Abstract
The term savings refers to faster motor adaptation upon reexposure to a previously experienced perturbation, a phenomenon thought to reflect the existence of a long-term motor memory. It is commonly assumed that sustained practice during the first perturbation exposure is necessary to create this memory. Here we sought to test this assumption by determining the minimum amount of experience necessary during initial adaptation to a visuomotor rotation to bring about savings the following day. Four groups of human subjects experienced 2, 5, 10, or 40 trials of a counterclockwise 30° cursor rotation during reaching movements on one day and were retested the following day to assay for savings. Groups that experienced five trials or more of adaptation on day 1 showed clear savings on day 2. Subjects in all groups learned significantly more from the first rotation trial on day 2 than on day 1, but this learning rate advantage was maintained only in groups that had reached asymptote during the initial exposure. Additional experiments revealed that savings occurred when the magnitude, but not the direction, of the rotation differed across exposures, and when a 5-min break, rather than an overnight one, separated the first and second exposure. The overall pattern of savings we observe across conditions can be explained as rapid retrieval of the state of learning attained during the first exposure rather than as modulation of sensitivity to error. We conclude that a long-term memory for compensating for a perturbation can be rapidly acquired and rapidly retrieved.
Keywords: adaptation, cognition, recall, savings, explicit memory
motor adaptation in humans serves to maintain movement accuracy in the presence of ever-changing environments and physiological states (Shadmehr and Mussa-Ivaldi 1994; Krakauer et al. 1999, 2000; Kording et al. 2007; van Beers 2009). One important observation related to adaptation is that human subjects learn to adapt to a perturbation in fewer trials when they have previously experienced that perturbation, a phenomenon referred to as “savings” (Lackner and Lobovits 1977; Brashers-Krug et al. 1996; Kojima et al. 2004; Krakauer et al. 2005; Zarahn et al. 2008; Huang et al. 2011; Villalta et al. 2015).
There have been a number of theories attempting to explain the phenomenon of savings during repeated perturbation exposures. These theories can broadly be categorized as appealing to recall, modulation of error sensitivity, and representational redundancy. One theory posits that savings emerges from recall of an action that had successfully countered a previously seen rotation (Huang et al. 2011; Xivry and Lefévre 2015). We have previously argued that repetition of an action during the initial rotation exposure is necessary to recall that action during a subsequent exposure (Huang et al. 2011). A second theory has proposed that savings comes about not through recall but through an increase in the sensitivity of the same error-driven process that is present during initial adaptation (Braun et al. 2009; Kobak and Mehring 2012; Yousif and Diedrichsen 2012; Gonzalez Castro et al. 2014; Herzfeld et al. 2014). These theories also tend to assume that savings increases with initial exposure to a perturbation. A third theory for savings appeals to the notion of redundant representations during adaptation, positing that although subjects may behave similarly at baseline from one day to the next, readaptation is faster during the second exposure because adaptation begins from a different underlying state (Medina et al. 2001; Smith et al. 2006; Kording et al. 2007; Berniker and Kording 2008; Ajemian et al. 2010). With these kinds of theories, prolonged practice is generally thought to be required during the initial exposure to engage slower learning processes that are assumed to underlie such changes in state. Although all of these explanations differ in important ways, they all assume that periods of prolonged prior exposure to a perturbation are necessary to elicit savings.
Recent work has shown that while overall adaptation relies on multiple processes, savings is attributable to a single component of learning (Hadjiosif and Smith 2013; Haith et al. 2015; Huberdeau et al. 2015) and that this component may be driven by explicit processes (Morehead et al. 2013). Adaptation is known to be supported by explicit processes, especially early in learning, which act in parallel with implicit, prediction-error-driven components (Redding and Wallace 2003; Mazzoni and Krakauer 2006; Keisler and Shadmehr 2010; Benson et al. 2011; Taylor and Ivry 2011; Taylor et al. 2014). This explicit process may involve, for instance, choosing to aim in a direction other than towards the target when adapting to a rotation (Taylor et al. 2014). Thus savings may plausibly result from recall of this explicit component of prior learning, rather than modulation of the implicit one. If so, savings may be obtainable following far less prior practice than has typically been thought, assuming that the memory subserving explicit processes can be acquired rapidly.
To gain further clarity on this topic, we sought to empirically determine the minimum amount of initial exposure to a perturbation that is sufficient to obtain savings. To do so, we varied the duration of initial exposure to a visuomotor rotation and tested for savings a day later.
MATERIALS AND METHODS
Subjects.
Eighty right-handed, neurologically healthy subjects participated in this study (18–40 yr old, 49 women), which was approved by the Johns Hopkins School of Medicine Institutional Review Board.
Experimental setup.
Subjects were seated at a glass-surfaced table with their right forearm supported by a splint equipped with air vents allowing near-frictionless planar arm movements. Subjects' arms were obstructed from their own view by a mirror, on which was projected a graphical interface from a downward-facing LCD monitor installed above the mirror (60-Hz refresh rate; LG). A cross-hair cursor presented on the screen represented the position of a subject's index finger, as reported by a Flock of Birds (130 Hz; Ascension, Shelburne, VT) magnetic sensor placed under the finger.
Task.
Subjects were instructed to make rapid “shooting” movements from a home position (a green circle, diameter: 0.7 cm) through a target (blue and grey concentric circles, diameter: 1.0 cm) located 8 cm away (Fig. 1A). After reaching to the target, subjects were instructed to return their hand and cursor to the start position again. The cursor indicating their hand position was not visible during this time, unless it was within 1 cm of the start position. On a specific predefined subset of trials, the cursor's instantaneous position was manipulated by imposing a 30° rotation of the cursor location about the start position in the counterclockwise direction (Fig. 1A). Any perturbation was turned off in the intertrial interval. The target location was fixed for each subject but was randomized across subjects to mitigate any biomechanical biases that may have been present at any individual target location.
Fig. 1.
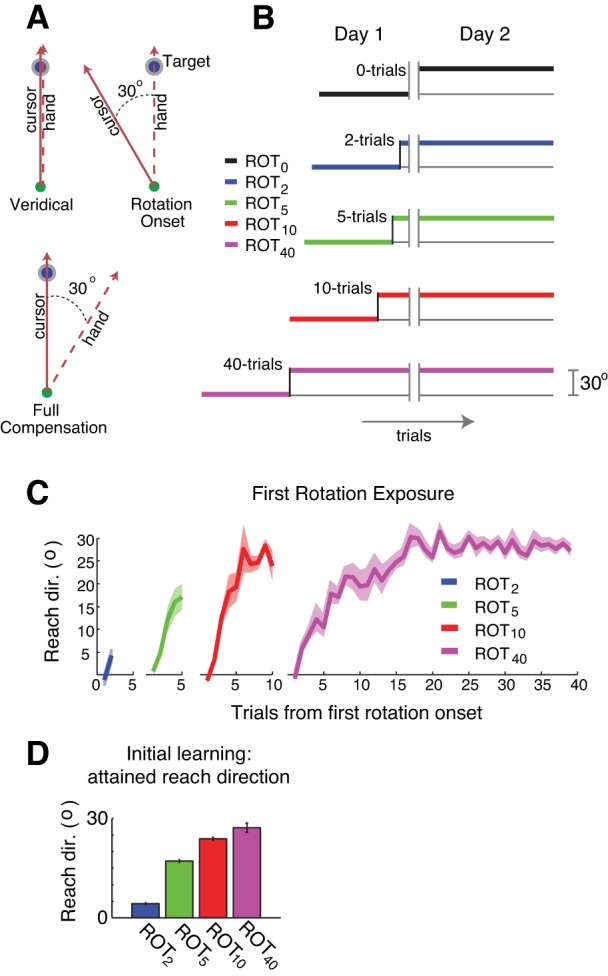
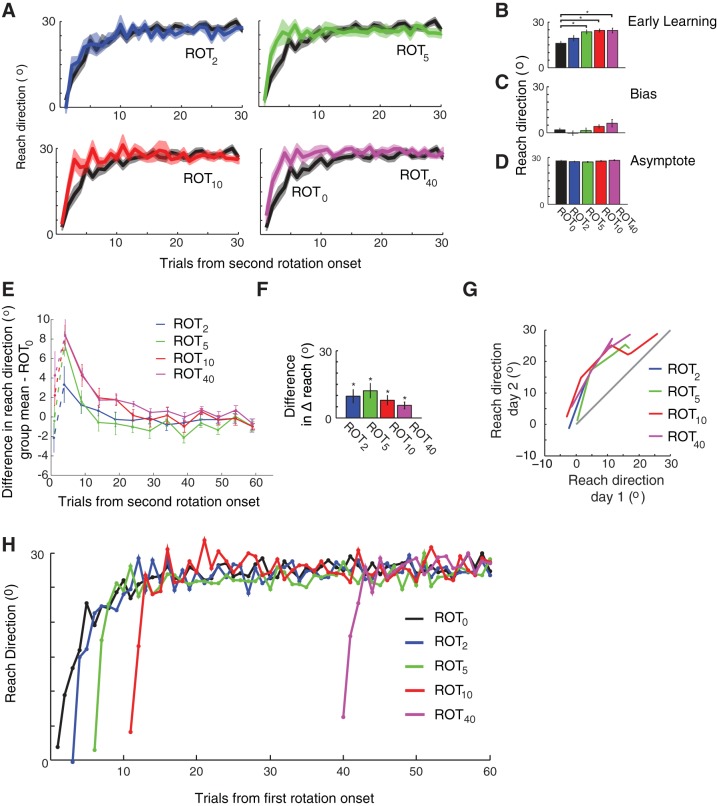
Experimental setup. A: during baseline movements, cursor feedback accurately reflects the position of the subject's hand [veridical, 0° rotation (ROT); see materials and methods for group definitions]. With onset of the 30° rotation, cursor feedback is rotated about the origin (the start position) by 30°. Dashed line: hand path, solid line: cursor path, green circle movement start position, blue circle with gray ring: target. B: perturbation schedule for principal groups and the control group (n = 10 per group). Double gray vertical lines indicate a break across days. C: subject-averaged learning curves from the initial rotation session for the four principal groups. Shaded regions indicate ± SE. Reach direction is abbreviated as “Reach dir.” D: mean attained reach direction for each group at the end of their initial exposure to the rotation. Values represent the mean reach direction across subjects on the last trial of adaptation within each group. Error bars indicate SE.
Fifty subjects were randomly assigned to one of four “principal” groups or a control group (Fig. 1B). The principal groups differed only in the number of trials of the initial rotation: 2, 5, 10, and 40 trials (n = 10 subjects per group). Note that subjects in the 40-trial group actually only received 39 trials of the rotation due to an implementational error; we nevertheless maintain the “40-trial” notation throughout. All subjects in each group made 59 reaching movements under “null” rotation conditions in which the cursor accurately reflected the location of the subjects' index finger. Both the initial and subsequent perturbations were 30° counterclockwise rotations for these principal groups. The training durations of the first rotation were chosen to vary the amount of adaptation achieved across groups during the initial exposure. We refer to these groups as ROT2, ROT5, ROT10, and ROT40, respectively. The control group, ROT0, did not experience a perturbation on the first day and thus served as a baseline against which to establish the existence of savings in the principal groups. ROT0 practiced reaching to the target under null-perturbation conditions (no rotation) on day 1 and then first encountered the rotation on day 2. Ten subjects who had never experienced a rotation were assigned to this group. All subjects in each group returned the next day to complete 65 trials of a 30° counterclockwise rotation.
Three additional groups, ROT15DEG, ROTCOUNTER, and ROT5MIN, were tested to further explore the conditions sufficient to achieve savings. Each group was composed of 10 new, naïve subjects. Group ROT15DEG tested whether the magnitude of the first and second rotations must be the same to bring about savings. For the first rotation, subjects experienced a 15° counterclockwise rotation for 39 trials to match the number of trials experienced by group ROT40. The second rotation was identical to that for the principal groups. A further group, ROTCOUNTER, was tested to determine whether the sign of the first and second rotations must be the same to observe savings. For the first rotation, subjects in this group received a 30° clockwise rotation for 5 trials and were tested for savings the next day by experiencing 65 trials of the opposite rotation, which was the same savings probe as in all the other groups. In the last group, ROT5MIN, we tested whether savings requires an overnight period between exposures or can be achieved with only a short break between sessions on a single day. Subjects in this group received five trials of a 30° counterclockwise rotation and then started session 2 5 min later, which again consisted of the same savings probe as all other groups.
Data analysis.
All data were analyzed offline using Matlab (The Mathworks, Natick, MA). Kinematic data were sampled at 130 Hz. These signals were filtered with a third-order Savitzky-Golay interpolation filter with a half-width of 35 ms. Reach direction was determined by computing the angle at which each movement passed a circle centered on the start position with a radius of 8 cm (the distance to the target). Each subject's reach direction bias, determined by taking the mean reach direction during a 59 trial practice block without a rotation was subtracted from the reach directions measured during the rest of the experiment to mitigate any potential biases due to biomechanical differences across subjects and target locations. Analysis results were qualitatively unchanged if the initial reach direction (the angle at which each movement was launched, measured at 200 ms after movement initiation) was used instead of the angle that the cursor passed the target radius.
There are at least three ways in which prior experience with the rotation can influence behavior in subsequent exposures. First is retention of adapted behavior, expressed as a reach direction bias on the first trial of the second exposure (Joiner and Smith 2008; Criscimagna-Hemminger and Shadmehr 2008). Second, and of primary interest to us, is savings, in the form of a faster relearning rate (Zarahn et al. 2008). Third is an asymptote effect, in which the mean steady-state reach direction after adaptation is closer to the direction that would fully cancel the rotation (Krakauer et al. 2005). We quantified each of these aspects of behavior as follows: The initial bias was defined as the measured reach direction on the first trial of the second rotation. Adaptation rate is reflected in subjects' average amount of learning early in adaptation, which was defined as the mean reach direction on trials 2–6 on day 2 or session 2. This range of trials was chosen a priori as it encompasses the period during which learning progresses most rapidly in prior studies (Huang et al. 2011; Kitago et al. 2013). Alternative trial boundaries for this measure (including the first trial or later trials) did not qualitatively alter the results. Finally, asymptote was defined as the mean reach direction over the last 40 trials of the second rotation.
The control group, ROT0, served as a basis of comparison for bias, savings, and asymptote effects measured in the other groups. Three one-way ANOVAs were conducted with group as the main factor and the relevant measure [i.e., initial bias (trial 1), early learning (mean of trials 2–6), and asymptotic learning (mean of trials 31–65)] as within group factors. In the event that the outcome of a test returned a significant main effect, we planned post hoc t-tests between group ROT0 and each of the other groups to detect which groups were significantly different from naïve, correcting for multiple comparisons using the Tukey-Kramer method. Additionally, a one-way ANOVA was used to test for differences in these three behavioral measures (bias, savings, and asymptote) among the four principal groups.
A single-trial analysis was also used to more closely examine behavior at the very beginning of reexposure to the rotation. This “single-trial learning rate” was defined as the change in reach direction from the first to the second trial of a rotation. The use of a single trial to determine an estimate for learning rate has been employed by others (Marko et al. 2012; Semrau et al. 2012; Gonzalez Castro et al. 2014; Herzfeld et al. 2014). To formally test this difference, a paired t-test was conducted for each group comparing the single-trial learning rate between the first and second rotation exposures within each subject.
Analysis for the three additional groups (ROT15DEG, ROTCOUNTER, and ROT5MIN) was the same as that described for the above groups with respect to quantifying bias, savings, and asymptote. These additional groups were compared against ROT0 with respect to the mean initial adaptation measure, and groups ROTCOUNTER and ROT5MIN were analyzed with respect to the single-trial learning rate. For this latter analysis, we reversed the sign of the reach direction for ROTCOUNTER for day 1 to compare across rotation sessions. The single-trial-learning-rate analysis was not performed for the ROT15DEG group because of the difference in perturbation magnitudes across sessions.
Group sizes of 10 were chosen based on a power analysis conducted using pilot data. Specifically, we used an estimate for the effect size of the initial adaptation measure of 6.5° and an estimated standard deviation of 6°, with a probability of a false negative result of 0.8. This results in an estimated minimum of eight subjects per group.
RESULTS
We sought to determine the minimum perturbation exposure necessary to instill a memory for adaptation that is expressible through savings. Four principal groups were exposed to a 30° counterclockwise rotation for varying numbers of trials on day 1 and were assayed for savings on day 2 by reexposing them to the same perturbation that they had experienced on day 1.
Varying the duration of rotation exposure had the desired effect of creating differing amounts of initial learning across groups (Fig. 1C). On average, ROT2 adapted only 3.8°. ROT5 partially adapted to the rotation (17.0°), ROT10 almost fully adapted but with little or no repetition of actions on asymptote (23.0°). ROT40 repeated their asymptotic behavior (26.8°) for ∼30 trials (Fig. 1D). Thus the predefined groups spanned a wide range of experiences during initial exposure.
Savings was observed even when initial adaptation was brief and incomplete.
Despite differing amounts of adaptation during the first session, groups ROT5, ROT10, and ROT40 all showed savings during the second rotation session, as evidenced by faster adaptation compared with the rotationally naïve group ROT0 (Fig. 2A). As expected, we found a significant difference across the five groups (principal groups and ROT0) according to mean performance during early learning [average reach direction on trials 2–6; ANOVA, F(4,45) = 6.0, P = 0.0006; Fig. 2B]. Post hoc tests comparing early learning in the principle groups to that for the control group ROT0 revealed that each principal group except ROT2 exhibited significant savings (ROT0 vs. ROT2: P = 0.49; each other comparison: P < 0.01). An ANOVA revealed a marginal difference across the four principle groups [ANOVA, F(3,36) = 2.76, P = 0.056], likely driven by ROT2.
Fig. 2.
Relearning data for the principal groups. A: adaptation curves for the second rotation exposure are shown for each principal group with group ROT0 superimposed. B–D: mean performance across groups. B: early learning (day 2, mean of trials 2–6). C: initial bias (day 2, trial 1). D: asymptote performance (day 2, mean of trials 26–65). E: difference in adaptation curves of each principal group from that of ROT0, binned by trials of 5 (except the initial bias, which is connected by a dashed line). F: difference in single-trial learning between day 2 and day 1. G: reach direction on day 2 vs. that on day 1. Grey line represents the unity line where data would be expected to lie if adaptation were unchanged from one day to the next. H: day 2 learning curves aligned by total number of perturbation trials experienced. Curves represent mean across subjects; error bars omitted for clarity. All error bars and shaded regions indicate ± 1 SE across subjects. *P < 0.05.
In addition to changes in the learning rate of adaptation, we examined how other characteristics of performance (i.e., bias and asymptote) in the second session varied with the duration of exposure in the first session. The initial bias exhibited no significant difference across the principal groups [ANOVA, F(4,45) = 1.88, = 0.13; ANOVA excluding ROT0, F(3,36) = 2.08, P = 0.12; Fig. 2C], and there was no difference at asymptote during the second session [ANOVA, F(4,45) = 1.37, P = 0.26; ANOVA excluding ROT0, F(3,36) = 1.80, P = 0.17; Fig. 2D]. We did observe a trend for the initial bias on day 2 to increase with the amount of initial exposure on day 1, in line with previous observations (Joiner and Smith 2008). This effect is unlikely, however, to account for the overall savings we observed. In particular, the initial bias for ROT5 was comparable to that for ROT0 (respectively, 1.5 and 2.0°), yet ROT5 nevertheless still showed clear savings. Therefore, the savings we observed cannot entirely be attributed to a residual bias.
Experiencing a rotation for two trials was sufficient to alter single-trial learning upon reexposure.
Plotting the data from all four principal groups on a single axis enables a more detailed comparison of the precise pattern of savings across groups (Fig. 2E). Specifically, Fig. 2E shows the mean difference in adaptation on day 2 between each principal group and group ROT0. Positive values in Fig. 2E indicate savings. The principal groups all exhibited a similar increase in learning over naïve subjects following the initial trial, apparent as a sharp positive rise in the difference in reach direction above zero (which is consistent with the single trial analysis described below). This accelerated learning was transient for ROT2 and ROT5, lasting just a few trials but was sustained until reaching asymptote for ROT10 and ROT40. These two groups in which savings was sustained were also the two groups in which subjects reached asymptote on day 1. Notably, however, the additional 30 trials on asymptote completed by ROT40 did not lead to stronger savings, compared with ROT10.
The observation that the early savings seen in group ROT2 (in Fig. 2E) was not sustained beyond the first few trials of reexposure prompted us to perform a finer-grained analysis of the differences in readaptation among the groups. Specifically, we examined the amount of learning from the first trial (the single-trial learning between trials 1 and 2). Since each principal group (ROT2, ROT5, ROT10, and ROT40) performed at least two trials on both days, this measure of learning rate yielded a within-subject measure of savings. All four principal groups had a greater single-trial learning rate in the second rotation exposure compared with the first exposure (4 paired t-tests, P < 0.05 for all groups, with Bonferroni correction for multiple comparisons; Fig. 2F). Moreover, the magnitude of this effect was comparable across groups [ANOVA, F(3,36) = 1.08, P = 0.37]. This analysis established that even very limited (as few as 2 trials) prior experience with a perturbation could lead to single-trial performance improvements during reexposure. This effect is clearly illustrated by plotting the reach direction on day 2 as a function of the reach direction on day 1 (Fig. 2G) and noting that all groups follow the same pattern of faster learning on day 2, at least for as many trials as had been experienced initially.
In summary, although all the principal groups exhibited some degree of savings, there was a difference in the pattern of savings across groups. Savings was equally strong at the single-trial level in all groups but was sustained for just a few trials in groups that had very limited initial exposure (i.e., ROT2, and ROT5). Only the groups in which subjects reached or nearly reached asymptote during the first session showed sustained savings (i.e., ROT10 and ROT40).
To determine the nature of the advantage that savings provided, we plotted the adaptation curves for the principle groups aligned according to the total number of rotation trials experienced (Fig. 2H). Viewing the data in this way reveals that varying patterns of savings seen in the data appear to be generated by subjects rapidly reacquiring the reach direction attained at the end of the initial exposure and then adapting at a naïve rate thereafter. Critically, we saw no evidence of performance on day 2 surpassing that of naïve learners who had experienced a comparable number of rotation trials in total. We also did not see a gradual exponential convergence towards the behavior of naïve subjects, as might be expected from a change in sensitivity to error. Instead, subjects appeared to rapidly reacquire the position on the adaptation curve they would have been at if the first exposure had been continued. This view of the data suggests that savings represents a process of rapid retrieval, or recall, rather than a change in the learning rate of the same initial acquisition process.
Savings was sensitive to the direction but not the magnitude of exposure.
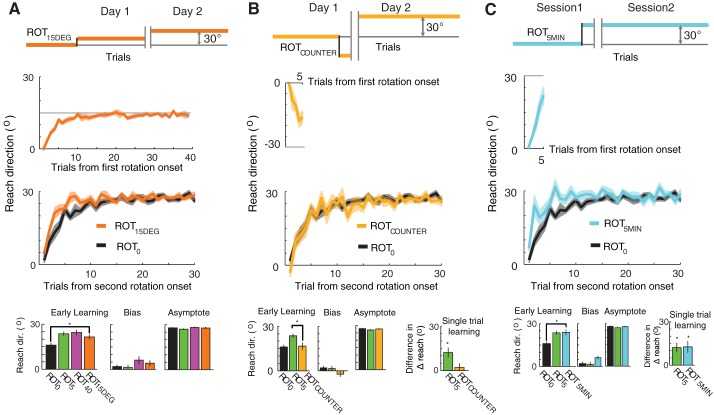
The results from the principal groups show that experiencing only a small number of trials without reaching asymptote is sufficient to elicit savings. To further explore the conditions necessary to bring about savings, we tested three additional groups. ROT15DEG experienced a 15° rotation on day 1 for 39 trials and was assayed for savings with a 30° rotation the next day. The purpose of including this group was to test whether the memory associated with savings is only associated with a specific perturbation or generalizes across perturbation directions (Haruno et al. 2001; Ingram et al. 2011). Savings did occur in this group (Fig. 2H); early learning (mean reach direction measured during trials 2–6) was faster than that for naïve participants (ROT0; t-test, P < 0.01). We compared the behavior of ROT15DEG with that of groups ROT5 and ROT40 because these were the principal groups matched for attained reach direction on day 1 (ROT5) and number of exposure trials on day 1 (ROT40; Fig. 3A). An ANOVA comparing these three groups revealed no difference in savings [ANOVA, F(2,27) = 1.14, P = 0.34]. Bias and asymptote measures also failed to show a difference across these three groups [bias: ANOVA, F(2,27) = 1.46, P = 0.24; asymptote: ANOVA, F(2,27) = 1.56, P = 0.22]. The single-trial learning rate analysis was not applicable for ROT15DEG because the rotations on days 1 and 2 differed in magnitude.
Fig. 3.
Results for additional groups. A, top: perturbation schedule for ROT15DEG. Middle 2 panels: initial adaptation and readaptation. A, bottom: early learning (day 2, mean of trials 2–6), initial bias (day 2, trial 1), and asymptotic performance (day 2, mean of trials 26–65). B and C: same as in A but for groups ROTCOUNTER and ROT5MIN, respectively. Relevant principal groups are included for comparison. All error bars and shaded regions indicate ± 1 SE across subjects. *P < 0.05.
ROTCOUNTER was added to test whether the perturbation needed to be in the same direction during the first and second rotation exposures to observe savings. Subjects in this group experienced a 30° clockwise rotation for five trials on day 1 and were tested on an opposite-direction, counterclockwise rotation on day 2. Comparison of performance during early learning (trials 2–6) with that of group ROT0 revealed no evidence for savings in group ROTCOUNTER (Fig. 3B; t-test, P = 0.84), indicating that the two rotation exposures must be in the same direction in order for the short first rotation exposure to influence the learning rate of the second exposure. Confirming this finding, a comparison of groups ROT5 and ROTCOUNTER, which are matched in number of initial adaptation trials, did show a significant difference according to this measure (t-test, P < 0.05). Finally, an analysis of the single-trial learning rate further supports there being no change in response to the perturbation from the first to the second day of adaptation (Fig. 3B; paired t-test, P = 0.46) in this group. These groups (ROT0, ROTCOUNTER, and ROT5) also failed to show a significant difference in bias and asymptote [bias: ANOVA, F(2,27) = 2.54, P = 0.10; asymptote: ANOVA, F(2,27) = 1.51, P = 0.24].
Savings was insensitive to the passage of time between the first and second exposures.
Finally, we tested whether an overnight break might be necessary to enable savings on reexposure via a possible consolidation mechanism (Krakauer et al. 2005). ROT5MIN experienced five trials of a 30° rotation and were reexposed to the perturbation following a 5-min break. This group exhibited savings compared with the naïve group (Fig. 3C), based on comparison of performance during early learning (trials 2–6; t-test, P < 0.01). This group also showed no detectable difference in early learning from group ROT5 (t-test; P = 0.88), which is the group matched for all conditions except time between initial and final perturbation exposures. The single-trial learning rate was also greater during the second exposure compared with the first (paired t-test, P < 0.05). Comparison of the biases across groups ROT0, ROT5, and ROT5MIN did show marginal significance [bias: ANOVA, F(2,27) = 3.37, P = 0.051; Fig. 3C] likely due to the comparatively short interval between the initial and second adaptation sessions in ROT5MIN, increasing residual bias in this group (Joiner and Smith 2008). Asymptotic performance on either day 2 or session 2 was not significantly different among these comparison groups [ANOVA, F(2,27) = 1.41, P = 0.26]. These results show that savings observed after five trials of exposure does not depend on an overnight consolidation period; comparable savings is evident even after a short 5-min break.
DISCUSSION
Theories regarding the formation of savings in adaptation tasks commonly assume that extended practice is required to instill a memory (Brashers-Krug et al. 1996; Smith et al. 2006; Braun et al. 2009; Huang et al. 2011; Joiner and Smith 2008). Knowing the lower bound on the duration of initial experience with a rotation required to obtain savings could provide important insight into the nature of the memory formed. We therefore sought to determine the minimum amount of exposure to a rotation that is sufficient to form a long-term memory for adaptation by varying the number of trials of initial exposure across four groups of subjects and assaying for savings a day later.
Notably, savings was present even after only two trials of initial exposure to a rotation. The specific pattern of savings differed, however, across groups: they all showed a similar benefit of prior experience according to the amount they learned from the first trial of reexposure, but this advantage over naïve learners was only sustained in groups that had initially reached asymptote during their first exposure. These differing patterns are most starkly illustrated by group ROT2. Depending on the analysis used, we could either conclude that ROT2 showed strong savings (based on single-trial learning rate) or no savings (based on mean reach direction during early learning) because this group rapidly jumped to the position on the adaptation curve it had previously acquired but then adapted as if naïve thereafter.
Further experiments revealed that the duration of the break between the first and second rotation exposures had little bearing on whether or not savings would be observed; participants exhibited the same amount of savings whether that break was overnight or only 5 min. Additionally, in order for savings to be observed, the direction of the rotation had to be consistent across exposures, but the magnitude could differ.
Savings as recall.
How can a long-term memory for adaptation be established if not via gradual processes requiring practice at asymptote? Recent evidence showing that explicit processes contribute to initial adaptation (Taylor et al. 2014; Redding and Wallace 2003; Benson et al. 2011; Fernandez-Ruiz et al. 2011) may provide a possible explanation. Specifically, since an explicit aiming component is present early in adaptation (Taylor et al. 2014), subjects might form a memory for this aiming strategy early during the initial exposure and recall it once they have identified that the rotation is present when tested again later.
Alternatively, subjects may form a memory for action (Huang et al. 2011; Xivry and Lefévre 2015), as opposed to a memory for an aiming direction (Taylor et al. 2014), or a memory for the perturbation (Herzfeld et al. 2014). We have previously suggested that this may occur through an implicit reinforcement learning mechanism that is established through experience (Huang et al. 2011). Specifically, repetition of a successful action on asymptote might be necessary to reinforce and remember it. Our new data suggest, instead, that subjects remember something about their prior rotation exposure even in the absence of such repetition on asymptote. That said, it is still possible that other latent mechanisms may be active in parallel with a recall mechanism when experimental conditions promote them, and thus either phenomenon or both may be active depending on the experimental conditions.
Potential mechanism supporting savings as recall.
Why would one remember an action or aiming direction that was ultimately unsuccessful (i.e., led to a target miss), as is often the case given the bias toward baseline often exhibited at asymptote (Kitago et al. 2013; van der Kooij et al. 2015; Vaswani et al. 2015)? It is plausible that a memory for action could be formed because of strong positive reward prediction errors experienced by subjects during the initial course of adaptation. However, as was just mentioned, performance is typically worse under a perturbation than during baseline. Thus, nominally, the reward prediction errors during adaptation would be negative (i.e., reward is less than expected), because performance is worse under the perturbation compared with at baseline. Whether subjects interpret a given action as an improvement (a positive reward prediction error) or continued failure (a negative reward prediction error) may depend on whether they detect that a change point had occurred in the experiment following the rotation onset (Wilson et al. 2013). If the imposed rotation is interpreted as a change, actions and/or strategies that reduce the initially large errors experienced after the onset of the perturbation may be associated with a positive reward prediction error and thus may be remembered.
Recall as a general mechanism of meta-learning in adaptation paradigms.
Other studies have also observed behavior that is consistent with the idea of recall as a mechanism for savings. For instance, two recent experiments have shown that if experience with a particular perturbation (e.g., a force field perturbation or a visuomotor rotation) is followed by a single episode of a novel perturbation, the first few actions under the new perturbation are directed in accordance with cancelling the previously experienced perturbation (Morehead et al. 2013; Gonzalez Castro et al. 2014). These findings are consistent with the idea that such actions were stored in memory and retrieved (albeit inappropriately) when another perturbation was experienced. Retrieval of actions previously used to counter a perturbation can even be triggered by withholding an expected visual reward (Pekny et al. 2011), suggesting that reward prediction error, rather than reexperiencing the same or a similar perturbation, may be the key trigger for retrieval.
A recent study suggested that savings may not be due to recall of prior actions but rather might be due to an underlying sensory-error-driven learning process increasing its sensitivity to previously experienced errors (Herzfeld et al. 2014). The authors of that study showed that experiencing a particular error at one time leads to a durable change in response to the same or similar errors in the future. These findings can alternatively be interpreted under a recall hypothesis if we posit that errors of a specific magnitude can augment reward prediction error to act as a cue for retrieval of an existing memory.
The sensitivity-to-error model proposed by Herzfeld et al. (2014) cannot account for all of our results, however. In particular, it predicts that adaptation rate upon reexposure to a given perturbation will steadily increase as the duration of the initial exposure to the perturbation increases, even after one reaches asymptote. In our data, however, the duration of initial exposure had little effect on the overall magnitude of savings. In particular, having just reached asymptote (as in ROT10) is sufficient to exhibit nearly identical savings behavior as having experienced nearly 30 trials on asymptote (as in ROT40). Similarly, only reaching halfway to asymptote (as in ROT5) produces nearly the same amount of savings as having reached asymptote (ROT10 & ROT40), as subjects rapidly reacquire the state of adaptation they had previously attained. Furthermore, the pattern of learning in all of the conditions we tested was different from that expected by a modulation of rate: we saw uniform rapid retrieval of the position previously attained during adaptation, rather than an increase in learning rate. We therefore suggest that savings is, in general, driven by recall of prior behavior rather than modulation of learning rate but that this recall process can potentially be cued by the observation of a specific error.
A further piece of evidence in support of the recall hypothesis is that Parkinson's disease (PD) patients are typically unimpaired in initial adaptation to a perturbation (Stern et al. 1988; Gutierrez-Garralda et al. 2013; Leow et al. 2013; Mongeon et al. 2013) but show impaired savings during readaptation (Marinelli et al. 2009; Bédard and Sanes 2011; Leow et al. 2013). This dissociation suggests that initial adaptation and savings depend on different processes. Models that posit that savings is due to upregulation of the rate of adaptation processes would need to explain how initial adaptation would be unaffected in PD but modulation of adaptation rate would be impaired. Alternatively, the model proposed here, in which savings is attributable to a separate recall mechanism, is entirely consistent with observed memory impairments in PD, in which deficits in the acquisition and/or retrieval of cognitive information have been observed (Chiaravalloti et al. 2014; Costa et al. 2014).
Conclusions and implications for motor skill learning.
Here we have suggested that savings in adaptation can be entirely accounted for by rapid retrieval of a component of learning that is acquired within a few trials. This component may be subserved by explicit memory, suggesting that savings actually reflects formation of declarative memory (Eichenbaum 2000), rather than formation of a motor memory. In particular, it has been shown that savings is absent when reaction time is constrained to be low, a condition that likely omits explicit or strategic components to adaptation (Haith et al. 2015). It is also thought that long-term motor learning requires extended practice over days and weeks to acquire (Shmuelof et al. 2012; Karni et al. 1998; Reis et al. 2009; Sampaio-Baptista et al. 2014) in contrast to our findings here. What, then, does adaptation serve as a model for: motor skill, or some other form of memory that is possibly declarative? The idea that initial acquisition and savings both have an explicit component is congruent with recent theories that contend that cognition and explicit knowledge are factors critical to learning and performing any motor task (Stanley and Krakauer 2013; Manley et al. 2014). Adaptation may therefore serve as a suitable model for how cognition and knowledge together may play a role in the formation of long-term motor memories.
The link between explicit processes and motor memory might be that long-term motor memory takes the form of a persistent explicit memory. Alternatively, long-term motor memory might be mediated by an implicit or procedural memory for a component of learning that was initially explicit or declarative. This process of transition from one type of memory to another has been suggested as a general mechanism for skill acquisition (Fitts 1964; Anderson 1982). If visuomotor adaptation serves as an example of the initial, explicit stage of this process, one task that may serve as a model for this process following more practice is adjustment of grip and load forces for lifting objects of unusual densities. This task is ostensibly similar to visuomotor adaptation, given that both show signs of long-term memory formation following only brief periods of initial practice (Gordon et al. 1993; Flanagan and Beltzner 2000; Flanagan et al. 2008). However, visuomotor adaptation is known to be subserved by both explicit and implicit processes (Mazzoni and Krakauer 2006; Taylor et al. 2014), while adjustment of grip and load forces during lifting seems to be largely implicit, evidenced by the fact that the size-weight illusion persists after appropriate motor adjustments have been made (Flanagan and Beltzner 2000). These tasks also differ in their dependence on the cerebellum; patients with cerebellar degeneration are impaired in adapting to visuomotor rotations (Martin et al. 1996; Maschke et al. 2004; Smith and Shadmehr 2005; Chen et al. 2006; Tseng et al. 2007; Rabe et al. 2009b) but show no apparent deficit in adjusting grip and load forces to objects of unusual densities (Rabe et al. 2009a).
We suggest that the core difference between visuomotor adaptation and grip/load force adjustments is in the duration of prior practice, given that subjects have a lifetime of experience lifting objects whose weight is difficult to predict, but generally do not have much prior experience with unusual visual manipulations like a cursor rotation. It might be that with prolonged experience adapting to rotations, adjusting to novel visuomotor mappings would no longer rely on explicit or cerebellar-mediated adaptation processes and thus would begin to more closely resemble adjusting grip and load forces for novel size and weight combinations. This prediction has yet to be tested.
GRANTS
This work was supported by funds from the Johns Hopkins University Science of Learning Institute and by National Science Foundation Grant 1358756.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.M.H., A.M.H., and J.W.K. conception and design of research; D.M.H. performed experiments; D.M.H. analyzed data; D.M.H., A.M.H., and J.W.K. interpreted results of experiments; D.M.H. prepared figures; D.M.H. drafted manuscript; D.M.H., A.M.H., and J.W.K. edited and revised manuscript; D.M.H., A.M.H., and J.W.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Aaron Wong, Jing Xu, and Amy Bastian for helpful comments on the manuscript.
REFERENCES
- Ajemian R, D'Ausilio A, Moorman H, Bizzi E. Why professional athletes need a prolonged period of warm-up and other peculiarities of human motor learning. J Mot Behav 42: 381–388, 2010. [DOI] [PubMed] [Google Scholar]
- Anderson J. Acquisition of cognitive skill. Psychol Rev 89: 369–406, 1982. [Google Scholar]
- Bédard P, Sanes JN. Basal ganglia-dependent processes in recalling learned visual-motor adaptations. Exp Brain Res 209: 385–393, 2011. [DOI] [PubMed] [Google Scholar]
- Benson BL, Anguera JA, Seidler RD. A spatial explicit strategy reduces error but interferes with sensorimotor adaptation. J Neurophysiol 105: 2843–2851, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berniker M, Kording K. Estimating the sources of motor errors for adaptation and generalization. Nat Neurosci 11: 1454–1461, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature 382: 252–255, 1996. [DOI] [PubMed] [Google Scholar]
- Braun DA, Aertsen A, Wolpert DM, Mehring C. Motor task variation induces structural learning. Curr Biol 19: 352–357, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hua SE, Smith MA, Lenz FA, Shadmehr R. Effects of human cerebellar thalamus disruption on adaptive control of reaching. Cereb Cortex 16: 1462–1473, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaravalloti ND, Ibarretxe-Bilbao N, DeLuca J, Rusu O, Pena J, García-Gorostiaga I, Ojeda N. The source of the memory impairment in Parkinson's disease: acquisition versus retrieval. Mov Disord 29: 765–771, 2014. [DOI] [PubMed] [Google Scholar]
- Costa A, Monaco M, Zabberoni S, Peppe A, Perri R, Fadda L, Iannarelli F, Caltagirone C, Carlesimo GA. Free and cued recall memory in parkinson's disease associated with amnestic mild cognitive impairment. PLoS One 9: e86233, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Shadmehr R. Consolidation patterns of human motor memory. J Neurosci 28: 9610–9618, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci 1: 41–50, 2000. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Wong W, Armstrong IT, Flanagan JR. Relation between reaction time and reach errors during visuomotor adaptation. Behav Brain Res 219: 8–14, 2011. [DOI] [PubMed] [Google Scholar]
- Fitts PM. Perceptual-motor skill learning. In: Categories of Human Learning, edited by Melton AW. New York: Academic Press, 1964, p. 244–283. [Google Scholar]
- Flanagan JR, Beltzner MA. Independence of perceptual and sensorimotor predictions in the size-weight illusion. Nat Neurosci 3: 737–741, 2000. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Bittner JP, Johansson RS. Experience can change distinct size-weight priors engaged in lifting objects and judging their weights. Curr Biol 18: 1742–1747, 2008. [DOI] [PubMed] [Google Scholar]
- Gonzalez Castro LN, Hadjiosif AM, Hemphill MA, Smith MA. Environmental consistency determines the rate of motor adaptation. Curr Biol 24: 1050–1061, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Westling G, Cole KJ, Johansson RS. Memory representations underlying motor commands used during manipulation of common and novel objects. J Neurophysiol 69: 1789–1796, 1993. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Garralda JM, Moreno-Briseno P, Boll MC, Morgado-Valle C, Campos-Romo A, Diaz R, Fernandez-Ruiz J. The effect of Parkinson's disease and Huntington's disease on human visuomotor learning. Eur J Neurosci 38: 2933–2940, 2013. [DOI] [PubMed] [Google Scholar]
- Hadjiosif A, Smith MA. Savings is restricted to the temporally labile component of motor adaptation. Proc Transl Comput Mot Control (San Diego, CA), 2013. [Google Scholar]
- Haith AM, Huberdeau DM, Krakauer JW. The influence of movement preparation time on the expression of visuomotor learning and savings. J Neurosci 35: 5109–5117, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M, Wolpert DM, Kawato M. Mosaic model for sensorimotor learning and control. Neural Comput 13: 2201–2220, 2001. [DOI] [PubMed] [Google Scholar]
- Herzfeld DJ, Vaswani PA, Marko MK, Shadmehr R. A memory of errors in sensorimotor learning. Science 345: 1349–1353, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron 70: 787–801, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberdeau DM, Krakauer JW, Haith AM. Dual-process decomposition in human sensorimotor adaptation. Curr Opin Neurobiol 33: 71–77, 2015. [DOI] [PubMed] [Google Scholar]
- Ingram JN, Howard IS, Flanagan JR, Wolpert DM. A single-rate context-dependent learning process underlies rapid adaptation to familiar object dynamics. PLoS Comput Biol 7: e1002196, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WM, Smith MA. Long-term retention explained by a model of short-term learning in the adaptive control of reaching. J Neurophysiol 100: 2948–2955, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA 95: 861–868, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keisler A, Shadmehr R. A shared resource between declarative memory and motor memory. J Neurosci 30: 14817–14823, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitago T, Ryan SL, Mazzoni P, Krakauer JW, Haith AM. Unlearning versus savings in visuomotor adaptation: comparing effects of washout, passage of time, and removal of errors on motor memory. Front Hum Neurosci 7: 307, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobak D, Mehring C. Adaptation paths to novel motor tasks are shaped by prior structure learning. J Neurosci 32: 9898–9908, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Iwamoto Y, Yoshida K. Memory of learning facilitates saccadic adaptation in the monkey. J Neurosci 24: 7531–7539, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kording KP, Tenenbaum JB, Shadmehr R. The dynamics of memory as a consequence of optimal adaptation to a changing body. Nat Neurosci 10: 779–786, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghez C, Ghilardi MF. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci 25: 473–478, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi MF, Ghez C. Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci 2: 1026–1031, 1999. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20: 8916–8924, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JR, Lobovits D. Adaptation to displaced vision: evidence for prolonged after-effects. Q J Exp Psychol 29: 65–69, 1977. [DOI] [PubMed] [Google Scholar]
- Leow LA, de Rugy A, Loftus AM, Hammond G. Different mechanisms contributing to savings and anterograde interference are impaired in Parkinson's disease. Front Hum Neurosci 7: 55, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley H, Dayan P, Diedrichsen J. When money is not enough: awareness, success, and variability in motor learning. PloS One 9: e86580, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli L, Crupi D, Di Rocco A, Bove M, Eidelberg D, Abbruzzese G, Ghilardi MF. Learning and consolidation of visuo-motor adaptation in Parkinson's disease. Parkinsonism Relat Disord 15: 6–11, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko MK, Haith AM, Harran MD, Shadmehr R. Sensitivity to prediction error in reach adaptation. J Neurophysiol 108: 1752–1763, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. Brain 119: 1199–1211, 1996. [DOI] [PubMed] [Google Scholar]
- Maschke M, Gomez CM, Ebner TJ, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol 91: 230–238, 2004. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26: 3642–3645, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Mauk MD. A mechanism for savings in the Cerebellum. J Neurosci 21: 4081–4089, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongeon D, Blanchet P, Messier J. Impact of Parkinson's disease and dopaminergic medication on adaptation to explicit and implicit visuomotor perturbations. Brain Cogn 81: 271–282, 2013. [DOI] [PubMed] [Google Scholar]
- Morehead JR, Crossley MJ, Ivry RB. Savings upon re-aiming in visuomotor adaptation. Proc Transl Comput Mot Control (San Diego, CA), 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny SE, Criscimagna-Hemminger SE, Shadmehr R. Protection and expression of human motor memories. J Neurosci 31: 13829–13839, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe K, Brandauer B, Li Y, Gizewski ER, Timmann D, Hermsdörfer J. Size-weight illusion, anticipation, and adaptation of fingertip forces in patients with cerebellar degeneration. J Neurophysiol 101: 569–579, 2009a. [DOI] [PubMed] [Google Scholar]
- Rabe K, Livne O, Gizewski ER, Aurich V, Beck A, Timmann D, Donchin O. Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol 101: 1961–1971, 2009b. [DOI] [PubMed] [Google Scholar]
- Redding GM, Wallace B. First-trial adaptation to prism exposure. J Mot Behav 35: 229–245, 2003. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA 106: 1590–1595, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Baptista C, Scholz J, Jenkinson M, Thomas AG, Filippini N, Smit G, Douaud G, Johansen-Berg H. Gray matter volume is associated with rate of subsequent skill learning after a long term training intervention. Neuroimage 96: 158–166, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semrau JA, Daitch AL, Thoroughman KA. Environmental experience within and across testing days determines the strength of human visuomotor adaptation. Exp Brain Res 216: 409–418, 2012. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuelof L, Krakauer JW, Mazzoni P. How is a motor skill learned? Change and invariance at the levels of task success and trajectory control. J Neurophysiol 108: 578–594, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol 4: e179, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in huntington's disease but not cerebellar degeneration. J Neurophysiol 93: 2809–2821, 2005. [DOI] [PubMed] [Google Scholar]
- Stanley J, Krakauer JW. Motor skill depends on knowledge of facts. Front Hum Neurosci 7: 503, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Mayeux R, Hermann A, Rosen J. Prism adaptation in Parkinson's disease. J Neurol Neurosurg Psychiatry 51: 1584–1587, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Ivry RB. Flexible cognitive strategies during motor learning. PLoS Comput Biol 7: e1001096, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Krakauer JW, Ivry RB. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J Neurosci 34: 3023–3032, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng Y, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007. [DOI] [PubMed] [Google Scholar]
- Van Beers RJ. Motor learning is optimally tuned to the properties of motor noise. Neuron 63: 406–417, 2009. [DOI] [PubMed] [Google Scholar]
- Van der Kooij K, Brenner E, van Beers RJ, Smeets JB. Visuomotor adaptation: how forgetting keeps us conservative. PLoS One 10: e0117901, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaswani PA, Shmuelof L, Haith AM, Delnicki RJ, Huang VS, Mazzoni P, Shadmehr R, Krakauer JW. Persistent residual errors in motor adaptation tasks: reversion and exploratory escape. J Neurosci 35: 6969–6977, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalta JI, Landi SM, Flo A, Della-Maggiore V. Extinction interferes with the retrieval of visuomotor memories through a mechanism involving the sensorimotor cortex. Cereb Cortex 25: 1535–1543, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Nassar MR, Gold JI. A mixture of delta-rules approximation to Bayesian inference in change-point problems. PLoS Comput Biol 9: e1003150, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xivry de JJ, Lefévre P. Formation of model-free motor memories during motor adaptation depends on perturbation schedule. J Neurophysiol 113: 2733–2741, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif N, Diedrichsen J. Structural learning in feedforward and feedback control. J Neurophysiol 108: 2373–2382, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarahn E, Weston GD, Liang J, Mazzoni P, Krakauer JW. Explaining savings for visuomotor adaptation: linear time-invariant state-space models are not sufficient. J Neurophysiol 100: 2537–2548, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]