Abstract
AIM: To check the utility of postcibal ultrasonography for the evaluation of reflux in relation to gastric emptying in infants with recurrent respiratory symptoms and to link imaging with clinical data.
METHODS: Esophageal reflux (hyperechoic retrograde filling) and gastric emptying (antral areas) were quantified before and after ingestion of a standard formula in 35 untreated infants (13 with chronic cough, 22 with recurrent bronchitis) and in 31 controls.
RESULTS: The prevalence of abnormal (≥8 episodes) postcibal refluxes was 74% in patients and 3% in controls. Number, duration of the longest episode and extent of refluxes were significantly higher in patients compared to controls. Number of refluxes was higher in patients with symptomatic refluxes than in those without. Infants with recurrent bronchitis had more refluxes than those with chronic cough and controls. Extent and timing of gastric emptying were similar in patients and controls.
CONCLUSION: Esophageal ultrasonography is a useful and physiological test in infants with recurrent respiratory diseases, which have a high prevalence of abnormal postcibal esophageal reflux and a gastric emptying similar to that of normal controls. Esophageal reflux is more severe in subjects with recurrent bronchitis than in those with chronic cough.
Keywords: Ultrasonography, Gastro-esophageal reflux, Gastric emptying, Recurrent respiratory disease
INTRODUCTION
Gastro-esophageal reflux has been frequently linked to several acute and chronic respiratory diseases in infants[1-4]. As ultrasonography may allow direct visualization of gastro-esophageal junction and of retrograde reflux episodes[5,6], it has been used as an alternative to invasive techniques in pediatric age[5-9]. Validation studies reported high sensitivity and specificity for ultrasonography in diagnosing gastro-esophageal reflux, as compared to standard barium swallow examination[6], esophageal pH monitoring[8,10], and esophagomanometry[10]. The application of ultrasonography in pediatric age has major advantages due to its non-invasiveness, wide availability, and optimal compliance. Thus, in the present study, we aimed to: (1) employ ultrasound for the evaluation of gastro-esophageal reflux in relation to postprandial gastric emptying in infants with or without recurrent respiratory diseases; and (2) relate the gastro-esophageal imaging with clinical data in the postcibal period.
MATERIALS AND METHODS
Subjects
The ultrasonographic test was part of a routine work-up abdominal assessment. Informed consent was obtained from parents for each infant participating in the study and the study protocol was approved by an institutional review board. The study comprised 66 non-preterm infants (age range, 1-12 mo). Characteristics of the subjects are summarized in Table 1. Thirty-five inpatient or outpatient infants with a recent history of recurrent respiratory diseases were recruited. Patients had at least two episodes of asthmatic bronchitis requiring hospital admission (group A, n = 22) or unexplained chronic cough (group B, n = 13). Patients, who were referred only for respiratory problems, underwent de novo evaluation without prior investigations or therapies. Parents denied, in all cases, the consent to invasive techniques. In all patients, immune deficiencies, cystic fibrosis, and allergopathies were excluded by clinical and biohumoral assessment. Parents were asked for the presence of daily regurgitation in all the patients.
Table 1.
Characteristics of enrolled subjects
| Patients | Controls | P | |
| N | 35 | 31 | |
| Age (mo) | 5.6±2.9 | 4.5±3.6 | NS |
| Weight (kg) | 7.4±2.0 | 6.7±1.7 | NS |
| Height (m) | 0.7±0.07 | 0.6±0.01 | NS |
Data are expressed as mean±SD. NS: not significantly different.
Additionally, 31 inpatient or outpatient infants, who never experienced respiratory or gastrointestinal diseases, were considered as controls. Patients were not examined during acute respiratory disease. Neither patients nor controls were under medications able to influence gastrointestinal motility or secretion and were not affected by conditions known to alter gastrointestinal tract functions.
Study design
Each subject underwent a simultaneous ultrasonographic study of the esophagus and of the antrum with a Toshiba Capasee scanner and a 3.5-MHz convex probe. Ultrasonography was performed in a sitting position before and after the ingestion of a low volume liquid standard meal (10 mL/kg standard infant formula). Subjects were fed in the upright position. The observation was carried out continuously for at least 20 min and in all cases until complete antral evacuation was recorded. All tests started in the morning, in the fasting state (at least 4 h) and were performed by a single examiner who was unaware of patient’s history and clinical examination. Ultrasonographic findings were analyzed while being done and events were recorded on a specific form. The infants were not burped after the feeding and pacifiers were not employed. All infants were awake during the examination. Subjects with excessive gas in the stomach or excessive crying time (able to interrupt the continuity of observation) were excluded by analysis due to technical limitations.
Gastro-esophageal reflux study
The employment of ultrasound in the diagnosis of gastro-esophageal reflux disease has previously been validated against other techniques[6,8,10]. The esophagus and the gastro-esophageal junction were studied by transverse and longitudinal scans at the epigastrium[5,6,11] at the level of the left lobe of the liver, slightly rotating up the probe. In all the subjects, this allowed the visualization of the esophagus like a three-layer canalicular structure posterior to the left lobe of the liver and anterior to the aorta, which were used as landmark points[11]. Immediately after the ingestion of the standard liquid meal, the esophageal lumen appeared dilated by hyperechoic content flowing towards the gastric lumen. First, a longitudinal scan was performed to show the emptying of the distal esophagus. Subsequently, a “real time” observation of the esophagus and the gastro-esophageal junction was performed[11] by splitting the image on the ultrasound monitor. In particular, the esophagus and the gastro-esophageal junction were imaged on one half of the monitor continuously during 20 min. At regular intervals (mentioned below), the observation was stopped shortly for 2-3 s to catch antral sections on the second half of the screen for subsequent antral area measurement.
Gastro-esophageal reflux, when present, was visible like a flow of hyperechoic material directed from gastric lumen to distal esophagus through gastro-esophageal junction.
In the present series, gastro-esophageal reflux was defined as abnormal when the total number of episodes lasting for more than 2 s was ≥8 (upper limit derived from mean+2SD in normal control group = 7.5). The presence/absence and the number of gastro-esophageal reflux episodes during 20 min were recorded and the frequency of gastro-esophageal reflux episodes (i.e., no. of episodes/time unit) was therefore calculated.
Immediately after the retrograde flow of hyperechoic material from gastric lumen to distal esophagus, the ultrasonographic image was frozen on one half of the monitor and the amount of gastro-esophageal reflux was subsequently quantified by measuring maximal esophageal lumen enlargement (distance between external esophageal layers). On the second half of the monitor (set as "real time" imaging), the duration of the reflux episode was counted by a timer. Maximal esophageal lumen enlargement and duration of the longest reflux episode for each examination were noted.
During the real-time continuous monitoring, all cough episodes were counted in both patients and controls. Cough episodes starting within 2 s from hyperechoic retrograde filling of esophageal lumen were therefore marked on a specific form and were considered related to reflux.
Gastric emptying study
Postprandial evacuation of the stomach was assessed by functional ultrasonography by monitoring antral areas, a well standardized technique in adults[11-15] and in infants[16-20]. Antral area was quantified in fasting infants and subsequently monitored at regular time intervals postprandially by longitudinal scans at the epigastrium at the level of antral-body connection in a single section. The superior mesenteric vein and the aorta were used as landmark points. Antral emptying was described by the following indices: (1) antral (basal) area (mean of two measurements between -5 and 0 min before meal, in cm2); (2) maximal postprandial antral area, measured 2 min after meal ingestion; (3) postprandial antral areas each taken at 5-min intervals from meal ingestion; (4) minimal postprandial antral area observed throughout the emptying curve; and (5) half-emptying time.
Antral emptying curves were obtained by plotting antral areas vs time. In order to obviate interindividual variability of antrum size[13], postprandial areas were normalized to maximal areas after subtracting basal areas: 100×(At-a)/(A2-a), where At = postprandial area at any given time; a = basal area; A2 = maximal antral area (measured at 2 min postprandially)[21]. Half-emptying time was the time at which 50% decrease of antral area occurred (T1/2, min), and was calculated by linear regression analysis from the linear part of antral emptying curve. This index closely correlates with the scintigraphic half-emptying time[14].
Statistical analysis
All calculations were performed with the NCSS2001 software (Kaysville, UT, USA). Results were expressed as mean±SE or mean±SD or median and range, where appropriate. Data were statistically compared using two-tailed Student's t-test for unpaired data or by non-parametric Mann-Whitney U test, where appropriate. Analysis of variance followed by Fisher’s LSD multiple comparison test was utilized to compare the differences among the groups. The χ2 test was used to compare proportions of categorical data. Two-tailed P values less than 0.05 were considered statistically significant[22].
RESULTS
Patients and controls were similar in age-weight and height distribution (Table 1) and all subjects were below the 95th percentile for body weight. Overall, patients had significantly greater prevalence of abnormal postcibal reflux (74% in patients vs 3% in controls, P<0.05), number and frequency of reflux episodes, extent of refluxes and duration of the longest reflux episode as compared to controls (Table 2). Stratification of patients by age (0-6 mo, n = 22 and 7-12 mo, n = 13) showed similar results in the two age groups that were examined (Table 3).
Table 2.
Ultrasonographic characteristics of gastro-esophageal reflux in infants with recurrent respiratory diseases and in healthy controls
| Patients | Controls | P | |
| Number of reflux episodes | 10 (2-21) | 3 (1-9) | <0.01 |
| Reflux frequency (reflux episodes/min) | 0.5 (0.1-1.05) | 0.15 (0.05-0.45) | <0.01 |
| Maximal esophageal lumen enlargement (mm) | 5 (1-17) | 1.5 (1-9) | <0.01 |
| Duration of the longest reflux episode (s) | 11 (2-18) | 4 (2-12) | <0.01 |
Results are expressed as median (range). Differences between groups were tested using Mann-Whitney U test.
Table 3.
Ultrasonographic characteristics of gastro-esophageal reflux in patients with recurrent respiratory diseases in two age groups
| 0-6 mo | 7-12 mo | P | |
| Number of reflux episodes | 9.5 (3-21) | 11 (2-18) | NS |
| Reflux frequency (reflux episodes/min) | 0.5 (0.15-1.05) | 0.6 (0.1-0.9) | NS |
| Maximal esophageal lumen enlargement (mm) | 5.5 (1-17) | 4 (1.6-11.5) | NS |
| Duration of the longest reflux episode (s) | 11 (2-17) | 7 (4-18) | NS |
Results are expressed as median (range). Differences between groups were tested by Mann-Whitney U test; NS: not significantly different.
The total number of cough episodes was significantly greater in patients than in controls (2.7±0.5 vs 0.03±0.03; P<0.01). Symptomatic reflux episodes (i.e., cough observed within 2 s from hyperechoic retrograde filling of esophageal lumen) were documented in 57% of patients (2.0±0.4 symptomatic refluxes in the observation period) and in none of the controls. Cough episodes not preceded by reflux were 0.5±0.3 in patients without symptomatic reflux episodes and 0.8±0.2 in patients with symptomatic refluxes.
The median number and frequency of gastro-esophageal reflux episodes were obviously higher in patients with symptomatic reflux episodes than in those without (Table 4).
Table 4.
Ultrasonographic characteristics of gastro-esophageal reflux in patients with or without symptomatic reflux episodes
| With symptomatic reflux | Without symptomatic reflux | P | |
| Subjects | 20 | 15 | |
| Number of reflux episodes | 11.5 (4-21) | 7 (2-18) | <0.02 |
| Reflux frequency (reflux episodes/min) | 0.58 (0.2-1.05) | 0.4 (0.1-0.9) | <0.05 |
| Maximal esophageal lumen enlargement (mm) | 5.5 (1.7-13.5) | 3.0 (1-17) | NS |
| Duration of the longest reflux episode (s) | 11 (6-18) | 8 (2-17) | NS |
Results are expressed as median (range). Differences between groups were tested using Mann-Whitney U test. NS: not significantly different.
The prevalence of abnormal refluxes was higher in patients with recurrent bronchitis (group A) and in patients with chronic cough (group B) as compared to controls (Table 5). Both subgroups of patients had more reflux episodes, higher reflux frequency, higher esophageal lumen enlargement and duration of the longest reflux episode as compared to controls (P<0.01, ANOVA, Figure 1 and Table 5). Patients with recurrent bronchitis also showed more reflux episodes, higher reflux frequency and higher duration of the longest reflux episode as compared to infants with chronic cough (Table 5). The number of symptomatic reflux episodes was similar in patients with recurrent bronchitis (2.6±0.6) and in those with chronic cough (1.2±0.4).
Table 5.
Ultrasonographic characteristics of gastro-esophageal reflux in patients with recurrent episodes of bronchitis (group A), chronic cough (group B), and in healthy controls
| Group A | Group B | Controls | ANOVA | |
| Reflux prevalence (%) | 82a | 54a | 3 | |
| Number of reflux episodes (N) | 12.0 (2-21)ac | 8.0 (2-12)a | 3.0 (1-9) | <0.00001 |
| Reflux frequency (N/min) | 0.6 (0.1-1.05)ac | 0.4 (0.1-0.6)a | 0.15 (0.05-0.45) | <0.00001 |
| Maximal esophageal lumen enlargement (mm) | 5 (1-17)a | 4 (1.5-11.8)a | 1.5 (1-9) | <0.0001 |
| Duration of the longest reflux episode (s) | 11.5 (2-18)ac | 7 (3-12)a | 4 (2-12) | <0.00001 |
Results are expressed as median (range). Differences between percentage reflux prevalence were tested by χ2 test. Differences between groups were examined by using ANOVA, followed by Fisher’s LSD multiple comparison test.
P<0.05 vs controls;
P<0.05 vs group B.
Figure 1.
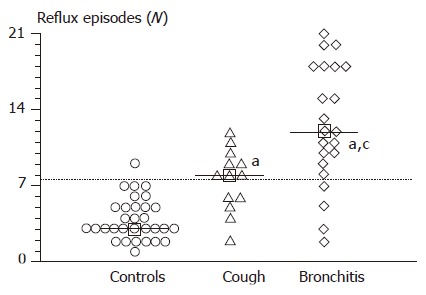
Number of postcibal gastro-esophageal reflux episodes, as determined by ultrasound, in controls (n = 31) and in infants with recurrent respiratory diseases (n = 35). Horizontal dotted line indicates the mean+2SD in the normal control group. Squares and continuous horizontal lines indicate medians. Differences between the groups were tested by using ANOVA, followed by Fisher’s LSD multiple comparison test. aP<0.05 vs controls; cP<0.05 vs chronic cough.
Daily regurgitation was present in 57% of patients with recurrent respiratory diseases, who showed a higher number, frequency, and duration of the longest reflux episode compared to patients without this symptom (Table 6). The number of symptomatic refluxes was similar in patients with (2.4±0.6) and without (1.7±0.6) daily regurgitation.
Table 6.
Ultrasonographic characteristics of gastro-esophageal reflux in infants with recurrent respiratory diseases, with or without daily regurgitation
| With daily regurgitation | Without daily regurgitation | P | |
| Subjects | 20 (57%) | 15 (43%) | NS |
| Number of reflux episodes (N) | 10.5 (5-21) | 8 (2-18) | <0.03 |
| Reflux frequency (N/min) | 0.5 (0.25-1.05) | 0.4 (0.1-0.9) | <0.03 |
| Maximal esophageal lumen enlargement (mm) | 5 (1.5-17) | 4 (1.0-11.5) | NS |
| Duration of the longest reflux episode (s) | 11.5 (3-17) | 7 (2-18) | <0.01 |
Results are expressed as median (range). Differences between groups were tested by using Mann–Whitney U test. NS: not significantly different.
The study of gastric emptying showed a rapid and continuous decrement of the antral areas starting immediately after meal ingestion in both patients and controls (Figure 2). Patients and controls showed comparable fasting (1.3±0.01 and 1.2±0.1 cm2, respectively) and maximal postprandial areas (5.7±0.3 vs 5.6±0.4 cm2), and similar postprandial emptying indices, in terms of both minimal postprandial areas (1.4±0.1 vs 1.3±0.2 cm2; 4.6±1.9% vs 3.5±1.4%) and half-emptying time (9±0.6 vs 9±0.5 min, respectively). No significant difference was observed in time to maximal emptying between patients (20±1 min) and controls (19±1 min). Invariably, gastric emptying was complete after 30 min in all cases. Gastric emptying speed was also similar in patients with and without symptomatic reflux episodes (half-emptying time 9±1 and 10±1 min, respectively) and in patients with and without daily regurgitation (half-emptying time 9±1 and 9±1 min, respectively).
Figure 2.
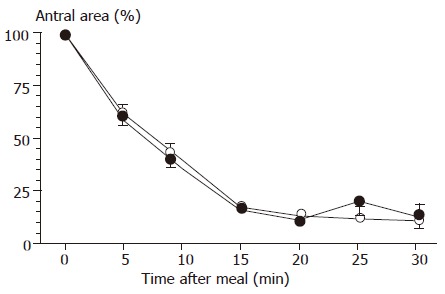
Postcibal gastric emptying curves, determined by ultrasound, in controls (n = 31) and in infants with recurrent respiratory diseases (n = 35). Symbols indicate means ( : controls; : patients), and vertical lines indicate SE. Differences between groups were tested by using ANOVA.
DISCUSSION
The present study shows that ultrasonography is useful in providing important information about the presence, extent, and duration of postcibal gastro-esophageal reflux in infants with recurrent respiratory diseases.
Gastro-esophageal reflux is the effortless retrograde flow of gastric content in the esophageal lumen and, when abnormal, it is a recognized clinical problem in infancy, where it has been frequently associated with respiratory diseases[1-4,23].
Respiratory diseases and gastro-esophageal reflux disease have long been linked in childhood by both physiopathological and epidemiological studies, and the complex interplay between these two frequent clinical conditions is still under debate[24,25].
By prolonged pH-metry, a high prevalence of abnormal esophageal pH was reported in infants with recurrent bronchopulmonary infections, suggesting that this technique may be useful for diagnosing gastro-esophageal reflux and its association with recurrent bronchopulmonary diseases[26]. pH-metry, however, has several pitfalls, since it does not help with alkaline gastric content as seen in a number of conditions, namely after the ingestion of normal infant formula, achlorhydria or duodenogastric reflux[27,28], and alkaline reflux episodes[29,30]. In a recent study, intraluminal impedance has been used in infants with respiratory phenomena and it has been shown that gastro-esophageal refluxes accompanied by respiratory symptoms can be detected only in a minority of cases by pH-metry[29]. Similar findings were also evident in a large ultrasonographic study in symptomatic infants undergoing pH-metry[7].
Impedance monitoring is a pH-independent method for detecting bolus movement within the esophagus. It might provide a valid alternative to esophageal pH-metry because it evaluates reflux occurring both in the interprandial and postprandial period, and it is sensitive to very small volumes of refluxate[29-33].
On the other hand, functional ultrasonography of the gastro-esophageal junction may allow direct visualization of lower esophageal sphincter relaxation and reflux episodes. The technique offers several advantages in that it has low costs, it is simple, non-invasive and is a more physiological alternative to invasive techniques[5,6,8-10]. In this respect, our results suggest that ultrasonography might represent a useful and physiological screening test in infants with recurrent respiratory diseases.
The importance of meal volume also needs to be addressed. We used a relatively low-volume liquid standard meal (i.e. of lower volume compared to that currently used during a physiological feeding). Such low volume would not affect the observed results, since in early studies, gastro-esophageal reflux was either unrelated to feed volume[17] or it was worsened by a high volume meal, through significant changes in lower esophageal sphincter pressure[20]. Here, we have observed several postcibal refluxes with a low-volume meal, suggesting that meal volume per se may not be a determinant in postcibal reflux occurrence.
The short postprandial observation period might be considered as a major pitfall of the ultrasonographic study. However, by using a 20-min postprandial time-window to check for persistence of intragastric content, we found significant differences for extent and timing of gastro-esophageal reflux between patients with recurrent respiratory diseases and controls, and an event/symptom relationship. Indeed, patients with recurrent involvement of lower respiratory tract (i.e. bronchitis) showed a higher number of postcibal reflux episodes compared to both patients with chronic cough and controls.
While interprandial refluxes could not be documented at ultrasonography, we underlined the importance of postcibal reflux in the pathogenesis of recurrent respiratory diseases in infants. Transient lower esophageal sphincter relaxation is the predominant mechanism of gastro-esophageal reflux[34] and postprandial reflux has been suggested to be a dynamic indicator of sphincter competency[20,35]; in this respect, postcibal ultrasonography allows direct visualization of both gastro-esophageal reflux and sphincter incompetency.
It has been reported that delayed clearance of refluxed material and clustering of reflux events may precipitate respiratory symptoms in infants[36,37]. Indeed, we found that amount, extent and number of postcibal gastro-esophageal reflux episodes were higher in subjects with symptomatic refluxes than in those without.
Although ultrasound visualization of reflux is limited to the distal esophagus, our results suggest the possibility that the involvement of the respiratory tract might be partly due to a greater chance for microaspiration of refluxed materials reaching the proximal esophagus. This possibility is more likely in subjects with a great amount and timing of gastro-esophageal reflux episodes. Interestingly, a recent study found a direct relation between incidence and duration of refluxes in the proximal and in the distal esophagus[38]. However, other mechanisms responsible for respiratory tract involvement secondary to gastro-esophageal reflux, particularly vagally mediated reflexes, may be taken into account[39].
The association of gastro-esophageal reflux disease and chronic cough in infants has been well demonstrated[2,23,40], and our results suggest that postcibal ultrasonography might be employed as a useful diagnostic approach also in patients with chronic cough.
Delayed gastric emptying has been frequently linked to gastro-esophageal reflux both in adults[41-43] and in pediatric age[16,44]. In a scintigraphic study in pediatric patients, it was found that delayed gastric emptying increased postprandial reflux by increasing the refluxate volume per episode of reflux through an underlying incompetent lower esophageal sphincter[44]. By using ultrasonography, we also performed the measurement of gastric emptying by a well standardized and validated procedure[11,16-20]. Our data demonstrated a similar extent and timing of postprandial gastric emptying in patients and controls. This is in accordance with a previous ultrasonographic study in infants with reflux disease, in which the speed of gastric emptying was unrelated to any interprandial or postprandial indices of gastro-esophageal reflux by a 24-h pH-metry[17]. Similar conclusions were also depicted by another recent study by (13)C-octanoic acid breath test, showing that gastric emptying was not delayed in infants with gastro-esophageal reflux[34].
In conclusion, postcibal esophageal ultrasonography is a useful and physiological screening test in patients with recurrent respiratory diseases, who show lower esophageal sphincter incompetence and high postprandial gastro-esophageal reflux prevalence, with a postprandial gastric emptying similar to controls. Subjects with recurrent bronchitis seem to have a greater amount of gastro-esophageal reflux episodes compared to those with chronic cough.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Andze GO, Brandt ML, St Vil D, Bensoussan AL, Blanchard H. Diagnosis and treatment of gastroesophageal reflux in 500 children with respiratory symptoms: the value of pH monitoring. J Pediatr Surg. 1991;26:295–299; discussion 295-299;. doi: 10.1016/0022-3468(91)90505-n. [DOI] [PubMed] [Google Scholar]
- 2.Burton DM, Pransky SM, Katz RM, Kearns DB, Seid AB. Pediatric airway manifestations of gastroesophageal reflux. Ann Otol Rhinol Laryngol. 1992;101:742–749. doi: 10.1177/000348949210100905. [DOI] [PubMed] [Google Scholar]
- 3.Orenstein SR, Shalaby TM, Cohn JF. Reflux symptoms in 100 normal infants: diagnostic validity of the infant gastroesophageal reflux questionnaire. Clin Pediatr (Phila) 1996;35:607–614. doi: 10.1177/000992289603501201. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd RW, Wren J, Evans S, Lander M, Ong TH. Gastroesophageal reflux in children. Clinical profile, course and outcome with active therapy in 126 cases. Clin Pediatr (Phila) 1987;26:55–60. doi: 10.1177/000992288702600201. [DOI] [PubMed] [Google Scholar]
- 5.Naik DR, Moore DJ. Ultrasound diagnosis of gastro-oesophageal reflux. Arch Dis Child. 1984;59:366–367. doi: 10.1136/adc.59.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naik DR, Bolia A, Moore DJ. Comparison of barium swallow and ultrasound in diagnosis of gastro-oesophageal reflux in children. Br Med J (Clin Res Ed) 1985;290:1943–1945. doi: 10.1136/bmj.290.6486.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes H. [Gastroesophageal reflux in infants: ultrasonographic reading of pHmetry] Arch Pediatr. 1994;1:639–645. [PubMed] [Google Scholar]
- 8.Westra SJ, Wolf BH, Staalman CR. Ultrasound diagnosis of gastroesophageal reflux and hiatal hernia in infants and young children. J Clin Ultrasound. 1990;18:477–485. doi: 10.1002/jcu.1870180605. [DOI] [PubMed] [Google Scholar]
- 9.Westra SJ, Derkx HH, Taminiau JA. Symptomatic gastroesophageal reflux: diagnosis with ultrasound. J Pediatr Gastroenterol Nutr. 1994;19:58–64. doi: 10.1097/00005176-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Riccabona M, Maurer U, Lackner H, Uray E, Ring E. The role of sonography in the evaluation of gastro-oesophageal reflux--correlation to pH-metry. Eur J Pediatr. 1992;151:655–657. doi: 10.1007/BF01957566. [DOI] [PubMed] [Google Scholar]
- 11.Portincasa P, Colecchia A, Di Ciaula A, Larocca A, Muraca M, Palasciano G, Roda E, Festi D. Standards for diagnosis of gastrointestinal motility disorders. Section: ultrasonography. A position statement from the Gruppo Italiano di Studio Motilità Apparato Digerente. Dig Liver Dis. 2000;32:160–172. doi: 10.1016/s1590-8658(00)80404-1. [DOI] [PubMed] [Google Scholar]
- 12.Bergmann JF, Chassany O, Petit A, Triki R, Caulin C, Segrestaa JM. Correlation between echographic gastric emptying and appetite: influence of psyllium. Gut. 1992;33:1042–1043. doi: 10.1136/gut.33.8.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolondi L, Bortolotti M, Santi V, Calletti T, Gaiani S, Labò G. Measurement of gastric emptying time by real-time ultrasonography. Gastroenterology. 1985;89:752–759. doi: 10.1016/0016-5085(85)90569-4. [DOI] [PubMed] [Google Scholar]
- 14.Hveem K, Jones KL, Chatterton BE, Horowitz M. Scintigraphic measurement of gastric emptying and ultrasonographic assessment of antral area: relation to appetite. Gut. 1996;38:816–821. doi: 10.1136/gut.38.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricci R, Bontempo I, Corazziari E, La Bella A, Torsoli A. Real time ultrasonography of the gastric antrum. Gut. 1993;34:173–176. doi: 10.1136/gut.34.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroccio A, Iacono G, Li Voti G, Montalto G, Cavataio F, Tulone V, Lorello D, Kazmierska I, Acierno C, Notarbartolo A. Gastric emptying in infants with gastroesophageal reflux. Ultrasound evaluation before and after cisapride administration. Scand J Gastroenterol. 1992;27:799–804. doi: 10.3109/00365529209011187. [DOI] [PubMed] [Google Scholar]
- 17.Ewer AK, Durbin GM, Morgan ME, Booth IW. Gastric emptying and gastro-oesophageal reflux in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;75:F117–F121. doi: 10.1136/fn.75.2.f117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabiani E, Bolli V, Pieroni G, Corrado G, Carlucci A, De Giacomo C, Catassi C. Effect of a water-soluble fiber (galactomannans)-enriched formula on gastric emptying time of regurgitating infants evaluated using an ultrasound technique. J Pediatr Gastroenterol Nutr. 2000;31:248–250. doi: 10.1097/00005176-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 19.LiVoti G, Tulone V, Bruno R, Cataliotti F, Iacono G, Cavataio F, Balsamo V. Ultrasonography and gastric emptying: evaluation in infants with gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 1992;14:397–399. doi: 10.1097/00005176-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Salvia G, De Vizia B, Manguso F, Iula VD, Terrin G, Spadaro R, Russo G, Cucchiara S. Effect of intragastric volume and osmolality on mechanisms of gastroesophageal reflux in children with gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:1725–1732. doi: 10.1111/j.1572-0241.2001.03865.x. [DOI] [PubMed] [Google Scholar]
- 21.Wedmann B, Schmidt G, Wegener M, Coenen C, Ricken D, Althoff J. Effects of age and gender on fat-induced gallbladder contraction and gastric emptying of a caloric liquid meal: a sonographic study. Am J Gastroenterol. 1991;86:1765–1770. [PubMed] [Google Scholar]
- 22.Armitage P, Berry G. Statistical methods in medical research. Oxford: Blackwell Science Ltd; 1994. [Google Scholar]
- 23.Megale SR, Scanavini AB, Andrade EC, Fernandes MI, Anselmo-Lima WT. Gastroesophageal reflux disease: its importance in ear, nose, and throat practice. Int J Pediatr Otorhinolaryngol. 2006;70:81–88. doi: 10.1016/j.ijporl.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Gold BD. Asthma and gastroesophageal reflux disease in children: exploring the relationship. J Pediatr. 2005;146:S13–S20. doi: 10.1016/j.jpeds.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 25.Scarupa MD, Mori N, Canning BJ. Gastroesophageal reflux disease in children with asthma: treatment implications. Paediatr Drugs. 2005;7:177–186. doi: 10.2165/00148581-200507030-00004. [DOI] [PubMed] [Google Scholar]
- 26.Chen PH, Chang MH, Hsu SC. Gastroesophageal reflux in children with chronic recurrent bronchopulmonary infection. J Pediatr Gastroenterol Nutr. 1991;13:16–22. doi: 10.1097/00005176-199107000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Sutphen JL, Dillard VL. pH-adjusted formula and gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 1991;12:48–51. doi: 10.1097/00005176-199101000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Tolia V, Kuhns L, Kauffman RE. Comparison of simultaneous esophageal pH monitoring and scintigraphy in infants with gastroesophageal reflux. Am J Gastroenterol. 1993;88:661–664. [PubMed] [Google Scholar]
- 29.Wenzl TG, Silny J, Schenke S, Peschgens T, Heimann G, Skopnik H. Gastroesophageal reflux and respiratory phenomena in infants: status of the intraluminal impedance technique. J Pediatr Gastroenterol Nutr. 1999;28:423–428. doi: 10.1097/00005176-199904000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Wenzl TG, Moroder C, Trachterna M, Thomson M, Silny J, Heimann G, Skopnik H. Esophageal pH monitoring and impedance measurement: a comparison of two diagnostic tests for gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2002;34:519–523. doi: 10.1097/00005176-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Peter CS, Wiechers C, Bohnhorst B, Silny J, Poets CF. Detection of small bolus volumes using multiple intraluminal impedance in preterm infants. J Pediatr Gastroenterol Nutr. 2003;36:381–384. doi: 10.1097/00005176-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Skopnik H, Silny J, Heiber O, Schulz J, Rau G, Heimann G. Gastroesophageal reflux in infants: evaluation of a new intraluminal impedance technique. J Pediatr Gastroenterol Nutr. 1996;23:591–598. doi: 10.1097/00005176-199612000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Wenzl TG, Skopnik H. Intraluminal impedance: an ideal technique for evaluation of pediatric gastroesophageal reflux disease. Curr Gastroenterol Rep. 2000;2:259–264. doi: 10.1007/s11894-000-0070-4. [DOI] [PubMed] [Google Scholar]
- 34.Omari TI, Barnett CP, Benninga MA, Lontis R, Goodchild L, Haslam RR, Dent J, Davidson GP. Mechanisms of gastro-oesophageal reflux in preterm and term infants with reflux disease. Gut. 2002;51:475–479. doi: 10.1136/gut.51.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mason RJ, Oberg S, Bremner CG, Peters JH, Gadenstätter M, Ritter M, DeMeester TR. Postprandial gastroesophageal reflux in normal volunteers and symptomatic patients. J Gastrointest Surg. 1998;2:342–349. doi: 10.1016/s1091-255x(98)80073-5. [DOI] [PubMed] [Google Scholar]
- 36.Gustafsson PM, Kjellman NI, Tibbling L. Bronchial asthma and acid reflux into the distal and proximal oesophagus. Arch Dis Child. 1990;65:1255–1258. doi: 10.1136/adc.65.11.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jolley SG, Herbst JJ, Johnson DG, Matlak ME, Book LS. Esophageal pH monitoring during sleep identifies children with respiratory symptoms from gastroesophageal reflux. Gastroenterology. 1981;80:1501–1506. [PubMed] [Google Scholar]
- 38.Bagucka B, Badriul H, Vandemaele K, Troch E, Vandenplas Y. Normal ranges of continuous pH monitoring in the proximal esophagus. J Pediatr Gastroenterol Nutr. 2000;31:244–247. doi: 10.1097/00005176-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Ricciardolo FL, Gaston B, Hunt J. Acid stress in the pathology of asthma. J Allergy Clin Immunol. 2004;113:610–619. doi: 10.1016/j.jaci.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 40.Contencin P, Narcy P. Gastropharyngeal reflux in infants and children. A pharyngeal pH monitoring study. Arch Otolaryngol Head Neck Surg. 1992;118:1028–1030. doi: 10.1001/archotol.1992.01880100018006. [DOI] [PubMed] [Google Scholar]
- 41.Baldi F, Corinaldesi R, Ferrarini F, Stanghellini V, Miglioli M, Barbara L. Gastric secretion and emptying of liquids in reflex esophagitis. Dig Dis Sci. 1981;26:886–889. doi: 10.1007/BF01309491. [DOI] [PubMed] [Google Scholar]
- 42.McCallum RW, Berkowitz DM, Lerner E. Gastric emptying in patients with gastroesophageal reflux. Gastroenterology. 1981;80:285–291. [PubMed] [Google Scholar]
- 43.Shay SS, Eggli D, McDonald C, Johnson LF. Gastric emptying of solid food in patients with gastroesophageal reflux. Gastroenterology. 1987;92:459–465. doi: 10.1016/0016-5085(87)90142-9. [DOI] [PubMed] [Google Scholar]
- 44.Estevão-Costa J, Campos M, Dias JA, Trindade E, Medina AM, Carvalho JL. Delayed gastric emptying and gastroesophageal reflux: a pathophysiologic relationship. J Pediatr Gastroenterol Nutr. 2001;32:471–474. doi: 10.1097/00005176-200104000-00015. [DOI] [PubMed] [Google Scholar]


