Abstract
The transient receptor potential (TRP) channels are widely distributed in the central nervous system (CNS) and peripheral nervous system. We examined the effects of TRP ankyrin 1 (TRPA1) agonists (cinnamaldehyde and allyl isothiocyanate) on respiratory rhythm generation in brainstem-spinal cord preparations from newborn rats [postnatal days 0–3 (P0–P3)] and in in situ-perfused preparations from juvenile rats (P11–P13). Preparations were superfused with modified Krebs solution at 25–26°C, and activity of inspiratory C4 ventral root (or phrenic nerve) was monitored. In the newborn rat, an in vitro preparation of cinnamaldehyde (0.5 mM) induced typically biphasic responses in C4 rate: an initial short increase and subsequent decrease, then a gradual recovery of rhythm during 15 min of bath application. After washout, the respiratory rhythm rate further increased, remaining 200% of control for >120 min, indicating long-lasting facilitation. Allyl isothiocyanate induced effects similar to those of cinnamaldehyde. The long-lasting facilitation of respiratory rhythm was partially antagonized by the TRPA1 antagonist HC-030031 (10 μM). We obtained similar long-lasting facilitation in an in situ-perfused reparation from P11–P13 rats. On the basis of results from transection experiments of the rostral medulla and whole-cell recordings from preinspiratory neurons in the parafacial respiratory group (pFRG), we suggest that the rostral medulla, including the pFRG, is important to the induction of long-lasting facilitation. A histochemical analysis demonstrated a wide distribution of TRPA1 channel-positive cells in the reticular formation of the medulla, including the pFRG. Our findings suggest that TRPA1 channel activation could induce long-lasting facilitation of respiratory rhythm and provide grounds for future study on the roles of TRPA1 channels in the CNS.
Keywords: transient receptor potential ankyrin 1, respiratory rhythm, facilitation
transient receptor potential (TRP) channels are widely distributed in the central nervous system (CNS) and the peripheral nervous system. They are suggested to participate in various functions such as neurite outgrowth, receptor signaling, and excitotoxic cell death resulting from anoxia (reviewed in Fernandes et al. 2012; Moran et al. 2004; Vennekens et al. 2012). In the respiratory center of the brainstem, it has been suggested that the TRPM/C channels (a TRP channel subtype) are involved in respiratory rhythm/pattern generation (Ben-Mabrouk and Tryba 2010; Crowder et al. 2007; Mironov 2008). TRP agonists or antagonists are useful tools for elucidating the functional role of the TRP channels. At present, however, it is not well understood whether the chemicals that are known as TRP channel agonists exert any effects on the medullary respiratory center. Previously, we reported that capsaicin (a heat-sensitive TRPV1 channel agonist) (Tani et al. 2014) and menthol (a cold-sensitive TRPM8 channel agonist) caused excitatory or inhibitory effects on respiratory rhythm generation in brainstem-spinal cord preparation from newborn rats (Tani et al. 2010, Tani et al. 2014).
The respiratory control system is sensitive to change in peripheral and central oxygen concentration. The role of the TRP ankyrin 1 (TRPA1) channels in respiratory control is therefore of particular interest, because TRPA1 channels are sensitive to both hypoxia and hyperoxia (Takahashi et al. 2011). In the present study, to elucidate the function of TRPA1 in the central respiratory control system, we examined the effects of TRPA1 agonists (cinnamaldehyde and allyl isothiocyanate) (Fernandes et al. 2012; Iwasaki et al. 2008; Macpherson et al. 2006) on respiratory rhythm generation in brainstem-spinal cord preparations from newborn rats [i.e., at postnatal days 0–3 (P0–P3)] and in an in situ-perfused preparation from juvenile rats aged P11–P13. Our findings suggest that activation of TRPA1 channels could induce long-lasting facilitation of the respiratory rhythm. Some of these findings have previously been presented in abstract form (Tani et al. 2013).
METHODS
The experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of Showa University.
In vitro preparation.
Experiments were performed with brainstem-spinal cord preparations from newborn P0–P3 Wistar rats. The newborn rats were deeply anesthetized with isoflurane. The brainstem and spinal cord were isolated and superfused at a rate of 3.0 ml/min with the following artificial cerebrospinal fluid (ACSF) (Suzue 1984) (in mM): 124 NaCl, 5.0 KCl, 1.2 KH2PO4, 2.4 CaCl2, 1.3 MgCl2, 26 NaHCO3, and 30 glucose, equilibrated with 95% O2 and 5% CO2, pH 7.4 at 26–27°C. In most experiments, the preparations were cut transversely at a level (line a in Fig. 1) just rostral to the anterior inferior cerebellar artery (AICA) (standard preparation). In some experiments, the preparations were cut at other levels (between lines b and c in Fig. 1) of more caudal medulla (caudal preparation).
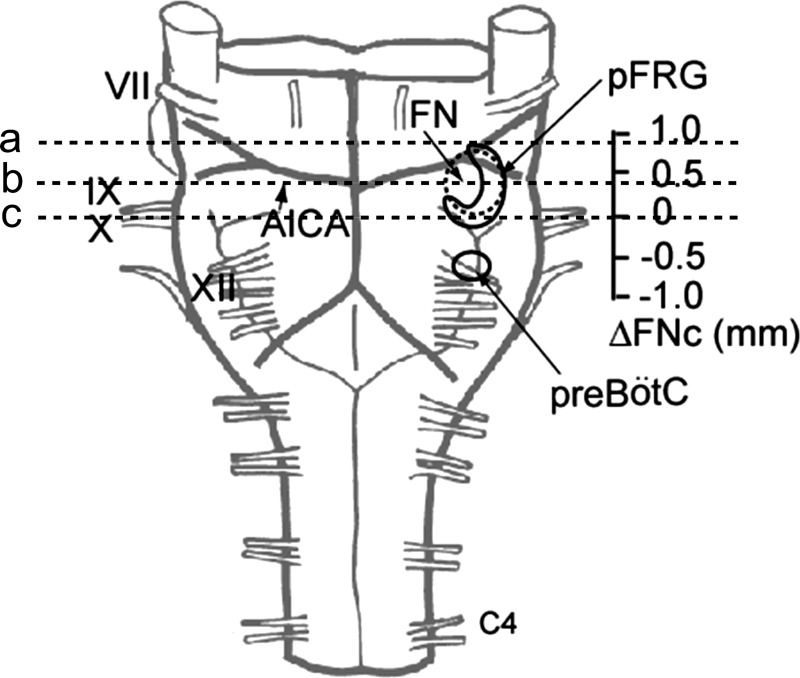
Fig. 1.
Ventral view of a brainstem-spinal cord preparation from a newborn rat and levels of transverse sections. The caudal end of the facial nucleus was referenced as the zero level (Ruangkittisakul et al. 2008). The pre-Bötzinger complex (pre-BötC) was centered at −0.5 mm (Ruangkittisakul et al. 2008) and the parafacial respiratory group (pFRG) was extended from −0.2 to +1 mm (Ballanyi et al. 2009). In a standard preparation, the brainstem was transversely cut at line a just rostral to the anterior inferior cerebellar artery (AICA). In the caudal preparation, the sections were between lines b and c, corresponding to positions of 0 to 0.4 mm rostral to the caudal end of the facial nucleus (ΔFNc). FN, facial nucleus; VII-XII, cranial nerves; C4, fourth cervical ventral root.
Drugs.
Cinnamaldehyde and allyl isothiocyanate were purchased from Wako Pure Medical (Tokyo, Japan). Cinnamaldehyde was stocked as a 1 M solution in 87% ethanol. Allyl isothiocyanate was stocked as a 1 M solution in 90% ethanol. HC-030031 was purchased from Sigma-Aldrich (Tokyo, Japan) and stocked as a 10 mM solution in 100% ethanol. All stock solutions were kept at 4°C. All drugs were dissolved with the ACSF and applied as a bath.
Electrophysiology.
Inspiratory activity corresponding to phrenic nerve activity was monitored from the fourth cervical ventral root (C4). Membrane potentials of preinspiratory (Pre-I) or inspiratory neurons in the rostral ventrolateral medulla corresponding to the rostral and caudal parts of the parafacial respiratory group (pFRG) (Onimaru and Homma 2003) were recorded by a blind, whole-cell patch-clamp method (Onimaru and Homma 1992). The rostral pFRG neurons were recorded by approach from the rostral cut surface at the level of 0.5–0.7 mm rostral to the caudal end of the facial nucleus (0 mm), and the caudal pFRG neurons were recorded by approach from the ventral surface at the level within ±100 μm rostro-caudal to the caudal end of the facial nucleus (Fig. 1) (Ballanyi et al. 2009; Ruangkittisakul et al. 2008). The electrodes, which had an inner tip diameter of 1.2–2.0 μm and a resistance of 4–8 MΩ, were filled with the following pipette solution (mM): 130 K-gluconate, 10 EGTA, 10 HEPES, 2 Na2-ATP, 1 CaCl2, and 1 MgCl2, with pH 7.2–7.3 adjusted with KOH. For histological analysis of the recorded cells, the electrode tips were filled with 0.5% Lucifer yellow (lithium salt). After experiments, preparations were fixed for 2–3 h at 4°C in 4% paraformaldehyde in 0.1 M PBS, transferred into 18% sucrose-PBS, and cut into 50-μm-thick transverse sections. A histological analysis was then performed. The membrane potential and input resistance were measured at the resting level between bursts (or between action potentials when tonic firings occurred).
Decerebrate and arterially perfused in situ rat preparation.
Experiments in a decerebrate and arterially perfused in situ rat preparation (Pickering and Paton 2006; Yazawa 2014) were performed on 12 juvenile Wistar rats (body wt 24.8–29.3 g) at age P11–P13. Each rat was initially sedated via inhalation of 5.0% isoflurane. During surgery, isoflurane concentration was maintained at 2.0%, and the depth of anesthesia was assessed by respiratory rate and tail pinch response. The same surgical procedure as described in our previous study (see Yazawa 2014; Yazawa and Shioda 2015) was then used to prepare the decerebrate and arterially perfused in situ rat preparation. While being held in the supine position in the recording chamber, a double-lumen catheter (DL-AS-040; Braintree Scientific, Braintree, MA) was inserted into the heart through an incision in the left ventricle. Arterial perfusion was immediately performed with carbogen-gassed, heparinized (10–20 U/liter) Ringer's solution containing Ficoll-PM70 (1.26%; Sigma-Aldrich, Tokyo, Japan), an oncotic agent, at 26°C. Subsequently, an incision was made in the right atrium. After the resumption of spontaneous breathing, at ≤5 min from the initiation of perfusion at 26°C, the muscle relaxant d-tubocurarine (2 μM; Sigma-Aldrich) was added to the perfusate to induce immobilization. The Ringer's solution was composed of the following (in mM): 125 NaCl, 3 KCl, 24 NaHCO3, 1.25 KH2PO4, 1.25 MgSO4, 2.5 CaCl2, and 10 glucose, equilibrated with 95% O2 and 5% CO2, pH 7.4 at 25–27°C. Using a peristaltic pump (323U pump, model 318MC pump head; Watson-Marlow, Wilmington, MA) to generate the perfusion flow, the perfusate was pumped from a reservoir flask into the aortic arch through a bubble trap and a nylon net filter, and then recycled from the recording chamber back to the reservoir. The flow rate was set above 5× the total blood volume (TBV) per minute at 26°C, with TBV calculated using 1/13 of the body weight as measured in grams. The left phrenic nerve was detached from the pulmonary pleura and cut at the distal end. Although bradycardia was pronounced at the initiation of perfusion, ventricular fibrillation never developed. Suction electrodes constructed of polyethylene tubing (PE 50; Becton-Dickinson, Franklin Lakes, NJ) were used to record neuronal discharge from the left phrenic nerve. Cinnamaldehyde, which was added to the perfusate, was applied to the (perfused) preparation with systemic perfusion.
Immunofluorescence and in situ hybridization.
The following primary antibodies were used for immunofluorescence staining: rabbit anti-TRPA1 (1:400 dilution, Novus Biologicals, Littleton, CO), and guinea pig anti-Phox2b (1:1000 dilution) (Onimaru et al. 2008). The secondary antibodies for fluorescence staining (1:1,000 dilution) were Alexa-Fluor 546 anti-rabbit IgG (Molecular Probes/Invitrogen, Carlsbad, CA), and Alexa-Fluor 633 anti-guinea pig IgG. Images of immunofluorescent samples were obtained with ×10 or ×20 magnification on an Olympus FV1000 confocal microscope or a conventional fluorescence microscope (BX60; Olympus, Tokyo, Japan).
In situ hybridization was performed on brainstems isolated from three independent Wistar rat neonates. The brainstem was isolated and further fixed at 4°C in fixation solution for 1–2 h. Samples were immersed in 18% sucrose/HBSS, embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA), then frozen on dry ice, and cut into 20-μm-thick cryosections. Partial cDNAs of rat Trpa1 (nucleotide number 2451–3378 of NM_207608) was obtained via RT-PCR using rat brain total RNA, subcloned into pGEM-T Easy Vector (Promega, Madison, WI), confirmed by sequencing, and used as a riboprobe template. In situ hybridization was performed as described previously with a digoxigenin-UTP (Roche Diagnostics, Basel, Switzerland)-labeled riboprobe (Ikeda et al. 2013). Proteinase K (1 μg/ml) was applied for 3–4 min at 26°C and the hybridization temperature was 50°C. Signals were detected using an anti-digoxigenin antibody conjugated with alkaline phosphatase (Roche) and NBT/BCIP (Roche) for chromogen.
Data analyses.
The initial data analyses were performed using the LabChart 7 Pro software program (ADInstruments, Castle Hill, Australia). To assess the effects of drugs on C4 or phrenic nerve activity, the burst rate (bursts/minute) was calculated from the mean rate for 3–5 min. The burst amplitude and duration of C4 activity were averaged from 5 to 10 consecutive respiratory cycles. An analysis of the consecutive time course of change in the respiratory rate was performed by the peak analysis program of LabChart 7 Pro. Data are presented as means ± SD for all preparations. Next, the significance of values was analyzed by a one-way ANOVA, followed by a Tukey-Kramer multiple comparisons test at a confidence level of P < 0.05 using the GraphPad InStat software program (GraphPad Software, La Jolla, CA).
RESULTS
First, we determined the lowest concentration (i.e., around 0.1–0.2 mM) of cinnamaldehyde that would cause significant effects on respiratory activity in the brainstem-spinal cord preparation. Bath application of 0.2 mM cinnamaldehyde induced the following reversible facilitation of the respiratory rhythm (bursts/min, n = 6): 5.1 ± 2.0 in control rats, 8.1 ± 1.9 in rats exposed to cinnamaldehyde for 15 min (P < 0.05), and 6.1 ± 1.3 in rats exposed to a 20-min washout period (not significant compared with control animals). At higher concentrations (0.5 or 1 mM) the effects were not reversible. Fig. 2A shows a typical example of the effects of 0.5 mM cinnamaldehyde (for 15 min) on C4 inspiratory activity in a standard preparation, and Fig. 3A shows the averaged time course of the effects (n = 7, solid circles). Cinnamaldehyde induced typically biphasic responses in C4 rate: an initial short increase (0.5–2 min) and a subsequent decrease, followed by a gradual recovery of rhythm during 15 min of bath application. After washout, the rate of respiratory rhythm increased and remained at ∼190% of control for more than 120 min (Fig. 2A, b and c). The group data (Table 1) indicate a decrease in burst duration and peak amplitude together with a significant increase in C4 rate after 90 min of washout. This C4 rate phenomenon is referred to as long-lasting facilitation of respiratory rhythm.
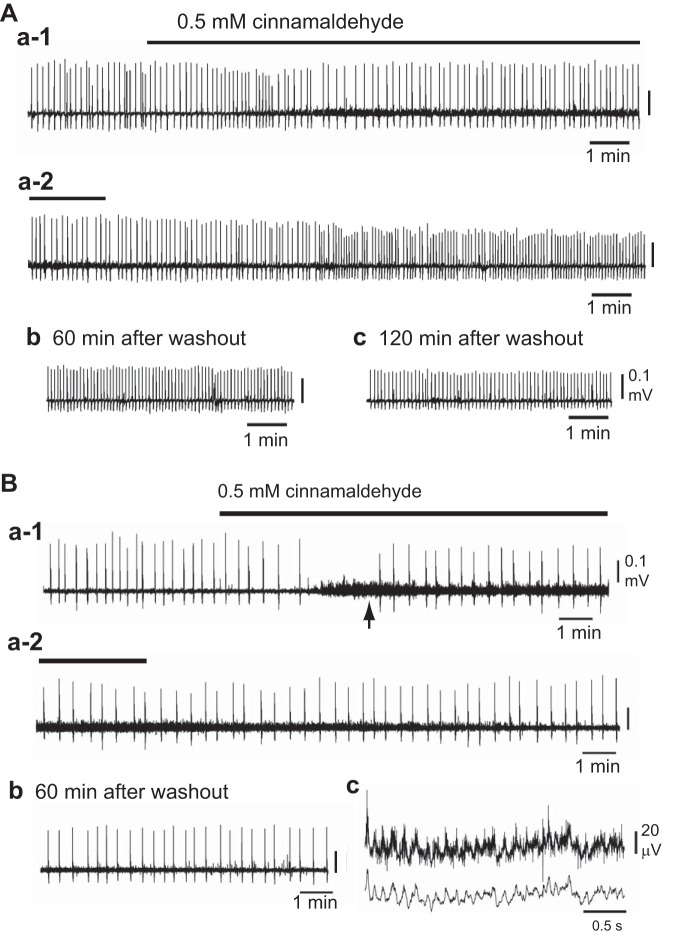
Fig. 2.
A typical example of the effects of the TRPA1 agonist cinnamaldehyde on C4 inspiratory activity. A: in a standard preparation, cinnamaldehyde (0.5 mM) was applied for 15 min (Aa-1). The C4 burst rate was initially increased and then decreased with a partial recovery (i.e., biphasic responses). Cinnamaldehyde also induced a strong tonic (nonrespiratory) discharge in C4. After washout, the C4 rate gradually increased (Aa-2). The higher frequency activity remained for more than 120 min after washout (Ab and Ac). This phenomenon was thus called long-lasting facilitation. B: a caudal preparation in which the rostral medulla was removed 0.2 mm rostral to the caudal end of the facial nucleus (see Fig.1). Cinnamaldehyde (0.5 mM) induced an initial decrease in C4 burst rate followed by a partial recovery (Ba-1, Ba-2), together with a strong tonic (nonrespiratory) discharge in C4 (arrow in Ba-1). Bc shows a faster sweep representation of tonic discharge in Ba-1 (arrow): upper trace, raw data; lower trace, after smoothing using the triangular window (window width 7) of LabChart 7 Pro. Long-lasting facilitation was not induced in this preparation (Bb).
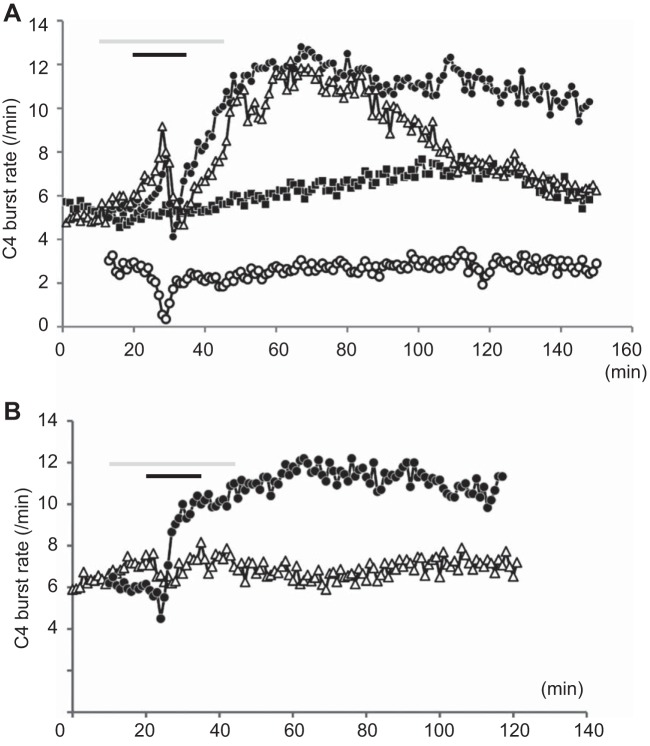
Fig. 3.
Time course of the effects of cinnamaldehyde (A) and allyl isothiocyanate (B). A: cinnamaldehyde (0.5 mM, average of seven preparations, solid circles) induced typically biphasic responses in C4 rate; an initial short increase (0.5–2 min) and a subsequent decrease, followed by a gradual recovery of rhythm during the 15-min bath application (black bar). After washout, the rate of respiratory rhythm further increased and remained at 190% of control for more than 120 min (long-lasting facilitation). The effects of cinnamaldehyde were partially antagonized by pretreatment (gray bar) with HC-030031 (10 μM, open triangles, n = 3). Long-lasting facilitation was not induced in the caudal preparations in which more than 50% of the rostral pFRG was removed (n = 4, open circles). Solid squares (time control) denote time-dependent changes in C4 burst rate of the control preparation without drug application (n = 7). B: allyl isothiocyanate (0.5 mM, average of five preparations, solid circles) induced an initial short decrease (0.5–2 min) and a subsequent increase in C4 burst rate during 15 min of bath application (black bar). After washout, the rate of respiratory rhythm further increased and remained at 200% of control for more than 90 min (long-lasting facilitation). The effects of allyl isothiocyanate were antagonized by pretreatment (gray bar) with HC-030031 (10 μM, open triangles, n = 3).
Table 1.
Effects of 0.5 mM cinnamaldehyde on C4 activity (in vitro) in standard preparation
| Burst Rate,/min | Duration, m | Peak, % | |
|---|---|---|---|
| Control, n = 8 | 5.6 ± 1.6 | 774 ± 132 | 100 |
| After application, 15 min | 9.5 ± 3.0† | 858 ± 172 | 87.7 ± 9.3 |
| After washout, 90 min | 10.8 ± 2.6‡ | 683 ± 124§ | 77.8 ± 24.2* |
P < 0.05,
P < 0.01,
P < 0.001 in comparison with control values.
P < 0.05 in comparison with 15-min values.
To elucidate whether the rostral medulla, including the pFRG, is involved in induction of long-lasting facilitation, the effects of cinnamaldehyde were examined in caudal preparations in which the rostral medulla was removed at the level between lines b and c in Fig. 1. Caudal preparation that harbored only the caudal region of the pFRG showed a lower burst rate than was observed in a standard preparation. The application of cinnamaldehyde did not induce long-lasting facilitation or initial transient excitation in these preparations (Figs. 2B and 3A, n = 4), but it induced a strong C4 tonic (nonrespiratory, short burst-like) discharge of around 10 Hz (Fig. 2Bc), similar to that in the standard preparation.
Pretreatment with the TRPA1 antagonist HC-030031 (10 μM, n = 3, open triangles in Fig. 3A) did not block the initial biphasic effects or the subsequent gradual increase in the C4 burst rate until ∼40 min after washout. Then, however, the facilitated rhythm began to return toward that of the control level (i.e., the normalizing of the inspiratory rhythm began earlier with the antagonist pretreatment than without the antagonist pretreatment).
We also examined the effects of another TRPA1 agonist, allyl isothiocyanate. Allyl isothiocyanate (0.5 mM, n = 5) induced an initial short decrease (0.5–2 min) and subsequent increase in C4 burst rate during 15 min of bath application. Similar to the effects of cinnamaldehyde, the rate of respiratory rhythm remained at ∼200% of control for more than 90 min after washout (long-lasting facilitation) (Fig. 3B). The long-lasting effects of cinnamaldehyde were antagonized by pretreatment with 10 μM HC-030031 (n = 3, Fig. 3B, open triangles).
Because our results suggested that the rostral medulla, including the pFRG, was important in the induction of long-lasting facilitation, we examined the effects of cinnamaldehyde on Pre-I neurons, which are a major component of the rostral pFRG. We recorded a total of five Pre-I neurons. The application of cinnamaldehyde (15 min) depolarized three of five Pre-I neurons by 6–8 mV but hyperpolarized the other two neurons by −3 or −6 mV. Figure 4A shows an example of a Pre-I neuron recording in the rostral parafacial region (0.5 mm rostral to the caudal end of facial nucleus). The firing initially changed to a tonic pattern (Fig. 4Ac) and then restored a rhythmic burst pattern with washout (Fig. 4Ad). This neuron was located in the rostral pFRG and was identified as Phox2b-positive by immunofluorescence staining after recording (Fig. 4a′). Two other Pre-I neurons that were depolarized by application of cinnamaldehyde showed a similar change of burst pattern to that shown in Fig. 4A. The firings of two other neurons that were hyperpolarized tended to be depressed (data not shown). The averaged membrane potential (n = 5) was −48.4 ± 3.0 mV in control, −45.8 ± 9.2 mV not significant vs. control) at 15 min application −45.0 ± 5.3 mV (not significant vs. control) at 20 min of washout. We also examined the membrane potential change in inspiratory neurons (n = 5) in the caudal pFRG (Fig. 4B), which is located at the level of line c in Fig. 1. All inspiratory neurons were depolarized by 2 to 9 mV. The averaged membrane potential (n = 5) was −45.3 ± 5.4 mV in control, −40.0 ± 4.8 mV (not significant vs. control) at 15 min application, and −38.8 ± 2.2 mV (P < 0.05 vs. control) at 20 min of washout. The timing of Pre-I and inspiratory neuron bursts basically corresponded to the timing of C4 bursts with a one-to-one manner.
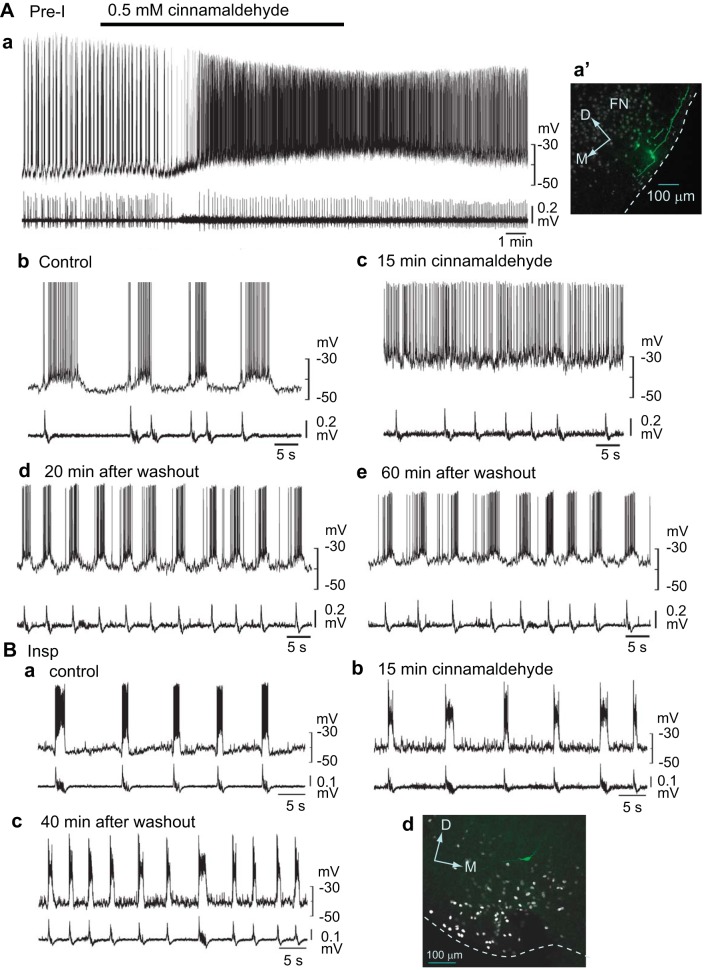
Fig. 4.
Effects of cinnamaldehyde on respiratory-related neurons in the medulla. Upper traces, membrane potentials; lower traces, C4 activity. A: a preinspiratory (Pre-I) neuron in the rostral parafacial region (0.5 mm rostral to the caudal end of facial nucleus). a: a slower sweep representation of the membrane potential response to the application of cinnamaldehyde (0.5 mM). Ab–e: faster sweep representations of the membrane potential responses. Application of cinnamaldehyde depolarized this neuron, and the burst discharge turned into a tonic firing pattern (Ac). Note that the facilitation of C4 burst rate after washout was accompanied by facilitation of the Pre-I neuron burst (Ad and Ae). This neuron was located in the rostral pFRG and was Phox2b-positive (Aa′). FN, facial nucleus; D, dorsal; M, medial; dotted line, ventral surface. B: an inspiratory (Insp) neuron in the caudal parafacial region. Ba: membrane potential trajectory in control. Bb: membrane potential responses after a 15-min application of 0.5 mM cinnamaldehyde. Bc: activity 40 min after washout. This neuron was located in the caudal pFRG. Note that facilitation of the C4 burst rate after washout was accompanied by facilitation of the inspiratory neuron burst (Bc).
We examined the effects of cinnamaldehyde on respiratory activity in an arterially perfused in situ rat preparation (P11–P13, n = 5). Cinnamaldehyde (0.5 mM) induced typically biphasic responses in phrenic burst rate: an initial short increase (0.5–2 min) and subsequent decrease, followed by a gradual recovery of rhythm during the 15-min bath application (Fig. 5A). After washout, the rate of respiratory rhythm increased and remained at ∼250% of control for more than 120 min. Thus a very similar response to an in vitro preparation from newborn rat was observed (Fig. 5B). Regarding other parameters, only the peak value decreased significantly at 15 min of application (Table 2).
Fig. 5.
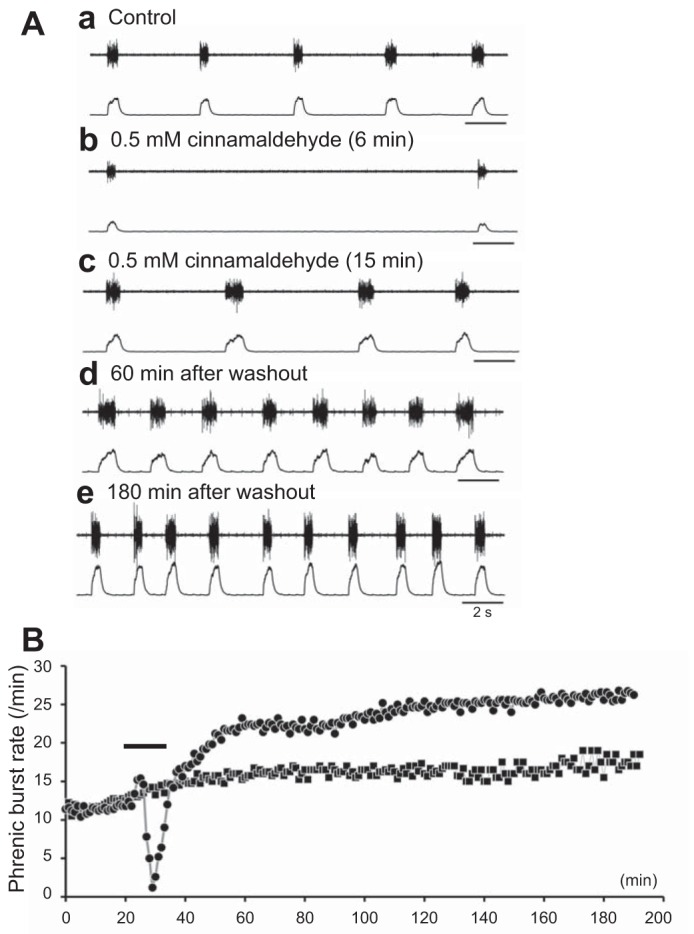
Effects of cinnamaldehyde on a decerebrate and arterially perfused rat in situ preparation. Upper traces, raw data; lower traces, integrated phrenic activity. A: a typical example of phrenic activity. Aa: control animals. Ab: activity 6 min after application of 0.5 mM cinnamaldehyde. Ac: activity 15 min after application cinnamaldehyde (0.5 mM). Ad: activity 60 min after returning to the standard solution. Ae: activity 180 min after returning to the standard solution. B: time course of effects of cinnamaldehyde in an arterially perfused in situ preparation of rats aged postnatal days 11 to 13. Shown is the average from five preparations (solid circles). Cinnamaldehyde (0.5 mM) induced typically biphasic responses in the phrenic burst rate: an initial short increase (0.5–2 min) and a subsequent decrease, followed by a gradual recovery of rhythm during 15 min of bath application (black bar). After washout, the rate of respiratory rhythm increased and remained at 250% of control levels for more than 120 min (long-lasting facilitation). Note that this was a very similar response to that observed in in vitro preparations from newborn rats (Fig. 3A). Solid squares (time control) denote time-dependent changes of the phrenic burst rate of the control preparation without application of the drug (n = 5).
Table 2.
Effects of 0.5 mM cinnamaldehyde on phrenic activity in arterially perfused in situ preparations
| Burst Rate,/min | Duration, ms | Peak, % | |
|---|---|---|---|
| Control, n = 5 | 12 ± 2.5 | 546 ± 74 | 100 |
| After application, 15 min | 19.2 ± 5.2* | 547 ± 133 | 65.5 ± 6.4 |
| After washout, 90 min | 26.2 ± 4.1† | 498 ± 41 | 140 ± 99 |
P < 0.05,
P < 0.001; in comparison with control values.
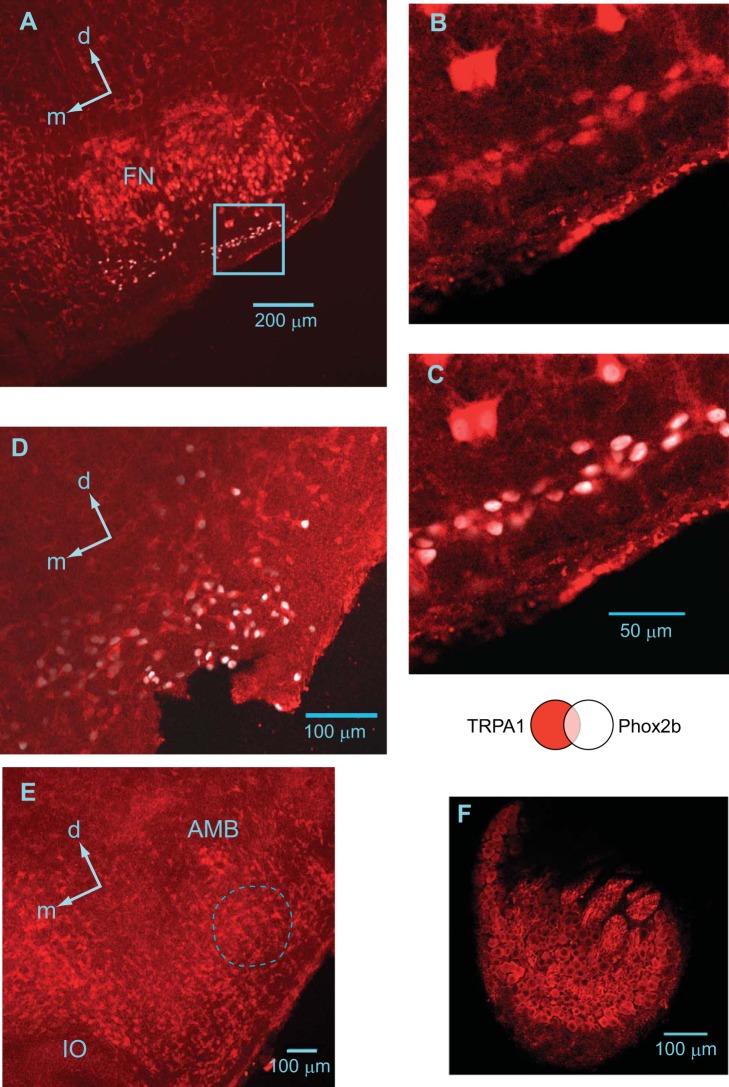
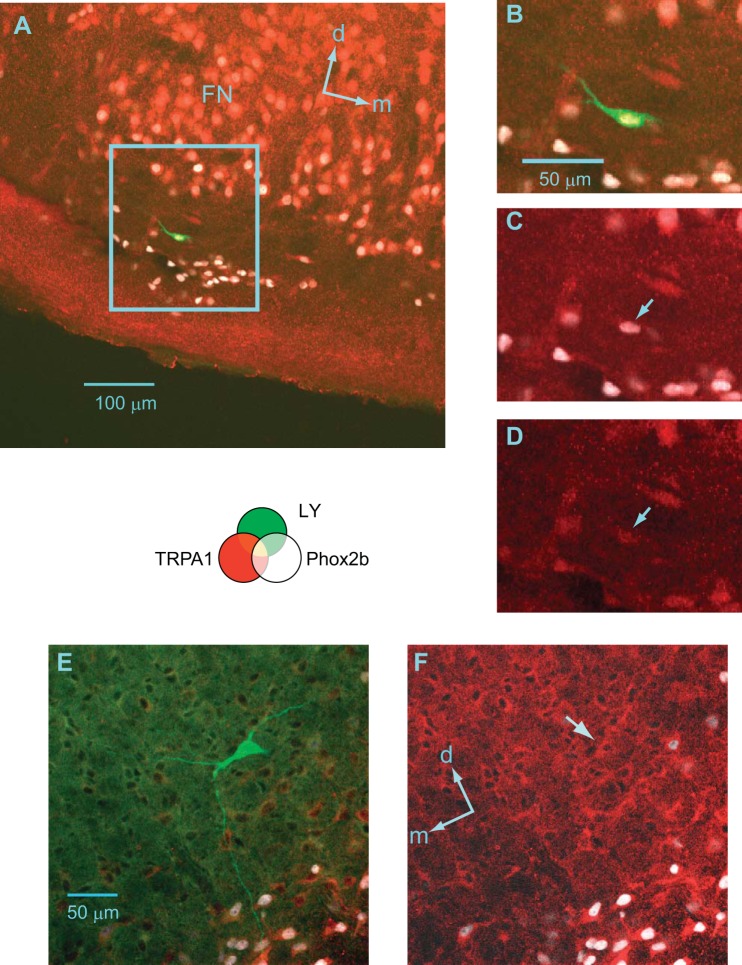
A histochemical analysis demonstrated the wide distribution of TRPA1 channel-positive cells in the reticular formation of the medulla, including the pFRG and pre-Bötzinger complex (preBötC), with stronger expression in the motor neuron pools (Fig. 6). It has been reported that a group of the pFRG-Pre-I neurons are Phox2b-positive (Onimaru et al. 2008). We confirmed that Phox2b-positive cells in the parafacial region expressed TRPA1 channel protein (Fig. 6, A–D). A similar distribution was also confirmed by in situ hybridization of mRNA for TRPA1 channel protein (Fig. 7). We examined TRPA1 channel protein expression in respiratory-related neurons (three Pre-I neurons, two inspiratory neurons) in the pFRG. All of these neurons were determined to be TRPA1-positive. Figure 8 shows examples of Pre-I neurons in the rostral pFRG (Fig. 8A) and caudal pFRG (Fig. 8E). The Pre-I neuron in the rostral pFRG was located in the cluster of Phox2b-positive cells and expressed Phox2b as well as TRPA1 (Fig. 8, A–D). The Pre-I neuron in the caudal pFRG was located outside the Phox2b-positive cell cluster at a depth of 320 μm from the ventral surface and was TRPA1-positive but Phox2b-negative (Fig. 8, E and F).
Fig. 6.
TRPA1 immunostaining in the ventral medulla of the neonatal rat (postnatal day 1). TRPA1 (red), Phox2b (white). A: at the level of the rostral pFRG (0.6 mm rostral to the caudal end of facial nucleus; see Fig. 1). FN, facial nucleus; d, dorsal; m, medial. B and C: higher magnification images of the parafacial region indicated as the highlighted square region in A. B: TRPA1 expression. C: merged TRPA1 (red) and Phox2b (white) expression. D: caudal pFRG (0.05 mm caudal to the caudal end of facial nucleus). Note the clear expression of TRPA1 in the parafacial region. E: pre-Bötzinger complex (0.4 mm caudal to the caudal end of facial nucleus). The dotted line denotes the approximate region of pre-Bötzinger complex in which TRPA1 is expressed. AMB, ambigual nucleus; IO, inferior olivary nucleus; d, dorsal; m, medial. F: a dorsal root ganglion as positive control.
Fig. 7.
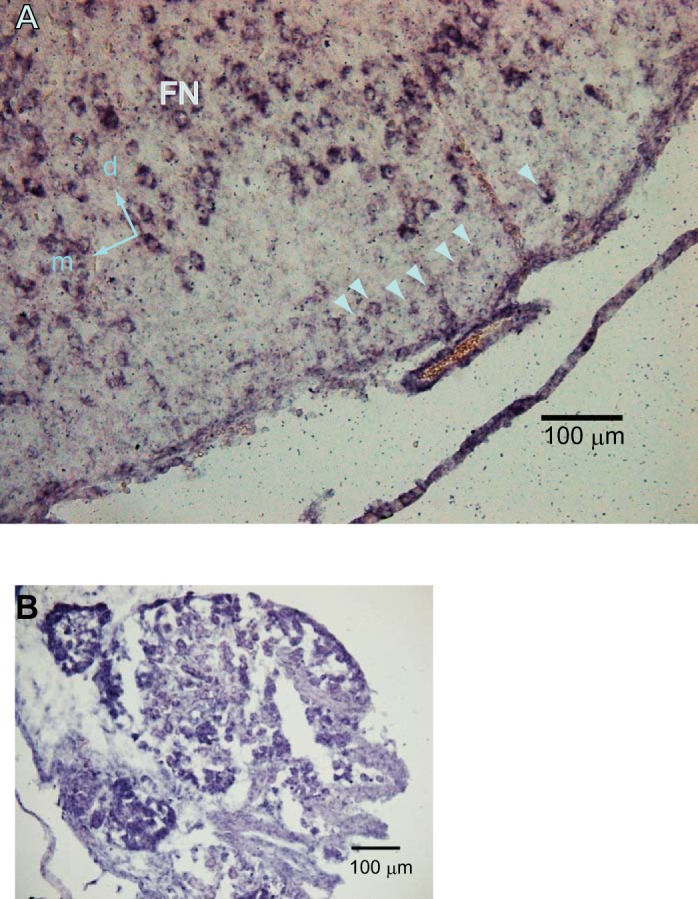
Expression of genes encoding TRPA1 in the region of the pFRG of a neonatal rat at postnatal day 1. A: in situ hybridization using a riboprobe for rat Trpa1 Arrowheads indicate trpa1-positive cells in the pFRG region. FN, facial nucleus; d, dorsal; m, medial. B: a dorsal root ganglion serving as a positive control.
Fig. 8.
TRPA1 immunoreactivity and Lucifer yellow staining of pre-inspiratory (Pre-I) neurons. Lucifer yellow (green), TRPA1 (red), Phox2b (white). A: a Pre-I neuron recorded in the rostral pFRG (0.6 mm rostral to the caudal end of facial nucleus); d, dorsal; m, medial. B–D: higher magnification of the parafacial region is indicated as the highlighted square region in A. The neuron was TRPA1-positive and Phox2b-positive (arrows in C and D). E and F: a Pre-I neuron recorded in the caudal pFRG (at the level of line c in Fig. 1). This neuron was TRPA1-positive and Phox2b-negative (arrow in F); d, dorsal; m, medial.
DISCUSSION
Cinnamaldehyde and allyl isothiocyanate induced the long-lasting facilitation of respiratory rhythm in brainstem-spinal cord preparations from newborn rats. These substances (0.5 mM) may stimulate TRPV1 (or other types of TRP channels) as well as TRPA1 (Macpherson et al. 2006). Long-lasting facilitation was at least partly blocked by the TRPA1 antagonist HC-030031. Therefore, activation of TRPA1 is involved in this phenomenon. Our preliminary results regarding the effects of TRPV1 stimulation by capsaicin did not induce such long-lasting facilitation, but it did induce the initial biphasic responses observed in the present study (Fig. 2). Because the initial biphasic responses induced by cinnamaldehyde were not blocked by HC-030031, they may depend on TRPV1 or other unknown mechanisms. In contrast, allyl isothiocyanate induced weaker initial biphasic responses than cinnamaldehyde, and HC-030031 effectively blocked the responses. This suggests a more specific action of allyl isothiocyanate on the TRPA1 channels compared with cinnamaldehyde. The long-lasting facilitation of the C4 burst rate after washout was accompanied by the facilitation of Pre-I and inspiratory neuron burst rates (Fig. 5); however, it was not induced in the preparation without the rostral medulla, including more than 50% of the pFRG. The structure of the rostral medulla, including the pFRG, may therefore be necessary for the induction of this response and for the initial transient excitatory response. Previous studies have reported that this region is important in central chemoreception (Guyenet and Mulkey 2010; Onimaru et al. 2008; Stornetta et al. 2006). Long-lasting facilitation of phrenic nerve activity was also induced in a decerebrate and arterially perfused in situ preparation from juvenile rats (P11–P13), indicating that this phenomenon is not specific to the en bloc preparations from neonatal rats and that it could be also observed in other (more retained) types of preparations from more developed animals. Our histochemical analysis using antibodies and mRNA for TRPA1 channel protein demonstrated, for the first time, a wide distribution of TRPA1 channel-positive cells in the reticular formation of the medulla, including the pFRG and preBötC.
In the CNS, TRPA1 channels are typically expressed in neurons of sensory afferent networks such as the trigeminal sensory nuclei and spinal dorsal horn (Kim et al. 2010). Several studies have reported that TRPA1 agonists induce changes in the excitability of cells in the CNS. TRPA1 agonists increased the frequency and amplitude of spontaneous excitatory postsynaptic currents (EPSCs) in the spinal dorsal horn neurons involved in the central modulation of sensory signals (Kosugi et al. 2007). In the medulla, the TRPA1 agonist allyl isothiocyanate increased glutamate release in the nuclei of tractus solitarii neurons, which are known to mediate various autonomic regulations, including respiratory control (Sun et al. 2009). These excitatory effects of TRPA1 agonists might have been one of the cellular mechanisms for the direct or indirect modulation of respiratory rhythm and the induction of tonic discharges that was observed in the present study.
It has been well established that the long-term facilitation (LTF) of respiratory motor pattern is induced by intermittent hypoxia (Mateika and Syed 2013; Mitchell et al. 2001; Xing et al. 2013). Although the cellular mechanism behind LTF is not yet well understood, serotonin is suggested to play a key role in its induction (Bach and Mitchell 1996; Millhorn et al. 1980; Pavlinac Dodig et al. 2012; Xing et al. 2013). The hallmark of LTF by intermittent hypoxia is an increase in the peak amplitude of phrenic nerve bursts (or tidal volume) with a small change in respiratory frequency (Baker-Herman and Mitchell 2008), although the effects on amplitude or frequency depend on the experimental conditions (including under anesthesia or awake; in vivo, reduced preparations; and other conditions). Thus there is basic difference between LTF induced by intermittent hypoxia and the present long-lasting facilitation of respiratory rhythm by TRPA1 agonists (but not in the motor output amplitude). In newborn mouse en bloc preparation, Berner et al. (2007) reported that intermittent anoxia induced LTF of respiratory rhythm and suggested the involvement of substance P (and/or neurokinin A) in anoxia-induced responses. TRPA1 channels are sensitive to both hypoxia and hyperoxia (Takahashi et al. 2011). This property of TRPA1 channels may underlie changes in respiratory activity that occur via responses to hypoxia in carotid body, airways, or other structures (Pokorski et al. 2014; Takahashi et al. 2011). Therefore, it would be interesting to investigate whether a hypoxia- or hyperoxia-sensitive mechanism of the TRPA1 channels is involved in the central mechanisms of the hypoxia-induced respiratory response or the LTF induced by intermittent hypoxia.
Recent studies have demonstrated that TRPA1 channels are expressed in astrocytes in the brain (Lee et al. 2012; Shigetomi et al. 2012). TRPA1 channels in hippocampal astrocytes play an essential role in the determination of astrocyte basal Ca2+ levels, and this system has been suggested to be involved in long-term potentiation via astrocyte-neuron interactions in the hippocampus (Shigetomi et al. 2012, Shigetomi et al.2013). Astrocyte-neuron interactions have also been suggested to be important in respiratory rhythm generation (Hulsmann et al. 2000; Okada et al. 2012). Moreover, Hirata and Oku (2010) reported that cells in glia-rich medullary cultures expressed the mRNA of TRPA1 as well as TRPV1 and TRPM8, and suggested a role for TRP channels in central chemoreception. Thus the contribution of astrocytes should be considered as one of the possible cellular mechanisms in the long-lasting facilitation of respiratory rhythm that was observed in the present study.
Some previous studies reported that cinnamon extracts (or cinnamaldehyde) inhibited Na/K ATPase activity in the kidney and jejunum (Kreydiyyeh et al. 2000; Usta et al. 2003). We previously reported that inhibition of Na/K ATPase by ouabain induced a long-lasting facilitation of respiratory rhythm accompanied by a depolarization of respiratory neurons (Tsuzawa et al. 2014). Thus this effect could be one of mechanisms for long-lasting facilitation by TRPA1 agonists. It should be noted that there were differences between the effects of ouabain and cinnamaldehyde on respiratory neurons; the former consistently depolarized Pre-I neurons, whereas the latter also induced hyperpolarization in some Pre-I neurons.
In conclusion, we found that TRPA1 agonists induced long-lasting facilitation of respiratory rhythm in a brainstem-spinal cord preparation from newborn rats. TRPA1 channels were expressed in the rostral medulla, which is involved in respiratory rhythm generation. Although the cellular mechanisms and physiological meaning of this remain to be clarified, our findings provide the grounds for future study of the functional roles of TRPA1 channels in the CNS.
GRANTS
This work was supported by Grants-in Aid for Scientific Research (KAKENHI) 22500296 and 25430012 from the Japan Society for the Promotion of Science.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
M.T. and H.O. conception and design of research; M.T., I.Y., K.I., K.K., and H.O. performed experiments; M.T., I.Y., K.I., K.K., and H.O. analyzed data; M.T., I.Y., K.I., K.K., and H.O. interpreted results of experiments; M.T., K.I., K.K., and H.O. prepared figures; M.T., I.Y., K.I., K.K., and H.O. drafted manuscript; M.T., I.Y., K.I., K.K., and H.O. edited and revised manuscript; M.T., I.Y., K.I., K.K., and H.O. approved final version of manuscript.
REFERENCES
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol 162: 8–17, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K, Ruangkittisakul A, Onimaru H. Opioids prolong and anoxia shortens delay between onset of preinspiratory (pFRG) and inspiratory (preBötC) network bursting in newborn rat brainstems. Pflugers Arch 458: 571–587, 2009. [DOI] [PubMed] [Google Scholar]
- Ben-Mabrouk F, Tryba AK. Substance P modulation of TRPC3/7 channels improves respiratory rhythm regularity and ICAN-dependent pacemaker activity. Eur J Neurosci 31: 1219–1232, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner J, Shvarev Y, Lagercrantz H, Bilkei-Gorzo A, Hökfelt T, Wickström R. Altered respiratory pattern and hypoxic response in transgenic newborn mice lacking the tachykinin-1 gene. J Appl Physiol 103: 552–559, 2007. [DOI] [PubMed] [Google Scholar]
- Crowder EA, Saha MS, Pace RW, Zhang H, Prestwich GD, Del Negro CA. Phosphatidylinositol 4,5-bisphosphate regulates inspiratory burst activity in the neonatal mouse preBötzinger complex. J Physiol 582: 1047–1058, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes ES, Fernandes MA, Keeble JE. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br J Pharmacol 166: 510–521, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK. Retrotrapezoid nucleus and parafacial respiratory group. Respir Physiol Neurobiol 173: 244–255, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y, Oku Y. TRP channels are involved in mediating hypercapnic Ca2+ responses in rat glia-rich medullary cultures independent of extracellular pH. Cell Calcium 48: 124–132, 2010. [DOI] [PubMed] [Google Scholar]
- Hülsmann S, Oku Y, Zhang W, Richter DW. Metabolic coupling between glia and neurons is necessary for maintaining respiratory activity in transverse medullary slices of neonatal mouse. Eur J Neurosci 12: 856–862, 2000. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Satake S, Onaka T, Sugimoto H, Takeda N, Imoto K, Kawakami K. Enhanced inhibitory neurotransmission in the cerebellar cortex of Atp1a3-deficient heterozygous mice. J Physiol 591: 3433–3449, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y, Tanabe M, Kobata K, Watanabe T. TRPA1 agonists–allyl isothiocyanate and cinnamaldehyde–induce adrenaline secretion. Biosci Biotechnol Biochem 72: 2608–2614, 2008. [DOI] [PubMed] [Google Scholar]
- Kim YS, Son JY, Kim TH, Paik SK, Dai Y, Noguchi K, Ahn DK, Bae YC. Expression of transient receptor potential ankyrin 1 (TRPA1) in the rat trigeminal sensory afferents and spinal dorsal horn. J Comp Neurol 518: 687–698, 2010. [DOI] [PubMed] [Google Scholar]
- Kosugi M, Nakatsuka T, Fujita T, Kuroda Y, Kumamoto E. Activation of TRPA1 channel facilitates excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. J Neurosci 27: 4443–4451, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreydiyyeh SI, Usta J, Copti R. Effect of cinnamon, clove and some of their constituents on the Na+-K+-ATPase activity and alanine absorption in the rat jejunum. Food Chem Toxicol 38: 755–762, 2000. [DOI] [PubMed] [Google Scholar]
- Lee SM, Cho YS, Kim TH, Jin MU, Ahn DK, Noguchi K, Bae YC. An ultrastructural evidence for the expression of transient receptor potential ankyrin 1 (TRPA1) in astrocytes in the rat trigeminal caudal nucleus. J Chem Neuroanat 45: 45–49, 2012. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci 32: 335–343, 2006. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Syed Z. Intermittent hypoxia, respiratory plasticity and sleep apnea in humans: present knowledge and future investigations. Respir Physiol Neurobiol 188: 289–300, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol 42: 171–188, 1980. [DOI] [PubMed] [Google Scholar]
- Mironov SL. Metabotropic glutamate receptors activate dendritic calcium waves and TRPM channels which drive rhythmic respiratory patterns in mice. J Physiol 586: 2277–2291, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB Jr. Invited review: intermittent hypoxia and respiratory plasticity. J Appl Physiol 90: 2466–2475, 2001. [DOI] [PubMed] [Google Scholar]
- Moran MM, Xu H, Clapham DE. TRP ion channels in the nervous system. Curr Opin Neurobiol 14: 362–369, 2004. [DOI] [PubMed] [Google Scholar]
- Okada Y, Sasaki T, Oku Y, Takahashi N, Seki M, Ujita S, Tanaka KF, Matsuki N, Ikegaya Y. Preinspiratory calcium rise in putative pre-Bötzinger complex astrocytes. J Physiol 590: 4933–4944, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci 23: 1478–1486, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Whole cell recordings from respiratory neurons in the medulla of brainstem-spinal cord preparations isolated from newborn rats. Pflugers Arch 420: 399–406, 1992. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci 28: 12845–12850, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlinac Dodig I, Pecotic R, Valic M, Dogas Z. Acute intermittent hypoxia induces phrenic long-term facilitation which is modulated by 5-HT1A receptor in the caudal raphe region of the rat. J Sleep Res 21: 195–203, 2012. [DOI] [PubMed] [Google Scholar]
- Pickering AE, Paton JF. A decerebrate, artificially-perfused in situ preparation of rat: utility for the study of autonomic and nociceptive processing. J Neurosci Methods 155: 260–271, 2006. [DOI] [PubMed] [Google Scholar]
- Pokorski M, Takeda K, Sato Y, Okada Y. The hypoxic ventilatory response and TRPA1 antagonism in conscious mice. Acta Physiol (Oxf) 210: 928–938, 2014. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Ma Y, Bobocea N, Poon BY, Funk GD, Ballanyi K. Generation of eupnea and sighs by a spatiochemically organized inspiratory network. J Neurosci 28: 2447–2458, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Jackson-Weaver O, Huckstepp RT, O'Dell TJ, Khakh BS. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J Neurosci 33: 10143–10153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci 15: 70–80, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26: 10305–10314, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Bang SI, Jin YH. Transient receptor potential A1 increase glutamate release on brain stem neurons. Neuroreport 20: 1002–1006, 2009. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol 354: 173–183, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, Yamamoto S, Naito S, Knevels E, Carmeliet P, Oga T, Kaneko S, Suga S, Nokami T, Yoshida J, Mori Y. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol 7: 701–711, 2011. [DOI] [PubMed] [Google Scholar]
- Tani M, Lin ST, Onimaru H. Cellular mechanisms of capsaicin actions on the respiratory neurons in brainstem-spinal cord preparations from the newborn rat. J Physiol Sci 64: s280, 2014. [Google Scholar]
- Tani M, Onimaru H, Ikeda K, Kawakami K, Homma I. Menthol inhibits the respiratory rhythm in brainstem preparations of the newborn rats. Neuroreport 21: 1095–1099, 2010. [DOI] [PubMed] [Google Scholar]
- Tani M, Yazawa I, Tsuzawa K, Onimaru H. Long-lasting facilitation of respiratory rhythm by treatment with TRPA1 agonist, cinnamaldehyde. J Physiol Sci 63: s221, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzawa K, Yazawa I, Shakuo T, Ikeda K, Kawakami K, Onimaru H. Effects of ouabain on respiratory rhythm generation in brainstem-spinal cord preparation from newborn rats and in decerebrate and arterially perfused in situ preparation from juvenile rats. Neuroscience 286: 404–411, 2014. [DOI] [PubMed] [Google Scholar]
- Usta J, Kreydiyyeh S, Barnabe P, Bou-Moughlabay Y, Nakkash-Chmaisse H. Comparative study on the effect of cinnamon and clove extracts and their main components on different types of ATPases. Hum Exp Toxicol 22: 355–362, 2003. [DOI] [PubMed] [Google Scholar]
- Vennekens R, Menigoz A, Nilius B. TRPs in the brain. Rev Physiol Biochem Pharmacol 163: 27–64, 2012. [DOI] [PubMed] [Google Scholar]
- Xing T, Fong AY, Bautista TG, Pilowsky PM. Acute intermittent hypoxia induced neural plasticity in respiratory motor control. Clin Exp Pharmacol Physiol 40: 602–609, 2013. [DOI] [PubMed] [Google Scholar]
- Yazawa I. Reciprocal functional interactions between the brainstem and the lower spinal cord. Front Neurosci 8: 124, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa I, Shioda S. Reciprocal functional interactions between the respiration/circulation center, the upper spinal cord, and the trigeminal system. Transl Neurosci 6: 87–102, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]