 37.5 ms for 16-Hz stimulation. Each trial consisted of 1-s light delivery followed by a 3-s intertrial interval. While long pulse-on durations may inhibit ChR2-mediated neural activity via depolarization block (Herman et al. 2014), SC responses in preliminary experiments suggested that 25 ms is an appropriate duration for reliably depolarizing PPTg neurons and eliciting ACh release. Frequencies above 16 Hz were initially tested in preliminary experiments; these frequencies were subsequently discontinued because of the failure of recorded neurons to “follow” such high-frequency stimulation, likely because a sufficiently long pulse-off duration was required for maximal subsequent light-elicited ACh release. One stimulation/recording session was performed per day, and a total of 48 sessions were recorded from 8 mice.
To record single-unit responses of SC neurons, electrical signals from the tetrodes were amplified and recorded with a multichannel Digital Lynx system (Neuralynx). We did not attempt to target our recordings to SC neurons of any particular type (e.g., excitatory or inhibitory). Single units were obtained by manually clustering waveform features (peak, valley, and energy) derived from the sampled waveforms with MClust software (A. David Redish, University of Minnesota). We excluded clusters with Lratio > 0.25, isolation distance < 15, and interstimulus intervals < 1 ms (Schmitzer-Torbert et al. 2005). In addition, clusters were qualitatively assessed with autocorrelograms and cross-correlograms. We stringently excluded data that may have come from the same neuron in different sessions by ensuring that response properties and waveforms changed across sessions, as previously reported (Thompson and Felsen 2013). For each light-responsive neuron, the average light-elicited and spontaneous waveforms were nearly identical (r > 0.98, P < 1.0 × 10−12).
Optetrode depth was adjusted daily (between 40 and 100 μm) to sample an independent population of neurons across sessions. The depth of each recording session was estimated based on measured turns of the optetrode drive thumb nut (Anikeeva et al. 2012) and later confirmed histologically based on electrolytic lesions and on the visible optetrode tracks (Fig. 2B).
RESULTS
Anatomy of the cholinergic projection from the PPTg to the SC in mice.
Our goal in this study was to determine how endogenous cholinergic input to the SC modulates SC activity in vivo. We first sought to examine whether the spatial extent of the cholinergic projection from the PPTg to the SC in mice is comparable to that reported in other species. To isolate this projection, we injected a Cre-dependent YFP virus (AAV5-Ef1a-DIO-EYFP) into the left PPTg of ChAT-Cre mice and examined cholinergic PPTg input as indicated by fluorescent labeling in the SC (Fig. 1).
We assessed this input in several ways: First, we examined the relationship between cholinergic terminals and the principal projection neurons thought to mediate orienting motor output from the SC (Isa and Hall 2009). These were retrogradely labeled by injecting the contralateral (right) mPRF, to which these principal projection neurons send orienting commands (Grantyn and Berthoz 1987; Isa et al. 2013; Isa and Sasaki 2002; Sooksawate et al. 2005, 2008), with CTB (Fig. 1, A and D; materials and methods). Qualitatively, we observed spatial overlap between these ipsilateral cholinergic terminals and SC principal projection neurons (Fig. 1E), as observed in rats (Beninato and Spencer 1986) and other species (Hall et al. 1989; Harting and Van Lieshout 1991). We next examined the laminar specificity of the cholinergic projection by quantifying YFP expression across mice in the superficial, intermediate, and deep layers separately (Fig. 1F; materials and methods). Briefly, throughout the mediolateral and anteroposterior extent of the SC, the boundaries of each layer were hand-traced based on the fluorescent Nissl stain. For each section, the mean and SD of the green RGB channel (ChAT-positive label) were calculated for the set of all pixels across layers. We then calculated the number of pixels exceeding threshold in the ChAT-positive channel (defined as the mean + 2SD of all pixels across layers). Finally, we derived the fraction expressing YFP per layer by dividing the area of expression by the total area of the layer (Fig. 1, G and H).
Using this method, we found that, as can be seen in Fig. 1F, cholinergic innervation was substantially denser in the intermediate than deep layers (P = 6.78 × 10−4, paired t-test) and was also denser in the deep than superficial layers (P = 6.83 × 10−21, paired t-test; Fig. 1G). These results are the first demonstration in mice that this laminar specificity, as well as the qualitative overlap between cholinergic PPTg axons and SC principal projection neurons, is consistent with findings in other species (Beninato and Spencer 1986; Hall et al. 1989; Harting and Van Lieshout 1991; Ma et al. 1991; Sooksawate et al. 2008).
Next we asked whether the density of cholinergic input to the SC varied as a function of distance from the midline. Thus we similarly quantified the density of YFP expression, separately for the intermediate and deep layers, across 40-μm sagittal sections. We found that within the intermediate layers YFP expression (and therefore cholinergic input) was strongest medially (Fig. 1H; r = 0.50, P = 1.97 × 10−7), while in the deep layers expression was strongest with increasing lateral distance (r = 0.31, P = 2.10 × 10−3). To our knowledge, this is the first study to quantify the laminar-specific density of PPTg cholinergic input to the SC along the mediolateral axis, which may have implications for the functional modulation of topographic representations in the SC (King 2004). In addition, these data allowed us to target our recordings, which we presently describe, to ACh-rich regions of the SC.
SC responses to stimulation of endogenous cholinergic terminals.
We next sought to quantify in vivo responses of SC neurons to endogenous cholinergic release. “Optetrodes,” consisting of an optical fiber for light delivery and tetrodes for neuronal recordings (Anikeeva et al. 2012) (materials and methods), were chronically implanted in ACh-recipient regions of the intermediate and deep layers of ChAT-Cre/ChR2 mice, allowing for optogenetic stimulation of cholinergic release and simultaneous extracellular recording of SC responses (Fig. 2A). At the completion of the final stimulation/recording session, we histologically confirmed that our drives targeted the desired regions of the SC (Fig. 2B). In a subset of ChAT-Cre/ChR2 mice, we confirmed that ChR2 expression was limited to cholinergic neurons (Fig. 2C; materials and methods). We recorded the responses of 90 well-isolated SC neurons during optical stimulation of cholinergic terminals at 2, 4, 8, and 16 Hz (Fig. 2D; materials and methods). To ensure that putative neuronal activity was not contaminated by noise induced by the direct illumination of our recording electrodes (Cardin et al. 2010), we verified that waveforms obtained during light delivery were identical to those obtained in the absence of light [materials and methods; Fig. 2D, right; correlation between “no light” and “+ light” waveforms: red, r = 0.999 (P = 1.49 × 10−58); green, r = 0.992 (P = 1.96 × 10−28)].
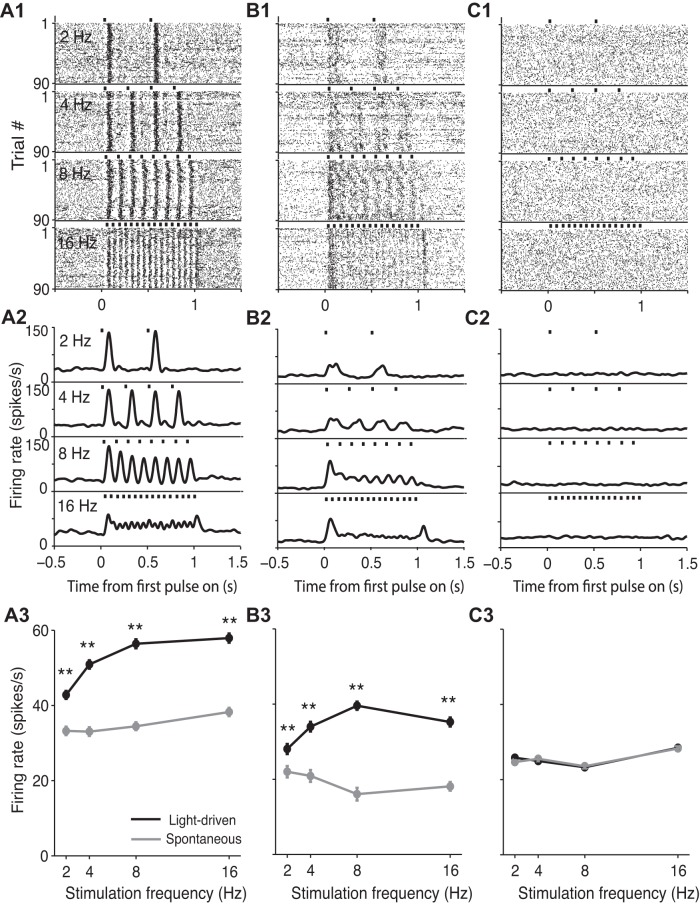
The responses of several representative SC neurons are shown in Fig. 3. Some neurons exhibited a clear and consistent increase in firing rate in response to light stimulation, visible in the temporal structure of the rasters (Fig. 3, A1 and B1) and peristimulus time histograms (PSTHs) (Fig. 3, A2 and B2) aligned to light onset. This increase is indicative of an excitatory effect of cholinergic release on SC activity at all frequencies tested (Fig. 3A3: 2 Hz, P = 0.01; 4 Hz, P = 2.70 × 10−7; 8 Hz, P = 5.55 × 10−17; 16 Hz, P = 1.48 × 10−20; Fig. 3B3: 2 Hz, P = 4.46 × 10−5; 4 Hz, P = 3.89 × 10−15; 8 Hz, P = 3.45 × 10−30; 16 Hz, P = 4.51 × 10−21; paired t-tests, 1-tailed). Other neurons, however, showed no change in firing rate in response to light stimulation (Fig. 3C3: 2 Hz, P = 0.05; 4 Hz, P = 0.77; 8 Hz, P = 0.65; 16 Hz, P = 0.40; paired t-test, 1-tailed). Notably, no SC neurons exhibited a consistent decrease in firing activity in response to light stimulation. However, the divergent response properties exhibited by these examples suggest that distinct groups of SC neurons may be differentially modulated by cholinergic input.
Fig. 3.
Responses of example SC neurons to endogenous cholinergic input. A1 and B1: rasters for 2 individual SC neurons demonstrating light-driven increases over the spontaneous firing rate at each frequency. A2 and B2: peristimulus time histograms for the 2 light-responsive neurons. Histograms are smoothed with a Gaussian filter (σ = 20 ms). A3 and B3: light-driven and spontaneous firing rates as a function of stimulation frequency for the 2 light-responsive neurons. Means ± SD. **P < 0.001, 1-tailed paired t-test comparing light-driven and spontaneous firing rates trial by trial. C1 and C2: rasters and peristimulus time histograms, respectively, for an SC neuron that did not respond to light at any stimulation frequency. C3: as in A3, for the neuron shown in C1 and C2. Light-driven and spontaneous firing rate did not differ at any stimulation frequency.
We therefore classified each recorded neuron as either “light-responsive” or “non-light-responsive” by comparing its firing rate during the 1.2 s following the onset of light delivery on each trial to the spontaneous firing rate for that trial during the 2 s immediately preceding light delivery, for all trials (including all stimulation frequencies). Across our population of neurons, we found that 28% exhibited higher firing rates in response to light delivery (Fig. 4A; P < 0.01, paired t-tests), and they were therefore considered light-responsive. The remaining neurons exhibited no difference in response to light delivery across frequencies; these neurons were therefore considered non-light-responsive (Fig. 4A). To determine how the firing rate of light-responsive neurons depended on stimulation frequency, we averaged (across neurons) the responses of each neuron normalized to its own mean spontaneous activity. We found that normalized activity monotonically increased as a function of stimulation frequency up to 8 Hz but that 16-Hz stimulation did not further increase activity (Fig. 4B; P = 0.8217, unpaired t-test). Across all frequencies, the normalized firing rates of light-responsive neurons were significantly higher than those of non-light-responsive neurons (Fig. 4B; 2 Hz, P = 0.005; 4 Hz, P = 2.05 × 10−8; 8 Hz, P = 7.47 × 10−13; 16 Hz, P = 3.46 × 10−15; unpaired t-tests).
Fig. 4.
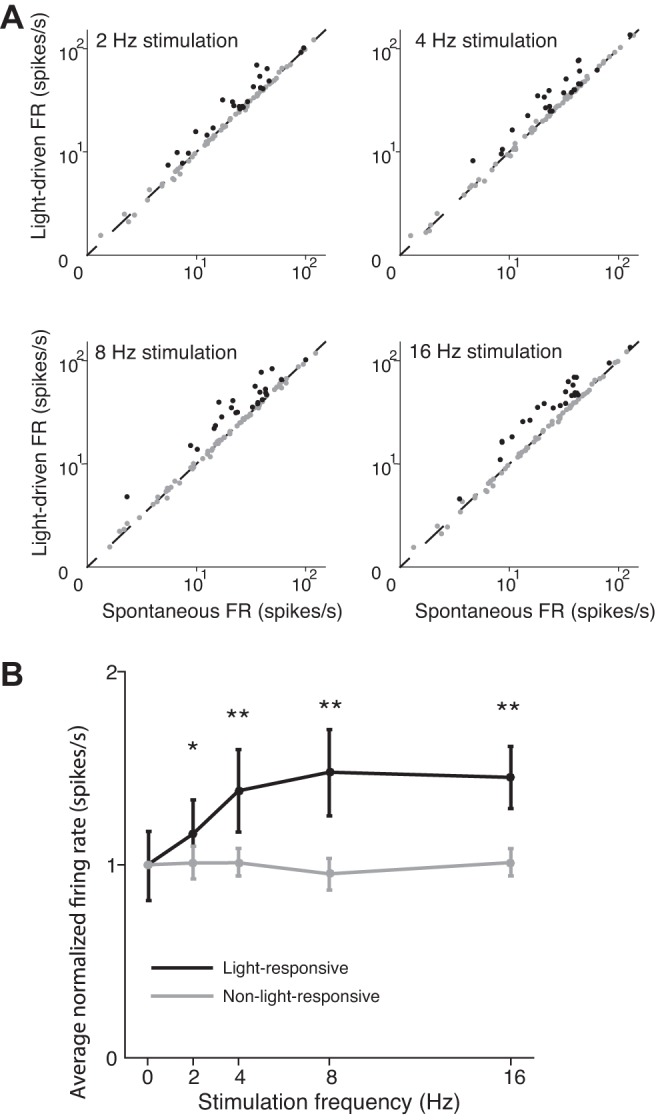
Summary of responses across the population of SC neurons to endogenous cholinergic input. A: for each neuron, mean light-driven and spontaneous firing rates (FR) were compared, separately for each stimulation frequency. Black, light-responsive neurons; gray, non-light-responsive neurons. B: for each trial, light-driven firing rate was normalized to the average spontaneous firing rate for the neuron. Normalized firing rate is shown as a function of stimulation frequency separately for light-responsive and non-light-responsive neurons. Means ± SD. *P ≤ 0.05, **P < 0.001, unpaired t-test, 1-tailed.
Diversity of SC responses to cholinergic input.
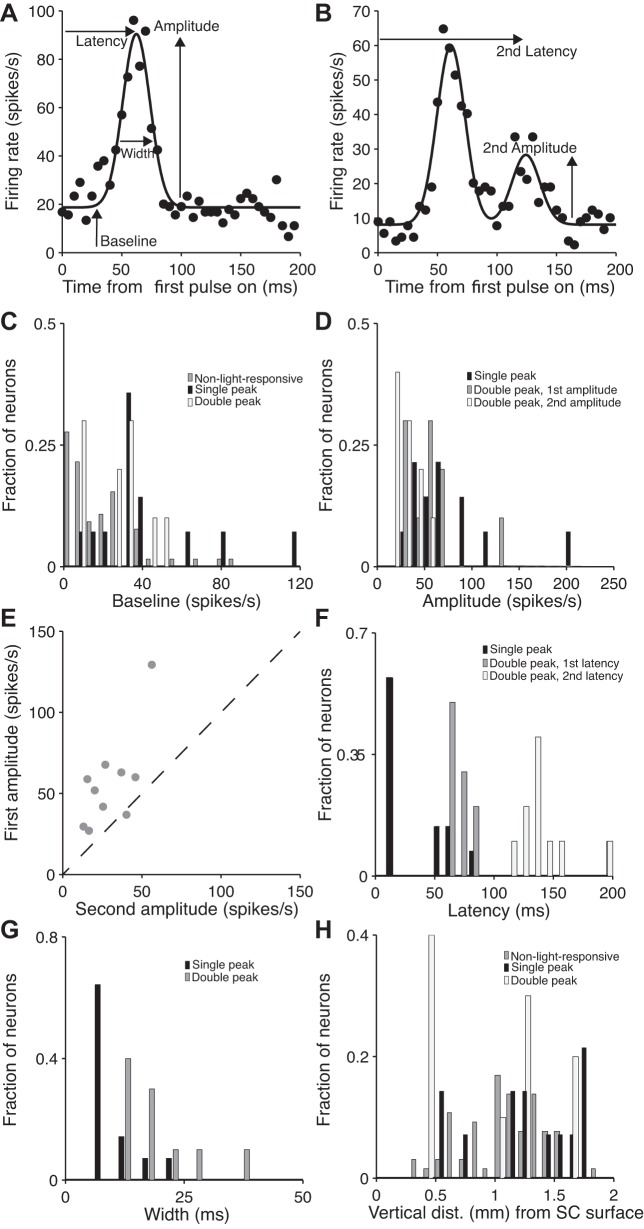
As can be seen from the example neurons (Fig. 3, A and B) and the population results (Fig. 4A), light-responsive neurons exhibit a range of response properties to endogenous cholinergic input. To quantify this diversity, for each neuron we fit the first 200 ms of the mean response to the first light pulse, in the 2 Hz and 4 Hz conditions, with a Gaussian function (Fig. 5A), r(t) = a + b, where r represents the firing rate as a function of time (t) and a, b, c, and d are free parameters (described below). This function provided a good fit (mean squared error of peak-normalized fit ≤ 0.01) for 58% of light-responsive neurons. The remaining light-responsive neurons were better fit to a mixture of two Gaussians (Fig. 5B), r(t) = a + b1 + b2, where r again represents the firing rate as a function of time (t) and a, b1, b2, c1, c2, and d are free parameters. These functions allowed us to extract several relevant characteristics about the response properties of each neuron. We first examined the baseline (i.e., non-light-elicited) firing rates (a). Consistent with the spontaneous firing rates calculated during the 2 s preceding light onset (Fig. 4A), these baselines ranged widely, from 0 to 120 spikes/s, but did not vary between light-responsive and non-light-responsive neurons (Fig. 5C; P = 0.989, ANOVA). We then examined the amplitudes of the fits (b for single-peaked neurons; b1 and b2 for 1st and 2nd amplitudes of double-peaked neurons), which correspond to the maximum responses to light delivery. We again found that the maximum response was variable across neurons, ranging from 27 to 205 spikes/s (Fig. 5D), and that among the neurons best fit by a mixture of two Gaussians 90% exhibited a larger first than second amplitude (Fig. 5E).
Fig. 5.
Diversity of SC responses to endogenous cholinergic input. A: for each neuron, the average firing rate was calculated in 5-ms bins for 200 ms after the first pulse across 2- and 4-Hz stimulation trials and fit to a Gaussian function. The baseline, amplitude, width, and latency to peak were estimated from the best-fit function, as shown for 1 example neuron. B: neurons that were poorly fit by a single Gaussian were better fit by a mixture of 2 Gaussians, in which an additional amplitude and latency were estimated, as shown for 1 example neuron. C: baselines for 3 populations of neurons: non-light-responsive, single-peaked, and double-peaked. D: amplitudes for single-peaked neurons and 1st and 2nd amplitudes for double-peaked neurons. E: 1st amplitude compared with 2nd amplitude for double-peaked neurons. F: latencies for single-peaked neurons and 1st and 2nd latencies for double-peaked neurons. G: widths for single-peaked and double-peaked neurons. H: depths at which non-light-responsive, single-peaked, and double-peaked neurons were recorded.
We next examined the latencies of responses (c for single-peaked neurons; c1 and c2 for the first and second latencies of double-peaked neurons) from light onset to peak firing rate. Interestingly, we observed a range of latencies, with the shortest latencies exhibited by single-peaked neurons (Fig. 5F). These data suggest that cholinergic input affects SC activity across multiple timescales, with the longer latencies perhaps due to polysynaptic innervation. We note that even the shortest timescales that we observed are sufficiently long (>5 ms) to ensure that none of our recordings was obtained from the axons of the photostimulated cholinergic neurons themselves. We then investigated the temporal variability of light-elicited responses (d), indicated by the full width (at half amplitude) of the fits. We found that the responses of some neurons were tightly coupled to light delivery, indicating high temporal fidelity in the response to cholinergic input (Fig. 5G), particularly for the single-peaked rather than double-peaked neurons.
To determine whether any systematic relationship existed between the baseline, amplitude, latency, and width of the best-fit Gaussian functions, we examined pairwise correlations between each of these parameters. We found that width was correlated with latency for single-peaked neurons (r = 0.77, P = 0.002), with the second latency for double-peaked neurons (r = 0.91, P = 2.22 × 10−4), and with the first amplitude for double-peaked neurons (r = 0.85, P = 0.002), suggesting that the temporal variability was related to the latency and magnitude of the light-elicited responses in some neurons. Otherwise, we found no pairwise correlations between baseline, amplitude, latency, and width.
Finally, we found no difference between the estimated depths of light-responsive and non-light-responsive neurons (Fig. 5H), suggesting that these populations are intermingled within the dorsoventral axis of the intermediate and deep layers. Together, these analyses suggest that the excitatory effects of cholinergic input on SC activity are not limited to a narrow range of predictable magnitudes, timescales, or depths but are instead considerably diverse.
DISCUSSION
In this study we examined how endogenous cholinergic input to the SC modulates the activity of intermediate- and deep-layer SC neurons. Toward this goal, we first examined, in the mouse, the topography of the cholinergic projection from the PPTg to the ipsilateral SC. We observed laminar specificity of the density of cholinergic innervation (intermediate > deep > superficial; Fig. 1, F and G), denser medial innervation of the intermediate layers, and denser lateral innervation of the deep layers (Fig. 1H). These results are consistent with findings in other species. Specifically, in both rat (Beninato and Spencer 1986) and cat (Hall et al. 1989; Harting and Van Lieshout 1991), the superficial layers receive less cholinergic input than the intermediate and deep layers. In addition, we observed heterogeneous innervation of the intermediate and deep layers by cholinergic terminals (Fig. 1F), akin to the “patchy” pattern of distribution of cholinergic input reported in previous studies (Beninato and Spencer 1986; Hall et al. 1989; Harting and Van Lieshout 1991). However, the laminar-specific dependence of cholinergic PPTg innervation on the mediolateral axis (Fig. 1H) has not been previously examined in other species, and its functional relevance remains to be determined. Staining in the cat SC for acetylcholine esterase, which localizes SC neurons that receive cholinergic input as well as the terminals of extrinsic cholinergic neurons, has been shown to be stronger medially than laterally (Illing and Graybiel 1985). This is consistent with our findings in the mouse with respect to the intermediate but not the deep layers.
While our Cre-dependent YFP injections successfully targeted the PPTg (Fig. 1, B and C), it is possible that a small volume of virus was taken up by the laterodorsal tegmental nucleus (LDTg), which has also been shown to supply a small fraction of the cholinergic input to the SC (Beninato and Spencer 1986; Hall et al. 1989; Jeon et al. 1993; McHaffie et al. 1991). We therefore cannot rule out the possibility that some of the fluorescence that we observed reflects LDTg input. Similarly, it is possible that our stimulation experiments (Figs. 2–5) coactivated LDTg terminals, since the mice used for these experiments expressed ChR2 in ChAT-positive neurons. It is highly unlikely, however, that our anatomical or physiological experiments engaged either SC interneurons, which are not known to be cholinergic, or the parabigeminal nucleus, the only other brain stem region known to provide cholinergic input to the SC. This nucleus is located distal to the PPTg, and its cholinergic input appears limited to the superficial layers (Feig et al. 1992; Graybiel 1978; Hall et al. 1989; Mufson et al. 1986) from which we did not record in this study. However, it is possible that cholinergic fibers originating in the parabigeminal nucleus were stimulated as they passed through the intermediate layers of the SC (Graybiel 1978) or that some light may have reflected into the superficial layers, which could have affected our recordings polysynaptically (Lee et al. 1997).
We next examined the physiological function of this projection by stimulating ACh release from cholinergic terminals in the SC and recording SC responses in awake mice (Fig. 2). Based on our anatomical results (Fig. 1H), we targeted our light stimulation to 0.9–1.0 mm from the midline in order to maximally drive cholinergic release in both the intermediate and deep layers across recording depths. We found that the firing rate of a subpopulation of intermediate- and deep-layer SC neurons reliably increased—while no neurons exhibited a decrease—in response to light delivery (Figs. 3 and 4), suggesting that these neurons are excited by release of endogenous ACh. These results are consistent with previous findings in slices and anesthetized animals (Bezdudnaya and Castro-Alamancos 2014; Isa and Hall 2009; Li et al. 2004; Sooksawate et al. 2008, 2011; Sooksawate and Isa 2006) and suggest that endogenous cholinergic input serves to excite activity in the SC circuits responsible for motor output. However, we found that the magnitude and timescale of this excitatory effect varied considerably across the population of light-responsive neurons (Fig. 5). The range of magnitudes and timescales of SC responses likely reflects variable ACh release from PPTg neurons (Takakusaki et al. 1997) and variable cholinergic receptor kinetics that have been previously reported in the SC (Sooksawate et al. 2008). In addition, the temporal diversity of SC responses may also reflect both monosynaptic and polysynaptic cholinergic input, although local network interactions within the SC may also contribute, and we therefore interpret this temporal diversity with caution.
Our application of optogenetics allowed us to examine neuronal responses to temporally precise, cell type-specific stimulation in awake animals—which often exhibit activity patterns distinct from anesthetized animals (Greenberg et al. 2008)—and was therefore invaluable for this study. However, some caveats to this approach are worth noting. 1) Were our SC recordings affected by light- or heat-induced artifacts (Cardin et al. 2010)? In addition to regularly calibrating our optical power output (materials and methods) and verifying that waveforms of spontaneous and light-evoked spikes were similar (Fig. 2D), we replicated our experiments in a set of control mice that did not express ChR2. In these mice, we found that all of the recorded neurons resembled the non-light-responsive neurons shown in Figs. 3 and 4 (data from control mice not shown). 2) In general, we found that 16-Hz stimulation did not increase SC firing rate above that elicited by 8-Hz stimulation (Figs. 3 and 4). Did this limit on firing rate result from an inability to drive cholinergic release more frequently than at 8 Hz, or because SC neurons were not able to “follow” cholinergic input above this frequency? The latter is unlikely, given that SC neurons are capable of achieving high firing rates (Fig. 5B). In addition, SC-projecting PPTg neurons exhibit spontaneous firing rates higher than 16 Hz (Dormont et al. 1998) and have been shown to follow antidromic electrical stimulation frequencies above 200 Hz (Krauthamer et al. 1995). Furthermore, previous studies have driven cholinergic neurons in other brain regions with frequencies > 16 Hz (Herman et al. 2014; Ma and Luo 2012; Ren et al. 2011; Witten et al. 2010). Thus this limit was likely due to the kinetics of activating the ChR2 expressed in the terminals of our ChAT-Cre/ChR2 mice (Jackman et al. 2014; Schoenenberger et al. 2011). 3) Related to this, the response to the final light pulse of 16-Hz stimulation appears to be larger than the responses to the preceding pulses (Fig. 3, A2 and B2). Is it possible that SC activity is due to the offset of ACh release, rather than its onset? This would be inconsistent with the observation that no SC neurons were inhibited by ACh release at lower frequencies. Instead, we suggest that, at sufficiently high frequencies, individual pulses may modulate the ACh release elicited by preceding pulses (leaving the ACh release elicited by the final pulse unaffected). However, given that pulse-on duration was fixed at 25 ms (materials and methods), it is formally impossible to disambiguate whether neural activity was more tightly correlated with the onset or offset of the pulse. 4) The proportion (28%) of SC neurons modulated by cholinergic activation was smaller in our study than in slice studies (Sooksawate et al. 2008; Sooksawate and Isa 2006). This difference may be due to ChR2 expression levels, or to subthreshold activation of SC neurons by cholinergic input. 5) The optetrode implants necessarily caused some tissue damage that could potentially affect SC function, particularly given the columnar organization of the SC. However, we did not observe any obvious behavioral deficits that would be associated with unilateral SC damage or inactivation in rodents, such as turning behavior or hemineglect (Felsen and Mainen 2008; Sinammon and Garcia 1988). 6) Our recordings may reflect indirect input from the PPTg to the SC via action potentials elicited in PPTg terminals that backpropagated to the soma, modulating the activity of PPTg-recipient structures that themselves project to the SC. While this mechanism may have contributed to the longer-latency responses that we observed, it cannot explain the short-latency responses, which likely reflect direct PPTg input. Nevertheless, the potentially distinct functional roles of monosynaptic and polysynaptic cholinergic input to the SC can be examined in future studies.
While the cholinergic projection to the SC is predominantly ipsilateral (Beninato and Spencer 1986), a minor contralateral component has been described (Krauthamer et al. 1995). Since we stimulated all cholinergic terminals entering one SC in mice that expressed ChR2 in all cholinergic neurons, it is possible that some of our responses were due to input from the contralateral side. However, data from awake, behaving recording experiments suggest that in rodents the ipsilateral projection is functionally dominant. Specifically, in separate studies, the firing rates of most PPTg and SC neurons were shown to increase prior to contralateral movements (Felsen and Mainen 2012; Thompson and Felsen 2013). While these recordings made no attempt to target SC-projecting PPTg neurons or PPTg-recipient SC neurons, the results suggest that the ipsilateral PPTg and SC function together to control motor output. Future experiments can examine the relative contribution of the ipsilateral and contralateral cholinergic projections to SC activity by restricting ChR2 expression to cholinergic neurons originating from one side of the brain stem.
Taken together, our results provide a first step toward understanding how cholinergic signaling modulates activity in the circuitry responsible for sensorimotor transformations in the SC. Although optical stimulation did not elicit an overt behavioral response (e.g., an orienting movement), we suggest that this cholinergic input may play a subtle, but critical, role in SC-dependent behavior, perhaps by facilitating the initiation of planned contralateral movements (Beninato and Spencer 1986; Kobayashi et al. 2001, 2002; Thompson and Felsen 2013). Since the SC appears to play similar functional roles in orienting movements in rodent and primate models (Felsen and Mainen 2008, 2011; Gandhi and Katnani 2011; Glimcher and Sparks 1992; Horwitz and Newsome 1999; McPeek and Keller 2004; Stubblefield et al. 2013; Wurtz and Goldberg 1971), future experiments in behaving mice can build upon the present study in order to elucidate the functional role of this well-conserved cholinergic input.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants F32 NS-080402 (E. A. Stubblefield) and R01 NS-079518 (G. Felsen), the Boettcher Foundation's Webb-Waring Biomedical Research Award (G. Felsen), and the University of Colorado Anschutz Medical Campus Optogenetics Pilot Program. Imaging experiments were performed in the University of Colorado Anschutz Medical Campus Advanced Light Microscopy Core supported by National Institutes of Health through the Rocky Mountain Neurological Disorders Core Center (P30 NS-048154) and the Colorado CTSI (UL1 TR-001082).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.A.S. and G.F. conception and design of research; E.A.S. and J.A.T. performed experiments; E.A.S., J.A.T., and G.F. analyzed data; E.A.S., J.A.T., and G.F. interpreted results of experiments; E.A.S., J.A.T., and G.F. prepared figures; E.A.S. and G.F. drafted manuscript; E.A.S. and G.F. edited and revised manuscript; E.A.S. and G.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jamie D. Costabile for technical support and Gabe J. Murphy, Mario J. Lintz, and Andrew Wolf for helpful comments on the manuscript.
REFERENCES
- Aizawa H, Kobayashi Y, Yamamoto M, Isa T. Injection of nicotine into the superior colliculus facilitates occurrence of express saccades in monkeys. J Neurophysiol 82: 1642–1646, 1999. [DOI] [PubMed] [Google Scholar]
- Al-Juboori SI, Dondzillo A, Stubblefield EA, Felsen G, Lei TC, Klug A. Light scattering properties vary across different regions of the adult mouse brain. PLoS One 8: e67626, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, Gunaydin LA, Frank LM, Deisseroth K. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat Neurosci 15: 163–170, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninato M, Spencer RF. A cholinergic projection to the rat superior colliculus demonstrated by retrograde transport of horseradish peroxidase and choline acetyltransferase immunohistochemistry. J Comp Neurol 253: 525–538, 1986. [DOI] [PubMed] [Google Scholar]
- Bezdudnaya T, Castro-Alamancos MA. Neuromodulation of whisking related neural activity in superior colliculus. J Neurosci 34: 7683–7695, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc 5: 247–254, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniau JM, Chevalier G, Feger J. Electrophysiological study of the nigro-tectal pathway in the rat. Neurosci Lett 10: 215–220, 1978. [DOI] [PubMed] [Google Scholar]
- Dormont JF, Condé H, Farin D. The role of the pedunculopontine tegmental nucleus in relation to conditioned motor performance in the cat. I. Context-dependent and reinforcement-related single unit activity. Exp Brain Res 121: 401–410, 1998. [DOI] [PubMed] [Google Scholar]
- Feig S, Van Lieshout DP, Harting JK. Ultrastructural studies of retinal, visual cortical (area 17), and parabigeminal terminals within the superior colliculus of Galago crassicaudatus. J Comp Neurol 319: 85–99, 1992. [DOI] [PubMed] [Google Scholar]
- Felsen G, Mainen ZF. Neural substrates of sensory-guided locomotor decisions in the rat superior colliculus. Neuron 60 137–148, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsen G, Mainen ZF. Midbrain contributions to sensorimotor decision making. J Neurophysiol 108: 135–147, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NJ, Katnani HA. Motor functions of the superior colliculus. Annu Rev Neurosci 34: 205–231, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW, Sparks DL. Movement selection in advance of action in the superior colliculus. Nature 355: 542–545, 1992. [DOI] [PubMed] [Google Scholar]
- Grantyn A, Berthoz A. Reticulo-spinal neurons participating in the control of synergic eye and head movements during orienting in the cat. I. Behavioral properties. Exp Brain Res 66: 339–354, 1987. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. A satellite system of the superior colliculus: the parabigeminal nucleus and its projections to the superficial collicular layers. Brain Res 145: 365–374, 1978. [DOI] [PubMed] [Google Scholar]
- Greenberg DS, Houweling AR, Kerr JN. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat Neurosci 11: 749–751, 2008. [DOI] [PubMed] [Google Scholar]
- Hall WC, Fitzpatrick D, Klatt LL, Raczkowski D. Cholinergic innervation of the superior colliculus in the cat. J Comp Neurol 287: 495–514, 1989. [DOI] [PubMed] [Google Scholar]
- Harting JK, Huerta MF, Hashikawa T, Weber JT, Van Leishout DP. Neuroanatomical studies of the nigrotectal projection in the cat. J Comp Neurol 278: 615–631, 1988 [DOI] [PubMed] [Google Scholar]
- Harting JK, Van Lieshout DP. Spatial relationships of axons arising from the substantia nigra, spinal trigeminal nucleus, and pedunculopontine tegmental nucleus within the intermediate gray of the cat superior colliculus. J Comp Neurol 305: 543–558, 1991. [DOI] [PubMed] [Google Scholar]
- Herman AM, Huang L, Murphey DK, Garcia I, Arenkiel BR. Cell type-specific and time-dependent light exposure contribute to silencing in neurons expressing Channelrhodopsin-2. Elife 3: e01481, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol 53: 266–291, 1985. [DOI] [PubMed] [Google Scholar]
- Hirokawa J, Sadakane O, Sakata S, Bosch M, Sakurai Y, Yamamori T. Multisensory information facilitates reaction speed by enlarging activity difference between superior colliculus hemispheres in rats. PloS One 6: e25283, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science 284: 1158–1161, 1999. [DOI] [PubMed] [Google Scholar]
- Illing RB, Graybiel AM. Convergence of afferents from frontal cortex and substantia nigra onto acetylcholinesterase-rich patches of the cat's superior colliculus. Neuroscience 14: 455–482, 1985. [DOI] [PubMed] [Google Scholar]
- Isa T, Hall WC. Exploring the superior colliculus in vitro. J Neurophysiol 102: 2581–2593, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Sasaki S. Brainstem control of head movements during orienting; organization of the premotor circuits. Prog Neurobiol 66: 205–241, 2002. [DOI] [PubMed] [Google Scholar]
- Isa T, Sooksawate T, Isa K, Matsui R, Kato S, Kinoshita M, Kobayashi K, Watanabe D, Kobayashi K. Viral vector-mediated selective and reversible blockade of the pathway for visual orienting in mice. Front Neural Circuits 7: 162, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Beneduce BM, Drew IR, Regehr WG. Achieving high-frequency optical control of synaptic transmission. J Neurosci 34: 7704–7714, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon CJ, Spencer RF, Mize RR. Organization and synaptic connections of cholinergic fibers in the cat superior colliculus. J Comp Neurol 333: 360–374, 1993. [DOI] [PubMed] [Google Scholar]
- Jones BE, Webster HH. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. I. Effects upon the cholinergic innervation of the brain. Brain Res 451: 13–32, 1988. [DOI] [PubMed] [Google Scholar]
- King AJ. The superior colliculus. Curr Biol 14: R335–R338, 2004. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Inoue Y, Yamamoto M, Isa T, Aizawa H. Contribution of pedunculopontine tegmental nucleus neurons to performance of visually guided saccade tasks in monkeys. J Neurophysiol 88: 715–731, 2002. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Isa T. Sensory-motor gating and cognitive control by the brainstem cholinergic system. Neural Netw 15: 731–741, 2002. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Saito Y, Isa T. Facilitation of saccade initiation by brainstem cholinergic system. Brain Dev 23, Supplement 1: S24–S27, 2001. [DOI] [PubMed] [Google Scholar]
- Krauthamer GM, Grunwerg BS, Krein H. Putative cholinergic neurons of the pedunculopontine tegmental nucleus projecting to the superior colliculus consist of sensory responsive and unresponsive populations which are functionally distinct from other mesopontine neurons. Neuroscience 69: 507–517, 1995. [DOI] [PubMed] [Google Scholar]
- Lee PH, Helms MC, Augustine GJ, Hall WC. Role of intrinsic synaptic circuitry in collicular sensorimotor integration. Proc Natl Acad Sci USA 94: 13299–13304, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Endo T, Isa T. Presynaptic muscarinic acetylcholine receptors suppress GABAergic synaptic transmission in the intermediate grey layer of mouse superior colliculus. Eur J Neurosci 20: 2079–2088, 2004. [DOI] [PubMed] [Google Scholar]
- Liu P, Basso MA. Substantia nigra stimulation influences monkey superior colliculus neuronal activity bilaterally. J Neurophysiol 100: 1098–1112, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Luo M. Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb. J Neurosci 32: 10105–10116, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TP, Graybiel AM, Wurtz RH. Location of saccade-related neurons in the macaque superior colliculus. Exp Brain Res 85: 21–35, 1991. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Beninato M, Stein BE, Spencer RF. Postnatal development of acetylcholinesterase in, and cholinergic projections to, the cat superior colliculus. J Comp Neurol 313: 113–131, 1991. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci 7: 757–763, 2004. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Martin TL, Mash DC, Wainer BH, Mesulam MM. Cholinergic projections from the parabigeminal nucleus (Ch8) to the superior colliculus in the mouse: a combined analysis of horseradish peroxidase transport and choline acetyltransferase immunohistochemistry. Brain Res 370: 144–148, 1986. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 2012. [Google Scholar]
- Redgrave P, Mitchell IJ, Dean P. Descending projections from the superior colliculus in rat: a study using orthograde transport of wheatgerm-agglutinin conjugated horseradish peroxidase. Exp Brain Res 68: 147–167, 1987. [DOI] [PubMed] [Google Scholar]
- Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Feng G, Luo M. Habenula “cholinergic” neurons corelease glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron 69: 445–452, 2011. [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Jackson J, Henze D, Harris K, Redish AD. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131: 1–11, 2005. [DOI] [PubMed] [Google Scholar]
- Schoenenberger P, Schärer YP, Oertner TG. Channelrhodopsin as a tool to investigate synaptic transmission and plasticity. Exp Physiol 96: 34–39, 2011. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM, Garcia EJ. Lateral neglect in a head movement task: more impairment with unilateral than bilateral lesions of the superior colliculus in the rat. Behav Brain Res 27: 131–143, 1988. [DOI] [PubMed] [Google Scholar]
- Sooksawate T, Isa K, Behan M, Yanagawa Y, Isa T. Organization of GABAergic inhibition in the motor output layer of the superior colliculus. Eur J Neurosci 33: 421–432, 2011. [DOI] [PubMed] [Google Scholar]
- Sooksawate T, Isa K, Isa T. Cholinergic responses in crossed tecto-reticular neurons of rat superior colliculus. J Neurophysiol 100: 2702–2711, 2008. [DOI] [PubMed] [Google Scholar]
- Sooksawate T, Isa T. Properties of cholinergic responses in neurons in the intermediate grey layer of rat superior colliculus. Eur J Neurosci 24: 3096–3108, 2006. [DOI] [PubMed] [Google Scholar]
- Sooksawate T, Saito Y, Isa T. Electrophysiological and morphological properties of identified crossed tecto-reticular neurons in the rat superior colliculus. Neurosci Res 52: 174–184, 2005. [DOI] [PubMed] [Google Scholar]
- Stubblefield EA, Costabile JD, Felsen G. Optogenetic investigation of the role of the superior colliculus in orienting movements. Behav Brain Res 255: 55–63, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, Shiroyama T, Kitai ST. Two types of cholinergic neurons in the rat tegmental pedunculopontine nucleus: electrophysiological and morphological characterization. Neuroscience 79: 1089–1109, 1997. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Felsen G. Activity in mouse pedunculopontine tegmental nucleus reflects action and outcome in a decision-making task. J Neurophysiol 110: 2817–2829, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JA, Salcedo E, Restrepo D, Finger TE. Second-order input to the medial amygdala from olfactory sensory neurons expressing the transduction channel TRPM5. J Comp Neurol 520: 1819–1830, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MN, Fredens K. Origin of high acetylcholinesterase activity in the mouse superior colliculus. Exp Brain Res 72: 335–346, 1988. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kobayashi Y, Inoue Y, Isa T. Effects of local nicotinic activation of the superior colliculus on saccades in monkeys. J Neurophysiol 93: 519–534, 2005. [DOI] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330: 1677–1681, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull 16: 603–637, 1986. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Superior colliculus cell responses related to eye movements in awake monkeys. Science 171: 82–84, 1971. [DOI] [PubMed] [Google Scholar]
- Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, Deisseroth K. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc 5: 439–456, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]