Abstract
Exposure to hand-transmitted vibration in the work-place can result in the loss of sensation and pain in workers. These effects may be exacerbated by pre-existing conditions such as diabetes or the presence of primary Raynaud's phenomena. The goal of these studies was to use an established model of vibration-induced injury in Zucker rats. Lean Zucker rats have a normal metabolic profile, while obese Zucker rats display symptoms of metabolic disorder or Type II diabetes. This study examined the effects of vibration in obese and lean rats. Zucker rats were exposed to 4 h of vibration for 10 consecutive days at a frequency of 125 Hz and acceleration of 49 m/s2 for 10 consecutive days. Sensory function was checked using transcutaneous electrical stimulation on days 1, 5 and 9 of the exposure. Once the study was complete the ventral tail nerves, dorsal root ganglia and spinal cord were dissected, and levels of various transcripts involved in sensorineural dysfunction were measured. Sensorineural dysfunction was assessed using transcutaneous electrical stimulation. Obese Zucker rats displayed very few changes in sensorineural function. However they did display significant changes in transcript levels for factors involved in synapse formation, peripheral nerve remodeling, and inflammation. The changes in transcript levels suggested that obese Zucker rats had some level of sensory nerve injury prior to exposure, and that exposure to vibration activated pathways involved in injury and re-innervation.
Keywords: Zucker rat, Metabolic disorder, Hyperalgesia, Neuropathic pain
1. Introduction
Workers using vibrating hand-tools may develop a disorder known as hand-arm vibration syndrome (HAVS). This disorder is characterized by cold-induced vasospasms that result in finger blanching, reductions in peripheral tactile sensitivity and grip strength, and pain (Griffin, 1990). To assess changes in sensorineural perception (including tactile perception and pain), workers can be tested for sensitivity to vibrotactile stimulation (Harazin et al., 2005; McGeoch et al., 2004), nerve conduction velocity (Bovenzi et al., 2000; Cherniack et al., 2004; House et al., 2008; Sakakibara et al., 1996), and pressure (Cederlund et al., 2003). These tests can be confounded by a number of factors including environmental temperature, posture, noise and/or a pre-existing disease state, such as hypertension, primary Raynaud's phenomenon and diabetes (McGeoch et al., 1994; Pelmear and Kusiak, 1994; Stromberg et al., 1999). Although the testing environment can be controlled, thus improving the ability to diagnosis HAVS, the presence of a pre-existing condition can only be noted. However, the effects of these pre-existing conditions on diagnosis of HAVS, or the development of the disorder cannot be determined (ISO, 2001; Krajnak et al., 2009).
In the United States, it is estimated that 29.1 million people over the age of 20 have Type II diabetes (Prevention, 2014). Left untreated, Type II diabetes serves as a significant risk factor for the development of cardiovascular disorders and neuropathic pain (McMillan, 1997; CDC, 2014; Saely et al., 2007; Tack et al., 1994). Because these symptoms are similar to those caused by occupational exposure to vibration, and the presence of these symptoms can confound tests used to diagnose HAVS, it is important to understand how vibration affects the sensorineural and peripheral vascular system in workers with diabetes.
As a first step to understanding how these factors interact to affect disease state and its diagnosis, we used lean and obese Zucker rats. Obese Zucker rats have an autosomal recessive mutation of the leptin receptor gene that disrupts leptin signaling and results in hyperphagia and weight gain throughout the life of the animal. These rats are overweight, have increased insulin and triglyceride levels and develop hypertension as they get older (Bray, 1977), and thus are used as a model of type II diabetes. We previously reported that in Zucker rats, glucose levels and obesity (both symptoms of type II diabetes and metabolic disorder), resulted in an increased risk of developing vascular symptoms that were associated with vibration exposure. In that study, we reported that in obese rats, the ability of acetylcholine to re-dilate arteries after vasoconstriction was reduced compared to their lean control counterparts (Krajnak et al., 2009). A second part of the same study assessed peripheral nerve function, and examined factors associated with changes in sensory perception and pain. We hypothesized that vibration-induced changes in peripheral nerve function and associated biological markers or sensory dysfunction would be more prominent in obese rats than in lean rats from the same strain. To perform these studies we used an animal model of vibration that was characterized at the National Institute for Occupational Safety and Health (NIOSH) (Welcome et al., 2008). Using this model, the tails of rats are exposed to vibration at the resonant frequency (i.e., the frequency that results in the greatest physical stress and strain in the tissue). The rat tail serves as a good model for studying vibration-induced changes in sensorineural and vascular function in human fingers because the resonant frequency of the tail falls in the same range as the resonant frequency of the human fingers, and long-term exposure of the tail to vibration causes physiological and anatomical changes that are similar to those seen in human fingers. In this study, lean and obese Zucker rats were used to examine the effects of high levels of circulating glucose on the responsiveness of sensory nerves to transcutaneous electrical stimulation and heat sensitivity. In addition, peripheral nerves, dorsal root and spinal cord sections were assessed for markers indicative of nerve injury and the development of markers of pain.
2. Results
2.1. Physiological tests
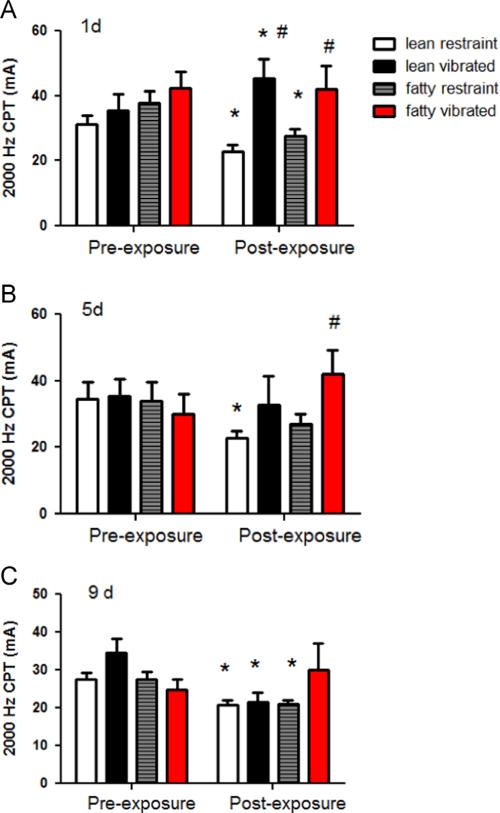
The results of the 2000 Hz CPT tests are presented in Fig. 1. On day 1, there were no significant differences between the groups in pre-exposure CPTs (Fig. 1A). However, following the exposure on day 1, control animals in both groups displayed a lower CPT value (i.e., indicative of an increased sensitivity to transcutaneous electrical stimulation). In contrast, exposure to vibration resulted in an increased CPT (i.e., reduced sensitivity) in both lean and obese rats. This increase was in comparison to pre-exposure levels.
Fig. 1.
These graphs show the current perception thresholds at 2000 Hz before and after. vibration exposures on days 1 (A), 5 (B) and 9 (C) of the experiment. On day 1, both lean and obese control rats showed an increased sensitivity to stimulation at 2000 Hz (the CPT was reduced as compared to pre-exposure values, * different than pre-exposure values, p<0.05). Lean rats exposed to vibration displayed a reduction in sensitivity to the 2000 Hz stimulus (i.e., an increase in the CPT value) after exposure to vibration, and obese rats did not display any significant changes pre- to post-exposure on day 1 of the experiment. However, both lean and obese rats exposed to vibration had higher CPTs as compared to control rats post-vibration exposure (# different than post-exposure controls, p<0.05). On day 5 of the experiment, lean rats exposed to vibration showed a reduction in the CPT pre- to post-vibration exposure, and obese rats showed an increased CPT after vibration exposure as compared to obese controls. On day 9 of the study, lean control, lean vibrated and obese control rats displayed a lower post-exposure CPT than pre-exposure CPT. However, obese rats did not display any significant changes in CPT.
On day 5 (Fig. 1B), all pre-exposure CPTs at 2000 Hz were similar (Table 1). However, after control exposure, lean rats displayed a reduction in sensitivity to 2000 Hz, but obese rats did not. Obese rats displayed a reduced sensitivity to the 2000 Hz CPT as compared to lean rats after vibration exposure. On day 9 of the study, all rats displayed similar responses to the 2000 Hz stimulation before vibration exposure (Fig. 1C). 2000 Hz CPT values were lower post-exposure than pre-exposure in all groups but the obese vibrated.
Table 1.
Transcripts examined by qPCR. This table shows the transcripts, tissue and primers used to assess transcript levels in various tissues.
| Transcript | Nerve | DRG | Spinal cord | Primary sequence |
|---|---|---|---|---|
| Ribosomal 18S | + | + | + | 18S-F AATCAGTTATGGTTCCTTTGTC |
| M11188.1 | 18S-R GCTCTAGAATTACCAGTTATCCAA | |||
| α2 A-adrenoreceptor (α2A) | – | + | – | 2A-F GTGTGTTGGTTCCCGTTCTT |
| NM_012739 | 2A-R CGGAAGTCGTGGTTGAAAAT | |||
| α2 C-adrenoreceptor (α2C) | – | + | – | 2C-F GGGTTTCCTCATCGTTTTCA |
| NM_138506 | 2C-R GAAAAGGGCATGACCAGTGT | |||
| Chemokine receptor type 2 (CCR2) | CCR2-F AAGAAGTATCCAAGAGCTTGATGAG | |||
| NM_021866.1 | + | + | – | CCR2-R TCACCATCATCATAGTCATACGG |
| Calcitonin gene related peptide | – | + | – | CGRP-F CCCAGAAGAGATCCTGCAAC |
| AY702025 | CGRP-R GTGGGCACAAAGTTGTCCTT | |||
| GTP cyclohydrolase (GCH1) | – | + | – | GCH1-F AGATTGCAGTGGCCATCAC |
| NM_024356.1 | GCH1-R ACCTCGCATGACCATACACA | |||
| Glial-derived nerve growth factor (GDNF) | – | + | + | GDNF-F GGCTGTCTGCCTGGTGTT |
| NM_019139 | GDNF-R TCAGGATAATTCTTCGGGCATA | |||
| Glutamate (N-methyl-D-Aspartate) receptor-1 (GRIN-1) | – | – | + | GRIN1-F GCTTTTGCAGCCGTAAC |
| NM_017010.1 | GRIN1-R GGGCTCTGCAGCCGTAAC | |||
| Hypoxia-induced factor-1 a (HIF1a) | – | + | + | Hif1a-F AAGCACTAGACAAAGCTCACCTG |
| AC_000074.1 | Hif1a-F CCATATCGCTGTCCACATCA | |||
| Insulin-like growth factor (IGF) | + | + | – | IGF-1-F AGGGCACAGGCTGGCTTTGTAC |
| M15481.1 | IGF-1-R CGATCCAAGTGGCAGCTCCTTC | |||
| Interleukin 1β (IL1β) | + | + | – | IL1b-F CAGGAAGGCAGTGTCACTCA |
| M98820 | IL1b-R AAAGAAGGTGCTTGGGTCCT | |||
| Monocyte chemoattractant protein-1 (MCP1) | + | + | – | MCP1-F AGCATCCACGTGCTGTCTC |
| M57441.1 | MCP1-R GATCATCTTGCCAGTGAATGAG | |||
| Nerve growth factor (NGFβ) | + | + | + | NGFβ-F GTGAGGCTCAGGCAGCAT |
| AC_000070.1 | NGFβ-R TGAGCTTGGGTCCAGCAT | |||
| Neuronal nitric oxide synthase-1 (NOS-1) | + | + | + | nNOS-F GATGAGGCACCCCAACTCT |
| AC_000080.1 | nNOS-R GGAAAGAAACGCAAGGGTTC | |||
| Neurotrophic kinase receptor-1 (nTkr-1) | – | + | – | NTrk1-F CTCGGCTCAGTCACCTGAA |
| NM_021589.1 | NTrk1-R GCACAGTTTTCCAGGAGAGG | |||
| Calcitonin gene-related peptide receptor (rCGRP) | – | + | – | rCGRP-F AGGTCCAGAGGATGAGCAGA |
| NM_053670.3 | rCGRP-R GCTTTGAGCATCACGAACAA | |||
| Tumor necrosis factor-α (TNFα) | + | + | + | TNFα-F ATGTGGAACTGGCAGAGGAG |
| NM_012675 | TNFα-R CAATCACCCCGAAGTTCAGT | |||
| Transient receptor potential cation channel subfamily V member 1 (TrpV1) | – | + | + | Trpv1-F GGTGTGCCTGCACCTAGC |
| Trpv1-R CTCTTGGGGTGGGGACTC | ||||
| NM_031982.1 | ||||
| Transient receptor potential cation channel subfamily V member 4 (TrpV4) | – | + | + | Trpv4-F CTGGTTTACAACAGCAAGATCG |
| Trpv4-R TGTCCCTCAGCAGTTCGTTA | ||||
| NM_023970.1 |
The CPTs collected using the 250 and 5 Hz transcutaneous stimuli are presented in Table 2. On day 1 of the experiment, there were no between group differences in the CPTs collected at 250 or 5 Hz. On day 5 of the experiment, lean rats exposed to vibration were more sensitive to transcutaneous stimulation at 250 Hz after the exposure than before the exposure. No other differences were seen on day 5 of the study. There also were no significant differences between the groups when tested with the 250 or 5 Hz electrical stimulus on day 9 of the experiment. Thermal thresholds were not affected at any of the time points measured (data not shown).
Table 2.
CPT responses in the various groups of rats to the 250 and 5 Hz stimulus. Values are the mean±sem values before and after vibration on 3 days during the exposure (* indicates significantly less than pre-exposure value).
| Test | 250 Hz |
5 Hz |
||
|---|---|---|---|---|
| Pre-exposure | Post-exposure | Pre-exposure | Post-exposure | |
| Lean control | ||||
| (day 1) | 8.44 (1.83) | 7.10 (1.60) | 8.03 (1.78) | 7.04 (1.61) |
| (day 5) | 11.15 (1.60) | 7.10 (1.60) | 11.28 (4.72) | 7.04 (1.61) |
| (day 9) | 8.40 (2.66) | 6.24 (1.52) | 9.96 (2.52) | 6.24 (1.50) |
| Obese control | ||||
| (day 1) | 11.71 (3.04) | 8.50 (2.34) | 10.94 (3.10) | 7.89 (2.48) |
| (day 5) | 9.91 (2.26) | 7.13 (1.53) | 7.87 (2.31) | 7.56 (1.50) |
| (day 9) | 7.28 (2.20) | 6.31 (1.67) | 6.83 (1.48) | 6.08 (1.70) |
| Lean vibrated | ||||
| (day 1) | 12.00 (1.50) | 11.55 (3.98) | 9.32 (1.50) | 13.76 (3.77) |
| (day 5) | 12.00 (1.54) | 7.04 (1.64)* | 9.32 (1.48) | 6.98 (1.84) |
| (day 9) | 11.93 (3.18) | 7.41 (1.85) | 10.62 (3.05) | 6.87 (1.88) |
| Obese vibrated | ||||
| (day 1) | 12.78 (4.56) | 11.08 (5.53) | 10.93 (4.64) | 11.15 (5.52) |
| (day 5) | 6.47 (1.73) | 11.08 (5.53) | 7.57 (1.73) | 11.15 (5.51) |
| (day 10) | 10.21 (2.71) | 9.03 (2.29) | 11.19 (2.81) | 7.68 (1.93) |
2.2. IHC and histology
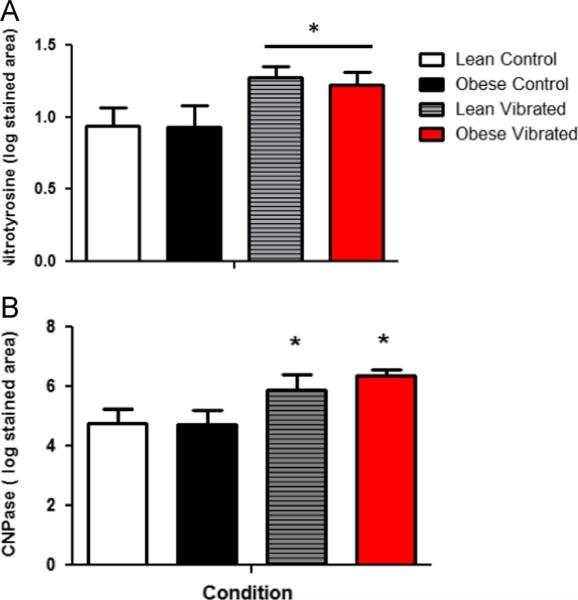
The average area stained by IHC within the ventral tail nerves is presented in Fig. 2(A and B). Staining for NT and CNPase is greater in nerves from vibrated rats than restrained rats, regardless of whether they were lean or obese (p<0.05). There were no significant differences in granulated mast cells within the nerve. In fact, very few mast cells were identified (mean±sem cells/section; lean restraint 2.57±0.43, lean vibrated 2.00±0.55, obese restraint 1.75±0.57; obese vibrated 1.07±0.57).
Fig. 2.
Area of the ventral tail nerve stained with nitrotyrosine (nTry; A) or CNPase (B) after. 10 days of vibration exposure. There were no differences in the area stained with nTry or CNPase in the lean control rats. However, exposure to vibration resulted in an increase in staining in nerves from both lean and obese rats.
2.3. qPCR
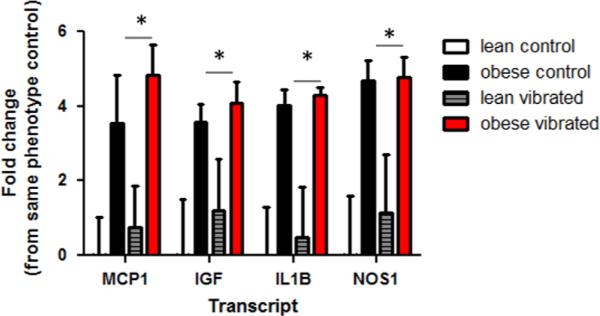
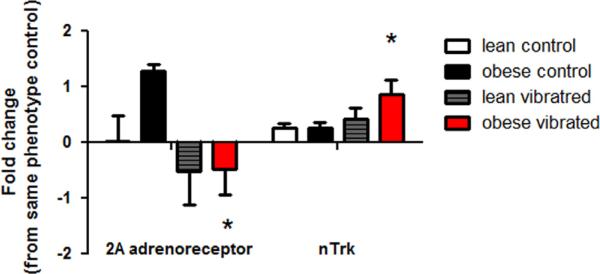
We examined genes previously shown to be altered by vibration exposure, (Krajnak et al., 2012), along with neurotrophic factors, and factors involved in peripheral nerve repair and regeneration. Exposure to vibration led to a number of changes in gene transcription in the nerve, dorsal root ganglia and spinal cord. Fig. 3 shows the transcripts that were significantly altered by vibration or phenotype in ventral tail nerves. These transcripts include monocyte-chemoattractant protein-1 (MCP1), insulin-like growth factor (IGF), interleukin-1β (IL1B) and neuronal nitric oxide synthase (NOS1). All of these genes were higher in obese than lean animals, regardless of exposure (p<0.05). Although the levels of these transcripts were also greater in tail nerves from vibrated than control animals, these differences were not significant. Transcript levels in the DRG are presented in Fig. 4. In obese rats, vibration resulted in a significant reduction in α2A-adrenoreceptor (ADRA2A) expression (p<0.05). The other transcript that showed a change was neurotrophic tyrosine kinase receptor-1 (NTRK). Expression of NTRK was significantly increased in the DRG of obese rats exposed to vibration (p<0.05).
Fig. 3.
Transcript levels were significantly different in the ventral tail nerves of lean and obese Zucker rats. MCP-1, IGF, IL1β and NOS1 expression was greater in the ventral tail nerves of obese rats than lean rats. Vibration did not affect transcript levels of any of the genes measured in the ventral tail nerve in this experiment (* different than lean rats, p<0.05).
Fig. 4.
Transcript levels that were different in the dorsal root ganglia of lean and obese Zucker rats. Vibration resulted in a reduction in α2A-adrenoreceptor gene expression, and an increase in nTrk expression in the DRG of obese rats exposed to vibration as compared to obese control rats (* different than obese controls, p<0.05).
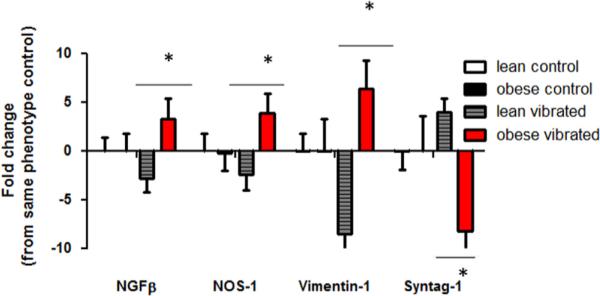
Fig. 5 shows transcripts that displayed significant changes in the spinal cord. Nerve growth factor (NGF)-β and vimentin-1 (VIM1) were significantly lower in the spinal cord of lean vibrated rats than lean control or obese vibrated rats. Vibration exposure resulted in an increase in the expression of those genes in the obese rats. NOS1 transcript levels were significantly greater in obese than lean animals regardless of the condition they were exposed to. Synaptotagmin-1 (SYNT1) transcript levels were significantly lower in obese-vibrated than obese–lean rats after 10 days of vibration exposure. All differences in transcript levels were significant if p<0.05.
Fig. 5.
Transcript levels that were significantly different in the spinal cord of lean and obese Zucker rats. Lean rats exposed to vibration displayed a reduction in NGFβ, NOS-1, and Vimentin-1. However these same rats displayed an increase in synaptotagmin-1. In obese rats exposed to vibration, NGF, NOS-1 and vimentin-1 were increased, while synaptotagmin-1 was reduced (*p<0.05).
3. Discussion
The goal of this study was to examine the effects of vibration in lean and obese Zucker rats to determine if the elevation in glucose (approximately 0.6 fold increase obese>lean rats regardless of treatment) and insulin (approximately 20 fold increase obese4lean regardless of treatment) in obese rats had a significant effect on sensation or peripheral nerve function, and if changes in peripheral nerve function were associated with physiological and biological markers that are indicative of peripheral sensory system injury and/or remodeling.
The greatest effects of vibration on responsiveness to electrical stimulation occurred at 2000 Hz. After the first exposure to vibration, lean and obese rats exposed to restraint displayed an increase in their responsiveness to electrical stimulation (i.e., a lower CPT value post-exposure). Sprague Dawley rats exposed to similar restraint conditions also display an increase in sensitivity to stimulation at 2000 Hz (Krajnak et al., 2007). This increased sensitivity is most likely the result of the stress associated with restraint. A number of studies have demonstrated that stress is associated with hyperalgesia (Jorum, 1988b; Vidal et al., 1984). This increased sensitivity to electrical stimulation in the restrained rats was seen on all three days measurements were collected, and appears to be temporary because the pre-exposure CPTs remained stable in the restraint groups on all days of the study.
The responses of rats exposed to tail vibration were different than those of rats in the restraint groups. Exposure to a single bout of vibration resulted in an increase (or reduced sensitivity) to stimulation at 2000 Hz (when compared to pre-exposure levels) in the lean rats. This is consistent with previous finding on the effects of a single exposure to vibration in rats (Krajnak et al., 2007). Although the obese rats exposed to vibration were less sensitive to stimulation at 2000 Hz than the restrained rats, there was no pre-post effect of vibration on responsiveness in these animals on any day. However, obese rats did display a reduction in sensitivity to stimulation at 2000 Hz between days 1 and 9. Thus, although there did not appear to be any acute effects of vibration on nerve function, there were more lasting effects that occurred after repeated exposures. Obese Zucker rats displayed a reduced sensitivity to vibration (i.e., increased CPT) on day 9 when compared to day 1. It is not clear why obese rats displayed acute changes in responsiveness to electrical stimulation at 2000 Hz when restrained, but not when exposed to vibration. This may be because restraint-induced changes in sensory thresholds are mediated by autonomic input (Jorum, 1988a, 1988b; McCormack et al., 1998; Vidal et al., 1984), while vibration-induced changes are likely mediated by both autonomic and mechanical/local changes in sensory function (Jorum, 1988a; Krajnak et al., 2012).
The vibration-induced changes in responsiveness to the 2000 Hz stimulation were associated with increases in both nTyr and CNPase immunostaining in the ventral tail nerve of both lean and obese Zucker rats (as compared to controls). We have previously shown that exposure to 10 days of tail vibration results in an increase in oxidative stress in Sprague Dawley rats, as measured by peroxynitrite (Krajnak et al., 2010). Other models that have been used to induce pain and sensory nerve dysfunction (i.e., sciatic nerve crush or sciatic pressure cuff) have demonstrated oxidative stress plays a role in both behavioral changes in responses to tactile stimulation and changes in cellular pathways that are associated with nerve death and/or repair. Thus, these findings are consistent with the results of other studies showing that peripheral nerve dysfunction and injury are associated with an increase in oxidative stress.
CNPase was also higher in vibrated than restrained rats. CNPase is an enzyme found in Schwann cells, microglia and circulating stem cells (LeBlanc et al., 1992). This has been used as a marker of myelination in peripheral nerves. It is also involved in mediating communication between glial cells and nerves, and, this enzyme helps guide regeneration and re-routing of nerves after injury (Jangouk et al., 2009). Because responsiveness to the CPT was different in lean and obese rats, it is difficult to make a direct correlation between changing CPT levels and changes in nerve function. However, the fact that CNPase was elevated in both vibrated groups indicates that there was some kind of nerve injury or dysfunction in the nerves of vibrated rats.
RNA transcript levels were measured by qPCR in the ventral tail nerve, DRG and spinal cord to determine if there were vibration, or phenotype-induced changes in pro-inflammatory factors, or factors indicative of injury and regeneration. In the ventral tail nerve, the pro-inflammatory factors monocytechemoattractant protein-1 (MCP-1), insulin-like growth factor-1 (IGF-1) and interleukin-1β (IL-1 β) were increased in obese rats. Vibration did not affect the expression of these transcripts in the nerve. This is consistent with the results of a number of previous studies showing that inflammatory markers are increased in obese Zucker rats (Frisbee et al., 2001; Krajnak et al., 2009; Xiang et al., 2008).
In the DRG, the only transcripts that showed changes were the α2 A-adrenoreceptor and the neurotrophic tyrosine kinase receptor (NTRK)-1. The transcript ADRA2A levels were lower in the DRG of ganglia from rats exposed to vibration than in ganglia from control rats. ADRA2A agonists have been shown to have analgesic properties (Terayama et al., 2015). Thus, if the reductions in transcript levels are indicative of a reduction in receptor number, it is possible that rats with reduced ADRA2A expression may be developing hyperalgesia.
In the ganglia of obese rats exposed to vibration, changes in 2A-adrenoreceptors were associated with an increase in NTRK-1 transcript expression. The NTRK-1 receptor is activated by numerous growth factors such as NGF (Frostick et al., 1998). This receptor has been associated with glial proliferation and signaling in the nervous system. During development or after neuronal/nerve injury, activation of cellular pathways associated with this receptor can help stimulate and guide reinnervation (van Biesen et al., 1995). For example, rats that had undergone L5 rhizotomy display a hyperalgesia that is associated with an increase in NGF and BDNF in the DRG (Obata et al., 2004). Thus, it is possible that the increase in NTRK-1 transcript expression in the DRG of obese Zucker rats may have been because these rats already displayed some kind of nerve injury.
Expression of NGFβ, NOS-1, vimentin-1 (VIM1) and synaptotagmin-1 were affected by vibration in the spinal cord. In lean animals, NGFβ, NOS-1 and VIM1 were reduced by vibration. However, in obese rats, vibration had the opposite effect. NGFβ, NOS-1 and VIM1 were increased. In addition, synaptotagmin-1 was decreased in the spinal cord of obese rats. These changes are consistent with the hypothesis that vibration and phenotypic changes associated with increased glucose and hyper-insulinemia may affect cellular responses of the peripheral nervous system.
Similar to its effects in the ganglia, NGF is involved in maintaining viability of the neurons in the spinal cord during development and after an injury. Although it is unlikely that tail vibration at 125 Hz resulted in any direct injury to the spinal cord, injury to peripheral nerves has been shown to induce changes in cell signaling in the dorsal horn of the spinal cord (Terayama et al., 2015), and these changes have been correlated with mechanisms that lead to neuropathic pain (Ahmed et al., 2014; Donnelly-Roberts et al., 2008; Tan et al., 2009). For example, after sciatic nerve injury there are changes in transcripts levels for neurotrophic factors, such as NGF, brain-derived neurotrophic factor and glial-derived neurotrophic factor (Barrette et al., 2010; Gray et al., 2007). VIM1 was also increased in obese rats exposed to vibration but reduced in lean rats. VIM1 is a protein expressed in glial cells and/or stem cells. This peptide helps guide growth of new nerve fibers. The increase in the expression of VIM1 in obese rats suggests that peripheral nerve damage may have stimulated the outgrowth of new fibers after vibration exposure, or guided the regeneration of existing, damaged fibers. In addition, factors involved in synaptogenesis, such as NOS-1 and synaptotagmin-1 also changed in response to vibration. A number of studies have demonstrated that after peripheral nerve injury, cellular changes similar to those seen during long-term potentiation can be induced, such as increases in synaptic number or synaptic strength occur and are involved in the development of neuropathic pain. These additional synapses and potential changes in neurotransmission could contribute to the development of neuropathic pain (Guo et al., 2007). The fact that the changes in gene expression in response to vibration were different in the lean and obese rats may have been due to 1) obese rats were already displaying signs of peripheral nerve injury, or 2) the rate at which the lean and obese rats were able to respond to injury was different because the higher glucose levels in the obese rats may have affected cellular processes that mediate repair and regeneration.
Taken together, the results of these studies indicate that the higher circulating glucose levels and hyperinsulinemia seen in obese Zucker rats may make them more susceptible to the negative health consequences of vibration exposure (Krajnak et al., 2009; Oltman et al., 2008). These findings suggest that humans with these conditions may also be more susceptible to vibration-induced nerve damage. It may be possible to incorporate information regarding a worker's health status when determining the maximal time a person should be exposed to vibration at work, thereby protecting workers that may be more susceptible to the negative health consequences of vibration.
The results of this study also suggest that it may be difficult to use traditional physiological measures to distinguish between peripheral neuropathies induced by vibration and those induced by type II diabetes. However, conducting studies using longer exposures and older animals may provide additional information regarding the effects of these two factors on the development of sensory neuropathies and identify additional changes that may allow clinicians to distinguish between diabetes and vibration induced nerve dysfunction/injury.
4. Experimental procedures
4.1. Animals
Male obese (n=16) and lean (n=16) Zucker rats were obtained at 6 wk of age (Charles River, Wilmington, MA) and were on average 12 wk of age when used in the study. Rats were maintained in a colony room with a 12:12 LD reverse light: dark cycle (lights off 0700 h) with Teklad 2918 rodent diet and tap water available ad libitum, at the NIOSH facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). All procedures were approved by the NIOSH Animal Care and Use Committee and were in compliance with the Public Health Service Policy on Human Care and Use of Laboratory Animals.
4.2. Vibration exposures
The equipment and protocol for exposing animals to vibration was described in previous publications (Welcome et al., 2008). Briefly, animals were acclimated to the animal care facilities for one week prior to the beginning of the experiment. They also were acclimated to restraint in Broome style restrainers for one week prior to the beginning of the study. Lean and obese Zucker rats were randomly assigned to the vibrated or restraint-control (restraint) group. During the exposures, the rats were restrained and their tail was secured to a vibrating platform described in Welcome et al. (2008). Rat tails were exposed to 4 h of sinusoidal vibration at 125 Hz, unweighted acceleration of 49 m/s2 r.m.s., between 0900 and 1300 h for 10 consecutive days. This frequency was utilized because this is the dominant frequency emitted by many tools including grinders, cement saws and chipping hammers (Griffin, 1990). This frequency is also in the resonant frequency range of both the tail and the human finger (Welcome et al., 2008). Control rats were treated in an identical manner except that their tails were secured to platforms mounted onto isolation blocks.
4.3. Physiological tests
Immediately prior to, and immediately following vibration exposure on days 1, 5 and 9 of the experiment, rats were restrained, and their tails were exposed to transcutaneous electrical stimuli at three different electrical frequencies to test functioning of different nerve fiber types. The 2000-Hz stimulus selectively activates Aß fibers (Koga et al., 2005). The 250-Hz stimulus activates Aδ and Aß nerve fibers. However, because stimulation of the Aδ fibers results in a noxious sensation, and elicits a response at a lower magnitude, it is likely that at 250 Hz rats are responding to Aδ activation over Aß activation (Koga et al., 2005). The 5-Hz frequency can activate Aß, Aδ and C fibers (Koga et al., 2005). However, because the responses of Aδ and Aß fibers to 5-Hz CPT stimulation occurs at a higher frequency, it is unlikely that any effects found are due to stimulation of those fibers, but instead, are due to C-fiber activation. The intensity of each stimulus was automatically increased in increments of 0.5 mA for the 2000-Hz stimulus, and 0.1 mA for the 250- and 5-Hz stimuli, until the rat flicked its tail. The intensity that elicited the tail-flick was recorded as the current perception threshold (CPT). CPTs were always measured in the same order: 2000, 250, and 5 Hz. After each set of frequencies was tested once, there was a 1-min rest interval before the next set of tests was performed. The tests at each frequency were repeated three times, and the mean CPT calculated for each frequency was used for statistical analyses.
Sensitivity to heat was also assessed prior to and following vibration or control exposures on days 1, 5 and 9 of the study. Changes in thermo-sensitivity were tested using a thermal analgesia meter from IITC Life Sciences (Woodland Hills, CA). To perform the test, rats were put into a plexiglass block and placed onto a glass table heated to 37 °C for 5 min, which warmed the tails to approximately the same temperature in all animals. A halogen light source was then pre-focused on the dorsal surface of the tip of the tail. Once the light was focused on the correct location, a radiant heat beam was turned on and the temperature was increased at a rate of 1°/s. When the animal flicked its tail away, the time and heat source were automatically shut off. If the animal did not flick its tail away from the light source within the 15 s cut-off time the light beam shuts off automatically avoiding tissue damage. Based on other studies in the literature, a cut off time of 15 s was set for the tail (Carstens and Wilson, 1993; D'Amour and Smith, 1941). A builtin timer displayed the reaction time in 0.01 s increments.
4.4. Tissue collection
One hour after the last exposure (day 10), rats were deeply anesthetized using pentobarbital (100 mg/kg) and exsanguinated by cardiac puncture. Blood glucose levels were immediately measured using a ReliOn® Ultima Blood Glucose Monitoring System (Solartek Products Inc, Alameda, CA, USA). Serum was isolated from the remaining blood and stored at −80 °C.
Ventral tail nerves were dissected from the C7–9 vertebrate regions of the tail, frozen in cryovials and stored at −80 °C. Nerves were dissected from these specific regions of the tail because the physical stress and strain of vibration is greatest in regions between the strap restraints (Welcome et al., 2008) and these regions display altered vascular responses after exposure to a single bout of vibration (Krajnak et al., 2006a). The dorsal root ganglia (DRG) between L3-5 and the lumbar spinal cord were also dissected from each animal, frozen in cryovials and stored at −80 C.
4.5. Quantitative RT-PCR
Quantitative RT-PCR (qRT-PCR) was performed on nerves from the C7–9 region of the tail using previously described methods (Krajnak et al., 2006b). The transcripts that were measured and the tissue they were measured in are presented in Table 1. For all tissues, ribosomal 18 s was used as an endogenous control.
Briefly, RNA was isolated and purified using previously described methods (Krajnak et al., 2006b) and first strand cDNA was synthesized from 1 μg of total RNA using Invitrogen's Reverse Transcription System (Invitrogen, Grand Island, NY). Control RNA from heart or brain was run at 10x dilutions for each transcript to establish a standard curve of relative transcript levels, and relative RNA levels were calculated using this curve. Samples that did not show a single defined melt peak in the 80 °C range were not included in the data set.
4.6. Histology and immunohistochemistry (IHC)
The ventral tail nerve was dissected, placed in liver tissue and frozen on dry ice. The liver tissue was used instead of OTC because previous studies in our laboratory demonstrated that better sections were obtained using this method (Krajnak et al., 2012). Cross sections of the nerve (40 μm) were cut on a cryostat and mounted onto Superfrost slides. There were 3–4 serial sections on a slide, and 5 slides were collected for each animal. Thus, there were approximately 200 μm of separation between each section on an individual slide. One set of slides was stained with toluidine blue using a previously described protocol (Krajnak et al., 2010, 2012). The stained sections were visualized at a total magnification of 400x. Granulated mast cells were identified as darkly stained cells within the section that were surrounded by granules. To count granulated mast cells, a 250 × 250 μm grid reticle was centered over the nerve. At this magnification the grid covered the majority of the nerve. The granulated mast cells within the grid area were then counted.
Two additional sets of sections were used for IHC, one for 2'3'-cyclic-nucleotide 3'-phosphodiesterase (CNPase) and one for nitrotyrosine (NTyr). The NTyr antibody was used at a final dilution of 1:600 (mouse anti-NTyr, 39B6, Santa Cruz, #sc-32757). The CNPase antibody was used at a final dilution of 1:500 (Mouse anti-CNPase, Sigma, #C5922). The secondary antibody was a donkey anti-mouse IgG-Cy3 (H&L, ML; Jackson, #715-165151) used at a dilution of 1:400 or 1:500 respectively. Procedures used to perform the IHC were similar to those that were previously published (Krajnak et al., 2010). To quantify the area of immunohistochemical staining, sections were visualized on a confocal microscope and photos were taken at 40x. All slides were stained with DAPI, coverslipped with Prolong Gold (Invitrogen), and digital images were taken using a Zeiss LSM510 laser scanning confocal microscope, with HeNe, Argon, and ultraviolet lasers, and integrated 2D and 3D image processing software. Images were imported into Scion Image (Scion Inc., Frederick MD), and density thresholds and brightness were set, and maintained for all tissue samples. Images were then imported into ImageJ (NIH), a threshold was set and the area and density of the stained regions that were above threshold were quantified. A similar method was used to quantify changes in CNPase staining. For CNPase, a picture of each nerve section was collected at 20x using a Leica light microscope. The density and area of the staining were measured as described. For both sets of IHC, the stained area in each section was averaged, and these averages were used for the analyses.
4.6.1. Data analyses
Physiological data (CPT and thermos-sensitivity) were analyzed using 2 (fat vs. lean) x 2 (vibrated vs. control) x 3 (day of exposure), repeated measures ANOVA. Significant interactions were further analyzed using appropriate ANOVAs. Biological and histological data were analyzed using 2 (fat vs. lean) x 2 (vibrated vs. control) ANOVAs. Data are presented as the mean±sem. Differences with p<0.05 were considered significant.
Acknowledments
This research was funded through intramural funding from the Health Effects Laboratory Division, National Institute for Occupational Safety and Health.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the author and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
REFERENCES
- Ahmed MM, Lee H, Clark Z, Miranpuri S, Nacht C, Patel K, Liu L, Joslin J, Kinter D, Resnick DK. Pathogenesis of spinal cord injury induced edma and neuropathic pain: expression of multiple isoforms of wnk1. Ann. Neurosci. 2014;21:97–103. doi: 10.5214/ans.0972.7531.210305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrette B, Calvo E, Vallières N, Lacroix S. Transcriptional profiling of the injured sciatic nerve of mice carrying the Wld(S) mutant gene: identification of genes involved in neuroprotection, neuroinflammation, and nerve regeneration. Brain Behav. Immun. 2010;24:1254–1267. doi: 10.1016/j.bbi.2010.07.249. [DOI] [PubMed] [Google Scholar]
- Bovenzi M, Giannini F, Rossi S. Vibration-induced multifocal neuropathy in forestry workers: electrophysiological findings in relation to vibration exposure and finger circulation. Int. Arch. Occup. Environ. Health. 2000;73:519–527. doi: 10.1007/s004200000177. [DOI] [PubMed] [Google Scholar]
- Bray GA. The Zucker-fatty rat: a review. Fed. Proc. 1977;36:148–153. [PubMed] [Google Scholar]
- Carstens E, Wilson C. Rat tail flick reflex: magnitude measurement of stimulus-response function, suppression by morphine and habituation. J. Neurophysiol. 1993;70:630–639. doi: 10.1152/jn.1993.70.2.630. [DOI] [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention . National diabetes statistics. Data, sources, methods and references for estimation of diabetes and its burden in the United States. US Department of Health and Human Services; Atlanta, GA.: 2014. [Google Scholar]
- Cederlund R, Iwarsson S, Lundborg G. Hand function tests and questions on hand symptoms as related to the Stockholm workshop scales for diagnosis of hand-arm vibration syndrome. J. Hand Surg. 2003;28:165–171. doi: 10.1016/s0266-7681(02)00361-3. [DOI] [PubMed] [Google Scholar]
- Cherniack M, Brammer AJ, Lundstrom R, Meyer J, Morse TF, Nealy G, Nilsson T, Peterson D, Toppilla E, Warren N, Fu RW, Bruneau H. Segmental nerve conduction velocity in vibration-exposed shipyard workers. Int. Arch. Occup. Environ. Health. 2004;77:159–176. doi: 10.1007/s00420-003-0486-x. [DOI] [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941;72:74–79. [Google Scholar]
- Donnelly-Roberts D, McGaraughty S, Shieh CC, Honore P, Jarvis MF. Painful purinergic receptors. J. Pharmacol. Exp. Ther. 2008;324:409–415. doi: 10.1124/jpet.106.105890. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, Stepp DW. NO-dependent dilation of skeletal muscle arterioles in hypertensive diabetic obese Zucker rats. Am. J. Physiol. Hear. Circ. Physiol. 2001;281 doi: 10.1152/ajpheart.2001.281.3.H1304. [DOI] [PubMed] [Google Scholar]
- Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Gray M, Palispis W, Popovich PG, van Rooijen N, Gupta R. Macrophage depletion alters the blood–nerve barrier without affecting Schwann cell function after neural injury. J. Neurosci. Res. 2007;85:766–777. doi: 10.1002/jnr.21166. [DOI] [PubMed] [Google Scholar]
- Griffin MJ. Handbook of Human Vibration. Academic Press; San Diego: 1990. [Google Scholar]
- Guo W, Wang HP, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J. Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harazin B, Harazin-Lechowska A, Kalamarz J, Zielinski G. Measurements of vibrotactile perception thresholds at the fingertips in Poland. Ind. Health. 2005;43:535–541. doi: 10.2486/indhealth.43.535. [DOI] [PubMed] [Google Scholar]
- House R, Krajnak K, Manno M. Proceedings of the 2nd American Conference on Human Vibration. NIOSH, ed. NIOSH; Chicago, IL.: 2008. Current perception threshold, nerve conduction studies and the Stockholm Sensorineucal scale in workers with HAVS. [Google Scholar]
- ISO . Mechanical Vibration – Measurement and Evaluation of Human Exposure to Hand-transmitted Vibration – Part 1: General Requirements. ed.\widehateds. International Organization for Standardization; 2001. ISO 5349-1. [Google Scholar]
- Jangouk P, Dehmel T, Meyer Zu Horste G, Ludwig A, Leh-mann HC, Kieseier BC. Involvement of ADAM10 in axonal outgrowth and myelination of the peripheral nerve. Glia. 2009;57:1765–1774. doi: 10.1002/glia.20889. [DOI] [PubMed] [Google Scholar]
- Jorum E. Noradrenergic mechanisms in mediation of stress-induced hyperalgesia in rats. Pain. 1988a;32:349–355. doi: 10.1016/0304-3959(88)90047-4. [DOI] [PubMed] [Google Scholar]
- Jorum E. Analgesia or hyperalgesia following stress correlates with emotional behavior in rats. Pain. 1988b;32:341–348. doi: 10.1016/0304-3959(88)90046-2. [DOI] [PubMed] [Google Scholar]
- Koga K, Furue H, Rashid MH, Takaki A, Katafuchi T, Yoshimura M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol. Pain. 2005;1 doi: 10.1186/1744-8069-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Dong RG, Flavahan S, Welcome DE, Flavahan NA. Acute vibration increases a2c-adrenergic smooth muscle constriction and alters thermosensitivity of cutaneous arteries. J. Appl. Physiol. 2006a;100:1230–1237. doi: 10.1152/japplphysiol.00761.2005. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Waugh S, Miller R, Baker B, Geronilla K, Alway SE, Cutlip RG. Proapoptotic factor Bax is increased in satellite cells in the tibialis anterior muscles of old rats. Muscle Nerve. 2006b;34:720–730. doi: 10.1002/mus.20656. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Waugh S, Wirth O, Kashon ML. Acute vibration reduces Aβ nerve fiber sensitivity and alters gene expression in the ventral tail nerves of rats. Muscle Nerve. 2007;36:197–205. doi: 10.1002/mus.20804. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Waugh S, Johnson C, Miller R, Kiedrowski M. Vibration disrupts vascular function in a model of metabolic syndrome. Ind. Health. 2009;47:533–542. doi: 10.2486/indhealth.47.533. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Miller GR, Waugh S, Johnson C, Li S, Kashon ML. Characterization of frequency-dependent response of the vascular system to reptitive vibration. J. Occup. Environ. Med. 2010;52:584–594. doi: 10.1097/JOM.0b013e3181e12b1f. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Miller GR, Johnson C, Waugh S, Kashon ML. Frequency-dependent effects of vibration on peripheral nerves and sensory nerve function in a rat model of hand-arm vibration syndrome. J. Occup. Environ. Med. 2012;54:1010–1016. doi: 10.1097/JOM.0b013e318255ba74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc AC, Pringle J, Lemieux J, Poduslo JF, Mezei C. Regulation of 2′,3′-cyclic nucleotide phosphodiesterase gene expressionn in expewrimental peripheral neuropathies. Brain Res. Mol. Brain Res. 1992;15:40–46. doi: 10.1016/0169-328x(92)90149-6. [DOI] [PubMed] [Google Scholar]
- McCormack K, Prather P, Chapleo C. Some new insights into the effects of opioids in phasic and tonic nociceptive tests. Pain. 1998;78:79–98. doi: 10.1016/S0304-3959(98)00146-8. [DOI] [PubMed] [Google Scholar]
- McGeoch KL, Gilmour WH, Taylor W. Sensorineural objective tests in the assessment of hand-arm vibration syndrome. Occup. Environ. Med. 1994;51:57–61. doi: 10.1136/oem.51.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch KL, Lawson IJ, Burke F, Proud G, Miles J. Use of sensorineural tests in a large volume of medico-legal compensation claims for HAVS. Occup. Med. (Lond.) 2004;54:528–534. doi: 10.1093/occmed/kqh112. [DOI] [PubMed] [Google Scholar]
- McMillan DE. Development of vascular complications in diabetes. Vasc. Med. 1997;2:132–142. doi: 10.1177/1358863X9700200209. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Dai y, Mizushima T, Fukuoka T, Tokunaga A, Yoshikawa H, Noguchi K. Contribution of degeneration of motor and sensory fibers to pain behavior and the changes in neurotrophic factors in rat dorsal root ganglion. Exp. Neurol. 2004:149–160. doi: 10.1016/j.expneurol.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Yorek MA. Attenuation of vascular/neural dys-function in Zucker rats treated with enalapril or rosuvastatin. Obesity. 2008;16:82–89. doi: 10.1038/oby.2007.19. [DOI] [PubMed] [Google Scholar]
- Pelmear PL, Kusiak R. Clinical assessment of hand-arm vibration syndrome. Nagoya J. Med. Sci. 1994;57:27–41. [PubMed] [Google Scholar]
- Saely CH, Rein P, Drexel H. The metabolic syndrome and risk of cardiovascular disease and diabetes: experiences with the new diagnostic criteria from the International Diabetes Federation. Horm. Metab. Res. 2007;39:642–650. doi: 10.1055/s-2007-985822. [DOI] [PubMed] [Google Scholar]
- Sakakibara H, Hirata M, Hashiguchi T, Toibana N, Koshiyama H, Zhu SK, Kondo T, Miyao M, Yamada S. Digital sensory nerve conduction velocity and vibration perception threshold in peripheral neurological test for hand-arm vibration syndrome. Am. J. Ind. Med. 1996;30:219–224. doi: 10.1002/(SICI)1097-0274(199608)30:2<219::AID-AJIM14>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Stromberg T, Dahlin LB, Rosen I, Lundborg G. Neuro-physiological findings in vibration-exposed male workers. J. Hand Surg. – Br. 1999;24:203–209. doi: 10.1054/jhsb.1998.0181. [DOI] [PubMed] [Google Scholar]
- Tack CJ, Netten PM, Scheepers MH, Meijer JW, Smits P, Lutterman J. Comparison of clinical examination, current and vibratory perception threshold in diabetic polyneuropathy. Neth. J. Med. 1994;44:41–49. [PubMed] [Google Scholar]
- Tan EC, Bahrami S, Kozlov AV, Kurvers HA, H.J., T.L., Nohl H, Redl H, Goris RJ. The oxidative response in the chronic constriction injury model of neuropathic pain. J. Surg. Res. 2009;152:84–88. doi: 10.1016/j.jss.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Terayama R, Yamamoto Y, Kishimoto N, Maruhama K, Mizutani M, Lida S, Sugimoto T. Peripheral nerve injury activates convergent nociceptive input to dorsal horn neurons. Exp. Brain Res. 2015;233(4):1201–1212. doi: 10.1007/s00221-015-4203-2. [DOI] [PubMed] [Google Scholar]
- van Biesen T, Hawes BE, Luttrell DK, Krueger KM, Touhara K, Porfiri E, Sakaue M, Luttrell LM, Lefkowitz RJ. Receptor-tyrosine-kinase- and G beta gamma-mediated MAP kinase activation by a common signalling pathway. Nature. 1995;376:781–784. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]
- Vidal C, Suaudeau C, Jacob J. Regulation of body temperature and nociception induced by non-noxious stress in rat. Brain Res. 1984;297:1–10. doi: 10.1016/0006-8993(84)90537-7. [DOI] [PubMed] [Google Scholar]
- Welcome DE, Krajnak K, Kashon ML, Dong RG. An investigation on the biodynamic foundation of a rat tail model. J. Eng. Med. (Proc. Inst. Mech. Eng. Part H) 2008;222:1127–1141. doi: 10.1243/09544119JEIM419. [DOI] [PubMed] [Google Scholar]
- Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1658–H1666. doi: 10.1152/ajpheart.01206.2007. [DOI] [PubMed] [Google Scholar]