Abstract
AIM: To clarify the efficacy and safety of three-dimensional conformal radiotherapy (3-D CRT) for this disease and to specify patient subgroups suitable for this treatment.
METHODS: Fifty-two patients with HCC received PVI-targeted radiation therapy from January 1995 through December 2003. Portal venous invasion (PVI) was found in the second or lower order branches of the portal vein in 6 patients, in the first branch in 24 patients and in the main trunk in 22 patients. Child classifications of liver function before radiation therapy were A, B, and C for 19, 24 and 2 patients, respectively. All patients received three-dimensional conformal radiotherapy with a total dose ranging from 39 to 60 Gy (57.0 Gy in average).
RESULTS: Overall survival rates at 1, 2, 3, 4, and 5 years were 45.1%, 25.3%, 15.2%, 10.1%, and 5.1%, respectively. Univariate analysis revealed that Child status, the number of tumor foci, tumor type, transcatheter arterial embolization (TAE) after radiation therapy were statistically significant prognostic factors. Multivariate analysis showed that the number of tumor foci and TAE after radiation therapy were statistically significant.
CONCLUSION: The results of this study strongly suggest the efficacy of 3-D CRT as treatment for PVI in HCC. 3-D CRT is recommended in combination with post-radiation TAE for PVI of HCC with 5 tumor foci or less in the liver and with Child A liver function.
Keywords: Hepatocellular carcinoma, Portal venous invasion, Radiation therapy
INTRODUCTION
Patients with primary hepatocellular carcinoma (HCC) often develop portal venous invasion (PVI)[1-6]. PVI is associated with a high probability of extensive tumor spread and an elevation of portal vein pressure, which subsequently may cause esophageal varices and liver dysfunction. Transcatheter arterial embolization (TAE) which is performed frequently for advanced HCC is not indicated when portal blood flow decreases due to PVI. It is, therefore, associated with a poor prognosis[2-3]. No treatment strategy for PVI has been established, and the median survival has been reported to range only from 5 to 11 mo[7-12]. Notably, the 1-year survival rate is less than 50% and there are only a few 3-year survivors of PVI affecting the first branch and/or the main trunk of the portal vein[3,13].
Few articles on the radiation therapy have been reported for the disease. The aim of the study is to clarify the efficacy and safety of three-dimensional conformal radiotherapy (3-D CRT) for PVI from HCC and to specify patient subgroups who are best benefited by this treatment strategy.
MATERIALS AND METHODS
Fifty-two patients with HCC received PVI-targeted radiation therapy from January 1995 through December 2003. The aim of the treatment was to prevent and/or improve liver dysfunction caused by PVI and re-actualize transcatheter arterial embolization (TAE) for intrahepatic tumors as well as to control PVI itself. All patients but one were male. A diagnosis of HCC was made using ultrasonography (US), computed tomography (CT), angiography, and liver biopsy, as previously reported. Histopathological diagnosis was confirmed in all the patients. Criteria of diagnostic imaging of PVT is a low-attenuation intraluminal mass that expanded the portal vein on enhanced CT or conventional US[14-16] and/or the detection of pulsatile flow in portal vein thrombi by Doppler US[17].
The observation period ranged between 17.4 mo and 123.6 mo (average, 60.4 mo; median, 58.4 mo). These patients ranged in age from 43 years to 82 years (average, 63.1 years; median, 64.0 years) when radiation therapy was started. Eight patients were hepatitis B virus (HBV) related, 40 patients were hepatitis C virus (HCV) related, and 45 had pathologically proven liver cirrhosis. Child classifications of liver function before radiation therapy were A, B, and C for 19, 24 and 2 patients, respectively. Values of the Karnofsky index ranged from 70 to 100 (average, 90.8; median 90). PVI was found in the second or lower order branches of the portal vein in 6 patients, in the first branch in 24 patients and in the main trunk in 22 patients. HCCs were classified as nodular, massive, and diffuse, according to the criteria of the Liver Cancer Study Group of Japan[18]. Thirty-six patients had nodular, 9 had massive, and 6 had diffuse HCCs. The size of the intrahepatic tumors varied patient to patient and no intrahepatic tumor was detected in one patient as summarized in Table 1. Thirteen patients had a single focus for the intrahepatic tumor, 7 had 2 to 5 foci, and 31 patients had 6 or more.
Table 1.
List of size of intrahepatic tumor
| Size of intrahepatic tumor (cm) | No. of patients |
| No HCC | 1 |
| 1-1.99 | 2 |
| 2-2.99 | 21 |
| 3-3.99 | 11 |
| 4-4.99 | 7 |
| 5-5.99 | 3 |
| 6-6.99 | 2 |
| ≥7 | 5 |
Regarding treatment for HCC before the detection of PVI, 31 patients had undergone percutaneous tumor ablation (PTA) such as percutaneous ethanol injection (PEIT) and percutaneous microwave coagulation therapy (PMCT), 40 patients had undergone TAE, and 22 patients had both procedures. Tumors were surgically resected in 7 patients.
All patients received 3-D CRT with a multi-leaf collimator (MLC) using 6 MV X-ray. Figure 1 is an example of dose distributions in one patient. Our goal to give a total dose of 60 Gy to each patient was achieved in 38 cases (mean total dose, 57.0 Gy, range: 39-60 Gy). A daily dose of 2 Gy was delivered 5 times a wk. Clinical tumor volume (CTV) was the tumor itself which was depicted on CT and/or US. Planning target volume (PTV) was defined as 1.5 to 2.0 cm beyond the enhanced lesion on CT scan. Radiation was performed while the patient was breathing shallowly without the use of breath control or respiratory gating. Initial effects of radiation therapy on PVI was evaluated 8 to 12 wk after the completion of radiation and expressed according to the WHO criteria[19].
Figure 1.
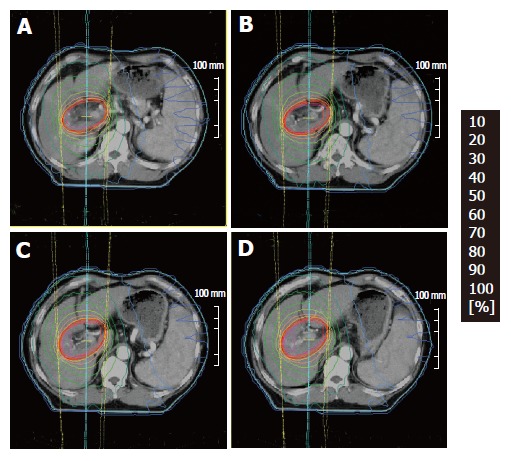
An example of dose distributions (A, B, C, D) in 3D-CRT.
After the completion of radiation therapy for PVI, 10 underwent PTA, 25 underwent TAE, and 8 patients had both procedures. No surgical operation was performed following radiotherapy for PVI.
The survival period was measured from first day of irradiation and the Kaplan-Meier technique was used to calculate the survival rate. The differences in survival rates were analyzed using the log rank test. Uni and multivariate analyses were performed by Cox’s proportional hazard model. Statistical analyses were considered significant if the P-value was 0.05 or less.
Written informed consent was obtained in all the patients in this study.
RESULTS
The initial effects of radiation therapy on PVI were complete regression (CR) in 2 patients, partial regression (PR) in 24 patients, stable disease (SD) in 18 patients and progressive disease (PD) in 8 patients. Figure 2 shows angiographs of the portal vein before (A) and after irradiation (B). Defect of the portal blood flow which was marked with arrows (A) disappeared after the treatment (B). Intrahepatic tumors increased in 31 patients (59.6%).
Figure 2.
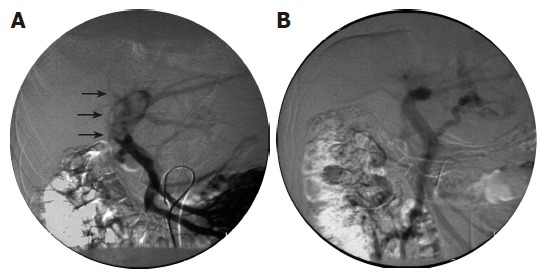
Angiographs of the portal vein showing disappearance of a tumoral embolus after irradiation therapy. Angiographs taken before and after treatment are shown to the left (A) and right (B), respectively.
Overall survival rates at 1, 2, 3, 4, and 5 years were 45.1%, 25.3%, 15.2%, 10.1%, and 5.1%, respectively (Figure 3). Neither age, nor Karnofsky performance index was related to the survival rate. Survival time greatly depended on the Child status as shown in Figure 4A (P = 0.0007). Diffuse type HCC showed a significant lower survival rate compared with nodular or massive type HCC (P = 0.0389). We also noted a higher survival rate in cases with 5 foci or less in the liver (P = 0.01). The spread of PVI (invasion of the main trunk, first branch, and second or lower order branches) was not relevant to the survival rate at all (P = 0.7679) (Figure 4B).
Figure 3.
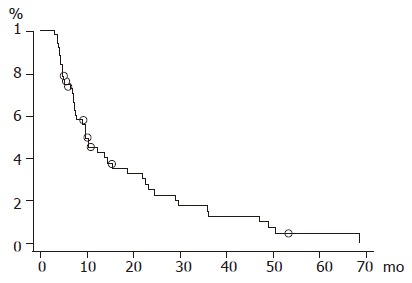
Overall survival curve.
Figure 4.
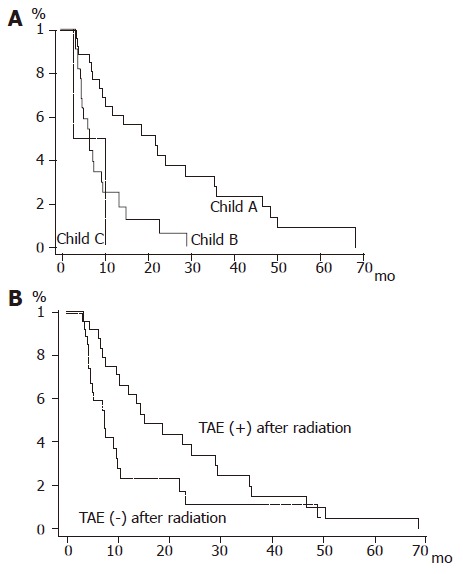
A: Survival curves according to Child classification. Solid line: Child A; bold line: Child B; dashed line: Child C; B: Survival curves for the patient group with and without post-radiation TAE. Solid line: post-radiation TAE (+); dashed line: post-radiation TAE (–).
Treatment preceding irradiation, namely, surgical resection, PTA and TAE, was irrelevant to the prognosis as well. We noted a significant longer survival periods in cases with favorable primary effects following radiation therapy (P = 0.0419). Regarding the effect of treatments given after radiation therapy, PTA had no influence on the survival rate (P = 0.3987). On the other hand, the survival rate was higher, albeit marginally, in the TAE-treated group than in the TAE-untreated group (P = 0.0465).
Univariate analysis by the Cox proportional hazard model revealed that Child status, the number of tumor foci, tumor type, TAE after radiation therapy were statistically significant prognostic factors (Table 2). Multivariate analysis by Cox’s proportional hazard model using five variables, namely, Child status, the number of tumor foci, tumor type, TAE after radiation therapy, initial effect showed that the number of tumor foci in the liver (P = 0.0285) were the most important factors contributing to the eligible prognosis of patients with HCC invading the portal vein, followed by TAE after radiation therapy (P = 0.0364) (Table 3).
Table 2.
Univariate analysis
| Hazard ratio | 95%CI | P value | |
| Child classification (A) | 0.217 | 0.048-0.978 | 0.0015 |
| No. of HCC (≤5) | 0.411 | 0.204-0.828 | 0.0129 |
| Tumor type (diffuse) | 2.897 | 1.147-7.313 | 0.0244 |
| No TAE after RT | 2.406 | 1.001-5.786 | 0.0498 |
| Initial effect (CR) | 0.194 | 0.043-0.878 | 0.0625 |
| No surgery before RT | 0.6 | 0.247-1.456 | 0.2589 |
| No PTA before RT | 1.373 | 0.741-2.544 | 0.3136 |
| No TAE before RT | 0.718 | 0.358-1.438 | 0.3494 |
| No PTA after RT | 1.372 | 0.654-2.880 | 0.4026 |
| KPS (<90) | 1.252 | 0.483-0.262 | 0.6457 |
| age | 1.008 | 0.971-1.046 | 0.681 |
| Main trunk invasion | 1.049 | 0.573-1.920 | 0.8772 |
CR: complete regression; PR: partial regression; SD: stable disease; PD: progressive disease; TAE: transcatheter arterial embolization; RT: radiation therapy; HCC: hepatocellular carcinoma; PTA: percutaneous tumor ablation; CI: confidence interval.
Table 3.
Multivariate analysis
| Hazard ratio | 95%CI | P value | |
| No. of HCC (≤5) | 0.414 | 0.188-0.912 | 0.0285 |
| No TAE after RT | 3.206 | 1.463-7.024 | 0.0364 |
| Child classification A | 0.729 | 0.111-4.667 | 0.0864 |
| Initial effect (CR) | 0.314 | 0.059-1.664 | 0.5783 |
| Tumor type (diffuse) | 1.309 | 0.190-9.011 | 0.7019 |
CR: complete regression; PR: partial regression; SD: stable disease; PD: progressive disease; TAE: transcatheter arterial embolization; RT: radiation therapy; HCC: hepatocellular carcinoma; CI: confidence interval.
Hepatic function, expressed using the Child classification, remained unaltered in 34 cases after radiation therapy. In the remaining 18 patients the classification changed from Child A to B in 13 cases, from Child B to C in two cases, and from Child B to A in three cases. A patient whose liver function deteriorated from Child B to C lapsed into a hepatic coma after radiation therapy. No other serious side effects of irradiation were observed.
DISCUSSION
PVI has been considered to be an important contributor to a poor prognosis in primary HCC, and no definitive treatment strategy has been established to it. The results of the present study demonstrated a relatively favorable survival following radiation therapy for PVI. The 3-year survival rate of 15.2 % among the present study patients is excellent in comparison with survival rates reported previously[8-9]. In contrast to the surgical series[3,13], in the present study, 3-year survival did not depend on whether PVI invaded the first branch and/or the main trunk of the portal vein. The authors consider, therefore, that radiation therapy seems to be superior to surgical intervention for the patients who developed PVI in the proximal portal vein.
Survival rates did not differ significantly among groups of patients who received one of three different treatments prior to radiation therapy, namely surgical resection, PTA and TAE. On the other hand, a significantly favorable outcome of TAE after radiation therapy was found. The TAE was performed preferentially in patients whose PVI was controlled sufficiently and whose liver function was kept relatively normal; thus, this bias in selecting patients for TAE was probably the main reason for the favorable outcome. However, 6 of 7 patients who survived for more than 2 years had undergone TAE after radiation therapy. Therefore, it appears beneficial to perform TAE in cases in which PVI is under sufficient control.
Radiation therapy for hepatic carcinoma is not performed widely, and reports of such treatment are infrequent[20-23]. Reports of radiotherapy for PVI from HCC is even rare[24-26] Radiation therapy markedly damages normal hepatic cells when used with conventional large fields. According to the experience in the present paper, however, decreases in liver function were within the acceptable range if only PVIs of the patients classified into Child A and B were treated with 3-D CRT. With 3-D CRT, high doses of radiation are confined to the target region while surrounding areas of the liver are exposed only to lower doses. Although clinical data have accumulated concerning hepatic dysfunction after whole or partial irradiation of the liver, those data are not directly applicable to predict hepatic dysfunction after conformal radiotherapy. The biological effect on the liver of such inhomogenous irradiation has not been elucidated fully. Further accumulation of clinical experience is required to determine the most appropriate method of irradiation for this disease.
The results of this study strongly suggest the efficacy of 3-D CRT as treatment for PVI in HCC. From the present analyses, the authors will recommend 3-D CRT in combination with post-radiation TAE for HCCs with 5 tumor foci or less in the liver and with Child A liver function.
Needless to say, because this study was retrospective, the presence of bias in the selection of treatments for individual patients cannot be excluded completely. To more fully clarify the significance of this therapy, a prospective randomized trial should be performed. We hope that the outcomes reported here may contribute to designing future clinical trials and optimizing treatment for this disease.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Albacete RA, Matthews MJ, Saini N. Portal vein thromboses in malignant hepatoma. Ann Intern Med. 1967;67:337–348. doi: 10.7326/0003-4819-67-2-337. [DOI] [PubMed] [Google Scholar]
- 2.Adachi E, Maeda T, Kajiyama K, Kinukawa N, Matsumata T, Sugimachi K, Tsuneyoshi M. Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments. Cancer. 1996;77:2022–2031. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2022::AID-CNCR9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Tobe T, Takayasu K, Kasugai F, Ikeya S, Muramatsu Y, Moriyama N. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Liver Cancer Study Group of Japan. Ann Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 4.Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, Imamura M, Yoshida H, Shiina S, Omata M. Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer. 2001;91:561–569. doi: 10.1002/1097-0142(20010201)91:3<561::aid-cncr1035>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer. 1996;77:2217–2222. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2217::AID-CNCR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–799; discussion 790-799;. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumada K, Ozawa K, Okamoto R, Takayasu T, Yamaguchi M, Yamamoto Y, Higashiyama H, Morikawa S, Sasaki H, Shimahara Y. Hepatic resection for advanced hepatocellular carcinoma with removal of portal vein tumor thrombi. Surgery. 1990;108:821–827. [PubMed] [Google Scholar]
- 8.Chen SC, Lian SL, Chang WY. The effect of external radiotherapy in treatment of portal vein invasion in hepatocellular carcinoma. Cancer Chemother Pharmacol. 1994;33 Suppl:S124–S127. doi: 10.1007/BF00686683. [DOI] [PubMed] [Google Scholar]
- 9.Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995;165:315–321. doi: 10.2214/ajr.165.2.7618547. [DOI] [PubMed] [Google Scholar]
- 10.Ando E, Yamashita F, Tanaka M, Tanikawa K. A novel chemotherapy for advanced hepatocellular carcinoma with tumor thrombosis of the main trunk of the portal vein. Cancer. 1997;79:1890–1896. doi: 10.1002/(sici)1097-0142(19970515)79:10<1890::aid-cncr8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 11.Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087–2094. [PubMed] [Google Scholar]
- 12.Yamakado K, Tanaka N, Nakatsuka A, Matsumura K, Takase K, Takeda K. Clinical efficacy of portal vein stent placement in patients with hepatocellular carcinoma invading the main portal vein. J Hepatol. 1999;30:660–668. doi: 10.1016/s0168-8278(99)80197-4. [DOI] [PubMed] [Google Scholar]
- 13.Yamanaka N, Okamoto E, Toyosaka A, Mitunobu M, Fujihara S, Kato T, Fujimoto J, Oriyama T, Furukawa K, Kawamura E. Prognostic factors after hepatectomy for hepatocellular carcinomas. A univariate and multivariate analysis. Cancer. 1990;65:1104–1110. doi: 10.1002/1097-0142(19900301)65:5<1104::aid-cncr2820650511>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 14.Inamoto K, Sugiki K, Yamasaki H, Miura T. CT of hepatoma: effects of portal vein obstruction. AJR Am J Roentgenol. 1981;136:349–353. doi: 10.2214/ajr.136.2.349. [DOI] [PubMed] [Google Scholar]
- 15.Mathieu D, Grenier P, Lardé D, Vasile N. Portal vein involvement in hepatocellular carcinoma: dynamic CT features. Radiology. 1984;152:127–132. doi: 10.1148/radiology.152.1.6328574. [DOI] [PubMed] [Google Scholar]
- 16.Van Gansbeke D, Avni EF, Delcour C, Engelholm L, Struyven J. Sonographic features of portal vein thrombosis. AJR Am J Roentgenol. 1985;144:749–752. doi: 10.2214/ajr.144.4.749. [DOI] [PubMed] [Google Scholar]
- 17.Dodd GD, Memel DS, Baron RL, Eichner L, Santiguida LA. Portal vein thrombosis in patients with cirrhosis: does sonographic detection of intrathrombus flow allow differentiation of benign and malignant thrombus? AJR Am J Roentgenol. 1995;165:573–577. doi: 10.2214/ajr.165.3.7645473. [DOI] [PubMed] [Google Scholar]
- 18.The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg. 1989;19:98–129. doi: 10.1007/BF02471576. [DOI] [PubMed] [Google Scholar]
- 19.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzaki Y, Osuga T, Saito Y, Chuganji Y, Tanaka N, Shoda J, Tsuji H, Tsujii H. A new, effective, and safe therapeutic option using proton irradiation for hepatocellular carcinoma. Gastroenterology. 1994;106:1032–1041. doi: 10.1016/0016-5085(94)90764-1. [DOI] [PubMed] [Google Scholar]
- 21.Thorn K, Williams J. Solitary osseus plasmacytoma as a cause of back pain in a young patient. Am J Emerg Med. 1999;17:615–617. doi: 10.1016/s0735-6757(99)90212-7. [DOI] [PubMed] [Google Scholar]
- 22.Cheng JC, Chuang VP, Cheng SH, Huang AT, Lin YM, Cheng TI, Yang PS, You DL, Jian JJ, Tsai SY, et al. Local radiotherapy with or without transcatheter arterial chemoembolization for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2000;47:435–442. doi: 10.1016/s0360-3016(00)00462-4. [DOI] [PubMed] [Google Scholar]
- 23.Seong J, Park HC, Han KH, Lee DY, Lee JT, Chon CY, Moon YM, Suh CO. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2000;47:1331–1335. doi: 10.1016/s0360-3016(00)00519-8. [DOI] [PubMed] [Google Scholar]
- 24.Fan J, Zhou J, Wu ZQ, Qiu SJ, Wang XY, Shi YH, Tang ZY. Efficacy of different treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2005;11:1215–1219. doi: 10.3748/wjg.v11.i8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada K, Izaki K, Sugimoto K, Mayahara H, Morita Y, Yoden E, Matsumoto S, Soejima T, Sugimura K. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113–119. doi: 10.1016/s0360-3016(03)00434-6. [DOI] [PubMed] [Google Scholar]
- 26.Ishikura S, Ogino T, Furuse J, Satake M, Baba S, Kawashima M, Nihei K, Ito Y, Maru Y, Ikeda H. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol. 2002;25:189–193. doi: 10.1097/00000421-200204000-00019. [DOI] [PubMed] [Google Scholar]


