Abstract
A widely accepted view is that wakefulness is a state in which the entire cortical mantle is persistently activated, and therefore desynchronized. Consequently, the EEG is dominated by low-amplitude, high-frequency fluctuations. This view is currently under revision because the 1–4 Hz delta rhythm is often evident during “quiet” wakefulness in rodents and nonhuman primates. Here we used intracranial EEG recordings to assess the occurrence of delta rhythm in 18 awake human beings. Our recordings reveal rhythmic delta during wakefulness at 10% of all recording sites. Delta rhythm could be observed in a single cortical lobe or in multiple lobes. Sites with high delta could flip between high and low delta power or could be in a persistently high delta state. Finally, these sites were rarely identified as the sites of seizure onset. Thus rhythmic delta can dominate the background operation and activity of some neocortical circuits in awake human beings.
Keywords: arousal, delta, sleep, wakefulness
a fundamental property of cortical circuits in vivo is that these circuits and the individual neurons that constitute them are spontaneously active and the levels and patterns of activity vary with cortical state. These differences in activity create different operating regimes for cortical neurons. For example, when cortical circuits are in a desynchronized state (evident in the EEG; Adrian and Matthews 1934; Berger 1929; Jasper and Carmichael 1935), individual cortical neurons are persistently depolarized close to threshold for action potentials, the local field potential (LFP) and EEG show low-amplitude, high-frequency components, and multiunit activity is maintained at a sustained level (Steriade et al. 2001). In contrast, during slow-wave sleep cortical neurons and circuits are in a different operating regime and the membrane potential of individual neurons fluctuates by 10–20 mV (Steriade et al. 2001). The LFP, EEG, and multiunit activity reflect this activity through large-amplitude, low-frequency synchronized fluctuations (Steriade et al. 2001). The key difference between the desynchronized states and states when delta dominates the EEG is that in the desynchronized states cortical circuits are constantly active and cortical neurons are persistently depolarized. In contrast, when low frequencies dominate, cortical neurons can be hyperpolarized or depolarized and cortical areas and circuits can be silent or active for hundreds of milliseconds.
Traditionally, the awake states—discerned from scalp EEG recordings in human beings—were thought of as a state dominated by high-frequency, low-amplitude fluctuations in the EEG (Adrian and Matthews 1934; Berger 1929; Jasper and Carmichael 1935; Steriade et al. 2001). The delta rhythm—high-amplitude, low-frequency fluctuations—in the awake human EEG is almost exclusively associated with sleep, pathology, or the use of antiepileptic drugs (AEDs) (Gaspard et al. 2013). Recent studies in rodents have challenged this notion by showing that the awake state can be divided into at least two states based on the occurrence of low-frequency (1–5 Hz) fluctuations in the membrane potential. One of these states is called quiet wakefulness, where the cortical EEG and LFP and the membrane potential of single neurons can be dominated by low-frequency fluctuations; the second state is the desynchronized, activated state (Crochet and Petersen 2006; Petersen et al. 2003; Vyazovskiy et al. 2011; Zagha et al. 2013). Finally, in visual, auditory, and somatosensory cortex, the membrane potential of single neurons is often dominated by low-frequency oscillations (in the 1–5 Hz frequency band) or by variable levels of power in the delta band (1–4 Hz) (Bennett et al. 2013; Haider et al. 2013; Hromadka et al. 2013; Okun et al. 2010; Polack et al. 2013; Zhou et al. 2014).
The question that then arises is whether the human awake state is fundamentally different from the rodent awake state with regard to the occurrence of delta rhythm. On one hand, scalp EEG recordings from human beings and intracellular recordings from awake cats point to the absence of delta power and a desynchronized EEG in the awake state (Adrian and Matthews 1934; Berger 1929; Jasper and Carmichael 1935; Steriade et al. 2001). On the other hand, intracellular recordings and intracranial LFP, multiunit, and EEG recordings in rodents paint a more varied picture for delta power: 1) delta rhythm can be evident in a single cortical area (Crochet and Petersen 2006; Petersen et al. 2003; Vyazovskiy et al. 2011; Zagha et al. 2013); 2) local delta rhythm can be abolished by movement or by activation of cortico-cortical connections (Crochet and Petersen 2006; Petersen et al. 2003; Zagha et al. 2013); 3) cortex can be in an inactive Down state with infrequent low-frequency activated Up states (Hromadka et al. 2013); 4) delta power evident in single-neuron membrane potential or LFP and EEG can vary in levels of activation and depolarization (Haider et al. 2013; Okun et al. 2010). Here we examined this question with fine-grained intracranial EEG (icEEG) recordings in human beings. Our recordings show that indeed, even in the awake human, a small percentage of recording sites can be in a low-frequency state.
MATERIALS AND METHODS
This study was approved by the Human Investigation Committee at Yale School of Medicine. Written informed consent for the study was obtained from all patients.
Subjects.
Subjects were 18 consecutive adult patients undergoing icEEG monitoring in the Yale Comprehensive Epilepsy Center/Yale New Haven Hospital for surgery for medically intractable localization-related epilepsy, in whom the intracranial monitoring was free of complications and the seizure onset area (SOA) could be identified and was unilateral. The average age of the subjects was 32.39 yr, and 8 of 18 subjects were women (see Table 2). Six of the subjects had medial temporal onset of seizures, and 12 had neocortical seizure onset.
Table 2.
Clinical information on the patients studied
| Patient | Sex | Age, yr | Brain Areas Sampled | No. of Contacts | Seizure Onset Location |
|---|---|---|---|---|---|
| 1 | M | 20 | IT, LT, O, P, F | 218 | L anterior medial frontal |
| 2 | M | 51 | IT, LT, O, P, F, MT | 174 | L medial temporal |
| 3 | F | 35 | IT, LT, O, P, F | 113 | L superior parietal |
| 4 | M | 27 | IT, LT, O, P, F, MT | 171 | L medial temporal |
| 5 | M | 26 | IT, LT, O, P, F, MT | 203 | L medial temporal |
| 6 | M | 27 | IT, LT, O, P, F, MT | 100 | R anterior superior lateral temporal |
| 7 | M | 23 | IT, LT, O, P, F, MT | 174 | R temporo-parietal |
| 8 | M | 54 | IT, LT, O, P, F, MT | 220 | R inferior temporal |
| 9 | F | 27 | IT, LT, O, P, F, MT | 261 | R medial temporal |
| 10 | F | 41 | IT, LT, O, P, F, MT | 201 | R parietal |
| 11 | M | 31 | IT, LT, O, P, F, MT | 146 | R inferior temporal |
| 12 | F | 24 | IT, LT, O, P, F, MT | 208 | L medial occipital |
| 13 | M | 35 | IT, LT, O, P, F, MT | 332 | L parieto-occipital |
| 14 | F | 31 | IT, LT, O, P, F, MT | 274 | R inf post temporo-occipital |
| 15 | F | 28 | IT, LT, O, P, F, MT | 228 | R medial temporal |
| 16 | F | 39 | IT, LT, O, P, F, MT | 216 | L medial temporal |
| 17 | F | 26 | IT, LT, O, P, F, MT | 194 | R parietal |
| 18 | M | 38 | IT, LT, O, P, F, MT | 252 | L post inferior temporal |
Location of seizure onset, sex, age, cortical areas sampled [inferior temporal (IT), medial temporal (MT), lateral temporal (LT), occipital (O), parietal (P) or frontal (F)], number of intracranial EEG electrode contacts implanted, and site of seizure onset are listed for each patient.
Intracranial electrode placement and localization.
Three types of icEEG electrodes were used for intracranial monitoring, subdural strip and grid electrodes and depth electrodes (AdTech Medical Instrument, Racine, WI). Depth electrodes were stereotactically implanted with MRI guidance and a computerized planning system that allows interactive previewing of electrode trajectories. Subdural strip and grid electrodes were implanted under visual guidance. Between 100 and 332 electrode contacts were placed in the 18 subjects. Most of the electrode contacts were subdural strip or grid electrode contacts, and a few were depth electrode contacts. To determine the location of electrode contacts, individual contacts were marked on a postoperative CT image. The CT image was then coregistered to a postoperative MR image with a six-parameter rigid transformation, and the postoperative MR image was coregistered with a preoperative MR image using a nonlinear grid-based transformation to account for the distortion of the brain that occurs as a result of craniotomy (Srikinjar et al. 2002). The SOA was identified by a standing multidisciplinary team in the Yale University Comprehensive Epilepsy Center using standard clinical criteria. The number of seizure onset contacts ranged from 1 to 12 in the 18 subjects.
Intracranial EEG acquisition.
Subjects were monitored continuously (for 5–8 days) with a clinical EEG acquisition and storage system (Natus Medical/Bio-logic Systems, San Carlos, CA). icEEGs were recorded with respect to a peg electrode placed within the skull at a distance from the icEEG electrodes. A contact on an inverted strip electrode placed in subgaleal space was used as the ground electrode. Up to 128 channels of icEEG, EEG, EMG, and EOG were recorded at a time and stored along with a time-synchronized video signal of the patient. The icEEGs were sampled at 256 Hz. The entire icEEG monitoring period was recorded for the 18 subjects. Subsequently we identified a 1-h epoch from day 2 or 3 of the monitoring, at least 6 h from a seizure, when the patient was awake and resting quietly, i.e., not talking or eating. The time period was identified after an examination of medication records, seizure times, the video, and icEEG. Day 2 or 3 was selected because it was considered to be sufficiently removed from the surgery and it was before the time that the subjects experienced seizures. Subsequently, a second epoch was similarly identified for each patient after the patient's AEDs had been tapered or stopped.
Analysis and power spectral density.
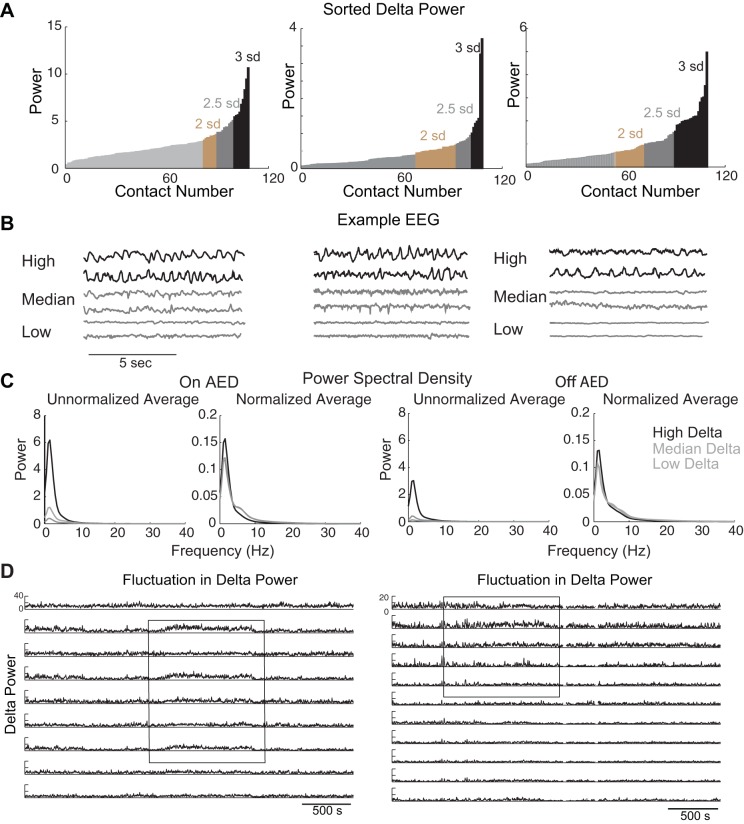
EEG epochs were segmented (segment length 1 s), the mean of each signal segment was subtracted, and the segments were weighted with a Hann window and zero-padded to 512 samples before calculation of the fast Fourier transform. The total power and power for the delta (0–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–25 Hz), gamma (25–55 Hz), and high (65–128 Hz) frequency bands were obtained for each 1-s icEEG segment by use of the fast Fourier transform. Unfiltered and filtered icEEGs were examined and marked for artifacts at a 1-s time resolution, and 1-s segments with artifacts were removed from the analysis. Power and band power estimates of artifact-free segments were obtained for each electrode contact studied and averaged over the 1-h epoch to obtain average measures for each contact. The electrode contacts were then sorted by the amount of delta power measured. The contacts with values below the median value were considered to be low values. We subsequently traversed the sorted value list, starting at the median value, and considered each next greater sorted value. If the next greater sorted value exceeded the mean + 3 SD of the current pool of values, that value was considered to be a threshold value for the detection of high delta electrode contacts. Values equal to and greater than the threshold value were considered to be the high delta values. For each patient and epoch, lower thresholds of 2 or 2.5 SDs were also examined (see Fig. 2). Delta power at each site was ranked and plotted from low to high delta power. Sites showing higher delta power (>3 SDs) than the mean delta power were considered to be sites with high delta power. Visual examination of these recording sites confirmed the occurrence of rhythmic delta at each site.
Fig. 2.
The nature of delta activity in icEEG recordings. A: distribution of delta power. For each patient, and each recording site, the delta power over 1 h of recording was estimated. The delta power at each site was ranked and plotted from lowest to highest value. Sites showing higher delta power (>3 SDs) than the mean delta power are highlighted in black, with >2.5 SDs in gray and >2 SDs in brown. Some recording sites have excessively high delta power (black bins). B: EEG traces. Representative EEG traces with high (black) and median and low (gray) delta power from 3 patients are shown here. Sites with high delta power show EEG with rhythmic delta. Drugs were still being tapered in patients 1 and 2 but had been completely withdrawn in patient 3. C: comparison of the power spectral density for 12 recording sites (in all 18 subjects) with the highest, median (n = 18), and lowest (n = 18) delta power, before (left) and after (right) AEDs had been tapered off. Even after normalizing for total power in each hour-long recording, the recording sites with high delta power contained significantly (n = 18 subjects, P < 0.01, paired t-test) more delta power than recording sites with low delta power. D: persistence of high delta. Each of the recording sites shown here had high delta power, but even in these sites delta power could fluctuate between high and low delta power. Note that only some recording sites show changes in delta power, i.e., some of the contacts are relatively flat in their delta power levels. Left: from the same patient as in B, left, and Fig. 1A. Right: from a different patient.
RESULTS
We recorded icEEG from ∼116 electrode contacts in 18 patients (range 101–118 electrode contacts in the 18 patients) placed over widespread neocortical sites, including prefrontal, frontal-parietal, temporal, and occipital cortices (Fig. 1; Tables 1 and 2). These recordings were obtained when patients were awake as scored by examination of the video and icEEG. Four criteria were used for selecting a 1-h epoch for analysis from each patient: the patient was not interacting with anyone else; the patient was not eating; the patient was not asleep as measured by the EEG and as scored in the video; and the patient had not been asleep (the hour) just before the recording began and did not fall asleep (the hour) immediately after this epoch ended.
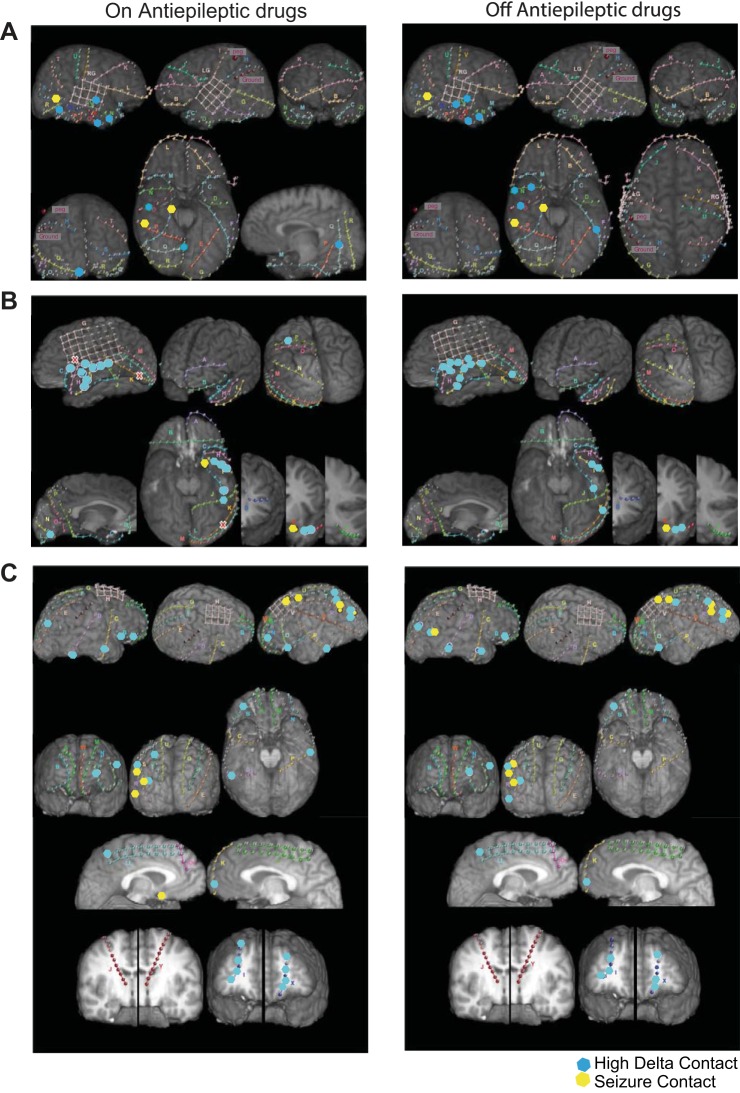
Fig. 1.
Sites with high delta in intracranial EEG (icEEG) recordings. A–C: recording sites in 3 patients before (left) and after (right) antiepileptic drugs (AEDs) had been tapered off. Subdural grid electrodes were positioned over temporal lobe primarily, and subdural strip electrodes were positioned over temporal, frontal, parietal and occipital cortices. Sites of seizures are marked with yellow dots; sites with high delta in excess of 3 SDs from the mean are marked with blue dots. Note that contacts with high delta can be clustered together (B) or separated into multiple cortical sites (A and C), even bilaterally. Furthermore, the sites with high delta can overlap the seizure onset area, but this is atypical. Finally, sites with high delta can show high delta power over 2 recording sessions separated by 3–4 days (before and after AEDs had been tapered off), though this is not necessarily the rule.
Table 1.
Number of seizure onset and high delta sites and number of recording sites monitored simultaneously
| Seizure Onset | High Delta | Seizure and High Delta | Total Number | |
|---|---|---|---|---|
| Minimum | 1 | 5 | 0 | 101 |
| Maximum | 12 | 32 | 3 | 118 |
| Mean | 3.7 | 12.2 | 0.6 | 116 |
The number of electrode contacts within the seizure onset area (1st column) and with high delta (2nd column) and the overlap between the two (3rd column) for the 18 patients studied are listed. The number of simultaneously recorded sites is shown in the last column.
Our recordings reveal that the icEEG in the awake human neocortex is not uniform. Individual recording sites in occipital and temporal lobe can show high levels of delta rhythm—at sites distant from the SOA (Fig. 1)—while other recording sites show no evidence of high delta, i.e., the raw icEEG traces at ∼90% of the recording sites show what appears to be an activated cortex, i.e., little evidence of runs of rhythmic low-frequency, 1–4 Hz, delta rhythm. Contacts with high delta could be isolated in a single cortical lobe (Fig. 1A) or could be scattered over the cortical mantle (Fig. 1C) or clustered together on multiple contacts in a single lobe (Fig. 1B).
Although most raw records show little evidence of low-frequency fluctuations, the distribution of delta power was skewed, with some recording sites containing high amounts of delta power (>3 SDs more than other contacts over the hour-long recording; Fig. 2A). This distribution of delta power—shown here for three patients—was typical. The histogram of delta power reveals that some recording sites have more delta power than others (sites on the right-hand side of the distribution, shown in black, display 3 SDs or more delta power than the mean delta power). As expected, lowering the threshold to 2.5 or 2 SDs included more recording sites that had a sample with high delta power.
An examination of the unprocessed EEG at these recordings sites reveals large-amplitude, rhythmic delta (Fig. 2B). While these ∼10% of recording sites (Table 1) show clear evidence of large-amplitude, rhythmic delta, the rest of the recording sites show what appears to be an activated state, in which the EEG has no large-amplitude, low-frequency fluctuations (Fig. 2B; median and low delta power in 3 patients). At these other recording sites, that is, at the majority of the recording sites, the cortex can be said to be in an activated state.
The average power spectral density function of delta power in all patients reveals that some sites do indeed contain significantly more delta power than others (Fig. 2) both while AEDs were being tapered off and after the drugs had been stopped (P < 0.01, paired t-test, comparing 12 recording sites in 18 patients with high delta to 12 recording sites with low delta power). Correcting for differences in average power at these recording sites does not affect the results: some cortical areas have significantly (P < 0.01, paired t-test) more delta power than others.
The possible implication of the presence of the delta rhythm even in 10% of the recording sites in awake people is that a portion of the brain is in a slow-wave sleep-like operational regime while the rest of the cortex is in an activated state. Is this portion of cortex permanently in high delta? To examine the persistence of delta, we looked at whether high delta persisted over days and whether high delta power was uniform during these hour-long epochs (Fig. 2D). We examined two separate hour-long recording sessions—the first when patients were still ostensibly on AEDs ∼2 days after surgery for implantation of intracranial electrodes and the second ∼5 days after surgery, when AEDs had been tapered or withdrawn. Our analysis of recording sites that have a highest amounts of delta in the 18 patients revealed that 69 of the 116 (∼60%) recording sites with high delta show persistently high delta rhythm, over both days (see Fig. 1 for examples). These data indicate that some cortical sites (60%) were in an operating regime dominated by low-frequency rhythms over days of monitoring.
Next we examined whether delta power fluctuated during the hour-long recordings. We measured delta power over 1-s-long epochs and looked at whether delta power fluctuated or was persistently elevated for minutes on end. Our estimate (deviation around mean delta power) revealed that at 34.4% (40 of 116 sites with high delta power) of the recording sites delta rhythm was not constant; it fluctuated in the course of the hour long recording (Fig. 2D). The fluctuation in delta power could reveal itself as a persistent elevation in delta power over minutes (Fig. 2D, left) or an increase in variance of the delta power (Fig. 2D, right, in a different patient). At these latter recording sites, the delta power in the EEG fluctuated considerably.
Taken together, these results indicate that at any one moment, even when human beings are ostensibly awake, some recording sites show considerably greater rhythmic delta than others. A longstanding observation from a variety of neurological disorders is that slowing—the occurrence of delta rhythm—in the awake and aroused EEG is a manifestation of pathology or disease (Gaspard et al. 2013; Gloor et al. 1977; Walter 1936). Undoubtedly, all of our recordings are in neurologically compromised humans, patients who have medically intractable epilepsy. Although the relationship between disease processes and cortical slowing cannot be ruled out, we can relate the location of delta in the cortex to the site of epilepsy. A close examination of the overlap between sites where delta was prevalent and the clinically and surgically determined locus of seizure onset revealed that in most patients there was no correspondence between sites that showed high delta and sites where seizures began (Fig. 1 and Table 1).
Finally, there was no anatomical segregation of sites with high delta. Sites with high delta varied from patient to patient. High delta contacts could be distributed across multiple cortical lobes or could occur on a single contact in a lobe or on multiple contacts in a single lobe. Delta could be observed bilaterally or in a single hemisphere.
DISCUSSION
Our results provide support for both the accepted idea that in the awake human being delta rhythm is not commonplace and the idea that there are indeed local cortical regions where slow waves and rhythmic delta can dominate. Our work suggests that these regions vary from patient to patient and are not necessarily associated with the clinically and surgically determined locus of seizures, although, by their very nature, intracranial recordings in human beings are only performed in cases where pathology is suspected and surgery is indicated. Nevertheless, our results suggest that just as in rodents, during conscious states, in the absence of seizures, irrespective of whether the person is on AEDs or not, rhythmic delta occurs in nonepileptic portions of the human cerebral cortex.
These results in concert with observations of delta rhythm in awake rodents and nonhuman primates suggest that rhythmic delta in the awake human being could sometimes be a manifestation of a normal operating regime of neocortical circuits. The exact level, frequency, and depth of delta power in the LFP, EEG, multiunit activity, and intracellular membrane potential vary [Haider et al. 2013; Hromadka et al. 2013; Okun et al. 2010; Petersen et al. 2003; although note that in cats wakefulness is not associated with delta power in the membrane potential (Steriade et al. 2001)]. The low-frequency fluctuation of quiet wakefulness evident in the LFP, EEG, and membrane potential is abolished by movement (Bennett et al. 2013; Crochet et al. 2006; Petersen et al. 2003; Polack et al. 2013), or by activity in motor cortex (Zagha et al. 2013). Although most of the recent work showing surprising amounts of delta in awake animals is in rodents, rhythmic delta has also been reported in awake nonhuman primates, where membrane potential, multiunit activity, and LFP fluctuate at low frequencies (Lakatos et al. 2005, 2008; Tan et al. 2014).
These results in animals are surprising because delta rhythm in the human EEG has almost completely been tied to sleep (Simon and Emmons 1956), loss of consciousness (Harvey et al. 1937; Loomis et al. 1938; Simon and Emmons 1956), or disease processes (Gaspard et al. 2013; Gloor et al. 1977; Walter 1935). However, some of the earliest EEG recordings from normal human subjects by Davis et al. show that delta rhythm can occur selectively in some portions of cortex in awake humans (Davis et al. 1937). During these epochs of local delta, subjects reported that they were completely conscious. One reason that local delta might be observed infrequently in human beings is related to the use of scalp EEGs. Although scalp EEGs are noninvasive, have high temporal resolution, and can be used for detecting normal and abnormal cortical function, scalp EEG also has limitations compared with intracranial recording: 1) scalp EEG records signals that are filtered by scalp and skull (Pacia and Ebersole 1997; Ray et al. 2007; Tao et al. 2005); 2) the power of the signal recorded at the scalp is much lower than the power of the same signal recorded intracranially (Tao et al. 2005, 2007); and 3) the scalp EEG reports only those events that occur synchronously over large cortical areas (Tao et al. 2005, 2007).
In principle, awake states where activity does not fluctuate between epochs of activity and inactivity, where membrane potential of single neurons remains relatively uniform and stable just below threshold for action potentials, have some important advantages over cortical states where activity fluctuates between active and inactive epochs. An activated low-amplitude, high-frequency EEG state implies less variability in background, and consequently less variability in the response to sensory or other inputs (Bennett et al. 2013; Haider et al. 2007; Hasenstaub et al. 2007; Lakatos et al. 2005; Petersen et al. 2003; Sachdev et al. 2004; Zhou et al. 2014). This desynchronized state implies that cortical neurons are maintained close to threshold for activity and that the levels of background input remain constant from cortical area to cortical area. In contrast, the occurrence of slow waves, intermixed with desynchronized activated states, implies more variability in responsiveness and more variance in perception and performance of the simplest behaviors. Although the rules governing the occurrence of delta in the awake state and switching between delta and activated states in human beings are still unknown, our work establishes that at any one moment—while most neocortical circuits are ostensibly in an activated, desynchronized state—some portion of the neocortical circuits can be in a state in which rhythmic delta dominates.
There are several distinct competing and somewhat overlapping ideas about the meaning and mechanisms of local delta rhythm in the awake state. The occurrence of local delta in a particular part of the brain could be related to inattentiveness, to unconsciousness, even to sleep in that part of the brain. Evidence that supports this hypothesis comes from studies—in dolphins (Mukhametov et al. 1977), seals (Lyamin et al. 2008), and birds (Lesku et al. 2011; Rattenborg and Amlaner 2010)—that show an entire hemisphere can show rhythmic delta, and slow-wave sleep, while other parts of the brain are in an activated state. In this state, the animals are conscious, active, and typically engaged in motor tasks. A different idea that has support in patients with epilepsy is that during seizures and in the immediate aftermath of seizures some cortical circuits can show slow-wave activity and delta rhythm (Blumenfeld 2005). In these moments, patients are unaware of their surroundings and unresponsive to stimuli. Thus, according to this hypothesis, local delta occurs when people are partially or completely unconscious (Blumenfeld 2005). Experiments in rodents suggest that seizures can effectively shut down the ascending activating system, specifically the cholinergic neurons in pedunculopontine nuclei (Motelow et al. 2015), and that this could explain both unconsciousness and cortical slowing. Separately, it has also been suggested that interictal regional delta activity may be colocated with the seizure onset and interictal spiking areas in neocortical temporal lobe epilepsy and reflect network areas involved in mesial temporal lobe epilepsy (Tao et al. 2011).
In contrast to these studies, there is evidence that local cortical circuits, even local columns, can show differential delta based on prior activity in the column, i.e., based on use and metabolism (Krueger and Obal 1993; Rector et al. 2005, 2009). According to this hypothesis, columns that have been active and engaged in a task can enter sleeplike rhythms while adjacent columns are still active. This hypothesis also has experimental support in rodents (Rector et al. 2005, 2009). However, it should be noted that this hypothesis posits that as opposed to being an abnormal rhythm of wakefulness, local delta is part of the normal use-dependent changes in cortical activity (Krueger et al. 2008; Krueger and Obal 1993; Krueger and Tononi 2011).
While we know that even during slow-wave sleep-like states cortical neurons and circuits can be activated by stimuli, the mechanisms that trigger rhythmic delta locally or in the entire cortical mantle are still largely unknown. Whether there is a causal relationship between delta, attention, consciousness, and metabolism is also not clear but will be the subject of future research.
GRANTS
Funding was provided by the C. G. Swebilius Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.N.S.S., N.G., L.J.H., D.D.S., and H.P.Z. conception and design of research; R.N.S.S. and H.P.Z. analyzed data; R.N.S.S., N.G., and H.P.Z. interpreted results of experiments; R.N.S.S. and H.P.Z. prepared figures; R.N.S.S. drafted manuscript; R.N.S.S., N.G., J.L.G., L.J.H., D.D.S., and H.P.Z. edited and revised manuscript; R.N.S.S., L.J.H., and H.P.Z. approved final version of manuscript; N.G., J.L.G., D.D.S., and H.P.Z. performed experiments.
REFERENCES
- Adrian ED, Matthews BH. The interpretation of potential waves in the cortex. J Physiol 81: 440–471, 1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C, Arroyo S, Hestrin S. Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron 80: 350–357, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Über das Elektrenkephalogramm des Menschen. Arch Psychiatr Nervenkr 87: 527–570, 1929. [Google Scholar]
- Blumenfeld H. Consciousness and epilepsy: why are patients with absence seizures absent? Prog Brain Res 150: 271–286, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci 9: 608–610, 2006. [DOI] [PubMed] [Google Scholar]
- Davis H, Davis PA, Loomis AL, Harvey EN, Hobart G. Changes in human brain potentials during the onset of sleep. Science 12: 448–450, 1937. [DOI] [PubMed] [Google Scholar]
- Gaspard N, Manganas L, Rampal N, Petroff OA, Hirsch LJ. Similarity of lateralized rhythmic delta activity to periodic lateralized epileptiform discharges in critically ill patients. JAMA Neurol 70: 1288–1295, 2013. [DOI] [PubMed] [Google Scholar]
- Gloor P, Ball G, Schaul N. Brain lesions that produce delta waves in the EEG. Neurology 27: 326–333, 1977. [DOI] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, Yu Y, McCormick DA. Enhancement of visual responsiveness by spontaneous local network activity in vivo. J Neurophysiol 97: 4186–4202, 2007. [DOI] [PubMed] [Google Scholar]
- Haider B, Häusser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature 493: 97–100, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EN, Loomis AL, Hobart GA. Cerebral states during sleep as studied by human brain potentials. Science 85: 443–444, 1937. [Google Scholar]
- Hasenstaub AR, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci 27: 9607–9622, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromádka T, Zador AM, DeWeese MR. Up states are rare in awake auditory cortex. J Neurophysiol 109: 1989–1995, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper HH, Carmichael L. Electrical potentials from the intact human brain. Science 81: 51–53, 1935. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obal F. A neuronal group theory of sleep function. J Sleep Res 2: 63–69, 1993. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci 9: 910–919, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Tononi G. Local use-dependent sleep; synthesis of the new paradigm. Curr Top Med Chem 11: 2490–2492, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 320: 110–113, 2008. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol 94: 1904–1911, 2005. [DOI] [PubMed] [Google Scholar]
- Lesku JA, Vyssotski AL, Martinez-Gonzalez D, Wilzeck C, Rattenborg NC. Local sleep homeostasis in the avian brain: convergence of sleep function in mammals and birds? Proc Biol Sci 278: 2419–2428, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis AL, Harvey N, Hobart GA 3rd. Distribution of disturbance-patterns in the human electroencephalogram, with special reference to sleep. J Neurophysiol 1: 413–430, 1938. [Google Scholar]
- Lyamin OI, Lapierre JL, Kosenko PO, Mukhametov LM, Siegel JM. Electroencephalogram asymmetry and spectral power during sleep in the northern fur seal. J Sleep Res 17: 154–165, 2008. [DOI] [PubMed] [Google Scholar]
- Motelow JE, Li W, Zhan Q, Mishra AM, Sachdev RN, Liu G, Gummadavelli A, Zayyad Z, Lee HS, Chu V, Andrews JP, Englot DJ, Herman P, Sanganahalli BG, Hyder F, Blumenfeld H. Decreased subcortical cholinergic arousal in focal seizures. Neuron 85: 561–572, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhametov LM, Supin AY, Polyakova IG. Interhemispheric asymmetry of the electroencephalographic sleep patterns in dolphins. Brain Res 134: 581–584, 1977. [DOI] [PubMed] [Google Scholar]
- Okun M, Naim A, Lampl I. The subthreshold relation between cortical local field potential and neuronal firing unveiled by intracellular recordings in awake rats. J Neurosci 30: 4440–4448, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacia SV, Ebersole JS. Intracranial EEG substrates of scalp ictal patterns from temporal lobe foci. Epilepsia 38: 642–654, 1997. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci USA 100: 13638–13643, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci 16: 1331–1339, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattenborg NC, Amlaner CJ. A bird's eye view of the function of sleep. In: Evolution of Sleep: Phylogenetic and Functional Perspectives, edited by McNamara P, Barton RA, Nunn CL. New York: Cambridge Univ. Press, 2010, p. 145–171. [Google Scholar]
- Ray A, Tao JX, Hawes-Ebersole SM, Ebersole JS. Localizing value of scalp EEG spikes: a simultaneous scalp and intracranial study. Clin Neurophysiol 118: 69–79, 2007. [DOI] [PubMed] [Google Scholar]
- Rector DM, Schei JL, Van Dongen HP, Belenky G, Krueger JM. Physiological markers of local sleep. Eur J Neurosci 29: 1771–1778, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain Res 1047: 45–55, 2005. [DOI] [PubMed] [Google Scholar]
- Sachdev RS, Ebner FF, Wilson CJ. Effect of subthreshold up and down states on the whisker-evoked response in somatosensory cortex. J Neurophysiol 92: 3511–3521, 2004. [DOI] [PubMed] [Google Scholar]
- Simon CW, Emmons WH. EEG, consciousness, and sleep. Science 30: 1066–1069, 1956. [DOI] [PubMed] [Google Scholar]
- Skrinjar O, Nabavi A, Duncan J. Model-driven brain shift compensation. Med Image Anal 6: 361–373, 2002. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85: 1969–1985, 2001. [DOI] [PubMed] [Google Scholar]
- Tan AY, Chen Y, Scholl B, Seidemann E, Priebe NJ. Sensory stimulation shifts visual cortex from synchronous to asynchronous states. Nature 509: 226–229, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao JX, Baldwin M, Hawes-Ebersole S, Ebersole JS. Cortical substrates of scalp EEG epileptiform discharges. J Clin Neurophysiol 24: 96–100, 2007. [DOI] [PubMed] [Google Scholar]
- Tao JX, Chen XJ, Baldwin M, Yung I, Rose S, Frim D, Hawes-Ebersole S, Ebersole JS. Interictal regional delta slowing is an EEG marker of epileptic network in temporal lobe epilepsy. Epilepsia 52: 467–476, 2011. [DOI] [PubMed] [Google Scholar]
- Tao JX, Ray A, Hawes-Ebersole S, Ebersole JS. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia 46: 669–676, 2005. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy W, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature 472: 443–447, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G. The location of cerebral tumours by electroencephalography. Lancet 227: 305–308, 1936. [Google Scholar]
- Zagha E, Casale AE, Sachdev RN, McGinley MJ, McCormick DA. Motor cortex feedback influences sensory processing by modulating network state. Neuron 79: 567–578, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Liang F, Xiong XR, Li L, Li H, Xiao Z, Tao HW, Zhang LI. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nat Neurosci 17: 841–850, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]