Abstract
Inhibitory neurons are known to play a vital role in defining the window for critical period plasticity during development, and it is increasingly apparent that they continue to exert powerful control over experience-dependent cortical plasticity in adulthood. Recent in vivo imaging studies demonstrate that long-term plasticity of inhibitory circuits is manifested at an anatomical level. Changes in sensory experience drive structural remodeling in inhibitory interneurons in a cell-type and circuit-specific manner. Inhibitory synapse formation and elimination can occur with a great deal of spatial and temporal precision and are locally coordinated with excitatory synaptic changes on the same neuron.We suggest that the specificity of inhibitory synapse dynamics may serve to differentially modulate activity across the dendritic arbor, to selectively tune parts of a local circuit, or potentially discriminate between activities in distinct local circuits. We further review evidence suggesting that inhibitory circuit structural changes instruct excitatory/inhibitory balance while enabling functional reorganization to occur through Hebbian forms of plasticity.
Keywords: inhibition, in vivo imaging, visual cortex, experience-dependent plasticity, structural remodeling
Introduction
In recent years, it has become increasingly clear that inhibitory cells play crucial roles in information processing in the brain. Inhibitory interneurons use γ-aminobutyric acid (GABA) as their neurotransmitter and represent approximately 20% of cortical neurons. They are highly diverse in their morphology, electrophysiological properties, and axonal targeting (Kawaguchi and Kubota 1997; Markram and others 2004; Somogyi and others 1998). Interneurons act locally to modulate the gain and synchrony of excitatory neurons and to shape their receptive field properties (Alitto and Dan 2010; Isaacson and Scanziani 2011). In addition, interneuron network connectivity and intrinsic properties allow them to generate and control the rhythmic and oscillatory activity of large neuronal ensembles, providing a temporal framework for binding together independent parts of a stimulus represented in different processing streams (Fino and others 2012; McBain and Fisahn 2001; Singer 1996).
Inhibitory circuits are also thought to play an important role in neural plasticity. During development, the maturation of GABAergic interneurons and the accompanying increase in intracortical inhibition has been shown to trigger the onset (Fagiolini and Hensch 2000) and closure (Hanover and others 1999; Huang and others 1999) of critical period plasticity. These findings, covered by several excellent reviews (Hensch 2005; Levelt and Hubener 2012), suggest that the inhibitory tone established at the end of developmental critical periods constrains further plasticity in the adult. Indeed, experimental manipulations that result in disinhibition of mature circuits can reinstate juvenile forms of plasticity (Baroncelli and others 2011; Bavelier and others 2010).
Neuronal plasticity can take many forms, including changes in intrinsic excitability, alterations in the strength of existing synapses, and structural changes that result in synapse formation or elimination (Feldman 2009). Mechanisms driving such changes can be broadly divided into two categories, Hebbian and non-Hebbian. Hebbian forms of plasticity such as spike-timing dependent plasticity (STDP) enable the strengthening or weakening of specific synapses based on the pattern of correlated activity between the pre- and postsynaptic cell. Inhibitory circuits can influence STDP by setting the spatial or temporal window for STDP induction. Non-Hebbian forms of plasticity, such as homeostatic plasticity, allow maintenance of stable neuronal function despite changes in sensory-driven or local network activity. This can be achieved either by global adjustment of synaptic strength or excitability across an individual neuron, or by network-wide modification of excitatory/inhibitory (E/I) balance.
Here, we review the potential role of inhibitory interneuron structural and synaptic rearrangements in adult cortical plasticity, with an emphasis on the primary visual cortex. The long-term nature of structural changes makes them particularly attractive as a cellular substrate for persistent changes in connectivity, such as might be required for learning and memory (Bailey and Kandel 1993) or changes in cortical map representation (Buonomano and Merzenich 1998). We first consider previous studies that suggest a role for interneurons in sensory map plasticity and then review recent evidence for inhibitory synapse-specific rearrangements and how these changes may influence plasticity on a circuit, cellular, or subcellular level.
Disinhibition and Adult Cortical Plasticity
Manipulations of the sensory periphery, particularly sensory deprivation, have been a standard tool for inducing and studying cortical plasticity. In the visual system, two common paradigms are monocular deprivation (MD) by eyelid suture and focal retinal lesions. In binocular regions of primary visual cortex (V1), MD during the developmental critical period causes a decrease in responses to the deprived eye followed by an increase in the nondeprived eye response (Wiesel and Hubel 1963). This ocular dominance (OD) plasticity is a conserved feature of mammalian development (Drager 1978; Hubel and others 1977; Wiesel and Hubel 1965). OD plasticity in rodents, unlike in felines and primates, can extend into adulthood (Frenkel and others 2006; He and others 2006; Hofer and others 2006; Sato and Stryker 2008; Sawtell and others 2003). Reorganization of retinotopic cortical maps has also been demonstrated in adults of several mammalian species in response to focal retinal lesions (Giannikopoulos and Eysel 2006; Gilbert and Wiesel 1992; Kaas and others 1990; Schmid and others 1996). An initial silencing in the cortical map region corresponding to the lesion (the lesion projection zone, LPZ) is followed by a period of recovery wherein neurons within the LPZ become responsive to visual input from intact retinal regions surrounding the lesion area. This functional “filling in” is a feature exclusive to cortex as the region corresponding to the lesion in the lateral geniculate nucleus (LGN) remains silent at a time when cortical reorganization is complete (Darian-Smith and Gilbert 1995).
A recurring theme among these and other visual deprivation protocols that elicit cortical plasticity is a depression of the inhibitory network in response to deprivation. Visual deprivation by MD decreases levels of GABA, the GABAA receptor, and the GABA synthesizing enzyme, glutamate decarboxylase, specifically in the deprived eye columns of monkey visual cortex (Hendry and Jones 1986). Focal retinal lesions produce decreased levels of extracellular GABA in the LPZ of cats (Arckens and others 2000; Massie and others 2003), and 10 days of dark adaptation in adult rats reduces the expression of GABAA receptors (He and others 2006). Interestingly, monocular enucleation and tetrodotoxin injection can also reduce GABA levels, suggesting that the general loss of activity, as opposed to noncorrelated activity, is sufficient to drive such changes (Hendry and Jones 1988; Hendry and others 1990; Hendry and others 1994). Thus, the depressed inhibition accompanying loss of visual input may be a homeostatic response for preserving cortical activity levels.
At the same time, reduced intracortical inhibition produces a milieu that is more permissive to Hebbian plasticity, normally quite limited in the adult as compared with developmental critical periods. In rats, reduction of GABAA receptor levels resulting from dark-adaptation enables a juvenile form of OD plasticity in the adult cortex (He, Hodos and others 2006). Similarly, direct blockade of GABAergic inhibition in visual cortex reactivates OD plasticity in response to MD (Harauzov and others 2010), and environmental enrichment that leads to a reduction in inhibitory tone also promotes full recovery from a normally irreversible OD shift induced during a juvenile MD (Sale and others 2007). Pharmacological treatment with fluoxetine, a selective serotonin reuptake inhibitor that reduces inhibition, restores juvenile OD plasticity in the adult rat cortex (Maya Vetencourt and others 2008).
GABA blockade and treatment with fluoxetine enable the induction of long-term potentiation (LTP) by white matter stimulation in L2/3 (Artola and Singer 1987; Maya Vetencourt and others 2008). This is perhaps not surprising given the potential temporal and spatial effects of disinhibition in the context of STDP and Hebbian plasticity. Disinhibition can relax the temporal window over which inputs are able to effectively cooperate (Dan and Poo 2004; Pouille and Scanziani 2001). Similarly, disinhibition along the dendrite or even at the soma would allow back-propagating action potentials (bAPs), a major source of depolarization in distal dendrites, to spread and initiate STDP at synapses located on dendrites that would otherwise be outside their spatial range (Magee and Johnston 1997).
Although direct in vivo evidence for the role of disinhibition in mediating STDP in V1 is lacking, a recent study in whisker barrel cortex (S1) showed that a deprivation paradigm in which all but two whiskers are trimmed produces a disinhibition-facilitated STDP from the spared surround whisker, potentially allowing correlation and experience-dependent receptive field plasticity (Gambino and Holtmaat 2012). In auditory cortex (A1), a form of disinhibition-mediated receptive field plasticity has been demonstrated in which paired auditory tone and nucleus basalis stimulation transiently decreases inhibition in A1 specific to the paired tone (Froemke and others 2007). Over the course of a few hours, this enables the remapping of excitatory connections to this new tone followed by an increase in inhibition to restore E/I balance. In summary, inhibitory circuits participate in adult cortical plasticity by a combination of mechanisms that serve to maintain E/I balance while enabling functional reorganization to occur through Hebbian forms of plasticity.
Structural Dynamics of Cortical Inhibitory Circuits
The recent advent of in vivo imaging technologies combined with the ability to genetically label and track inhibitory neurons has provided new insight into their structural dynamics and revealed a unique capacity for plasticity that is mechanistically distinct from that of their excitatory counterparts (Fig. 1). Pioneering structural imaging studies of excitatory pyramidal neurons in vivo revealed that their dendritic arbors are stable (Mizrahi and Katz 2003; Trachtenberg and others 2002). Yet the dendritic spines that stud pyramidal neuron arbors and represent the excitatory inputs to these neurons are quite dynamic. Depending on the functional region of cortex imaged, a varied fraction of dendritic spines can turn over on the order of days during normal experience, indicating ongoing remodeling of excitatory synaptic connections (Holtmaat and others 2005; Majewska and others 2006; Zuo and others 2005).
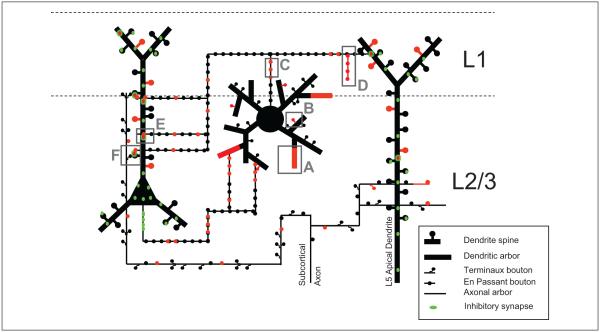
Figure 1.
Diversity of inhibitory circuit plasticity in the adult brain. A schematic of the types of structural rearrangements in inhibitory circuits observed in vivo (dynamic events are indicated in red).This includes (A) dendritic arbor, (B) dendritic spines, (C) axonal boutons, (D) axonal arbors, (E) inhibitory dendritic shaft synapses, and (f) inhibitory dendritic spine synapses.Axonal bouton and dendritic spine rearrangements in excitatory pyramidal neurons are also indicated.
The majority of inhibitory interneurons possess aspiny dendrites, receiving a dense amount of excitatory synaptic input directly onto the dendritic shaft (Kawaguchi and others 2006). Unlike excitatory pyramidal neurons, branch tips of inhibitory interneuron dendritic arbors can grow or retract on the order of tens of microns over a week in the adult mouse cortex during normal experience (Fig. 2A) ((Chen, Flanders, and others 2011; Lee and others 2006; Lee and others 2008). Retrospective electron microscopy (EM) shows that their branch tip elongation is accompanied by new excitatory synapse formation (Chen, Lin, and others 2011). Dendritic remodeling is observed in all known interneuron subtypes but is restricted to a superficial region of layer 2/3 (L2/3) (Lee and others 2008), a lamina ideally situated to integrate feedforward L4 → L2/3 input with L1 feedback input arriving from other cortical areas. Interneuron dendritic tip dynamics are indicative of significant alterations in excitatory input onto specific segments of their dendritic arbors during normal experience.
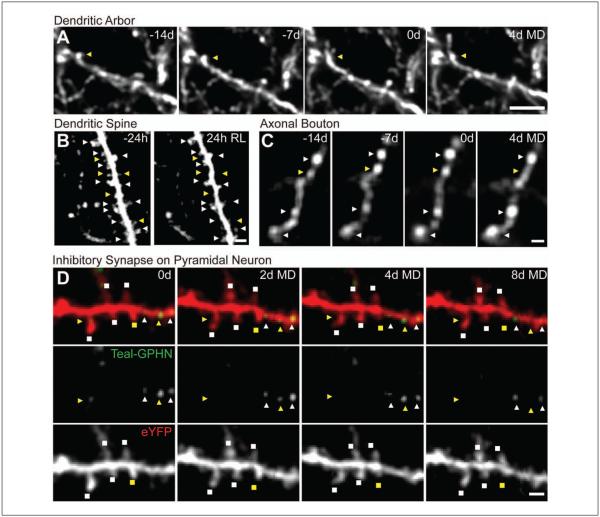
Figure 2.
Experience-dependent structural and synaptic plasticity in inhibitory circuits. Chronic two-photon in vivo images showing remodeling of (A) a dendritic branch tip, (B) dendritic spines, and (C) axonal boutons on inhibitory neurons as well as (D) inhibitory synapses and dendritic spines on excitatory pyramidal neurons in response to monocular deprivation (MD) or retinal lesion (RL) in primary visual cortex. Dynamic events (yellow) of inhibitory neurons (triangles) and pyramidal neurons (squares) with stable counterparts (white) are indicated. Scale bars: (A) 5 μm, (B-D) 2 μm. (B) from Keck and others (unpublished), with permission.
A small subpopulation of interneurons does bear dendritic spines that can also be followed as a marker of alterations in excitatory connections onto interneurons. These have been identified as Martinotti cells and subtypes that express neuropeptide Y (NPY+) as well as a fraction that express calretinin (CR+) and somatostatin (SOM+) (Keck, Scheuss and others 2011). While these dendritic spines receive excitatory input, a large fraction of excitatory synapses on these same interneurons are still found on the dendritic shaft (Kawaguchi and others 2006). Similar to their pyramidal counterparts, dendritic spines on inhibitory interneurons also exhibit a baseline turnover on the order of days (Figure 2B) (Keck and others 2011).
Remodeling of inhibitory inputs onto pyramidal neurons has mostly been inferred from imaging of inhibitory axons. Imaged axon arbors seem stable during normal experience (Keck and others 2011; Marik and others 2010), but a fraction of axonal boutons representing pre-synaptic connections do turnover with time (Figure 2C) (Chen, Lin, and others 2011; Keck and others 2011). Simultaneous in vitro imaging of inhibitory axons and pyramidal dendrites indicates that GABAergic synapses are formed exclusively by new boutons appearing at pre-existing axonal–dendritic crossings without the involvement of any dendritic or axonal protrusions (Wierenga and others 2008).
Inhibitory inputs onto excitatory neurons target a variety of subcellular domains, including the cell body, axon initial segment, and dendritic shaft, as well as some dendritic spines (Markram and others 2004; Somogyi and others 1998). This diversity suggests that inhibitory synapses can regulate neuronal activity in multiple ways. Perisomatic synapses, predominantly formed by parvalbumin (PV+) cells, block sodium (Na+) activity (Miles and others 1996). Axo-axonic synapses, predominantly formed by Chandelier cells, have been shown to have both hyperpolarizing and depolarizing effects (Woodruff and others 2010). Dendritic inhibitory synapses, mostly innervated by SOM+ neurons (Kawaguchi and Kubota 1998), suppress calcium (Ca+)-related dendritic activity (Miles and others 1996).
Although remodeling of perisomatic and axo-axonic synapses in the adult cortex has yet to be examined, recent in vivo imaging studies have looked at the dynamics of inhibitory synapses targeting the dendrites of L2/3 pyramidal neurons by fluorescent tagging of gephyrin, a post-synaptic scaffolding protein exclusive to inhibitory synapses (Chen and others 2012; van Versendaal and others 2012). These studies revealed for the first time the distributions of both inhibitory shaft and spine synapses, their relation to dendritic spine numbers, and their structural dynamics (Figure 2D) (Chen and others 2012). Inhibitory shaft synapses were found uniformly distributed across the dendritic tree at an abundance approximately one third that of dendritic spines. Surprisingly, inhibitory spine synapses sharing a dendritic spine with an excitatory synapse were found to be not just anecdotal (Beaulieu and Somogyi 1990; Jones and Powell 1969; Knott and others 2002; Kubota and others 2007) but rather to comprise almost a third of all dendrite targeting inhibitory inputs. Rather than being uniformly distributed, these inhibitory spine synapses are at particularly high density on distal apical dendrites, and their fractional turnover rates are about three times higher than that of dendritic spines and inhibitory shaft synapses. The excitatory synapses on dually innervated spines are also distinct in that they are positive for VGlut2, a presynaptic terminal marker associated with subcortical-to-cortical afferents (Kubota and others 2007). Interestingly, these dually innervated spines are highly persistent, suggesting that excitatory synapses receiving subcortical inputs are particularly stable but that excitatory transmission through these connections can be dynamically regulated by the plasticity of the co-innervating inhibitory synapses.
Structural Changes in Inhibitory Interneurons as a Substrate of Functional Plasticity
Visual deprivation alters the baseline structural and synaptic remodeling of cortical inhibitory circuits in a way that parallels the deprivation-induced functional changes (Figure 2). MD induces an initial 4-day period of dendritic branch tip retractions in interneurons in binocular visual cortex, a region innervated by both deprived and nondeprived eye inputs (Chen, Lin, and others 2011). The loss of excitatory drive due to these branch tip retractions results in a decrease in visual responsiveness in these neurons as measured by calcium imaging (Kameyama and others 2010). In the case of inhibitory interneurons with dendritic spines, focal retinal lesion results in a rapid and dramatic decrease in dendritic spines in the LPZ within the first 2 days after lesion, paralleling the silencing that occurs in this region (Keck and others 2011). These findings indicate that excitatory inputs onto inhibitory interneurons are highly and acutely responsive to sensory deprivation. Additional experiments demonstrate that these changes are not driven by competition as dendrite retractions are observed in binocular visual cortex even during binocular deprivation (Chen, Lin, and others 2011). Furthermore, complete binocular lesions still produce dendritic spine loss on inhibitory neurons (Keck and others 2011). These structural rearrangements are consistent with physiological observations that deprivation produces a reduction in excitatory drive onto interneurons.
However, not all interneuron structural dynamics are necessarily mere responses to changes in activity levels. Deprivation in the presence of instructive sensory input can guide dendritic branch tip growth and promote excitatory synapse formation. Interneuron dendritic branch tip elongations are observed in the binocular visual cortex, where nondeprived eye inputs are present, in later periods of MD but not during binocular deprivation (Chen, Lin, and others 2011). This occurs as visual responses to the nondeprived eye in this area are increasing. Pharmacological disinhibition by fluoxetine treatment leads to the immediate potentiation of nondeprived eye responses on MD (Maya Vetencourt, Sale and others 2008) as well as the immediate elongation of dendritic tips (Chen, Lin, and others 2011). This demonstrates that structural changes in inhibitory interneurons can themselves be driven by competition and are sensitive to changes in inhibitory tone.
The reduction in inhibitory tone achieved by the loss of excitatory inputs onto inhibitory interneurons is manifested not only as a decrease in inhibitory firing but also as changes in axonal arbors and boutons. In S1, whisker plucking leads to a retraction of inhibitory axons from the deprived barrel column (Marik and others 2010). In V1, inhibitory bouton loss has been observed during both MD and retinal lesions (Chen, Lin, and others 2011; Keck and others 2011). In the case of retinal lesions, inhibitory spine loss has been shown to precede bouton loss, suggesting that the loss of feed-forward deprived eye excitatory inputs to interneurons leads to loss of local inhibitory drive onto neighboring pyramidal cells and a disinhibition of the local circuit. Interestingly, monitoring of inhibitory synapse dynamics in response to MD shows that the MD-induced loss of local inhibitory drive to L2/3 pyramidal cells is manifested differently at inhibitory shaft and inhibitory spine synapses. An acute transient elimination of inhibitory spine synapses is observed within the 24 to 48 hours after MD (Chen and others 2012; van Versendaal and others 2012). In contrast, inhibitory synapses on the dendritic shaft are persistently eliminated throughout the 7-day duration of MD. This persistent elimination on MD suggests that inhibitory shaft synapse plasticity may serve to regulate overall dendritic excitability during this period of reduced sensory-evoked activity whereas inhibitory spine synapse elimination might be a first response to ungate subcortical excitatory synapses innervating that same spine.
The structural dynamics of the inhibitory circuit in response to MD are consistent with an overall preservation of E/I balance after a brief, intermediate period of disinhibition that would allow for sensory-guided rearrangements. Of course, homeostatic responses within excitatory neurons could also affect E/I balance. Whereas evidence for this exists during development (Maffei and others 2004; Maffei and Turrigiano 2008), it remains to be observed in adults.
Consequences of Inhibitory Synaptic Changes at the Circuit and Cellular Levels
Recent studies of structural rearrangements in inhibitory synapse connectivity onto excitatory pyramidal neurons have revealed an unexpected degree of spatial specificity. The local specificity of inhibitory synapse dynamics in terms of target cell type and dendritic location suggests that dendritic inhibitory synapses do not simply control the excitatory current flow to the soma for AP generation, but rather, they can influence the activity of the target cell on a much finer scale than previously imagined. Inhibitory synapse gain and loss can differentially modulate activity on different parts of the dendritic arbor, giving more or less weight to inputs at a specific locale. Similarly, selective rearrangements only on a subset of potential targets can tune specific parts of a local circuit or potentially discriminate between activities of different local circuits.
For example, L2/3 inhibitory interneurons target both L2/3 pyramidal neurons and the distal apical dendrites of L5 pyramidal cells traversing L2/3, but inhibitory synapse and dendritic spine dynamics are quite different on these two cell types. Post hoc immunostaining of inhibitory presynaptic terminals using the marker VGAT onto GFP-labeled dendrites of L5 pyramidal neurons show that MD and retinal lesions both result in a significant reduction in inhibitory synapse density that can be observed even in population comparisons (Chen, Lin, and others 2011; Keck and others 2011). This degree of dynamics was not observed in L2/3 pyramidal neurons where inhibitory synapse loss during MD could only be observed by longitudinal observations and not by comparing across populations (Chen and others 2012; van Versendaal and others 2012).
Whereas MD produced significant dendritic spine gain in L5 pyramidal neurons, such effects were not observed in L2/3 pyramidal neurons (Hofer and others 2009). However, simultaneous in vivo imaging of inhibitory synapse and dendritic spine dynamics show that their rearrangements are clustered within 10 μm of each other and that the frequency of these clustered events can increase with MD (Chen and others 2012). Thus, whereas the total number of dendritic spine changes on L2/3 pyramidal neurons is not influenced by MD, more of them are coordinated with inhibitory synapse dynamics within a 10 μm distance. The functional relevance of this distance is demonstrated by experiments where application of GABA uncaging or optogenetic stimulation of SOM+ inhibitory inputs result in selective inhibition of calcium transients in dendritic regions less than 20 μm from the uncaging or stimulation site (Chiu and others 2012; Kanemoto and others 2011). Activation of excitatory inputs can also induce translocation of calcium-dependent signaling molecules to inhibitory synapses resulting in locally enhanced GABAA receptor surface expression (Marsden and others 2010). Thus, a tight coupling exists between structural plasticity of inhibitory synapses and neighboring excitatory synapses within a distance of mutual influence.
The specificity of inhibitory synapse plasticity with respect to the dendritic tree must also be considered. One recent modeling study that takes into account the active properties of dendrites has proposed that dendritic inhibition may be optimized to control local excitability and plasticity processes in the dendritic tree (Gidon and Segev 2012). A key aspect of this model is that the strategic placement of inhibitory synapses spatially along the dendritic tree is critical. For example, it may be possible to effectively dampen excitatory dendritic currents by surrounding a dendritic region with a few inhibitory contacts rather than placing excitatory and inhibitory synapses adjacently in a one-to-one manner. In addition, the study also suggests that inhibitory synapses targeting the distal end of dendrites are likely to be more effective in controlling nonlinear processing in these dendritic segments. This raises the possibility that rearrangements of a small of number of well-placed inhibitory synapses can have significant influence over the way a neuron computes information in dendritic segments, effectively generating segment- or branch-specific computations (Chklovskii and others 2004; Poirazi and Mel 2001).
Inhibitory synapse location-dependent effects on plasticity also have implications for STDP rules along the dendritic tree, largely due to the fact that bAPs decrease in strength as they propagate out from the soma to distal dendrites. This gradient in postsynaptic activity coupled with a requirement for dendritic spiking activity results in different STDP rules as a function of dendritic distance from the soma (Feldman 2012). In the case of synapses at distal apical tufts where bAPs typically do not reach, STDP is likely to be absent and plasticity is induced by cooperative firing of neighboring inputs producing local dendritic spikes (Spruston 2008). Thus, modulation of dendritic excitability by inhibitory synapse plasticity has the capacity to regulate both the type of STDP and the relative contribution of STDP to local, associative forms of plasticity at any given point along the dendritic tree.
Conclusion and Outlook
In conclusion, long-term structural and synaptic rearrangements of inhibitory interneurons represent a substrate for inhibitory circuit participation in adult cortical plasticity through a combination of mechanisms serving to maintain homeostatic balance while enabling functional reorganization to occur through Hebbian forms of plasticity.
Looking forward, we still lack a great deal of basic information that would allow the complete dissection of inhibitory circuit contribution to adult cortical plasticity. The overall dense, unspecific inhibitory network connectivity observed by some (Fino and others 2012) begs reconciliation with the specificity of structural plasticity described here. What is needed is a closer examination of inhibitory synapse placement and dynamics with respect to various interneuron cell types, a characterization of the perisomatic and axo-axonic inhibitory synapse dynamics, as well as of inhibitory synapses onto interneurons. Inhibitory synapse plasticity in L4 may prove to be quite distinct from L2/3 or L5. How inhibitory synapse structural plasticity can influence local Hebbian plasticity rules on dendrites must also be directly investigated and demonstrated either in vitro or in vivo.
Given their varied and critical role in information processing as well as plasticity, it is not surprising that inhibitory interneurons have also been implicated in a variety of genetic disorders that present cognitive deficits, such as autism, Rett and Down syndromes (Dani and others 2005; Kleschevnikov and others 2004; Rubenstein and Merzenich 2003), as well as genetic mutations that give rise to E/I imbalances resulting in seizures and epilepsy (Bozzi and others 2012; Catterall and others 2008). Molecular and genetic targets that combine to reduce cortical inhibition or promote structural plasticity in the presence of instructive rehabilitative experience are excellent candidates for aiding recovery and restoring cognitive function in adults with such developmental disorders or acute brain injury (Baroncelli and others 2011; Bavelier and others 2010; Castrén and others 2012).
Acknowledgments
We thank members of the Nedivi lab, D. Feldman and B.M. Kampa, for comments on the article, as well as T. Keck, M. Hubener, and T. Bonhoeffer for contribution to figures.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grant RO1 EY017656 (EN) and fellowships from the U.S. National Science Foundation, International Research Fellowship Program, Grant No. 1158914, and Forschungskredit of the University of Zurich, Grant No. 54151805 (JLC).
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Alitto HJ, Dan Y. Function of inhibition in visual cortical processing. Curr Opin Neurobiol. 2010;20(3):340–6. doi: 10.1016/j.conb.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arckens L, Schweigart G, Qu Y, Wouters G, Pow DV, Vandesande F. Cooperative changes in GABA, glutamate and activity levels: the missing link in cortical plasticity. Eur J Neurosci. 2000;12(12):4222–32. doi: 10.1046/j.0953-816x.2000.01328.x. others. [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330(6149):649–52. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Baroncelli L, Braschi C, Spolidoro M, Begenisic T, Maffei L, Sale A. Brain plasticity and disease: a matter of inhibition. Neural Plast. 2011;2011;2011:286073. doi: 10.1155/2011/286073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30(45):14964–71. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C, Somogyi P. Targets and quantitative distribution of GABAergic synapses in the visual cortex of the cat. Eur J Neurosci. 1990;2(4):296–303. doi: 10.1111/j.1460-9568.1990.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Bozzi Y, Casarosa S, Caleo M. Epilepsy as a neurodevelopmental disorder. Front Psychiatry. 2012;3:19. doi: 10.3389/fpsyt.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–86. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Castrén E, Elgersma Y, Maffei L, Hagerman R. Treatment of neurodevelopmental disorders in adulthood. J Neurosci. 2012;32(41):14074–9. doi: 10.1523/JNEUROSCI.3287-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Dib-Hajj S, Meisler MH, Pietrobon D. Inherited neuronal ion channelopathies: new windows on complex neurological diseases. J Neurosci. 2008;28(46):11768–77. doi: 10.1523/JNEUROSCI.3901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Flanders GH, Lee WC, Lin WC, Nedivi E. Inhibitory dendrite dynamics as a general feature of the adult cortical microcircuit. J Neurosci. 2011;31(35):12437–43. doi: 10.1523/JNEUROSCI.0420-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Lin WC, Cha JW, So PT, Kubota Y, Nedivi E. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat Neurosci. 2011;14(5):587–94. doi: 10.1038/nn.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Villa KL, Cha JW, So PT, Kubota Y, Nedivi E. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron. 2012;74(2):361–73. doi: 10.1016/j.neuron.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Lur G, Higley MJ. 2012 Neuroscience Meeting Planner. Society for Neuroscience; New Orleans, LA: 2012. Compartmentalized GABAergic inhibition of dendritic calcium signaling in the neocortex. Program No. 335.08/E7. Online. [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431(7010):782–8. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44(1):23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2005;102(35):12560–5. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith C, Gilbert CD. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J Neurosci. 1995;15(3):1631–47. doi: 10.1523/JNEUROSCI.15-03-01631.1995. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager UC. Observations on monocular deprivation in mice. J Neurophysiol. 1978;41(1):28–42. doi: 10.1152/jn.1978.41.1.28. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404(6774):183–6. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75(4):556–71. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Packer AM, Yuste R. The logic of inhibitory connectivity in the neocortex. Neuroscientist. 2012 Aug 24; doi: 10.1177/1073858412456743. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel MY, Sawtell NB, Diogo AC, Yoon B, Neve RL, Bear MF. Instructive effect of visual experience in mouse visual cortex. Neuron. 2006;51(3):339–49. doi: 10.1016/j.neuron.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450(7168):425–9. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Gambino F, Holtmaat A. Spike-timing-dependent potentiation of sensory surround in the somatosensory cortex is facilitated by deprivation-mediated disinhibition. Neuron. 2012;75(3):490–502. doi: 10.1016/j.neuron.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Giannikopoulos DV, Eysel UR. Dynamics and specificity of cortical map reorganization after retinal lesions. Proc Natl Acad Sci U S A. 2006;103(28):10805–10. doi: 10.1073/pnas.0604539103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidon A, Segev I. Principles governing the operation of synaptic inhibition in dendrites. Neuron. 2012;75(2):330–41. doi: 10.1016/j.neuron.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature. 1992;356(6365):150–2. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19(22):RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harauzov A, Spolidoro M, DiCristo G, De Pasquale R, Cancedda L, Pizzorusso T. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J Neurosci. 2010;30(1):361–71. doi: 10.1523/JNEUROSCI.2233-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci. 2006;26(11):2951–5. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Fuchs J, deBlas AL, Jones EG. Distribution and plasticity of immunocytochemically localized GABAA receptors in adult monkey visual cortex. J Neurosci. 1990;10(7):2438–50. doi: 10.1523/JNEUROSCI.10-07-02438.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Huntsman MM, Viñuela A, Möhler H, de Blas AL, Jones EG. GABAA receptor subunit immunoreactivity in primate visual cortex: distribution in macaques and humans and regulation by visual input in adulthood. J Neurosci. 1994;14(4):2383–401. doi: 10.1523/JNEUROSCI.14-04-02383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Jones EG. Reduction in number of immunostained GABAergic neurones in deprived-eye dominance columns of monkey area 17. Nature. 1986;320(6064):750–3. doi: 10.1038/320750a0. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG. Activity-dependent regulation of GABA expression in the visual cortex of adult monkeys. Neuron. 1988;1(8):701–12. doi: 10.1016/0896-6273(88)90169-9. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–88. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Prior experience enhances plasticity in adult visual cortex. Nat Neurosci. 2006;9(1):127–32. doi: 10.1038/nn1610. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457(7227):313–7. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45(2):279–91. doi: 10.1016/j.neuron.2005.01.003. others. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98(6):739–55. doi: 10.1016/s0092-8674(00)81509-3. others. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72(2):231–43. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Powell TP. Morphological variations in the dendritic spines of the neocortex. J Cell Sci. 1969;5(2):509–29. doi: 10.1242/jcs.5.2.509. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Krubitzer LA, Chino YM, Langston AL, Polley EH, Blair N. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science. 1990;248(4952):229–31. doi: 10.1126/science.2326637. [DOI] [PubMed] [Google Scholar]
- Kameyama K, Sohya K, Ebina T, Fukuda A, Yanagawa Y, Tsumoto T. Difference in binocularity and ocular dominance plasticity between GABAergic and excitatory cortical neurons. J Neurosci. 2010;30(4):1551–9. doi: 10.1523/JNEUROSCI.5025-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto Y, Matsuzaki M, Morita S, Hayama T, Noguchi J, Senda N. Spatial distributions of GABA receptors and local inhibition of Ca2+ transients studied with GABA uncaging in the dendrites of CA1 pyramidal neurons. PloS One. 2011;6(7):e22652. doi: 10.1371/journal.pone.0022652. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Karube F, Kubota Y. Dendritic branch typing and spine expression patterns in cortical nonpyramidal cells. Cereb Cortex. 2006;16(5):696–711. doi: 10.1093/cercor/bhj015. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7(6):476–86. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience. 1998;85(3):677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- Keck T, Scheuss V, Jacobsen RI, Wierenga CJ, Eysel UT, Bonhoeffer T. Loss of sensory input causes rapid structural changes of inhibitory neurons in adult mouse visual cortex. Neuron. 2011;71(5):869–82. doi: 10.1016/j.neuron.2011.06.034. others. [DOI] [PubMed] [Google Scholar]
- Kleschevnikov AM, Belichenko PV, Villar AJ, Epstein CJ, Malenka RC, Mobley WC. Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. J Neurosci. 2004;24(37):8153–60. doi: 10.1523/JNEUROSCI.1766-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34(2):265–73. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Hatada S, Kondo S, Karube F, Kawaguchi Y. Neocortical inhibitory terminals innervate dendritic spines targeted by thalamocortical afferents. J Neurosci. 2007;27(5):1139–50. doi: 10.1523/JNEUROSCI.3846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Chen JL, Huang H, Leslie JH, Amitai Y, So PT. A dynamic zone defines interneuron remodeling in the adult neocortex. Proc Natl Acad Sci U S A. 2008;105(50):19968–73. doi: 10.1073/pnas.0810149105. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Huang H, Feng G, Sanes JR, Brown EN, So PT. Dynamic remodeling of dendritic arbors in GAB-Aergic interneurons of adult visual cortex. PLoS Biol. 2006;4(2):e29. doi: 10.1371/journal.pbio.0040029. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt CN, Hubener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–30. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7(12):1353–9. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28(17):4377–84. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275(5297):209–13. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Majewska AK, Newton JR, Sur M. Remodeling of synaptic structure in sensory cortical areas in vivo. J Neurosci. 2006;26(11):3021–9. doi: 10.1523/JNEUROSCI.4454-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik SA, Yamahachi H, McManus JN, Szabo G, Gilbert CD. Axonal dynamics of excitatory and inhibitory neurons in somatosensory cortex. PLoS Biol. 2010;8(6):e1000395. doi: 10.1371/journal.pbio.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Marsden KC, Shemesh A, Bayer KU, Carroll RC. Selective translocation of Ca2+/calmodulin protein kinase IIalpha (CaMKIIalpha) to inhibitory synapses. Proc Natl Acad Sci U S A. 2010;107(47):20559–64. doi: 10.1073/pnas.1010346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie A, Cnops L, Smolders I, Van Damme K, Vandenbussche E, Vandesande F. Extracellular GABA concentrations in area 17 of cat visual cortex during topographic map reorganization following binocular central retinal lesioning. Brain Res. 2003;976(1):100–8. doi: 10.1016/s0006-8993(03)02717-3. others. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320(5874):385–8. doi: 10.1126/science.1150516. others. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2(1):11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- Miles R, Tóth K, Gulyás AI, Hájos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16(4):815–23. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Mizrahi A, Katz LC. Dendritic stability in the adult olfactory bulb. Nat Neurosci. 2003;6(11):1201–7. doi: 10.1038/nn1133. [DOI] [PubMed] [Google Scholar]
- Poirazi P, Mel BW. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron. 2001;29(3):779–96. doi: 10.1016/s0896-6273(01)00252-5. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293(5532):1159–63. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–67. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10(6):679–81. doi: 10.1038/nn1899. others. [DOI] [PubMed] [Google Scholar]
- Sato M, Stryker MP. Distinctive features of adult ocular dominance plasticity. J Neurosci. 2008;28(41):10278–86. doi: 10.1523/JNEUROSCI.2451-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38(6):977–85. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Schmid LM, Rosa MG, Calford MB, Ambler JS. Visuotopic reorganization in the primary visual cortex of adult cats following monocular and binocular retinal lesions. Cereb Cortex. 1996;6(3):388–405. doi: 10.1093/cercor/6.3.388. [DOI] [PubMed] [Google Scholar]
- Singer W. Neurophysiology: the changing face of inhibition. Curr Biol. 1996;6(4):395–7. doi: 10.1016/s0960-9822(02)00505-5. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamás G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26(2–3):113–35. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9(3):206–21. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420(6917):788–94. doi: 10.1038/nature01273. others. [DOI] [PubMed] [Google Scholar]
- van Versendaal D, Rajendran R, Saiepour MH, Klooster J, Smit-Rigter L, Sommeijer JP. Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron. 2012;74(2):374–83. doi: 10.1016/j.neuron.2012.03.015. others. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Becker N, Bonhoeffer T. GABAergic synapses are formed without the involvement of dendritic protrusions. Nat Neurosci. 2008;11(9):1044–52. doi: 10.1038/nn.2180. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–17. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28(6):1029–40. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Anderson SA, Yuste R. The enigmatic function of chandelier cells. Front Neurosci. 2010;4:201. doi: 10.3389/fnins.2010.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. Development of longterm dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46(2):181–9. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]