Abstract
AIM: To investigate the therapeutic effects of DA-9601 on sodium taurocholate (TCA)-induced chronic reflux gastritis in SD rats.
METHODS: In this study, we have investigated the therapeutic effects of DA-9601 on chronic erosive and atrophic gastritis induced by 6 mo of TCA administration (5 mmol/L in drinking water) in SD rats.
RESULTS: Four weeks of DA-9601 administration (0.065%, 0.216% in rat chow), following the withdrawal of TCA treatment, resulted in a significant decrease in total length of erosions in rats in a dose-dependent manner. Furthermore, the indicators of atrophic gastritis, such as reduced mucosal thickness and reduction in the number of parietal cells, were improved by the administration of DA-9601 in a dose-related manner. DA-9601 also attenuated inflammatory cell infiltration and the proliferation of collagenous fiber in the gastric mucosa. The improvement in the reduction of the gastric mucus was observed in the rats receiving a high dose of DA-9601 (0.216%). The therapeutic effect of DA-9601 on experimental chronic erosive gastritis was superior to that of rebamipide (1.08% in rat chow). Biochemical analyses showed increased mucosal prostaglandin E2 and reduced glutathione levels by DA-9601 treatment.
CONCLUSION: We suggest that DA-9601 is a promising agent for the treatment of chronic erosive and atrophic gastritis with an etiological factor of bile reflux. Increased mucosal prostaglandin E2 and reduced glutathione by DA-9601 treatment may be therapeutic mechanisms for chronic erosive and atrophic gastritis.
Keywords: DA-9601, Reflux gastritis, Erosive gastritis, Atrophic gastritis, Sodium taurocholate
INTRODUCTION
Reflux gastritis is a chronic disease in which the duodenal contents, particularly bile acid, are flown back into the stomach. It is associated with various symptoms, such as epigastric pain, dyspepsia, loss of appetite, nausea or vomiting[1,2]. While reflux gastritis is an occasional occurrence, it is primarily found in patients with pyloric insufficiency or delayed gastric emptying. It can also be found in patients who have previously undergone cholecystectomy or gastrectomy[3]. The reflux of intestinal fluid containing bile juice to the residual stomach has been considered as a primary pathogenic factor in this type of gastritis[4].
Although a number of treatments, such as improved gastric emptying, reduction of hydrochloric acid secretion and bile salt binding are available, a considerable portion of patients do not achieve the complete mucosal healing or suffer from sustained symptoms. Some studies have suggested the possible participation of bile acid in the development of erosive and atrophic gastritis[1,2]. Sodium taurocholate (TCA), a component of bile acid, induces erosive and atrophic gastritis, and is increasingly utilized in animal models for the study of bile reflux gastritis because of its simplicity and reproducibility[5]. Chronic exposure of the gastric mucosa of rats to TCA induces chronic erosive gastritis characterized by mucosal erosions, inflammatory cell infiltration, decreases in the number of parietal cells and mucosal thickness, and interstitial fibrosis. These characterizations are similar to those observed in human chronic erosive and reflux gastritis[6].
DA-9601 was developed for the treatment of gastritis[7-9]. DA-9601 was demonstrated to possess cytoprotective actions in various experimental models, including ethanol-induced gastric mucosal damage, and trinitrobenzene sulfonic acid (TNBS)-induced colitis[10]. Recently, DA-9601 has been reported to be effective in reflux esophagitis[11,12]. Although the mechanism via which DA-9601 exerts its mucosal protective effect has not been fully elucidated, stimulation of the mucus, mucosal prostaglandins (PG), and reduced glutathione (GSH) are thought to play crucial roles in producing the gastric mucosal protective effect. The present study was undertaken to evaluate the effects of DA-9601 on TCA-induced chronic and atrophic gastritis in rats.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (weighing 210-230 g, aged 7 wk) were purchased from Charles River Japan (Kanagawa, Japan). Experimental procedures were performed in conformity with the Institutional Standard Procedure for Animal Care and Experiment (SOP-ANC) of Dong-A Pharmaceutical Company. The animals were kept under standard laboratory conditions and allowed free access to rodent chow (Cheil, Korea) and UV-sterilized tap water ad libitum in standard wire cage under a 12:12-h light-dark (LD) cycle. After a 5 d acclimation period, rats were treated according to the experimental protocols. Each group contained 20 rats, of them 10 rats were used for histological evaluation and the other rats were used for molecular assay.
Materials
DA-9601 was produced by Dong-A Pharmaceutical Co. Ltd. (Yongin, Korea). TCA was purchased from Sigma (St. Louis, USA), and rebamipide was obtained from Korea Otsuka (Seoul, Korea). DA-9601 (0.065% and 0.0216%) and rebamipide (1.08%) were mixed with rodent chow and then administered to the rats.
Sodium taurocholate (TCA)-induced gastritis and administration of DA-9601
Chronic gastritis was induced in rats by the administration of 5 mmol/L TCA dissolved in distilled water for 6 mo[13,14]. After discontinuation of TCA treatment, rats were allowed to drink plain tap water. To investigate the effects of the treatment, rats were divided into five groups, each consisting of 10 rats. In group 1, rats were fed a standard pellet meal and tap water for 7 mo. In group 2, rats were treated with TCA for 6 mo and then given the same standard pellet meals as used in the previous 6 mo and tap water alone for 1 mo. In groups 3, 4, and 5, rats were treated with TCA for 6 mo and then fed a standard pellet meal which was formulated to contain 0.065%, 0.216% of DA-9601 and 1.08% of rebamipide, respectively for 1 mo. Group 3 (0.065% of DA-9601), group 4 (0.216% of DA-9601), and group 5 (1.08% of rebamipide) all received tap water, following discontinuation of TCA treatment.
Histological evaluation
Rats were fasted for 24 h prior to the experiment and killed by decapitation under light ether anesthesia. The abdomen was opened and the stomach was then excised, opened along the greater curvature, laid flat so as not to cause any damage, and examined carefully for any evidence of macroscopic damage. The flattened stomach was divided into five parts, and each part was rolled in a Swiss manner for observation from the cardiac to the pyloric region. The Swiss-rolled tissue specimens were fixed in Bouin’s solution for 6 h at 4 °C, and paraffin-embedded blocks were prepared by the routine method. After sectioning, each specimen was stained with H&E and Masson's trichrome and examined under a light microscope (BH-2, Olympus, Japan) by a pathologist who was blinded to the study. The length of mucosal surface injury was measured on the entire length of each tissue section in a visual field of 100-fold magnification. The total length of mucosal surface injury for the five parts per stomach was expressed as a measured length. The mucosal thickness was measured in a visual field of 100-fold magnification. The number of parietal cells per unit area (mm2) in a visual field of 400-fold magnification was counted. The extents of inflammatory cell infiltration, the proliferation of collagenous fiber and stained gastric mucosa were evaluated in a visual field of 100-fold magnification.
Tissue malondialdehyde (MDA) and reduced glutathione (GSH)
The content of hepatic MDA was determined using the method described by Ohkawa et al[15]. In brief, after mincing and trimming, the pieces of liver were homogenized with four volumes of an ice-cold 0.1 mol/L potassium phosphate buffer (pH 7.4) solution. Then, the reaction mixture containing 0.2 mL of the homogenate, 81 g/L sodium dodecyl sulfate, 200 g/L acetate buffer (pH 3.5), and 8 g/L thiobarbituric acid (TBA) solution was mixed well for 3 min and incubated at 95 °C for 60 min. TBA reactive substance, MDA, was extracted with a butanol-pyrimidine mixture solution. The absorbance measured at 532 nm was expressed as nmol/mg protein. The content of hepatic glutathione (GSH) was determined by the spectrophotometric method of Griffith and expressed as nmol/mg protein[16]. Protein content was determined by the method described by Lowry et al[17]. using BSA as the standard.
Level of prostaglandin E2
Using a commercially available EIA kits (Amersham, UK), gastric mucosal PGE2 levels were measured. Immediately after biopsy and gross observation, mucosal specimens were frozen in liquid nitrogen and stored at -70 °C until the measurement of PGE2. Tissue specimens were processed for the assay according to the method described previously by Lipscomb and Rees[18]. The final PGE2 level was expressed in pg/mg of wet biopsy weight.
Statistical analysis
All data were expressed as mean±SE. Scheffe’s t-test was used for comparing body weight, tissue MDA, tissue GSH, and endoscopic scores between the control group and experimental groups. Rank transformation and Kruskal-Wallis test were performed to determine the inter-group difference of non-parametric data, and Bonferroni’s test was used for multiple pair-wise comparison. A P<0.05 was considered statistically significant.
RESULTS
Inhibitory effect of DA-9601 on chronic gastritis
Macroscopic lesions were not observed on the surface of mucosa in any of the groups, while marked changes were observed microscopically in the gastric mucosa of the rats treated with TCA for 6 mo (Figure 1). A significant increase in the length of erosion was observed in the control animals as compared to normal level (Figure 2). However, the microscopic appearance of the gastric mucosa taken from group 3 or 4, treated with 0.065% and 0.216% DA-9601, respectively, showed a significant decrease in the length of erosion. DA-9601 significantly decreased the length of erosion in a dose-dependent manner. Although treatment with rebamipide also caused a significant decrease of erosive lesion, this protective effect by rebamipide was not as prominent as observed in group 4 (treated with 0.216% DA-9601). To explore the mechanism underlying DA-9601-induced protection against gastric lesions, the thickness of the gastric mucus layer was measured (Figure 3). We observed that 0.065% DA-9601 ameliorated the TCA-induced reduction of mucosal thickness in the antrum, while 1.08% rebamipide significantly thickened the mucus layer in the fundus.
Figure 1.
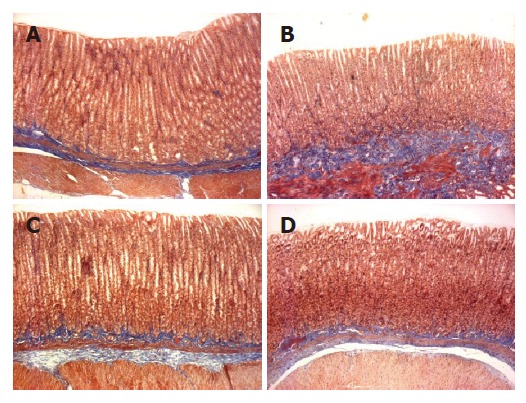
Microscopic appearance of gastric mucosa showing the effect of DA-9601 and rebamipide on sodium taurocholate (TCA)-induced erosive and atrophic gastritis in rats (×100). Rats were administered with 5 mmol/L TCA for
Figure 2.
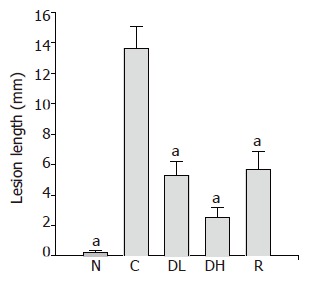
Inhibitory effects of DA-9601 and rebamipide on the length of mucosal lesion of TCA-induced erosive and atrophic gastritis in rats. DA-9601 (0.065% and 0.0.216%) and rebamipide (1.08%) were mixed with rodent chow and then administered to the rats. N: Normal; C: control; DL: 0.065% DA-9601; DH: 0.216% DA-9601; R: 1.08% rebamipide. Data are expressed as mean±SE. aP<0.05 vs controls.
Figure 3.
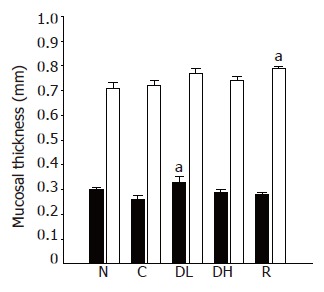
Effects of DA-9601 and rebamipide on the mucosal thickness of TCA-induced erosive and atrophic gastritis in rats. N: Normal; C: control; DL: 0.065% DA-9601; DH: 0.216% DA-9601; R: 1.08% rebamipide. Data are expressed as mean±SE. aP<0.05 vs controls.
Effect of DA-9601 on parietal cells, infiltration of inflammatory cells and intestinal fibrosis
The number of parietal cells in the controls (99.1±2.2 cells/0.25 cm2) was significantly lower than that of the normal level (107.5±2.8 cells/0.25 cm2). Furthermore, 0.065% DA-9601 (112.8±3.2 cells/0.25 cm2), 0.216% DA-9601 (115.4±2.9 cm2) and 1.08% rebamipide (113.9±2.9 cm2) significantly increased the number of parietal cells in TCA-induced chronic gastritis as compared to the control (Figure 4).
Figure 4.
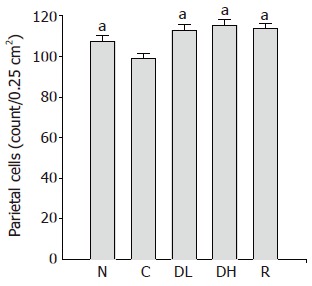
Effects of DA-9601 and rebamipide on the number of parietal cells in TCA-induced erosive and atrophic gastritis in rats. N: Normal; C: control; DL: 0.065% DA-9601; DH: 0.216% DA-9601; R: 1.08% rebamipide. Data are expressed as mean±SE. aP<0.05 vs controls.
The number of infiltrating inflammatory cells in the gastric mucosa of animals in the control group (4.7±0.86) was compared to the normal level (0.9±0.11). We observed significantly decreased number of infiltrating inflammatory cells in the gastric mucosa of the animals treated with 0.065% DA-9601 (1.4±0.27), 0.216% DA-9601 (2.0±0.35) and 1.08% rebamipide (3.0±0.37) as compared to the controls (P<0.05) (Figure 5).
Figure 5.
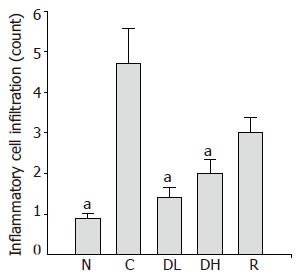
Effects of DA-9601 and rebamipide on the infiltration of inflammatory cells induced by TCA. N: Normal; C: control; DL: 0.065% DA-9601; DH: 0.216% DA-9601; R: 1.08% rebamipide. Data are expressed as mean±SE. aP<0.05 vs controls.
In addition, 0.065% DA-9601 (0.4±0.1) significantly reduced TCA-induced intestinal fibrosis (Figure 6). We found an obvious increase in the score of intestinal fibrosis in controls (1.1±0.2) as compared to normal level (0.3±0.2). On contrary, 0.216% DA-9601 (0.6±0.1) and 1.08% rebamipide (0.6±0.1) showed a decreasing tendency in the score of intestinal fibrosis (Figure 6).
Figure 6.
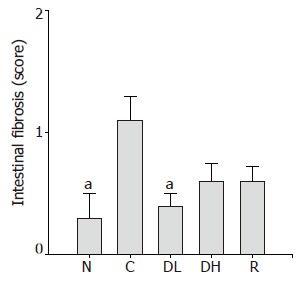
Effects of DA-9601 and rebamipide on the proliferation of collagenous fiber induced by TCA in rats. N: Normal; C: control; DL: 0.065% DA-9601; DH: 0.216% DA-9601; R: 1.08% rebamipide. Data are expressed as mean±SE. aP<0.05 vs controls.
Effects of DA-9601 on gastric mucosal MDA, PGE2, and GSH
Lipid peroxidation is a well-established mechanism of cellular injury, and the accumulation of MDA is used as an indicator of oxidative stress in cells and tissues. The mean level of MDA in animals treated with DA-9601 and rebamipide was not significantly different from that of controls (Figure 7).
Figure 7.
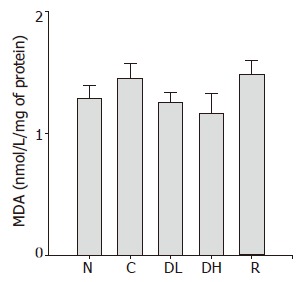
Effects of DA-9601 and rebamipide on malondialdehyde (MDA) induced by TCA in rat gastric mucosa. N: Normal; C: control; DL: 0.065% DA-9601; DH: 0.216% DA-9601; R: 1.08% rebamipide. Data are expressed as mean±SE. aP<0.05 vs controls.
PGE2 plays an important role in the regulation of gastric mucus secretion. As shown in Figure 8, we detected a significant decrease in PGE2 contents in the controls (215.2±84.4 pg/mg of protein) as compared to the normal level (374.0±64.9 pg/mg of protein). The treatment with 0.065% DA-9601 (440.3±93.0 pg/mg of protein) and 0.216% DA-9601 (538.7±78.5 pg/mg of protein) could significantly increase the PGE2 contents in a dose-dependent manner, and 1.08% rebamipide (421.6±88.4 pg/mg of protein) could significantly ameliorate the PGE2 production (Figure 8).
Figure 8.
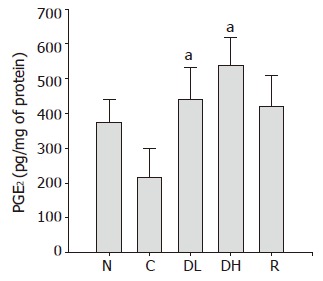
Effects of DA-9601 and rebamipide on PGE2 induced by TCA in rat gastric mucosa. N: normal; C: control; DL: 0.065% DA-9601; DH: 0.216% DA-9601; R: 1.08% rebamipide. Data are expressed as mean±SE. aP<0.05 vs controls.
As shown in Figure 9, we found a significant decrease in the GSH contents in the controls (23.5±4.3 nmol/mg of protein) as compared to normal level (37.0±4.6 nmol/mg of protein). We observed that the treatment with 0.065% DA-9601 (42.7±6.1 nmol/mg of protein) and 0.216% DA-9601 (48.5±4.5 nmol/mg of protein) significantly increased the GSH contents in a dose-dependent manner, and 1.08% rebamipide (43.2±5.3 nmol/mg of protein) significantly ameliorated the GSH production.
Figure 9.
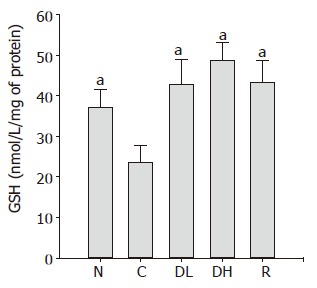
Effects of DA-9601 and rebamipide on glutathione (GSH) induced by TCA in rat gastric mucosa. N: Normal; C: control; DL: 0.065% DA-9601; DH: 0.216% DA-9601; R: 1.08% rebamipide. Data are expressed as mean±SE. aP<0.05 vs controls.
DISCUSSION
In the present study, 6 mo of DA-9601 administration resulted in a significant improvement of the experimental chronic erosive and atrophic gastritis induced by 6 mo of TCA treatment. The therapeutic effects of DA-9601 on experimental gastritis were superior to those of rebamipide observed in clinical practice for the treatment of gastritis. Six months of oral administration of TCA solution as drinking water induced the mucosal surface erosions and gastric mucosal thinning, and decreased parietal cell numbers, inflammatory cell infiltration in the lamina propria of the mucosa and mucosal fibrosis in rats. These characterizations were similar to those observed in chronic gastritis caused by bile reflux in human beings[19,20]. DA-9601 ameliorated the reduction in mucosal thickness and the decrease in the number of parietal cells induced by TCA treatment. Furthermore, DA-9601 increased the stimulation of PGE2 and GSH in the gastric mucosa. These results suggest that DA-9601 promotes the healing of TCA-induced chronic erosive and atrophic gastritis with an etiological factor of the bile reflux.
The TCA-induced infiltration of inflammatory cells in the gastric mucosa was ameliorated by the administration of DA-9601. Tissue damage causes the infiltration of inflammatory cells that have migrated from the capillary vessels. Therefore, this amelioration of inflammatory cell infiltration by DA-9601 may be due to a decreased inflammatory response against damaging tissues through the healing of gastric surface injury. The interstitial space in the gastric mucosa may play an important role as a regulative factor in local microcirculation, and changes in this area may influence the gastric gland, particularly the glandular portion and the cell proliferative zone. Mizumachi et al[21]. reported that interstitial fibrosis was associated with decreased gastric mucosal blood flow. In this study, DA-9601 markedly ameliorated the TCA-induced proliferation of collagenous fiber. This change appears to be associated with the improvements in microcirculation via DA-9601-induced increased PGE2 level.
In fact, reactive oxygen species has been suggested to be involved in the pathogenesis of gastric lesion caused by indomethacin[22,23]. In addition, previous studies have reported that the levels of scavengers and mucosal SOD activity were decreased and the lipid peroxide concentration was increased in the gastric mucosa before the sign of tissue injury appeared[24,25]. Some drugs that are capable of scavenging or inhibiting the generation of ROS have been introduced as effective agents to prevent the gastric mucosal injuries induced by various agents[26,27]. Recently, DA-9601 has been demonstrated to inhibit xanthine oxidase, a major source of ROS generation[28].
One of the causes of TCA-induced gastric lesions is that taurocholate selectively inhibits the synthesis of the vasodilator, PGI2, an endogenous gastroprotector[29]. The suppression of PGI2 production decreases the PGI2/TXA2 ratio, suggesting that the action of TXA2 overcomes the action of PGI2. This decreased ratio may reflect the pathogenesis of gastric ischemia due to vasoconstriction[30,31].
The mechanism by which DA-9601 produces such a therapeutic effect is possibly through its actions that elevate mucosal concentrations of PGE2, increase the gastric mucosal blood flow, and stimulate re-epithelization and the formation of glandular tissue. PGE2 has been reported to exert its cytoprotective effect on gastric mucosal epithelial cells by increasing gastric mucus secretion and stimulating cell proliferation in pyloric glands in rats[32]. It has been suggested that DA-9601 promotes the compensatory elevation in proliferative activity of generative cells through the possible increase in PG, which would be available to repair the gastric mucosa in erosive and atrophic gastritis. Gastric mucosal blood flow is essential for the homeostasis of the mucosal structure and function[33]. Whittle et al[30]. reported that the administration of (15s)-15-methyl PGE2 decreased acid back diffusion, increased mucosal blood flow, and significantly reduced the lesions formed by topical TCA plus HCl and indomethacin administration. Kishimoto et al[5]. reported that mucosal blood flow was decreased by the administration of TCA. DA-9601 may improve the decreased mucosal blood flow induced by TCA administration although the mucosal blood flow was not measured in this study.
Another possible mechanism is that DA-9601, an antioxidant, neutralizes reactive free oxygen metabolites in the mucosa and attenuates the inflammation. Our results demonstrated that gastric mucosal GSH concentrations were depleted in TCA-induced chronic erosive and atrophic gastritis. The depletion of gastric mucosal GSH in rats with chronic gastritis is likely to render the gastric mucosa more susceptible to oxidative damages and the injurious effects of reactive oxygen species. GSH acts as a scavenger of free radicals and toxic substances ingested with foods or produced directly in the gastrointestinal tract[34].
Reflux gastritis, which occasionally occurs after gastrectomy, is associated with various symptoms, such as abdominal pain, nausea, emesis, and loss of appetite. However, the pathogenic mechanism responsible for the development of gastritis due to bile regurgitation in the stomach has not been fully elucidated. Treating patients exhibiting alkaline reflux symptoms with bile acid binding agents, such as cholestyramine or sucralfate, has been reported to be successful in some cases, but not uniformly successful. It is possible that the presence of lysolecithin, pancreatic enzymes, and cholic acids is responsible for causing erosive changes in the stomach wall[11]. A study has suggested that these factors destroy the mucosal barrier and increase its permeability to hydrogen ions, which can initiate inflammation through a mechanism similar to the one attributed to the erosive effects of salicylates[12].
In conclusion, DA-9601 is a promising agent for the treatment of chronic erosive and atrophic gastritis with an etiological factor of bile reflux. Increased mucosal PGE2 and reduced glutathione levels by DA-9601 may be its therapeutic mechanisms for chronic erosive and atrophic gastritis.
Footnotes
Supported by the grant from Korean Ministry of Health and Welfare, HMP-98-D-1-0007
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
References
- 1.van Heerden JA, Phillips SF, Adson MA, McIlrath DC. Postoperative reflux gastritis. Am J Surg. 1975;129:82–88. doi: 10.1016/0002-9610(75)90172-5. [DOI] [PubMed] [Google Scholar]
- 2.Mackman S, Lemmer KE, Morrissey JF. Postoperative reflux alkali gastritis and esophagitis. Am J Surg. 1971;121:694–697. doi: 10.1016/0002-9610(71)90048-1. [DOI] [PubMed] [Google Scholar]
- 3.Madura JA. Primary bile reflux gastritis: diagnosis and surgical treatment. Am J Surg. 2003;186:269–273. doi: 10.1016/s0002-9610(03)00213-7. [DOI] [PubMed] [Google Scholar]
- 4.Ritchie WP. Alkaline reflux gastritis: a critical reappraisal. Gut. 1984;25:975–987. doi: 10.1136/gut.25.9.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishimoto S, Ogawa M, Takaba N, Kambara A, Okamoto K, Shimizu S, Kunita T, Daitoku K, Kajiyama G. Experimental model of gastritis induced by sodium taurocholate in rats. Hiroshima J Med Sci. 1986;35:143–147. [PubMed] [Google Scholar]
- 6.Kishimoto S, Kobuke H, Kobayashi H, Kajiyama G, Miyoshi A. Treatment of established taurocholate-induced chronic erosive gastritis in rats with cimetidine. Res Commun Chem Pathol Pharmacol. 1991;71:273–292. [PubMed] [Google Scholar]
- 7.Oh TY, Ryu BK, Park JB, Lee SD, Kim WB, Yang J, Lee EB. Studies on antiulcer effects of DA-9601, an Artemisia Herba extract against experimental gastric ulcers and its mechanism. Korean J Appl Pharmacol. 1996;4:111–121. [Google Scholar]
- 8.Ahn BO, Ryu BK, Ko JI, Oh TY, Kim SH, Kim WB, Yang J, Lee EB, Hahm KB. Beneficial effect of DA-9601, an extract of Artemisiae Herba, on animal models of inflammatory bowel disease. Korean J Appl Pharmacol. 1997;5:165–173. [Google Scholar]
- 9.Ahn BO, Oh TY, Ryu BK, Kim SH, Kim WB, Kang SH, Chun MS, Yoon JH. Protective effect of DA-9601, an Artemisia Herba extract, on radiation-induced colitis in wistar rats. Korean J Appl Pharmacol. 1998;6:37–44. [Google Scholar]
- 10.Oh TY, Ryu BK, Ko JI, Ahn BO, Kim SH, Kim WB, Lee EB, Jin JH, Hahm KB. Protective effect of DA-9601, an extract ofArtemisiae Herba, against naproxen-induced gastric damage in arthritic rats. Arch Pharm Res. 1997;20:414–419. doi: 10.1007/BF02973932. [DOI] [PubMed] [Google Scholar]
- 11.Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Lee KM, Hahm KB. Oxidative stress is more important than acid in the pathogenesis of reflux oesophagitis in rats. Gut. 2001;49:364–371. doi: 10.1136/gut.49.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JS, Oh TY, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Kim HJ, Hahm KB. Involvement of oxidative stress in experimentally induced reflux esophagitis and Barrett's esophagus: clue for the chemoprevention of esophageal carcinoma by antioxidants. Mutat Res. 2001;480-481:189–200. doi: 10.1016/s0027-5107(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 13.Kishimoto S, Konemori R, Kambara A, Okamoto K, Shimizu S, Koh H, Kunita T, Daitoku K, Kajiyama G. Endocrine profile in rats with postgastrectomy malabsorption: a pilot study. Hiroshima J Med Sci. 1985;34:209–213. [PubMed] [Google Scholar]
- 14.Kishimoto S, Kato R, Mukai T, Kanbara A, Okamoto K, Shimizu S, Daitoku K, Kajiyama G. Distribution and endocrine morphology of polypeptide YY (PYY) containing cells in the human gut. Hiroshima J Med Sci. 1985;34:155–160. [PubMed] [Google Scholar]
- 15.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Lipscomb GR, Rees WD. Gastric mucosal injury and adaptation to oral and rectal administration of naproxen. Aliment Pharmacol Ther. 1996;10:133–138. doi: 10.1046/j.1365-2036.1996.726118000.x. [DOI] [PubMed] [Google Scholar]
- 19.Jerzy Glass GB, Pitchumoni CS. Structural and ultrastructural alterations, exfoliative cytology and enzyme cytochemistry and histochemistry, proliferation kinetics, immunological derangements and other causes, and clinical associations and sequallae. Hum Pathol. 1975;6:219–250. [PubMed] [Google Scholar]
- 20.Morson BC, Dawson IMP. Chronic atrophic gastritis. In: Morson BC Gastrointestinal pathology., editor. Oxford: Blackwell Sci Pub; 1979. pp. 99–101. [Google Scholar]
- 21.Mizumachi S, Takeuchi K, Matsuda K, Harada H, Shimata M, Tada M, Saito M, Sakaki N, Iida Y, Okazaki Y. [The histological and functional studies of experimental gastritis in rats] Nihon Shokakibyo Gakkai Zasshi. 1986;83:7–16. [PubMed] [Google Scholar]
- 22.Vaananen PM, Meddings JB, Wallace JL. Role of oxygen-derived free radicals in indomethacin-induced gastric injury. Am J Physiol. 1991;261:G470–G475. doi: 10.1152/ajpgi.1991.261.3.G470. [DOI] [PubMed] [Google Scholar]
- 23.Yoshikawa T, Naito Y, Kishi A, Tomii T, Kaneko T, Iinuma S, Ichikawa H, Yasuda M, Takahashi S, Kondo M. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut. 1993;34:732–737. doi: 10.1136/gut.34.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oka S, Ogino K, Hobara T, Yoshimura S, Yanai H, Okazaki Y, Takemoto T, Ishiyama H, Imaizumi T, Yamasaki K. Role of active oxygen species in diethyldithiocarbamate-induced gastric ulcer in the rat. Experientia. 1990;46:281–283. doi: 10.1007/BF01951766. [DOI] [PubMed] [Google Scholar]
- 25.Ogino K, Hobara T, Kawamoto T, Kobayashi H, Iwamoto S, Oka S, Okazaki Y. Mechanism of diethyldithiocarbamate-induced gastric ulcer formation in the rat. Pharmacol Toxicol. 1990;66:133–137. doi: 10.1111/j.1600-0773.1990.tb00719.x. [DOI] [PubMed] [Google Scholar]
- 26.Cho CH, Pfeiffer CJ, Misra HP. Ulcerogenic mechanism of ethanol and the action of sulphanilyl fluoride on the rat stomach in-vivo. J Pharm Pharmacol. 1991;43:495–498. doi: 10.1111/j.2042-7158.1991.tb03521.x. [DOI] [PubMed] [Google Scholar]
- 27.Hearse DJ, Manning AS, Downey JM, Yellon DM. Xanthine oxidase: a critical mediator of myocardial injury during ischemia and reperfusion? Acta Physiol Scand Suppl. 1986;548:65–78. [PubMed] [Google Scholar]
- 28.Huh K, Kwon TH, Shin US, Kim WB, Ahn BO, Oh TY, Kim JA. Inhibitory effects of DA-9601 on ethanol-induced gastrohemorrhagic lesions and gastric xanthine oxidase activity in rats. J Ethnopharmacol. 2003;88:269–273. doi: 10.1016/s0378-8741(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 29.Calcamuggi G, Lanzio M, Babini G, Martini S, Anfossi G, Emanuelli G. Sodium taurocholate affects prostacyclin constitutive production by cultured human vascular endothelial cells. J Lab Clin Med. 1990;115:756–760. [PubMed] [Google Scholar]
- 30.Whittle BJ, Kauffman GL, Moncada S. Vasoconstriction with thromboxane A2 induces ulceration of the gastric mucosa. Nature. 1981;292:472–474. doi: 10.1038/292472a0. [DOI] [PubMed] [Google Scholar]
- 31.Esplugues JV, Whittle BJ. Gastric mucosal damage induced by local intra-arterial administration of Paf in the rat. Br J Pharmacol. 1988;93:222–228. doi: 10.1111/j.1476-5381.1988.tb11425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki Z, Murakami M, Misaki F, Kawai K. [Turnover of the superficial epithelial cells of the gastric mucosa] Nihon Rinsho. 1984;42:95–102. [PubMed] [Google Scholar]
- 33.Gannon B, Browning J, O'Brien P, Rogers P. Mucosal microvascular architecture of the fundus and body of human stomach. Gastroenterology. 1984;86:866–875. [PubMed] [Google Scholar]
- 34.Shirin H, Pinto JT, Liu LU, Merzianu M, Sordillo EM, Moss SF. Helicobacter pylori decreases gastric mucosal glutathione. Cancer Lett. 2001;164:127–133. doi: 10.1016/s0304-3835(01)00383-4. [DOI] [PubMed] [Google Scholar]


