Abstract
Much of our understanding of gut-microbial interactions has come from mouse models. Intestinal immunity is complex and a combination of host genetics and environmental factors play a significant role in regulating intestinal immunity. Due to this complexity, no mouse model to date gives a complete and accurate representation of human intestinal diseases, such as inflammatory bowel diseases. However, intestinal tissue from patients undergoing bowel resection reflects a condition of severe disease that has failed treatment, hence a more dynamic perspective of varying inflammatory states in IBD could be obtained through the analyses of pinch biopsy material. Here we describe our protocol for analyzing mucosal pinch biopsies collected predominantly during colonoscopies. We have optimized flow cytometry panels to analyze up to 8 cytokines produced by CD4+ and CD8+ cells, as well as for characterizing nuclear proteins and transcription factors such as Ki67 and Foxp3. Furthermore, we have optimized approaches to analyze the production of cytokines, including TGF-beta from direct ex vivo cultures of pinch biopsies and LPMCs isolated from biopsies. These approaches are part of our workflow to try and understand the role of the gut microbiota in complex and dynamic human intestinal diseases.
Introduction
Ulcerative colitis and Crohn’s disease are the two conditions that comprise inflammatory bowel diseases. Collectively they affect approximately 1.4 million people in North America alone with prevalence on the increase [1, 2]. IBD is a complex disease and there is a poor understanding of its etiology. Host genetics and immune responses combine somehow with environmental factors to contribute toward the onset of IBD [3]. Genetically susceptible individuals eventually mount an aberrant immune response against intestinal flora and/or dietary antigens causing the archetypal pathology associated with IBD [4–6]. Activated CD4+ T helper cells in the lamina propria and epithelium of the gut mucosa are key mediators of intestinal inflammation [7, 8] and we are performing in-depth analysis of their cytokine production to draw comparisons between active and inactive disease states. [9]
Mouse models of IBD have improved our understanding of intestinal immunity but none are a perfect representation of the human diseases [10–13]. Characterization of human samples from both diseased and healthy tissues is critical for our understanding of human intestinal immunity. Unlike mouse experiments, where the entire length of the colon can be dissected, human tissue samples are difficult to obtain and can be much more scarce. Analysis of tissue from the human gastrointestinal tract requires harvesting cells from either surgical specimens or pinch biopsies. While surgical specimens provide larger amount of tissue for greater cell yield, they represent a patient population that has failed treatment and does not provide a dynamic picture of all the disease states in IBD. Pinch biopsies allow us to analyze specific areas of the intestine without surgical intervention and thus, mucosal pinch biopsies can provide researchers with a better picture of varying disease conditions; remission, active and inactive colitis. Furthermore, pinch biopsies allow us to sample a single patient multiple times over the course of months or even years providing valuable longitudinal data. However, the major drawback of working with pinch biopsies is that the amount of tissue obtained is limited. It is, therefore, paramount to optimize protocols to ensure maximum cell yield to allow for accurate analysis without compromising the functional properties of the isolated cells, and also to obtain the maximum amount of information from the isolated cells.
Here we describe our optimized protocol for analyzing pinch biopsies obtained during colonoscopies. We now analyze up to 8 cytokines by flow cytometry, gating on CD4+, CD8+ and CD3+ and CD3- cells in a single panel. We utilize a second panel that allows us to examine nuclear proteins and transcription factors such as Ki67 and Foxp3. Furthermore, we have optimized approaches to analyze the production of cytokines, including TGF-beta from direct ex vivo cultures of pinch biopsies and LPMCs isolated from biopsies.
Materials and Methods
Isolation of lamina propria mononuclear cells (LPMCs) from biopsy tissue
Abbreviations
LPMC – lamina propria mononuclear cells, RT – room temperature, DMSO – dimethyl sulfoxide
PMA – phorbol 12-myristate 13-acetate
Materials
Supplies
| Item | Source |
|---|---|
| 15 mL conical tube | BD Falcon |
| 50 mL conical tube | BD Falcon |
| 100 μm cell strainer | BD Falcon |
| 1 mL syringe | BD Falcon |
| 3 mL transfer pipet | BD Falcon |
| 96-well tissue culture V-bottom plates | Corning |
Reagents
| Item | Source |
|---|---|
| Collagenase VIII | Sigma-Aldrich |
| DNase | Sigma-Aldrich |
| Percoll | GE Healthcare |
| 10X PBS | Gibco |
| 1X PBS | Gibco |
| RPMI-1640 medium with L-glutamine, 500 mL | Mediatech |
| Heat Inactivated Fetal Bovine Serum | BenchMark |
| 100X Penicillin/Streptomycin/Glutamine | Invitrogen |
| 2-mercaptoethanol | Sigma-Aldrich |
| Glycerol | Sigma-Aldrich |
Solution and Media Preparation
Complete RPMI
500 mL RPMI-1640
10% Fetal Bovine Serum
100× Penicillin/Streptomycin/Glutamine
50μM 2-mercaptoethanol
FACS Buffer
500 mL PBS
5 mL Fetal Bovine Serum
0.05% NaN3
PROCEDURE
Before Starting
Note that biopsy tissue should be collected in 15 mL conical tubes with complete media.
Resuspend collagenase VIII powder in RPMI-1640 at a concentration of 100,000 units/mL and resuspend DNase in 10% glycerol at a concentration of 150 mg/mL.
Steps 1–19: Isolation of LPMCs
-
1
Prepare collagenase-DNase digestion mix with 100 units/mL of collagenase and 150 μg/mL DNase in 10 mL complete RPMI per sample.
-
2
Transfer 10 mL of the collagenase-DNase digestion mix to a 50 mL conical tube.
-
3
Transfer biopsy tissue to the collagenase-DNAse digestion mix tube and shake vigorously.
-
4
Incubate at 37°C for one hour.
-
5
During the one hour incubation, prepare the 100% Percoll solution (9 parts Percoll, 1 part 10X PBS). Dilute the 100% Percoll solution to a 40% solution and an 80% solution with complete media. Note: By making the Percoll solution at this time, the solutions will then be at RT by the time the Percoll step arrives.
-
6
Take out the 50 mL conical tube from the incubator and shake vigorously.
-
7
Place a 100 μm cell strainer over a new 50 mL conical tube.
-
8
Pour the collagenase-DNase digestion mix through the 100 μm cell strainer.
-
9
Mash any undigested tissue through the filter using the bottom of a 1 mL syringe.
-
10
Add 10 mL 1X PBS through the 100 μm cell strainer to wash out any cells stuck on the filter.
-
11
Spin tube for 10 minutes at 600 RCF at RT.
-
12
Aspirate supernatant carefully down to the pellet level.
-
13
Resuspend pellet in 5 mL of the 40% Percoll solution and pipet up and down to mix. Transfer mix into a new 15 mL conical tube.
-
14
Very slowly, underlay 5 mL of the 80% Percoll solution below the 40% Percoll solution. Note: Do not pipet out all of the solution from the pipet. Save a small amount of liquid inside the pipet, as pipetting out the complete 5 mL solution will cause bubbles, which will interfere with the interphase.
-
15
Spin tubes for 20 minutes at 600 RCF at RT with the brake off.
-
16
Aspirate out 3 mL of the top layer. With a 3 mL transfer pipet, extract the interphase and transfer to a new 15 mL conical tube with 10 mL 1X PBS.
-
17
Spin the cells for 10 minutes at 600 RCF at 4°C.
-
18
Aspirate supernatant carefully down to the pellet level.
-
19
Resuspend pellet in 200 μL of complete RPMI.
Steps 20–48: Extracellular Staining for intracellular and intranuclear staining
MATERIALS
Supplies
| Item | Source |
|---|---|
| 6-well non-tissue culture treated plate | BD Falcon |
| 15 mL conical tube | BD Falcon |
| 50 mL conical tube | BD Falcon |
| 96-well tissue culture V-bottom plates | Corning |
Antibodies
| Antibodies | Source | Clone | Dilution |
|---|---|---|---|
| APC-Cy7-conjugated anti-human CD3 | BD Pharmingen | SK7 | 5 μL / test |
| PE-TR-conjugated anti-human CD4 | Invitrogen | S3.5 | 1 μL / test |
| V500-conjugated anti-human CD8 | BD Horizon | RPA-T8 | 0.5 μL / test |
| PE-Cy7-conjugated anti-human CD25 | BD Pharmingen | M-A251 | 1 μL / test |
| Human FcR Binding Inhibitor | eBioscience | N/A | 5 μL / test |
| LIVE/DEAD Fixable Blue Dead Cell Stain Kit | Invitrogen | N/A | 5 μL / test |
Other reagents
| Item | Source |
|---|---|
| PMA | Sigma-Aldrich |
| Ionomycin | Sigma-Aldrich |
| Golgi Plug | BD |
| 1X PBS | Gibco |
| RPMI-1640 | Mediatech |
| DMSO | Sigma-Aldrich |
| Paraformaldehyde | Electron Microscopy Sciences |
| Foxp3 Fixation/Permeabilization Buffer | eBioscience |
Solution and Media preparation
FACS Buffer
500 mL PBS
5 mL Fetal Bovine Serum
0.05% NaN3
PROCEDURE
Before Starting
-
20
Resuspend both the PMA and Ionomycin powder in DMSO at a concentration of 1 mg/mL.
-
21
Resuspend the Live/Dead Blue in 12.5 μL DMSO.
Extracellular Staining
-
22
Prepare PMA/Ionomycin/Golgi Plug stimulation mix with 50 ng/mL PMA, 500 ng/mL Ionomycin and Golgi Plug (1000×) in 6 mL complete RPMI per sample.
-
23
Add 6 mL of the stimulation mix to a 6-well non-tissue culture treated plate.
-
24
Add in cells from step 19.
-
25
Incubate at 37°C for 4 hours.
-
26
Transfer the stimulation mix and cells into a new 15 mL conical tube.
-
27
Add 5 mL 1X PBS into the same well and pipet up and down. Transfer to the same 15 mL conical tube as above.
-
28
Spin tubes for 10 minutes at 970 RCF at 4°C.
-
29
Aspirate supernatant carefully down to the pellet level.
-
30
Resuspend pellet in 200 μL 1X PBS.
-
31
Split cells and transfer 100 μL of cells into a 96-well V-bottom plate for cytokine staining and transfer the other 100 μL of cells into another well for nuclear staining.
-
32
Add 100 μL of 1X PBS to all wells and pipet up and down to mix.
-
33
Spin plate for 5 minutes at 1750 rpm at 4°C. Flick plate upside down over a sink to remove supernatant. Blot plate on a paper towel.
-
34
Take 1 μL of Live/Dead Blue and dilute it 1:120 in 1X PBS. Add 5 μL to each well (both cytokine and nuclear stains).
-
35
Incubate for 10 minutes at 4°C protected from light.
-
36
Add 50 μL of appropriate fluorescent antibodies and the FcR Binding Inhibitor diluted in FACS buffer to each of the wells.
-
37
Note: The cytokine panel will include CD3, CD4, and CD8. The nuclear panel will include CD3, CD4, CD8, and CD25.
-
38
Incubate for 30 minutes at 4°C protected from light.
-
39
Add 100 μL FACS buffer to each of the samples and pipet up and down to mix.
-
40
Spin plate for 5 minutes at 970 RCF at 4°C. Flick plate upside down over a sink to remove supernatant. Blot plate on a paper towel.
-
41
Add 200 μL FACS buffer to each of the samples and pipet up and down to mix.
-
42
Spin plate for 5 minutes at 970 RCF at 4°C. Flick plate upside down over a sink to remove supernatant. Blot plate on a paper towel.
-
43
Resuspend the wells that will be used in cytokine staining in 75 μL 4% paraformaldehyde and resuspend the wells that will be used in nuclear staining in 200 μL FACS buffer.
-
44
Incubate for 10 minutes at 4°C protected from light.
-
45
Spin plate for 5 minutes at 970 RCF at 4°C. Flick plate upside down over a sink to remove supernatant. Blot plate on a paper towel.
-
46
Resuspend the wells that will be used in cytokine staining in 100 μL PBS and resuspend the wells that will be used in nuclear staining in 100 μL FoxP3 Fixation buffer (dilute the concentrate 1 to 4 with the diluent). Pipet up and down.
-
47
Incubate at 4°C overnight protected from light.
-
48
Continue with the intracellular cytokine (steps 50–63) and nuclear (steps 64–76) staining on the next day. Note: for step 48 you can incubate for 20 minutes and then continue with the steps, if you prefer, instead of doing it the next day.
Steps 50–63: Intracellular Cytokine Staining
MATERIALS
| Antibodies | Source | Clone | Dilution |
|---|---|---|---|
| BV650-conjugated anti-human IFNg | Biolegend | 4S.B3 | 2 μL/test |
| FITC-conjugated anti-human TNFa | Biolegend | MAb11 | 2 μL/test |
| PE-conjugated anti-human IL-22 | R&D | 142928 | 1 μL/test |
| BV570-conjugated anti-human IL-17 | Biolegend | BL168 | 2 μL/test |
| BV421-conjugated anti-human IL-4 | Biolegend | MP4-25D2 | 2 μL/test |
| PerCP-Cy5.5-conjugated anti-human IL-13 | Biolegend | JES10.5A2 | 5 μL/test |
| Pe-Cy7-conjugated anti-human IL-10 | Biolegend | JES3-9D7 | 5 μL/test |
| BV605-conjugated anti-human IL-2 | Biolegend | MQ1-17H12 | 2 μL/test |
| Item | Source |
|---|---|
| DMSO | Sigma-Aldrich |
| 10X BD Perm/Wash | BD Biosciences |
| APC-conjugated anti-human CD107a | BD Biosciences |
Solution and Media preparation
FACS Buffer
500 mL PBS
5 mL Fetal Bovine Serum
0.05% NaN3
PROCEDURE
Before Starting
Note: This step can be done simultaneously with the intracellular nuclear stain (steps 50–63).
-
50
Prepare 1X PermWash solution by diluting the 10X BD PermWash with MilliQ water.
Intracellular Cytokine Staining
-
51
Add 100 μL of 1X BD PermWash to each of the wells used for cytokine staining. Pipet up and down to mix.
-
52
Incubate for 20 minutes at 4°C protected from light.
-
53
Spin plate for 5 minutes at 970 RCF at 4°C. Flick plate upside down over a sink to remove supernatant. Blot plate on a paper towel.
-
54
Add 200 μL of 1X BD PermWash to each of the wells used for cytokine staining. Pipet up and down to mix.
-
55
Spin plate for 5 minutes at 970 RCF at 4°C. Flick plate upside down over a sink to remove supernatant. Blot plate on a paper towel.
-
56
Add 50 μL of appropriately fluorescent antibodies diluted in 1X BD PermWash buffer per sample to each of the wells.
-
57
Incubate for 30 minutes at 4°C protected from light.
-
58
Add 100 μL of 1X BD PermWash buffer to each of the wells and pipet up and down to mix.
-
59
Spin plate for 5 minutes at 970 RCF at 4°C. Flick plate upside down over a sink to remove supernatant. Blot plate on a paper towel.
-
60
Add 200 μL of 1X BD PermWash buffer to each of the wells and pipet up and down to mix.
-
61
Spin plate for 5 minutes at 970 RCF at 4°C. Flick plate upside down over a sink to remove supernatant. Blot plate on a paper towel.
-
62
Resuspend cells in 150 μL FACS buffer.
-
63
Keep samples at 4°C until ready to be analyzed by flow cytometry.
Steps 64–76: Intracellular Nuclear Staining
MATERIALS
| Item | Source | Clone | Dilution |
|---|---|---|---|
| APC-conjugated anti-human FoxP3 | eBioscience | PCH101 | 1 μL/test |
| FITC-conjugated anti-human Ki67 | BD Biosciences | B56 | 1 μL/test |
| Foxp3 10× Permeabilization Buffer | eBioscience | N/A | 1 μL/test |
Solution and Media preparation
FACS Buffer
500 mL PBS
5 mL Fetal Bovine Serum
0.05% NaN3
PROCEDURE
Before Starting
Note: This step can be done simultaneously with the intracellular cytokine staining (1.3)
Prepare 1X PermWash solution by diluting the 10X Permeabilization Buffer (eBio PermWash) with MilliQ water.
Intracellular Cytokine Staining
-
64
Add 100 μL of 1X eBioscience PermWash to each of the wells used for cytokine staining. Pipet up and down to mix.
-
65
Incubate for 20 minutes at 4°C protected from light.
-
66
Spin plate for 5 minutes at 970 RCF at 4°C. Flick plate upside down over a sink to remove supernatant. Blot plate on a paper towel.
-
67
Add 200 μL of 1X eBio PermWash to each of the wells used for cytokine staining. Pipet up and down to mix.
-
68
Spin plate for 5 minutes at 970 RCF at 4°C. Flick plate upside down over a sink to remove supernatant. Blot plate on a paper towel.
-
69
Add 50 μL of appropriately fluorescent antibodies diluted in 1X eBio PermWash buffer per sample to each of the wells.
-
70
Incubate for 30 minutes at 4°C protected from light.
-
71
Add 100 μL of 1X eBio PermWash buffer to each of the wells and pipet up and down to mix.
-
72
Spin plate for 5 minutes at 970 RCF at 4°C. Flick plate upside down over a sink to remove supernatant. Blot plate on a paper towel.
-
73
Add 200 μL of 1X eBio PermWash buffer to each of the wells and pipet up and down to mix.
-
74
Spin plate for 5 minutes at 970 RCF at 4°C. Flick plate upside down over a sink to remove supernatant. Blot plate on a paper towel.
-
75
Resuspend cells in 150 μL FACS buffer.
-
76
Keep samples at 4°C until ready to be analyzed by flow cytometry.
Culturing of whole mucosal biopsy tissue for Cytokine analysis
MATERIALS
Supplies
| Item | Source | Bead |
|---|---|---|
| 48 well tissue culture plate | BD Biosciences | |
| Weighing scales (readability 0.0001g) | ||
| Eppendorf tubes | Phenix Research Products |
Other Reagents
| Item | Source | Bead |
|---|---|---|
| BD human CBA master buffer kit | BD Biosciences | |
| IL-2 | BD Biosciences | A4 |
| IL-4 | BD Biosciences | A5 |
| IL-5 | BD Biosciences | A6 |
| IL-6 | BD Biosciences | A7 |
| IL-17 | BD Biosciences | B5 |
| IL-10 | BD Biosciences | B7 |
| IFNγ | BD Biosciences | B8 |
| TNF | BD Biosciences | D9 |
| IL12p70 | BD Biosciences | E5 |
| IL-13 | BD Biosciences | E6 |
Solution and Media Preparation
Complete RPMI
500 mL RPMI-1640
10% Fetal Bovine Serum
100× Penicillin/Streptomycin/Glutamine
50μM 2-mercaptoethanol
Before starting
Steps 1–4: Determine the weight of each biopsy
-
1
Place individual biopsies in 1ml of complete RPMI in an 1.5ml Eppendorf tube.
-
2
Weigh the Eppendorf tube on a scale that has a readability of 0.0001g and record the weight of the tube containing the biopsy in RPMI.
-
3
Carefully remove the biopsy into fresh RPMI, re-weigh the Eppendorf tube and record the weight of the Eppendorf with RPMI only.
-
4
Subtract the weight of the Eppendorf with media alone from the weight of the Eppendorf with biopsy to get the weight of the biopsy.
Steps 5–9: Procedure
-
5
Place one pinch biopsy in 500μL complete RPMI in a well of a 48 well tissue culture treated plate. Note: Run duplicates of each intestinal site. If you have enough tissue triplicates can be run instead.
-
6
Place the plate in an incubator for 6 hours at 37°C and 5% CO2.
-
7
After 6 hours harvest the supernatant and store at −80°C until needed for the Cytometric Bead Array.
-
8
Determine the levels of IL-2, IL-4, IL-5, IL-6, IL-17, IL-10, IFNγ, TNF, IL12p70 and IL-13 by Cytometric Bead Array – refer to manufacturer’s instructions (BD Biosciences). Data were acquired on a LSRII (BD) and analyzed using Flowjo and Graphpad prism software. Amount of cytokine/gram of tissue can be calculated using the biopsy weight.
TGF beta assay on isolated biopsy cells
Supplies
| Item | Source | Concentration |
|---|---|---|
| SEAP Reporter Gene Assay system | Applied Biosystems | - |
| TGF-beta inhibitor | BD Biosciences | 2pg/ml |
| Recombinant TGF-beta | R and D systems | 260pg/ml |
| 96 well tissue culture plate | BD Biosciences | - |
Solution and Media Preparation
Complete DMEM
500 mL DMEM
10% Fetal Bovine Serum
100× Penicillin/Streptomycin/Glutamine
Note: MFB-F11 cells were maintained in culture as previously described [14]
Steps 1–5: Procedure
-
1
5mm biopsies were digested as previously described in section 1 (Steps 1–11) Note: No Percoll step for this assay.
-
2
Filter cells through a 50-micron filter and flush the filter with with 5 ml PBS to dislodge any cells on the filter.
-
3
Spin tube for 10 minutes at 600 RCF at RT to pellet the cells.
-
4
Re-suspend in 2 ml of PBS.
-
5
Count cells on a hemocytometer - For this assay you will need 25000 biopsy cells/ well.
-
6
Co-culture 25000 MFB-F11 [14] cells with 25000 biopsy cells in DMEM (Gibco) in triplicate for 48 hours at 37°C and 5% CO2. Include the following control wells in triplicate;
Biopsy and MFB-F11 co-cultured cells in the presence of a TGF-Beta inhibitor (2pg/ml),
MFB-F11 cells alone,
MFB-F11 cells with recombinant TGF-beta (260pg/ml).
-
4
Using the SEAP Reporter Gene assay system, determine the amount of SEAP Activity (TGF-beta) in the co-culture supernatants.
-
5
Analyze data and plot results using Graphpad Prism software.
Results
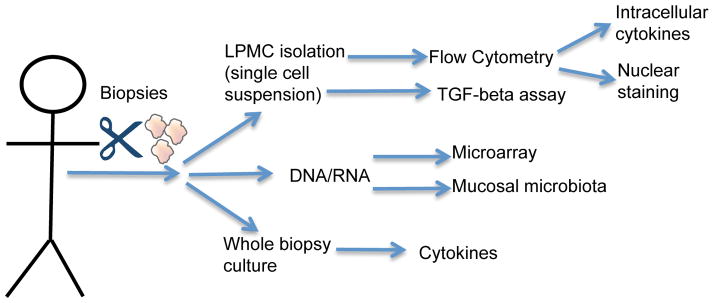
We have taken 5mm pinch biopsies from the human colon and analyzed the cytokine production by flow cytometry, Cytometric Bead Array and SEAP reporter assays (Figure 1). We can also extract DNA and RNA from these pinch biopsies to; 1) sequence 16S rRNA to detect mucosal associated bacteria; 2) analyze gene expression profiles (data not shown). On average we can isolate 1×105 cells/biopsy from IBD patients, which equates to approximately 6×106 cells/gram of tissue (Table 1). For non-IBD control patients we are able to isolate between 2×105 and 6×105 cells/biopsy and 1×107 and 3×107 cells/gram of tissue.
Figure 1.
Schematic representation of the types of analyses we can perform with mucosal biopsies.
Table 1. Number of cells isolated from pinch biopsies.
Lymphocytes were isolated from pinch biopsies taken from the cecum, one inflamed region and one non-inflamed region of the colon. Biopsies were digested with collagenase and DNase for 1 hour at 37 degrees Celsius. Cells were pelleted, washed and counted. The number of cells from 5 biopsies was divided by 5 to give an estimate of how many cells can be isolated from 1 biopsy. Average biopsy weight is 0.02g. This was used to determine the average number of cells that can be isolated per gram of tissue.
| Biopsy location | Cells from 5 biopsies | Average no of cells/biopsy | Average no of cells/gram of tissue | |
|---|---|---|---|---|
| Patient 1 (IBD) | ||||
| Cecum | 500000 | 100000 | 5×106 | |
| 10cm | 600000 | 120000 | 6×106 | |
| 20cm | 680000 | 136000 | 6.8×106 | |
| Patient 2 (Control) | ||||
| Cecum | 3040000 | 608000 | 3.04×107 | |
| 10cm | 1320000 | 264000 | 1.32×107 | |
| 20cm | 1080000 | 216000 | 1.08×107 |
Our previously published data showed accurate analysis of up to 5 cytokines by flow cytometry [9] and here we show analyses of 8 cytokines (Figure 2). By gating on live CD3+CD4+ cells we are able to detect positive staining for TNF, IFN-g, IL-17, IL-2 IL-4, IL-13, IL-22 and IL-10 (Figure 2B). The single cytokine analysis shown in figure 2B can depict the gross production of individual cytokines from CD4+ and CD8+ T cells; however, since cells are able to produce a variety of cytokines, we also visualized the composition of all 8 cytokines through Boolean Gating analysis (Figure 2C). Using this new cytokine panel we find that the largest population of cells are negative for the cytokines in this panel, which is consistent with our previously published data [9, 15]. Further corroborating our published work [9], we see an increase in mono-cytokine-producing IL-17+ CD4+ and TNFα+ CD4+ cells in tissue with active colitis compared with the cecum and un-inflamed tissue of the same patient.
Figure 2. Flow cytometry on mucosal biopsies.
5mm pinch biopsies were taken from cecum, 1 inflamed region and 1 uninflamed region of the human colon. 6–8 biopsies were isolated from each of these regions of which 5 were used for this assay. To isolate lymphocytes biopsies were digested with collagenase and DNase for 1 hour at 37 degrees Celsius. LPMCs were isolated by density gradient centrifugation using a 40% and 80% Percoll solution. LPMCs were isolated from the interphase and washed. The collected LMPCs were then stimulated with phorbol 12-myristate 13-acetate (PMA), ionomycin, and golgi plug for four hours. Cells were harvested, washed and resuspended in 200μl PBS. 100μl of cells were used for the cytokine panel and 100μl of cells were used for the nuclear staining panel. All cells were stained for the surface markers CD3, CD4, CD8 and cells for nuclear staining were also stained with CD25. Cytokine cells were fixed with 4% PFA for 10 minutes. Cells used for nuclear staining were fixed with Foxp3 fixation buffer overnight. Cells in the cytokine panel were stained with anti-TNFα, anti-IFNγ, anti-IL-17, anti-IL-2, anti-IL-4 anti-IL-13, anti-IL-22 and anti-IL-10. Nuclear cells were stained with anti-Foxp3 and anti-Ki67. Gating strategy is show in A. Data shown in B is from one patient and shows individual cytokine frequencies in the three regions of the colon. We performed Boolean gating analysis on the lymphocyte populations (C). Visualization via pie charts of the combination of cytokine-producing CD4+ LPL from pinch biopsies. A key for the main cytokine combinations is shown. Flow plots from cells stained with the nuclear staining panel is shown in D.
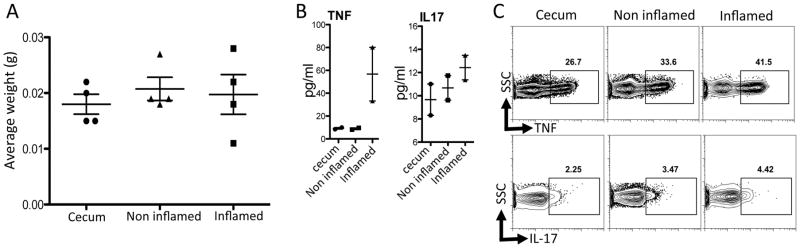
We have further developed cytokine assays to include analysis of secreted cytokines by whole biopsies in culture (Figure 3), avoiding stimulation with PMA and Ionomycin. We took whole biopsies and placed them in culture medium for 6 hours. Culture supernatants were harvested and we used CBA to detect secreted cytokines in the supernatants. We were able to detect cytokines in the cecum, inflamed and un-inflamed regions of the colon. Importantly, our results obtained from this assay are consistent with the ex vivo FACs data with PMA/Ionomycin activation (Figure 3).
Figure 3. Detection of cytokines from cultured biopsies.
The weight of each biopsy was determined by weighing an Eppendorf tube with 1ml of media with and without the biopsy (A). Biopsies were placed individually in 500μl of complete RPMI at 37 degrees Celsius and 5% CO2 for 6 hours. (B) Supernatants were harvested and the levels of TNF and IL-17 were determined by Cytometric Bead Array (BD Biosciences), data were acquired on a LSRII (BD) and analyzed using Flowjo and Graphpad prism software. Data are presented in pg/ml but the amount of cytokine/gram of tissue can also be calculated using the biopsy weight. Paired biopsy samples from the same site were analyzed by intracellular cytokine staining and FACS. Plots shown are gated on CD4+ cells (C).
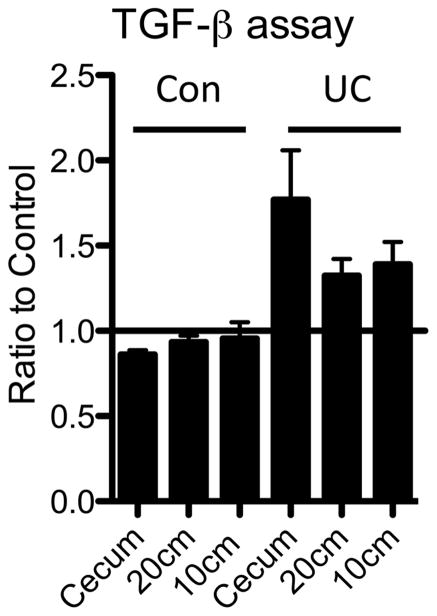
We have also developed techniques to detect TGF-beta in mucosal biopsies since TGF-beta has been shown to promote Th17 cell differentiation and may inhibit IL-22 production. Thus, if we can accurately detect TGF-beta activity in biopsy tissue we may be able to correlate this activity with IL-22. Indeed, our preliminary data showed that there is increased TGF-beta transcription in mucosal biopsies from inflamed regions of the intestine compared with non-inflamed regions [9]. However as transcription is not demonstrative of increased TGF-beta activity we sought to establish if TGF-beta activity is increased during active UC inflammation. We have now established an assay to measure TGF-beta activity from biopsies (Fig. 4), using the well established MFB-F11 reporter cell line[14, 16–19]. MFB-F11 cells secrete alkaline phosphatase in response to TGF-beta and activity is determined by measuring alkaline phosphatase activity in the medium. As an example, we show here data from a patient with inflammation throughout the entire length of their colon, which has TGF-beta activity increased compared to healthy controls (Figure 4). The data is shown as a ratio to control (reporter cells alone) however, a standard curve can be generated using recombinant TGF-beta which will allow absolute values of TGF-beta to be determined.
Figure 4. Analysis of TGF-beta activity in cells isolated from mucosal biopsies.
Lymphocytes were isolated from pinch biopsies taken from the cecum, one inflamed region and one non-inflamed region of the colon. Biopsies were digested with collagenase and DNase for 1 hour at 37 degrees Celsius. Cells were pelleted, washed and counted. TGF-beta activity was measured by co-culturing 25000 cells isolated from biopsies with 25000 cells from a TGF-beta sensitive alkaline phosphatase secreting cell line (MFB-F11 cells). MFB-F11 cells secrete alkaline phosphates in response to TGFbeta. Alkaline phosphatase activity is measured using a SEAP reporter gene assay system. MFB-F11 cells were co-cultured with LPMCs isolated from a UC patient and a non-IBD control.
Conclusion
Human intestinal lymphocytes are key mediators in IBD; thus the characterization of these cells and their cytokines is paramount for the understanding of this disease. In many previous IBD studies, the gut specimens used for isolating intestinal lymphocytes came from patients undergoing bowel resection for IBD, which reflects a condition of severe disease that has failed treatment. A more dynamic perspective of varying inflammatory states in IBD could be obtained through the analyses of pinch biopsy material taken during surveillance endoscopy. We have optimized analyses of intestinal lymphocytes from pinch biopsies taken from CD and UC patients using histo-pathological data from paired mucosal biopsies to inform us of the inflammatory state of the biopsied site[9]. Many previous methods for cellular isolation from biopsies have first used dithiothreitol (DTT) and/or ethylene diamine-tetraacetic (EDTA) to strip intraepithethial lymphocytes (IELs) from the mucosa [20]. Our method simply digests biopsies with collagenase and DNase and the subsequent Percoll step separates out lymphocytes from the remaining epithelial cells and debris. We then stimulate the cells with PMA and Ionomycin for cytokine staining and analysis by flow cytometry.
Initially, Th1 and Th2 cells were thought to be associated with Crohn’s and UC respectively [21, 22]; however, recent evidence has shown an increasing role for TH17 cells and their related cytokines interleukin 17A, IL-17F and IL-22 in the pathogenesis of both diseases [23]. Not all TH17 cells produce IL-22. Another subset of CD4+ T helper cells, termed TH22 cells, have been shown to solely produce IL-22 [24]. The literature to date suggests that IL-17 and TH22 have apposing roles within the gut mucosa. While IL-17 mediates a strong inflammatory response via the recruitment of neutrophils, IL-22 has an anti-inflammatory role promoting mucosal healing through epithelial proliferation and increased mucus production [25, 26]. Thus, IL-17 and IL-22 producing CD4+ helper T cells are undoubtedly important in regulating mucosal barrier function and intestinal homeostasis; however, their exact role in the development of Crohn’s disease and UC remains to be clearly defined [23]. We are interested in dissecting these relationships and have used flow cytometry to analyze the lymphocyte populations in the human intestine. By intracellular cytokine analysis, we have characterized production of TNF, IFNγ, IL-17, IL-2 IL-4, IL-13, IL-22 and IL-10 by various lymphocyte populations, which are all cytokines pertinent to IBD biology. Subsequent Boolean gating analysis provides further examination of the combination of cytokines produced by the cells in the mucosa. Additionally, we can successfully detect cytokines from the supernatant of whole biopsy cultures. Analysis of paired biopsy samples from the same site analyzed by intracellular cytokine analysis by flow cytometry and supernatant analysis of biopsy cultures show that these methods are corroborative. While biopsy culture assays are less labor intensive than the cell isolation and flow cytometry and requires fewer biopsies, it cannot identify the cellular source of cytokines as total tissue is analyzed.
TGF-β is a pleiotropic cytokine with complex biology [27] that has also been associated with IBD [28] [29] and may contribute to the TH17 –TH22 axis. In the presence of inflammatory cytokines such as IL-6, IL-23 and IL-1β, TGF-beta has been shown to promote TH17 differentiation [30]. However, it was shown recently in mice that TGF-beta could inhibit IL-22 production in TH17 cells [31]. Methods of detection have relied on enzyme-linked immunosorbent assays (ELISA) or qPCR, which do not accurately represent active physiological levels. Here we show a method that could be used to detect TGF-beta from mucosal biopsies using a bioassay for active TGF-beta. Preliminary results from a couple of individuals show, that TGF-beta levels may be higher in biopsies from IBD biopsies in comparison to health controls. However, much greater numbers of IBD patients and healthy subjects need to be investigated with this assay in order to draw any conclusions. An important caveat is that cells need to be isolated from biopsies for the assay to work and there are differences in cell numbers isolated from IBD patients and controls. At this stage it is not clear whether these differences in cell numbers are a reflection of the in vivo environment or a technical problem. One possibility would be that plating isolated cells at equal numbers for the in vitro assay may not reflect the true differences in TGF-beta present in the in vivo environment.
We have provided here our methodology for analysis of one parameter in the human intestine – the mucosal pinch biopsy – however results from these analyses only make up part of the picture. Integrated analyses of PBMCs, serum, biopsies, fecal and urine samples are needed in the future to build a portrait of the disease pathology to enable personalized treatment for IBD patients in the future [32].
References
- 1.Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006;12(38):6102–8. doi: 10.3748/wjg.v12.i38.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 3.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3(7):390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 4.Duchmann R, et al. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102(3):448–55. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maul J, Duchmann R. Can loss of immune tolerance cause IBD? Inflamm Bowel Dis. 2008;14(Suppl 2):S115–6. doi: 10.1002/ibd.20679. [DOI] [PubMed] [Google Scholar]
- 6.Mow WS, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology. 2004;126(2):414–24. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Funderburg NT, et al. Circulating CD4(+) and CD8(+) T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology. 2013;140(1):87–97. doi: 10.1111/imm.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009;15(5):199–207. doi: 10.1016/j.molmed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Leung JM, et al. IL-22-producing CD4+ cells are depleted in actively inflamed colitis tissue. Mucosal Immunol. 2014;7(1):124–33. doi: 10.1038/mi.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones-Hall YL, Grisham MB. Immunopathological characterization of selected mouse models of inflammatory bowel disease: Comparison to human disease. Pathophysiology. 2014 doi: 10.1016/j.pathophys.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn R, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 12.Ostanin DV, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296(2):G135–46. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okayasu I, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98(3):694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 14.Tesseur I, et al. Highly sensitive and specific bioassay for measuring bioactive TGF-beta. BMC Cell Biol. 2006;7:15. doi: 10.1186/1471-2121-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff MJ, et al. TH17, TH22 and Treg cells are enriched in the healthy human cecum. PLoS One. 2012;7(7):e41373. doi: 10.1371/journal.pone.0041373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blois SM, et al. Pregnancy-specific glycoprotein 1 (PSG1) activates TGF-beta and prevents dextran sodium sulfate (DSS)-induced colitis in mice. Mucosal Immunol. 2014;7(2):348–58. doi: 10.1038/mi.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumura Y, et al. Selective expansion of foxp3-positive regulatory T cells and immunosuppression by suppressors of cytokine signaling 3-deficient dendritic cells. J Immunol. 2007;179(4):2170–9. doi: 10.4049/jimmunol.179.4.2170. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura Y, et al. The latent form of transforming growth factor-beta administered orally is activated by gastric acid in mice. J Nutr. 2009;139(8):1463–8. doi: 10.3945/jn.109.108761. [DOI] [PubMed] [Google Scholar]
- 19.Pscherer S, et al. Anti-diabetic treatment regulates pro-fibrotic TGF-beta serum levels in type 2 diabetics. Diabetol Metab Syndr. 2013;5(1):48. doi: 10.1186/1758-5996-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leon F, Roy G. Isolation of human small bowel intraepithelial lymphocytes by annexin V-coated magnetic beads. Lab Invest. 2004;84(6):804–9. doi: 10.1038/labinvest.3700099. [DOI] [PubMed] [Google Scholar]
- 21.Mayer L. Evolving paradigms in the pathogenesis of IBD. J Gastroenterol. 2010;45(1):9–16. doi: 10.1007/s00535-009-0138-3. [DOI] [PubMed] [Google Scholar]
- 22.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 24.Trifari S, Spits H. IL-22-producing CD4+ T cells: middle-men between the immune system and its environment. Eur J Immunol. 2010;40(9):2369–71. doi: 10.1002/eji.201040848. [DOI] [PubMed] [Google Scholar]
- 25.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28(4):454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 12(5):383–90. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 27.Achyut BR, Yang L. Transforming growth factor-beta in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology. 141(4):1167–78. doi: 10.1053/j.gastro.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feagins LA. Role of transforming growth factor-beta in inflammatory bowel disease and colitis-associated colon cancer. Inflamm Bowel Dis. 2010;16(11):1963–8. doi: 10.1002/ibd.21281. [DOI] [PubMed] [Google Scholar]
- 29.Monteleone G, et al. TGF-beta1 and Smad7 in the regulation of IBD. Mucosal Immunol. 2008;1(Suppl 1):S50–3. doi: 10.1038/mi.2008.55. [DOI] [PubMed] [Google Scholar]
- 30.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 140(6):845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Rutz S, et al. Transcription factor c-Maf mediates the TGF-beta-dependent suppression of IL-22 production in T(H)17 cells. Nat Immunol. 2011;12(12):1238–45. doi: 10.1038/ni.2134. [DOI] [PubMed] [Google Scholar]
- 32.Huttenhower C, Kostic AD, Xavier RJ. Inflammatory Bowel Disease as a Model for Translating the Microbiome. Immunity. 2014;40(6):843–854. doi: 10.1016/j.immuni.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]