Abstract
AIM: To assess the accuracy of a model in diagnosing severe fibrosis/cirrhosis in chronic hepatitis C virus (HCV) infection.
METHODS: The model, based on the sequential combination of the Bonacini score (BS: ALT/AST ratio, platelet count and INR) and ultrasonography liver surface characteristics, was applied to 176 patients with chronic HCV infection. Assuming a pre-test probability of 35%, the model defined four levels of post-test probability of severe fibrosis/cirrhosis: <10% (low), 10-74% (not diagnostic), 75-90% (high) and >90% (almost absolute). The predicted probabilities were compared with the observed patients’ distribution according to the histology (METAVIR).
RESULTS: Severe fibrosis/cirrhosis was found in 67 patients (38%). The model discriminated patients in three comparable groups: 34% with a very high (>90%) or low (<10%) probability of severe fibrosis, 33% with a probability ranging from 75% to 90%, and 33% with an uncertain diagnosis (i.e., a probability ranging from 10% to 74%). The observed frequency of severe fibrosis/cirrhosis was within the predefined ranges.
CONCLUSION: The model can correctly identify 67% of patients with a high (>75%) or low (<10%) probability of cirrhosis, leaving only 33% of the patients still requiring liver biopsy.
Keywords: Liver fibrosis, Ultrasonography, Bonacini score, Liver biopsy, Hepatitis C
INTRODUCTION
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease worldwide: nearly 80% of HCV-infected patients develop chronic infection, and about 20% progress to cirrhosis[1]. Liver histology is currently considered as the “reference standard” for evaluating hepatic damage on the basis of the degree of necrotic inflammatory activity and fibrosis, with the latter having prognostic significance and playing a major role in therapeutic decision making[2,3].
However, liver biopsy is an invasive procedure with mild and severe complications of 20% and 0.5%, respectively[4,5]. Furthermore, its sensitivity in diagnosing liver cirrhosis is not absolute and the rate of false negative results is approximately 30%[6]. In addition, as it has been well evidenced by the recent studies, an adequate liver specimen should be at least 2.5 cm long[7] and 1.4 mm wide[8] including at least 6-8 portal tracts[9]. This has recently led various groups to investigate the non-invasive methods of detecting severe fibrosis/cirrhosis, including only biochemical (ALT, AST, GGT) tests[10-14] or in combination with hematological (platelet count) tests[15], test panels[16-25], serum “markers” of fibrosis (such as hyaluronic acid or procollagen peptides)[26-30] and ultrasonographic parameters (e.g., liver surface nodularity (LSN), portal blood flow)[31-34], but none of which have proved to be capable of avoiding liver biopsy.
The aim of this prospective study was to evaluate the accuracy of a model based on the sequential combination of a set of simple biochemical tests (Bonacini score, BS)[19,20] and liver surface ultrasound (US) examination[32] in diagnosing severe fibrosis/cirrhosis in a large series of consecutive patients with chronic HCV infection undergoing liver biopsy.
MATERIALS AND METHODS
All anti-HCV positive patients (EIA III, Abbott Laboratories, Chicago, IL, USA) with detectable HCV-RNA serum levels (Amplicor HCV kit, Roche, Molecular Systems, Basel, Switzerland) were evaluated for enrollment between September 2001 and June 2004.
The presence of decompensated liver disease (i.e., portosystemic encephalopathy, jaundice or ascites detected by means of US) and or an absolute contraindication to liver biopsy (PLT <60 000/mm3 , PT <60%) excluded patients from the study, whereas patients with an increase of ≥1.5 UNL in the serum alanine-aminotransferase (ALT) levels recorded twice in the previous 6 months, were included in the study, whose protocol was approved by the pertinent ethics committee after having obtained their written informed consent. Socio-demographic and clinical data were recorded as those on past and/or current alcohol intake for which a semi-quantitative questionnaire with three pre-defined levels of daily consumption (<30, 30-80 and >80 g) was used. At the time of liver biopsy, the laboratory data included AST, ALT (IU/L r.v. <40), platelet count (106/μL r.v. 130 000-400 000), the international normalized ratio (INR 0.8-1.2), level of albumin (g/dL r.v. 3.5-5.0), total bilirubin (mg/dL r.v. 0.3-1.0) and hemoglobin (g/dL r.v. 14-18 for men and 12-16 for women), as well as HCV genotyping by means of restriction fragment length polymorphism after amplification of the 5’ non-coding region of the HCV genome[35]. As detailed in Table 1, the “cirrhosis discriminant score” (range 0-11) of each patient was calculated according to the method of Bonacini et al[19], based on ALT/AST ratio, platelet count and INR. After an overnight fast, a US liver scan was performed using an ATL HDI 5000 equipment (Advanced Technology Laboratories, Bothell, WA, USA) and both 3.5 and a 5-12 MHz transducer by one of three gastroenterologists (AC, SM or MA) blinded to the clinical, biochemical and histologic data. A 5-12 MHz transducer was used to obtain multiple scans of the outer 2-3 cm of the liver parenchyma and of both lobes. LSN was considered positive when the liver surface appeared as a dotted or an irregular line and/or the liver parenchyma was not homogeneous, with areas of different echogenicity, reflecting an underlying nodularity. The interobserver agreement for this sign was calculated according to K statistics.
Table 1.
Determinants of Bonacini score
| Laboratory | Score | ||||||
| parameters | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| INR | <1.1 | 1.1-1.4 | >1.4 | ||||
| ALT/AST ratio | >1.7 | 1.7-1.2 | 1.19-0.6 | <0.6 | |||
| PLT ×1 000 /mm3 | >340 | 340-280 | 279-220 | 219-160 | 159-100 | 99-40 | <40 |
A US-guided transcostal or subcostal liver biopsy was performed (by AC or MF) using an 18-gauge needle (Biomol Hospital Service, Pomezia, Rome, Italy). We considered acceptable only specimens ≥2.0 cm long including >12 portal tracts, that were fixed in formalin and stained with hematoxylin-eosin, silver impregnation for reticulin, and Masson’s trichrome or picrosirius red for collagen. In case of inadequacy of the sample, a second biopsy was obtained.
The specimens evaluated by the same pathologist (VM) unaware of the patients’ characteristics were staged according to METAVIR scoring system[36], where F0 indicates the lack of fibrosis, F1 corresponds to portal fibrosis without septa, F2 to the presence of few septa, F3 and F4 to the finding of numerous septa without or with cirrhosis, respectively. Histologic findings were considered as the reference standard for the presence and degree of fibrosis.
Statistical analysis
The pre-test probability of severe fibrosis/cirrhosis (i.e., a staging score 3-4) was estimated to be about 35% on the basis of recent data from comparable series[2,32,37-39]. A predictive model was obtained by the sequential application of the BS and US liver surface examination, which could obtain the post-test probability of severe fibrosis/cirrhosis. As the BS and US signs of LSN could be considered conditionally independent, the probability of severe fibrosis/cirrhosis obtained by calculating the BS has been considered as the pre-test probability before the US examination. The post-test probabilities were calculated on the basis of post-test odds as previously described[40,41]. The values of sensitivity, specificity, positive and negative likelihood ratios (LR+ and LR-) of both BS and US in diagnosing severe fibrosis have been previously defined[19,32].
In detail, three possibilities had to be taken into account. Firstly, for a staging score 0-2, a BS ≤3 had a sensitivity of 58% and a specificity of 85%, accounting for a LR+ of 4. Accordingly, in our model in the presence of a BS ≤3, the probability of stage 0-2 increased from a pre-test one of 65% to 88%. Secondly, the sensitivity and specificity for severe fibrosis in the presence of a BS of 4-7 were 50%, making it impossible to modify the 35% pre-test probability of severe fibrosis/cirrhosis on the basis of a LR+ and a LR- of about one. Thirdly, the specificity of 100% for a BS >7 led to an almost absolute post-test probability of staging score 3-4, with a LR+ higher than 100. As far as the US finding of LSN was concerned, an inter-observer variability of 0.80 was reported. Our own previous data[32] demonstrated a sensitivity of 54% and a specificity of 95% for a staging score 3-4, with a LR+ and a LR- of 11.6 and 0.5, respectively. By combining the BS and the US signs of LSN, five groups of patients (I-V) were defined (Figure 1) with different post-test probability of severe fibrosis/cirrhosis. In the last group, with BS ≥7 the post-test probability of a staging score of 3-4 was close to 100% regardless of the US results. Finally, as detailed in Figure 1, to test the predictive accuracy and clinical usefulness of the model, four clinically acceptable ranges of post-test probability of severe fibrosis/cirrhosis were predefined based on BS and US findings: low probability (<10%), not diagnostic (10-74%), high probability (75-90%) and almost absolute probability (>90%).
Figure 1.
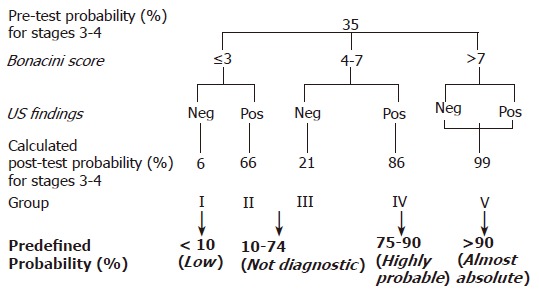
Post-test probability of severe fibrosis/cirrhosis calculated on the basis of both BS and US findings (starting from the pre-test value of 35%), and ranges of post-test probability for severe fibrosis/cirrhosis predefined as “clinically relevant” (in bold).
RESULTS
During the recruitment period, 1 089 patients were investigated for liver disease, and 363 were chronically infected with HCV. Of them, 184 were excluded for the refusal of liver biopsy (#11) because of already established cirrhosis with Child-Pugh score >5 (#67), or because of normal or slightly elevated ALT levels (<1.5 times the upper normal limit) or uncompleted ALT data (less than six distinct determinations) (#106). The remaining 179 fulfilled the inclusion criteria. Based on the inadequate US findings, three were excluded accounting for an overall recruitment of 176 patients, whose characteristics are detailed in Table 2. The inter-observer agreement for the detection of the LSN sign was 0.77. The average length of liver specimens was 4.1±0.4 cm and in 18 cases a second liver sample was obtained because of the inadequacy of the first one. At liver biopsy, well tolerated in all the cases, 67 patients (38%) had severe fibrosis (#52) or cirrhosis (#15, all in Child’s A class). Of the remaining 109 (62%), fibrosis was absent in 6, mild (score 1) in 40 and moderate (score 2) in 63. LSN was present in 9 out of the 109 (8%) patients with a fibrosis score of 0-2 and in 40 out of 67 (60%) with F 3-4 with a sensitivity, specificity, LR+ and LR- of 60%, 92% and 7.5 and 0.5, respectively. Patients’ distribution according to the BS and LSN, and corresponding observed frequency (%) of severe fibrosis/cirrhosis (stage 3-4) vs expected post-test probability calculated by the model, are shown in Table 3. Overall, the diagnostic accuracy of the model was 86.5% in groups I, IV, and V, accounting for 67% of the patients. Sixteen of one hundred and nineteen patients were incorrectly defined (1 in group I with fibrosis stage ≥3, 15 in group IV with fibrosis stage <3). In groups II and III (33% of the total), the model did not show any predictive role and did not significantly modify the pre-test probability of severe fibrosis/cirrhosis.
Table 2.
Clinical and biochemical characteristics of 176 consecutive patients with chronic hepatitis C included in
| # | % | Mean±SD | |
| Age (yr) | 53.5±21.2 | ||
| Sex: Male | 96 | 55 | |
| Female | 80 | 45 | |
| BMI (kg/m2) | 25.9±2.9 | ||
| <25 | 90 | 51 | |
| ≥25 | 86 | 49 | |
| Alcohol intake (g/d) | |||
| ≤30 | 142 | 80 | |
| 31-80 | 24 | 14 | |
| >80 | 10 | 6 | |
| HCV genotype | |||
| 1 | 92 | 52 | |
| 2 | 60 | 34 | |
| 3 | 19 | 11 | |
| 4 | 5 | 3 | |
| Platelet count | 181±128 | ||
| AST (IU/L) | 78.5±101 | ||
| ALT (IU/L) | 125±178 | ||
| AST/ALT | 1.6±1.1 | ||
| INR | 1.05±0.1 | ||
| Hemoglobin (g/dL) | 14.5±2.1 | ||
| Albumin (g/dL) | 4.5±0.6 | ||
| Bilirubin (mg/dL) | 0.9±0.2 |
Table 3.
Distribution of 176 patients according to Bonacini score and pre-defined grouping
DISCUSSION
In this prospective study, we have evaluated the accuracy of a non-invasive predictive model, consisting of the sequential combination of laboratory data score (BS) and US finding of a nodular liver surface, in detecting severe liver fibrosis or cirrhosis in patients with chronic hepatitis C. In clinical practice, it is easy to use the two elements with their operative characteristics individually validated in previous studies[19,20,32]. The BS is based on simple and widely available laboratory tests (ALT, AST, INR and platelet count) that assure its repeatability, and LSN can be easily detected using commercially available US equipment.
Previous series using platelet count or ALT and AST level or BS or US can precisely predict the presence or absence of fibrosis but with a low discriminating power; thus, few patients have a very low or high probability of fibrosis, and most of them are in the gray zone of uncertainty[19,20,32]. On the contrary, the sequential model, which is applicable to the vast majority of enrolled patients (98%, because the US examination was technically inadequate in three cases), has been proved to have an adequate discriminating power and an accurate calibration. Its discriminating power is demonstrated by the fact that we were able to divide the patients into three comparable groups: 34% with a very high (>90%) or low (<10%) probability of severe fibrosis, 33% with a probability ranging between 75% and 90%, and 33% with an uncertain diagnosis (i.e., with a fibrosis score 3-4 probability between 10% and 74%). The precise calibration of the model is demonstrated by the fact that the observed frequency of severe fibrosis/cirrhosis was within the predefined ranges for each group (Table 3).
The validity of the model is further supported by the finding that the 38% frequency of severe fibrosis/cirrhosis in the study population was similar to the theoretical value of 35% assumed by considering series of patients with comparable characteristics, represented by a ≥1.5 times increase in ALT levels recorded twice during a period of 6 months or more[2,32,37-39].
The operative characteristics of US are slightly different from those indicated by our own recent data[32], which were used for the model. However, the variations in LR+ and LR- remained within the broad confidence intervals of the original estimates and did not critically challenge the calibration of the model. Further studies are needed to assess the generalizability of the model, i.e. its reproducibility and transportability, for which independent validations are advisable.
Although our simple model could detect the presence or absence of severe fibrosis in nearly two-thirds of the patients, thus avoiding the need for liver biopsy, some limitations of the study require further discussion. Because of its intrinsic characteristics, the model was unable to detect moderate (stage 2) fibrosis, which together with grading is relevant in therapeutic decision making. Furthermore, the study seemed to underestimate the diagnostic role of liver biopsy, which was used not only to define staging and grading, but also to detect concomitant pathologies. However, the latter role which is already questionable in patients with liver disease of uncertain etiology[42] was irrelevant in our series of patients with chronic HCV infection. Conversely, liver biopsy still has some limitations in clinical practice, such as the difficulty in obtaining a liver sample of adequate size, as recently reported by some studies[7,8], and which can be considered as representative of the whole liver, given the patchy distribution of liver fibrosis in chronic hepatitis C. The above limitations in our opinion are a major issue in evaluating the diagnostic accuracy of non invasive diagnostic tests compared to liver biopsy. In fact in these types of studies, the major drawback is the imperfection of the reference standard itself, which can significantly affect the estimates of the operative characteristics of the test under investigation. To reduce the possible sample size effect, in the present study we obtained all the liver specimens >3 cm with >12 portal tracts from patients.
Previous studies assessing the individual role of BS and US findings of LSN in detecting severe fibrosis have found that they are inaccurate because their low power of discrimination means that a large proportion of patients still require liver biopsy[19,20,32]. It has recently been shown that the use of laboratory tests[10-16] and specific fibrosis markers, such as hyaluronic acid[26-30], is highly accurate in diagnosing cirrhosis. However, the considerable differences in the study design and the particularly high prevalence of symptomatic cirrhosis sometimes make it difficult to compare these data to our own. It has also recently been shown that relatively complex laboratory scores are accurate in discriminating stage 0-1 from stage 2-4, but fail to confirm severe fibrosis[17-25] although the aim of these studies was to detect patients with minimal or absent fibrosis with stage 3-4 found in only 15% (vs 37% in our series), possibly because they included patients with normal ALT levels and a younger age.
Recently, preliminary data from patients with chronic HCV liver disease[43] suggest a promising role of fibroscan [one-dimensional (1-D) transient elastography] in predicting the degree of liver fibrosis with a good accuracy in discriminating stage 0-2 from 3-4, and a LR+ and LR- of 5.7 and 0.16, respectively, similar to those observed in the present series for the US sign of LSN (LR+ and LR- of 7.5 and 0.5, respectively).
In conclusion, our data suggest that the use of liver biopsy to detect severe fibrosis can be avoided in about two-thirds of patients. Furthermore, the model can also be used in patients aged more than 65 years for whom liver biopsy could be questioned.
Footnotes
Supported by the “Research Competition Award 2002” from IRCCS Ospedale Maggiore, Milan, and “Associazione Amici della Gastroenterologia del Granelli”, Milan, Italy
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Seeff LB, Hoofnagle JH. National Institutes of Health Consensus Development Conference: management of hepatitis C: 2002. Hepatology. 2002;36:S1–S2. doi: 10.1053/jhep.2002.36992. [DOI] [PubMed] [Google Scholar]
- 2.Khan MH, Farrell GC, Byth K, Lin R, Weltman M, George J, Samarasinghe D, Kench J, Kaba S, Crewe E, et al. Which patients with hepatitis C develop liver complications? Hepatology. 2000;31:513–520. doi: 10.1002/hep.510310236. [DOI] [PubMed] [Google Scholar]
- 3.Poynard T, Ratziu V, Benmanov Y, Di Martino V, Bedossa P, Opolon P. Fibrosis in patients with chronic hepatitis C: detection and significance. Semin Liver Dis. 2000;20:47–55. doi: 10.1055/s-2000-9258. [DOI] [PubMed] [Google Scholar]
- 4.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 5.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165–173. doi: 10.1016/s0168-8278(86)80075-7. [DOI] [PubMed] [Google Scholar]
- 6.Poniachik J, Bernstein DE, Reddy KR, Jeffers LJ, Coelho-Little ME, Civantos F, Schiff ER. The role of laparoscopy in the diagnosis of cirrhosis. Gastrointest Endosc. 1996;43:568–571. doi: 10.1016/s0016-5107(96)70192-x. [DOI] [PubMed] [Google Scholar]
- 7.Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239–244. doi: 10.1016/s0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 9.Scheuer PJ. Liver biopsy size matters in chronic hepatitis: bigger is better. Hepatology. 2003;38:1356–1358. doi: 10.1016/j.hep.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Giannini E, Botta F, Fasoli A, Ceppa P, Risso D, Lantieri PB, Celle G, Testa R. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci. 1999;44:1249–1253. doi: 10.1023/a:1026609231094. [DOI] [PubMed] [Google Scholar]
- 11.Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93:44–48. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- 12.Haber MM, West AB, Haber AD, Reuben A. Relationship of aminotransferases to liver histological status in chronic hepatitis C. Am J Gastroenterol. 1995;90:1250–1257. [PubMed] [Google Scholar]
- 13.Assy N, Minuk GY. Serum aspartate but not alanine aminotransferase levels help to predict the histological features of chronic hepatitis C viral infections in adults. Am J Gastroenterol. 2000;95:1545–1550. doi: 10.1111/j.1572-0241.2000.02027.x. [DOI] [PubMed] [Google Scholar]
- 14.Park GJ, Lin BP, Ngu MC, Jones DB, Katelaris PH. Aspartate aminotransferase: alanine aminotransferase ratio in chronic hepatitis C infection: is it a useful predictor of cirrhosis? J Gastroenterol Hepatol. 2000;15:386–390. doi: 10.1046/j.1440-1746.2000.02172.x. [DOI] [PubMed] [Google Scholar]
- 15.Pohl A, Behling C, Oliver D, Kilani M, Monson P, Hassanein T. Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol. 2001;96:3142–3146. doi: 10.1111/j.1572-0241.2001.05268.x. [DOI] [PubMed] [Google Scholar]
- 16.Myers RP, De Torres M, Imbert-Bismut F, Ratziu V, Charlotte F, Poynard T. Biochemical markers of fibrosis in patients with chronic hepatitis C: a comparison with prothrombin time, platelet count, and age-platelet index. Dig Dis Sci. 2003;48:146–153. doi: 10.1023/a:1021702902681. [DOI] [PubMed] [Google Scholar]
- 17.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 18.Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 19.Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1997;92:1302–1304. [PubMed] [Google Scholar]
- 20.Saadeh S, Cammell G, Carey WD, Younossi Z, Barnes D, Easley K. The role of liver biopsy in chronic hepatitis C. Hepatology. 2001;33:196–200. doi: 10.1053/jhep.2001.20534. [DOI] [PubMed] [Google Scholar]
- 21.Poynard T, Munteanu M, Imbert-Bismut F, Charlotte F, Thabut D, Le Calvez S, Messous D, Thibault V, Benhamou Y, Moussalli J, et al. Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin Chem. 2004;50:1344–1355. doi: 10.1373/clinchem.2004.032227. [DOI] [PubMed] [Google Scholar]
- 22.Le Calvez S, Thabut D, Messous D, Munteanu M, Ratziu V, Imbert-Bismut F, Poynard T. The predictive value of Fibrotest vs. APRI for the diagnosis of fibrosis in chronic hepatitis C. Hepatology. 2004;39:862–863; author reply 863. doi: 10.1002/hep.20099. [DOI] [PubMed] [Google Scholar]
- 23.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 24.Sud A, Hui JM, Farrell GC, Bandara P, Kench JG, Fung C, Lin R, Samarasinghe D, Liddle C, McCaughan GW, et al. Improved prediction of fibrosis in chronic hepatitis C using measures of insulin resistance in a probability index. Hepatology. 2004;39:1239–1247. doi: 10.1002/hep.20207. [DOI] [PubMed] [Google Scholar]
- 25.Rossi E, Adams L, Prins A, Bulsara M, de Boer B, Garas G, MacQuillan G, Speers D, Jeffrey G. Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients. Clin Chem. 2003;49:450–454. doi: 10.1373/49.3.450. [DOI] [PubMed] [Google Scholar]
- 26.Oberti F, Valsesia E, Pilette C, Rousselet MC, Bedossa P, Aubé C, Gallois Y, Rifflet H, Maïga MY, Penneau-Fontbonne D, et al. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology. 1997;113:1609–1616. doi: 10.1053/gast.1997.v113.pm9352863. [DOI] [PubMed] [Google Scholar]
- 27.Murawaki Y, Ikuta Y, Okamoto K, Koda M, Kawasaki H. Diagnostic value of serum markers of connective tissue turnover for predicting histological staging and grading in patients with chronic hepatitis C. J Gastroenterol. 2001;36:399–406. doi: 10.1007/s005350170084. [DOI] [PubMed] [Google Scholar]
- 28.Murawaki Y, Koda M, Okamoto K, Mimura K, Kawasaki H. Diagnostic value of serum type IV collagen test in comparison with platelet count for predicting the fibrotic stage in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2001;16:777–781. doi: 10.1046/j.1440-1746.2001.02515.x. [DOI] [PubMed] [Google Scholar]
- 29.McHutchison JG, Blatt LM, de Medina M, Craig JR, Conrad A, Schiff ER, Tong MJ. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J Gastroenterol Hepatol. 2000;15:945–951. doi: 10.1046/j.1440-1746.2000.02233.x. [DOI] [PubMed] [Google Scholar]
- 30.Xie SB, Yao JL, Zheng RQ, Peng XM, Gao ZL. Serum hyaluronic acid, procollagen type III and IV in histological diagnosis of liver fibrosis. Hepatobiliary Pancreat Dis Int. 2003;2:69–72. [PubMed] [Google Scholar]
- 31.Gaiani S, Gramantieri L, Venturoli N, Piscaglia F, Siringo S, D'Errico A, Zironi G, Grigioni W, Bolondi L. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol. 1997;27:979–985 DOI : 10.1016/S0168-8278(97)80140-7. doi: 10.1016/s0168-8278(97)80140-7. [DOI] [PubMed] [Google Scholar]
- 32.Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection--analysis of 300 cases. Radiology. 2003;227:89–94. doi: 10.1148/radiol.2272020193. [DOI] [PubMed] [Google Scholar]
- 33.Hirata M, Akbar SM, Horiike N, Onji M. Noninvasive diagnosis of the degree of hepatic fibrosis using ultrasonography in patients with chronic liver disease due to hepatitis C virus. Eur J Clin Invest. 2001;31:528–535. doi: 10.1046/j.1365-2362.2001.00840.x. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Wang B, Cao H. An ultrasound scoring system for the diagnosis of liver fibrosis and cirrhosis. Chin Med J (Engl) 1999;112:1125–1128. [PubMed] [Google Scholar]
- 35.Lopez-Labrador FX, Ampurdanes S, Forns X, Castells A, Saiz JC, Costa J, Bruix J, Sanchez Tapias JM, Jimenez de Anta MT, Rodes J. Hepatitis C virus (HCV) genotypes in Spanish patients with HCV infection: relationship between HCV genotype 1b, cirrhosis and hepatocellular carcinoma. J Hepatol. 1997;27:959–965 DOI : 10.1016/S0168-8278(97)80137-7. doi: 10.1016/s0168-8278(97)80137-7. [DOI] [PubMed] [Google Scholar]
- 36.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 37.Niederau C, Lange S, Heintges T, Erhardt A, Buschkamp M, Hürter D, Nawrocki M, Kruska L, Hensel F, Petry W, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28:1687–1695. doi: 10.1002/hep.510280632. [DOI] [PubMed] [Google Scholar]
- 38.Patel K, Gordon SC, Jacobson I, Hézode C, Oh E, Smith KM, Pawlotsky JM, McHutchison JG. Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. J Hepatol. 2004;41:935–942. doi: 10.1016/j.jhep.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Cammà C, Di Bona D, Schepis F, Heathcote EJ, Zeuzem S, Pockros PJ, Marcellin P, Balart L, Alberti A, Craxì A. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology. 2004;39:333–342. doi: 10.1002/hep.20073. [DOI] [PubMed] [Google Scholar]
- 40.Black ER, Panzer RJ, Mayewski RJ, Griner PF. Characteristics of diagnostic tests and principles for their use in quantitative decision making in diagnostic strategies for common medical problems. In: Black ER, editor. Bordley DR., Tape TG., Panzer RJ eds. Diagnostic strategies for common medical problems. 2nd ed. Philadelphia: American College of Physicians; 1999. pp. 1–17. [Google Scholar]
- 41.Suchman AL, Dolan JG. Odds and likelihood ratios. In: Black ER, editor. Bordley DR., Tape TG., Panzer RJ. Eds. 2nd ed. Diagnostic strategies for common medical problems. Philadelphia: American College of Physicians; 1999. pp. 31–36. [Google Scholar]
- 42.Sorbi D, McGill DB, Thistle JL, Therneau TM, Henry J, Lindor KD. An assessment of the role of liver biopsies in asymptomatic patients with chronic liver test abnormalities. Am J Gastroenterol. 2000;95:3206–3210. doi: 10.1111/j.1572-0241.2000.03293.x. [DOI] [PubMed] [Google Scholar]
- 43.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]


