Abstract
Olfactory sensory axons target well-defined intermediate targets in the zebrafish olfactory bulb called protoglomeruli well before they form odorant receptor-specific glomeruli. A subset of olfactory sensory neurons are labeled by expression of the or111-7:IRES:GAL4 transgene whose axons terminate in the central zone (CZ) protoglomerulus. Previous work has shown that some of these axons misproject to the more dorsal and anterior dorsal zone (DZ) protoglomerulus in the absence of Netrin 1/Dcc signaling. In search of additional cues that guide these axons to the CZ, we found that Semaphorin 3D (Sema3D) is expressed in the anterior bulb and acts as a repellent that pushes them towards the CZ. Further analysis indicates that Sema3D signaling is mediated through Nrp1a, while Nrp2b also promotes CZ targeting but in a Sema3D-independent manner. nrp1a, nrp2b and dcc transcripts are detected in or111-7 transgene-expressing neurons early in development and both Nrp1a and Dcc act cell-autonomously in sensory neurons to promote accurate targeting to the CZ. dcc and nrp1a double mutants have significantly more DZ misprojections than either single mutant, suggesting that the two signaling systems act independently and in parallel to direct a specific subset of sensory axons to their initial protoglomerular target.
KEY WORDS: Axon guidance, Olfactory development, Protoglomeruli, Sema3D, Semaphorin, Zebrafish
Summary: In the zebrafish olfactory system, Sema3D-mediated repulsion and Netrin-mediated attraction act in parallel to direct a specific subset of sensory axons to their initial targets.
INTRODUCTION
The targeting of axons to their correct postsynaptic partners is a critical step in circuit formation. Studying this process is made challenging by the extraordinary degree of neuronal diversity within the nervous system and the specificity with which neuronal partners interact. The olfactory system is an attractive model for the study of axon targeting because each olfactory sensory neuron (OSN) assumes a specific identity as it chooses a single odorant receptor (OR) from a large gene repertoire and since OR choice is closely coordinated with axonal targeting (Alioto and Ngai, 2005; Zhang and Firestein, 2002). Although sensory neurons expressing a particular OR are broadly dispersed in the olfactory epithelium (OE), their axons converge upon one or a few reproducibly located OR-specific glomeruli in the olfactory bulb (OB); there, they synapse with the dendrites of second order neurons that communicate olfactory information deeper into the brain (Ramon y Cajal, 1892; Ressler et al., 1994; Vassar et al., 1994; Mombaerts et al., 1996; Sosulski et al., 2011; Miyasaka et al., 2014). The OR-specific glomerular map relates odorant experience to neuronal activity at specific locations within the OB, providing a basis for the identification and discrimination of odorants (Bozza et al., 2002). This mapping is, in a sense, an early stage of olfactory information processing.
Our working hypothesis is that the development of the olfactory projection can be divided into at least two broad stages. In the first stage, rather than projecting directly to OR-specific glomeruli, early axons diffusely target larger intermediate regions called protoglomeruli that contain axons from neurons that express a specific subset of different ORs. In a second stage, later in development, OR-specific glomeruli segregate from these larger regions (Royal and Key, 1999; Treloar et al., 1999; Conzelmann et al., 2001; Potter et al., 2001; Li et al., 2005). In the mouse, the first olfactory sensory axons enter the bulb at ∼E15, while glomeruli are only detectable postnatally (Doucette, 1989; Royal and Key, 1999; Conzelmann et al., 2001; Potter et al., 2001). Targeting of sensory axons from the OE to the bulb has been studied extensively in mice (Wang et al., 1998; Schwarting et al., 2000; Vassalli et al., 2002; Cutforth et al., 2003; Imai et al., 2006, 2009; Serizawa et al., 2006; Cho et al., 2007; Col et al., 2007; Kaneko-Goto et al., 2008; Nguyen-Ba-Charvet et al., 2008; Takeuchi et al., 2010). With few exceptions, most of this work has concentrated on glomerular formation near the time of birth or thereafter. The relative accessibility of postpartum animals and the convenience of using glomerulus formation to define target position has facilitated significant advances. Much less attention has been devoted to the initial acquisition of targets before glomeruli form. One important question yet to be addressed is the identity of the molecular cues that define the initial intermediate protoglomerular targets in the bulb and how are they recognized by sensory axons.
The zebrafish is an ideal vertebrate model system for identifying the cues that guide sensory axons as they first reach the bulb. Embryos are accessible at all embryonic stages and have a relatively simple olfactory system. More importantly, sensory axons initially target 12 protoglomerular neuropilar regions, which are discrete, identifiable and spatially reproducible (Dynes and Ngai, 1998; Li et al., 2005; Lakhina et al., 2012). OSN axons exiting the OE project dorsally and anteriorly into the telencephalon, entering the nascent OB by 24 hpf (Wilson et al., 1990; Hansen and Zeiske, 1993; Whitlock and Westerfield, 1998). By 48 hpf, three distinct projection branches can be observed that are thought to correspond to what will develop into the dorsal, central, and medial protoglomeruli (Dynes and Ngai, 1998). Twelve individually identifiable protoglomeruli emerge by 72 hpf (Dynes and Ngai, 1998; Li et al., 2005; Lakhina et al., 2012). Two classes of sensory neurons project to mutually exclusive protoglomeruli in each OB (Sato et al., 2005). Ciliated sensory neurons express classical main OB-type ORs along with olfactory marker protein (OMP). They innervate the central zone (CZ), dorsal zone (DZ), lateral protoglomerulus 3 (LG3), and also sparsely innervate the medial protoglomeruli (MG). Microvillous sensory neurons express V2R-type vomeronasal receptors along with the transient receptor potential channel C2 (Trpc2). They innervate lateral protoglomeruli 1, 2 and 4 (LG1, LG2 and LG4), the ventral posterior glomerulus (VPG) and the olfactory plexus (OP) (Celik et al., 2002; Sato et al., 2005; Lakhina et al., 2012). Individual glomeruli are seen to bud off from protoglomeruli starting at ∼96 hpf (Li et al., 2005). Zebrafish OSNs are first responsive to odorants between 60 and 96 hpf (Li et al., 2005). A long-term aim of our studies is to relate developmental mechanisms to the functional architecture of the bulb.
Previous work has shown that Netrins expressed near the ventral border and the midline of the OB help to guide a subset of olfactory sensory axons into the bulb and towards the CZ protoglomerulus (Lakhina et al., 2012). This subset of sensory neurons is labeled by expression of a transgene in which the or111-7 coding sequence and an IRES:GAL4 reporter are embedded in a minigene construct containing the non-coding sequences surrounding or111-7 along with an enhancer element that is near the or111 gene cluster (Lakhina et al., 2012). In this study we show that sensory axons labeled by the same transgene are repelled by Sema3D expressed at the anterior margin of the OB, pushing them towards the more posteriorly positioned CZ protoglomerulus. We show that the Semaphorin receptor component Neuropilin 1a (Nrp1a) is required for Sema3D repulsion of or111-7 transgene-labeled sensory neurons, and that Nrp2b contributes to their targeting through an independent signaling pathway mediated by an as yet unidentified ligand. Our results identify multiple parallel signaling pathways that cooperate to guide a subset of sensory axons to a particular protoglomerulus.
RESULTS
sema3d and netrin 1b have complementary expression patterns in the OB
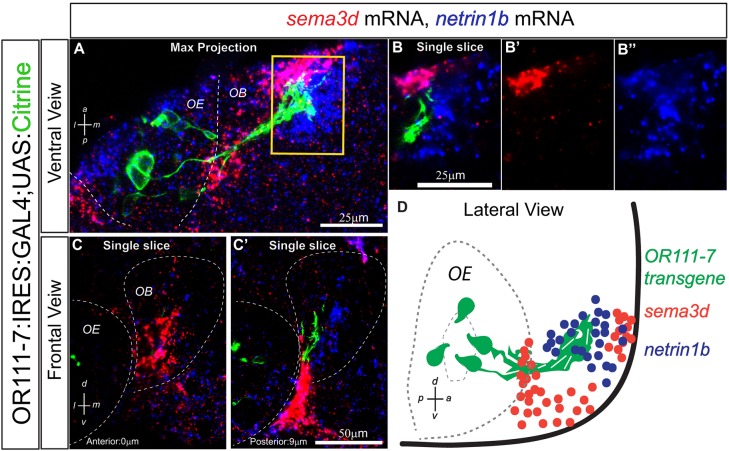
Semaphorins play key roles in the regional targeting of OSNs in both fly and mouse (reviewed by Mori and Sakano, 2011; Lattemann et al., 2007; Joo et al., 2013). Sema3A and Sema3F, secreted members of the larger semaphorin family of axon guidance molecules, are important determinants of olfactory sensory axon targeting in vertebrate systems (Schwarting et al., 2000; Cloutier et al., 2004; Imai et al., 2009; Takeuchi et al., 2010). The expression of additional class 3 semaphorins has been reported in mouse (Sema3C and Sema3B) and in chick (Sema3C, Sema3D and Sema3E) during olfactory system development (Giger et al., 2000; Renzi et al., 2000; Cloutier et al., 2002). As a first step towards understanding their contribution to protoglomerular targeting in the zebrafish, we examined the patterns of class 3 semaphorin mRNA expression in the olfactory system at 36 hpf, when sensory axons have reached the OB but are not yet organized into protoglomeruli. There are 12 identified class 3 zebrafish semaphorins: 3Aa, 3Ab, 3B, 3Bl, 3C, 3D, 3E, 3Fa, 3Fb, 3Ga, 3Gb and 3H (Amores, 1998; Halloran et al., 1999; Roos et al., 1999; Yee et al., 1999; Stevens and Halloran, 2005; Yu and Moens, 2005). Although many are expressed in distinct patterns (Table S1) within the bulb, sema3d is expressed in a complementary pattern to netrin 1b, an attractant that helps guide sensory neurons expressing an or111-7 transgene to the CZ protoglomerulus (Lakhina et al., 2012). At 36 hpf, sema3d mRNA is not detected in OSNs but is strongly expressed in the anteriormost region of the primordial OB (Fig. 1). The axons of OSNs expressing the or111-7 transgene project just posteriorly to sema3d-expressing regions of the bulb. By contrast, netrin 1b mRNA expression overlaps sema3d expression in the anterior bulb, but extends further posteriorly where it is coincident with or111-7 transgene-expressing axons (Fig. 1). Thus, sema3d expression is temporally and spatially positioned in a way that could direct the early axon pathfinding of or111-7 transgene-expressing axons, perhaps by working in opposition to netrin 1b.
Fig. 1.
sema3d and netrin 1b have complementary expression patterns in the zebrafish OB at 36 hpf. (A) Maximum intensity projection spanning 30 µm through a 36 hpf or111-7:IRES:GAL4:UAS:citrine embryo. Ventral view, anterior is up and medial is to the right. Axons are shown in green, sema3d mRNA in red and netrin 1b mRNA in blue. (B-B″) A single optical section through the inset in A. (C,C′) Single optical sections through a 36 hpf embryo. Frontal view, dorsal is up and medial is to the right. Sections are arranged from anterior (left) to posterior (right). The distance from each section to the anteriormost part of the telencephalon is denoted in bottom left. (D) Diagram of a 36 hpf embryo in lateral view. sema3d is expressed in the anterior OB. Some netrin 1b is detected in the anterior bulb but it extends further posteriorly. or111-7 transgene-expressing axons are positioned posterior to sema3d expression but within the netrin 1b expression domain. or111-7 transgenic axons are not present in the anteriormost portion of the telencephalon. sema3d expression wraps around the edge of the olfactory pit and is also present between the OE and nascent OB. OE, olfactory epithelium; OB, olfactory bulb.
or111-7 transgene-expressing axons project ectopically into the DZ protoglomerulus in sema3d mutants
To test whether Sema3D is required in the guidance of or111-7 transgene-expressing sensory axons, we analyzed the olfactory projections in larvae harboring the presumptive null allele sema3dsa1661. This allele was identified by the Sanger Centre Zebrafish Mutation Project and contains a nonsense codon that generates a premature stop at amino acid 257. This stop is within the Sema domain (amino acids 74-521; Ensembl genome browser 75, ENSDARG00000017369), which is required for the interaction of class 3 semaphorins with their receptors (Feiner et al., 1997; Koppel et al., 1997); it is in an exon that cannot be skipped without throwing the translated sequence out of frame. sema3d homozygous mutants were obtained at a frequency of ∼23% and are viable. Because protoglomeruli are composed exclusively of neuropil, propidium iodide staining allows their visualization as pronounced acellular regions in the OB. There were no gross differences in OB morphology or protoglomerular pattern in sema3d mutants as compared with wild-type larvae at 72 hpf (Fig. 2A,B).
Fig. 2.
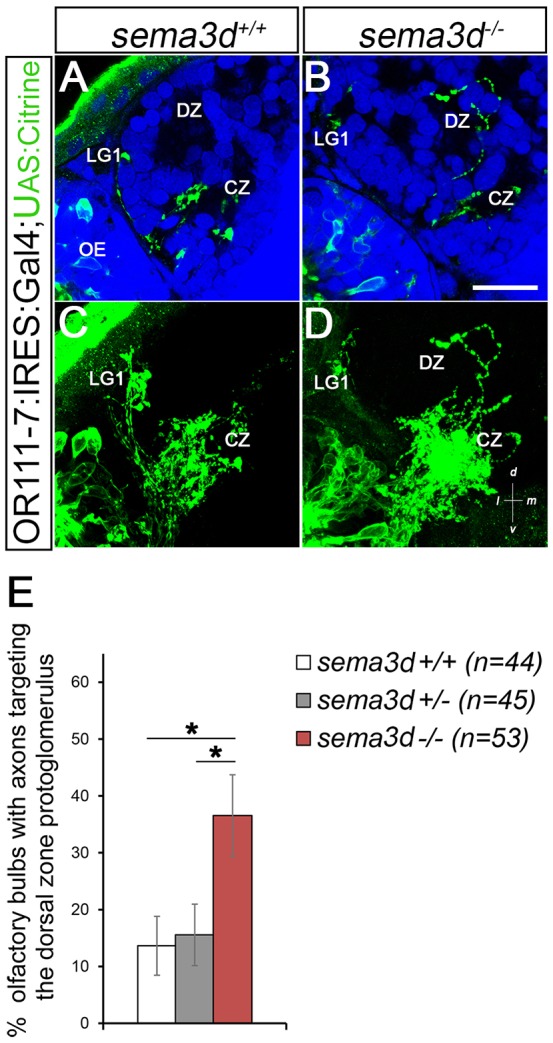
or111-7 transgenic axons misproject to the DZ in sema3d mutants. (A,B) Single optical sections through 72 hpf or111-7:IRES:GAL4;UAS:citrine larvae (frontal view). Axons are in green. Dorsal is up and medial is to the right. Propidium iodide (blue) labels cell bodies, revealing protoglomeruli as cell-free regions. (C,D) Maximum intensity projections of serial optical sections from the larvae shown in A,B. (A,C) Wild-type or111-7 transgenic axons project to the CZ and LG1. (B,D) In sema3d mutants, a subset of or111-7 transgenic axons inappropriately projects to the DZ. Scale bar: 25 µm. (E) The percentage of OBs that have a labeled projection to the DZ protoglomerulus. sema3d homozygous mutants are compared with heterozygous and wild-type siblings. Statistical significance was estimated using two-tailed Fisher's exact test (P<0.05*). Error bars represent s.e. of the sample proportion. CZ, central zone; DZ, dorsal zone; LG1, lateral protoglomerulus 1; OE, olfactory epithelium.
or111-7 transgene-expressing axons projected to the CZ in wild-type, sema3d+/− and sema3d−/− larvae (Fig. 2C,D). However, in sema3d−/− bulbs, one or more or111-7 transgene-expressing axons inappropriately targeted the DZ in 37% of bulbs (n=53) as compared with 14% in wild-type siblings (n=44) and 16% in sema3d+/− siblings (n=45). Using Fisher's exact test we estimate the chance of axons projecting to the DZ at the same frequency in sema3d−/− and wild-type larvae as P=0.02; similarly, when comparing sema3d−/− with sema3d+/− larvae as P=0.02 (Fig. 2). No additional significant misprojections to other protoglomerular targets were observed. Ectopic projections that did not terminate in a protoglomerulus were scored as ‘other’. or111-7 transgenic axons do not display an increase in non-protoglomerular errors in sema3d−/− mutants (Fig. S1G).
To determine whether the loss of sema3d has any broader effects on OSN pathfinding, we incrossed sema3d+/−;Omp:RFP or sema3d+/−;Trpc2:venus lines. Omp-expressing OSN axons project to the CZ, DZ, LG3, and MG protoglomeruli with the same frequency in sema3d−/− as compared with controls and there were no apparent differences in innervation density between mutants and controls (Fig. S1A,B,E). Nor do Omp-expressing sensory axons display any increase in non-protoglomerular targeting errors in sema3d−/− mutants (Fig. S1E). Similarly, there were no significant differences in the protoglomerular or non-protoglomerular targeting of Trpc2-expressing sensory axons in sema3d−/− as compared with controls (Fig. S1C,D,F). The or111-7 transgene also labels a small subset of Omp-negative sensory neurons that project to the Trpc2-specific protoglomerulus LG1 (Lakhina et al., 2012). The lack of a sema3d−/− phenotype in Trpc2-expressing axons indicates that the errors observed in the or111-7 transgenic background originate from Omp-positive neurons that normally target the CZ. Furthermore, there was no significant difference in the number of bulbs with projections to LG1 in sema3d−/− mutants (Fig. S1G).
Thus, Sema3D is required to specifically direct protoglomerular targeting of a subset of Omp-positive axons that project to the CZ. Since the Omp:RFP line does not permit analysis of specific protoglomerular targeting errors, it remains possible that Sema3D influences the targeting of other subsets of axons within this class; for example, those that normally target the DZ or LG3 protoglomeruli.
sema3d is strongly expressed in the anterior OB at 36 hpf. Although Sema3D protein may perdure longer, mRNA expression decreases markedly by 48 hpf, and is undetectable in the anterior OB by 72 hpf (data not shown). We examined whether errors in axon positioning could be observed at very early time points. We examined the or111-7 transgene-expressing projection at 36 hpf and 48 hpf. We found no obvious differences at these early stages in axon trajectories between mutants and controls (data not shown). At these time points, we occasionally saw a small subset of or111-7 transgene-expressing axons that project toward the anterior OB in wild-type larvae (Fig. 1; data not shown). It is possible that, in the absence of Sema3D, these projections fail to be redirected posteriorward and persist in abnormal anterior territories through 72 hpf. Protoglomeruli are not detectable before 72 hpf and, without a transgene that labels the DZ projection, it is unclear whether these axons are intermingled with those that will ultimately target the DZ. Our findings support a model whereby the early repellent actions of Sema3D in the anterior olfactory OB direct or111-7 transgene-labeled axons posteriorly away from the nascent DZ (see Fig. 7).
Fig. 7.
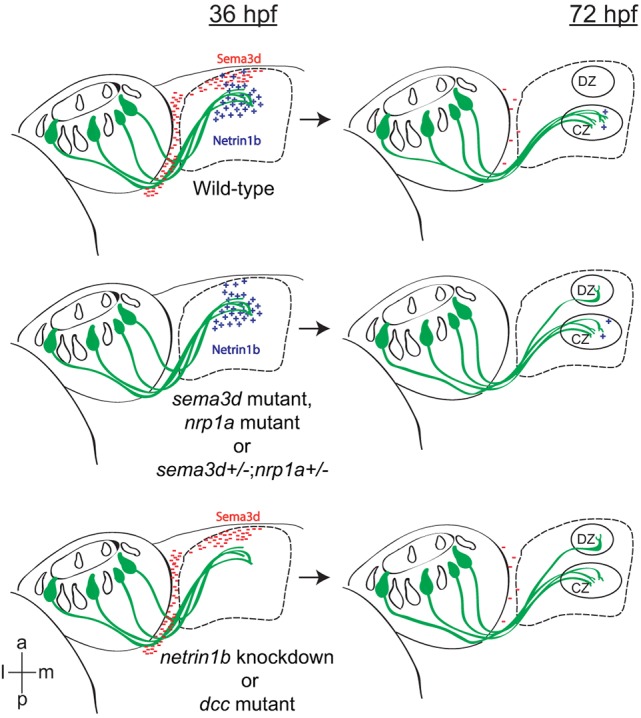
Semaphorin/Neuropilin-mediated repulsion and Netrin/Dcc-mediated attraction guide or111-7 transgene-expressing axons to their initial target in the OB, the CZ. or111-7 transgene-expressing neurons (green) project to the CZ protoglomerulus. Early in development (36 hpf), sema3d is expressed in the anterior OB and in the boundary between the olfactory pit and OB (red). netrin 1b (blue) is expressed more posteriorly in the OB. or111-7 transgene-expressing axons invade netrin 1b-expressing but not sema3d-expressing areas of the bulb. Loss of either Sema3D-mediated repulsion or Netrin-mediated attraction induces a subset of or111-7 transgene-expressing axons to target the more dorsally and anteriorly located DZ protoglomerulus at 72 hpf. Ventral views, anterior is up and medial is to the left. CZ, central zone; DZ, dorsal zone.
All four zebrafish neuropilins are expressed in the developing olfactory system
Class 3 semaphorins signal through neuropilin/plexin A receptor complexes (Sharma et al., 2012). There are two mammalian neuropilins, Nrp1 and Nrp2, which differ in their binding affinities for various class 3 semaphorins (Chen et al., 1997). Although mouse Sema3D has been reported to bind Nrp1 and not Nrp2 in vitro, experiments in zebrafish suggest that Sema3d can signal through Nrp1a/Nrp2b complexes (Wolman et al., 2004; Degenhardt et al., 2013).
To identify potential candidate receptors for Sema3D, we examined neuropilin expression in the olfactory system at 36 hpf. There are four zebrafish neuropilins: nrp1a, nrp1b, nrp2a and nrp2b (Bovenkamp et al., 2004; Yu et al., 2004). or111-7 transgene-expressing neurons express detectable nrp1a (66%), nrp1b (54%) and nrp2b (46%) (Fig. 3). nrp2a was detected in only 17% of or111-7 transgene-expressing neurons at this stage (Fig. 3C,E). The presence of nrp1a, nrp1b and nrp2b mRNA in a substantial proportion of or111-7 transgene-expressing neurons makes each a good candidate for mediating the effects of Sema3D in or111-7 transgene-expressing OSNs.
Fig. 3.
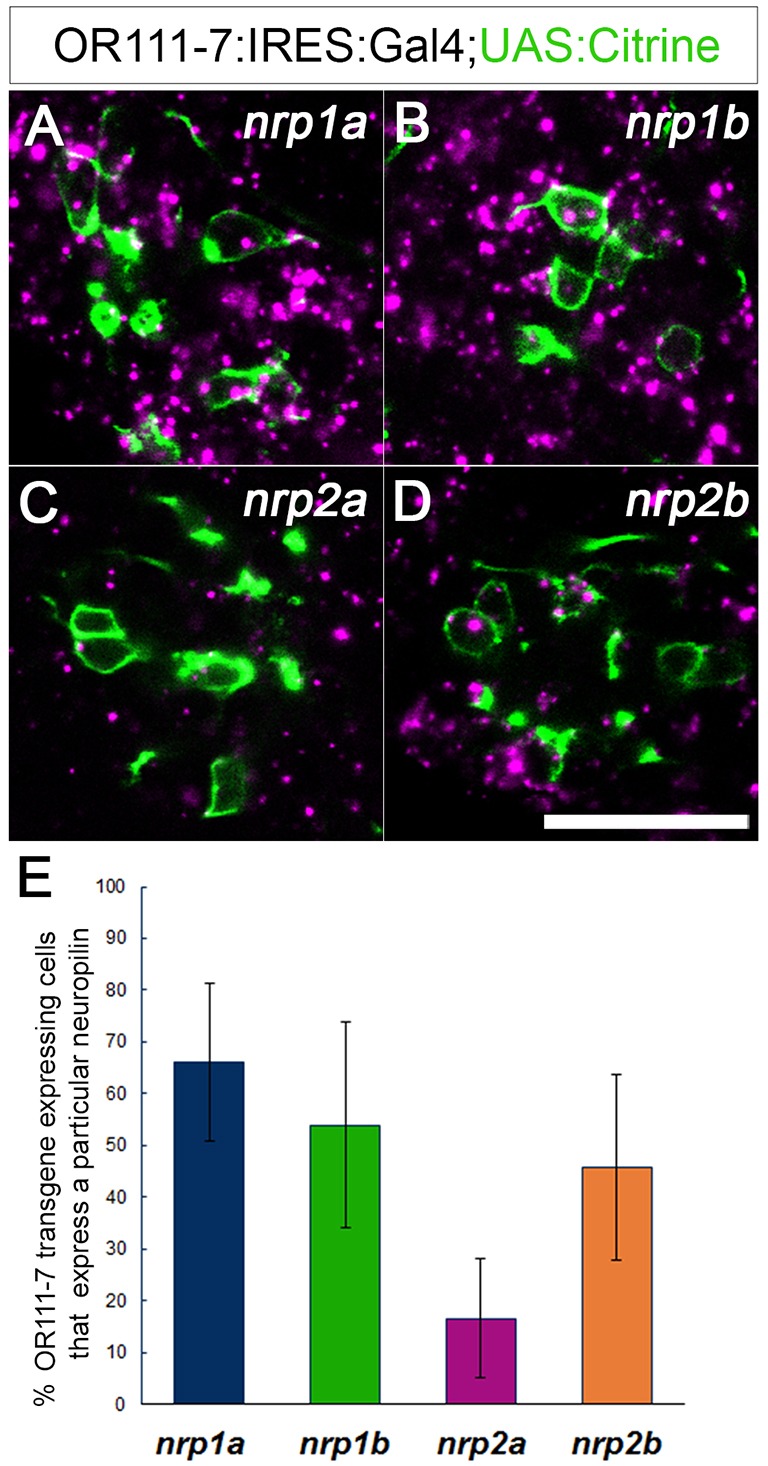
Four zebrafish neuropilins are expressed in or111-7 transgene-labeled neurons during development. (A-D) Single optical sections through the OE of 36 hpf embryos (frontal view). mRNA is in magenta. Subsets of or111-7 transgene-labeled neurons (green) express (A) nrp1a (N=56 pits/764 cells), (B) nrp1b (N=16 pits/191 cells), (C) nrp2a (N=29 pits/610 cells) and (D) nrp2b (N=26 pits/384 cells). Scale bar: 25 µm. (E) The percentage of or111-7 transgene-expressing cells expressing a given neuropilin at 36 hpf. Error bars represent s.d.
Loss of nrp1a, nrp1b or nrp2b induces protoglomerular mistargeting
To further identify candidate receptors for Sema3D, we examined or111-7 transgene-expressing OSN axon targeting in nrp1a, nrp1b or nrp2b presumptive null mutants. The nrp1asa1485 allele contains a nonsense mutation that results in a premature stop codon at amino acid 206 within the second CUB domain (Ensembl genome browser 75, ENSDARG00000071865). The nrp1bfh278 allele contains a nonsense mutation that results in a premature stop codon at amino acid 116 within the first CUB domain (Ensembl genome browser 75, ENSDARG00000027290). Skipping the exons harboring the nonsense mutations in either mutant would throw the translated sequences out of frame. The nrp2bmn0126GT allele contains an RFP and a polyadenylation site inserted into the coding sequence at amino acid 427 just before the second Coagulation factor homology domain (Clark et al., 2011).
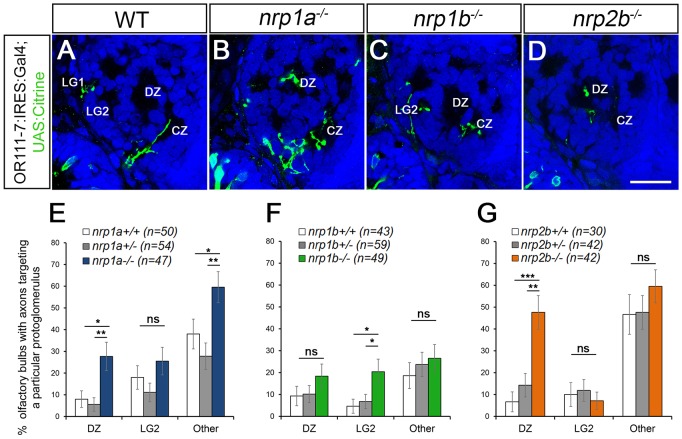
OB morphology and protoglomerular positioning appear normal in all three mutants at 72 hpf (Fig. 4). Similar to sema3d−/− mutants, or111-7 transgene-expressing axons misproject to the DZ in nrp1a−/− mutants. Mistargeting to the DZ was observed in 28% (n=47) of nrp1a−/− bulbs as compared with 8% in wild-type siblings (n=50, P=0.02) and 6% in heterozygote siblings (n=54, P=0.003) (Fig. 4B,E). In contrast to sema3d−/− mutants, or111-7 transgene-expressing axons also misproject to non-protoglomerular locations in nrp1a−/− mutants (Fig. 4E). Axons misproject to regions of the bulb that are dorsal to the CZ in 51% of mutants as compared with 24% of heterozygous siblings (P=0.007). Additionally, we found that or111-7 transgene-expressing axons inappropriately targeted the DZ in 48% (n=42) of nrp2b−/− bulbs as compared with only 7% (n=30, P=0.0002) in wild-type siblings and 14% (n=42, P=0.002) in heterozygous sibling larvae (Fig. 4D,G). No non-protoglomerular targeting errors were detected in nrp2b−/− larvae (Fig. 4G). or111-7 transgene-expressing axons do not have a significant increase in projections to the DZ in nrp1b−/− mutants (Fig. 4C,F). They do, however, have increased projections to LG2 (Fig. 4C,F). These data indicate that Nrp1a or Nrp2b could serve as a Sema3D receptor component in or111-7 transgene-expressing OSNs.
Fig. 4.
Loss of nrp1a phenocopies sema3d mutants. (A-D) Single optical sections through 72 hpf or111-7 transgenic larvae (frontal view). Axons are in green. Dorsal is up and medial is to the right. Propidium iodide (blue) labels cell bodies revealing protoglomeruli as cell-free regions. WT, wild type. Scale bar: 25 µm. (E-G) Percentage of OBs displaying a projection to a particular protoglomerulus or all non-protoglomerular regions (other) is shown. Statistical significance was estimated using two-tailed Fisher's exact tests (P<0.05*, P<0.01**, P<0.001***; ns, not significant). Homozygous mutants are compared with wild-type and heterozygous siblings. Error bars represent s.e. of the sample proportion. (B,E) A subset of or111-7 transgene-labeled axons misproject to the DZ and to non-protoglomerular areas in nrp1a mutants. (C,F) nrp1b mutants do not have increased mistargeting errors to the DZ but do have increased misprojections to LG2. (D,G) A subset of or111-7 transgene-labeled axons also inappropriately targets the DZ in nrp2b mutants. CZ, central zone; DZ, dorsal zone; LG1, lateral protoglomerulus 1; LG2, lateral protoglomerulus 2.
sema3d interacts with nrp1a to promote targeting of or111-7 transgene-expressing axons to the CZ
We next tested whether nrp1a or nrp2b genetically interact with sema3d. If either of these neuropilins acts as a receptor component for Sema3D, larvae that are heterozygous for both nrp1a or nrp2b and sema3d might have a sufficient reduction in signaling activity to induce ectopic misprojections to the DZ, similar to those observed in sema3d−/− mutants. Larvae carrying heterozygous mutations for either sema3d, nrp1a or nrp2b alone had no detectable DZ targeting errors (Fig. 5A,B,D,E,G). or111-7 transgene-expressing axons terminated inappropriately in the DZ in 29% of sema3d+/−;nrp1a+/− transheterozygotes (n=68, P=0.003) as compared with 10% in sema3d+/− or 8% in nrp1a+/− siblings (Fig. 5C,F,G). Similarly, we tested for interactions between sema3d and nrp2b. In contrast to the sema3d+/−;nrp1a+/− transheterozygotes, no excess DZ misprojections were observed in sema3d+/−;nrp2b+/− fish (Fig. 5G). Thus, a genetic interaction is detected between sema3d and nrp1a but not between sema3d and nrp2b.
Fig. 5.
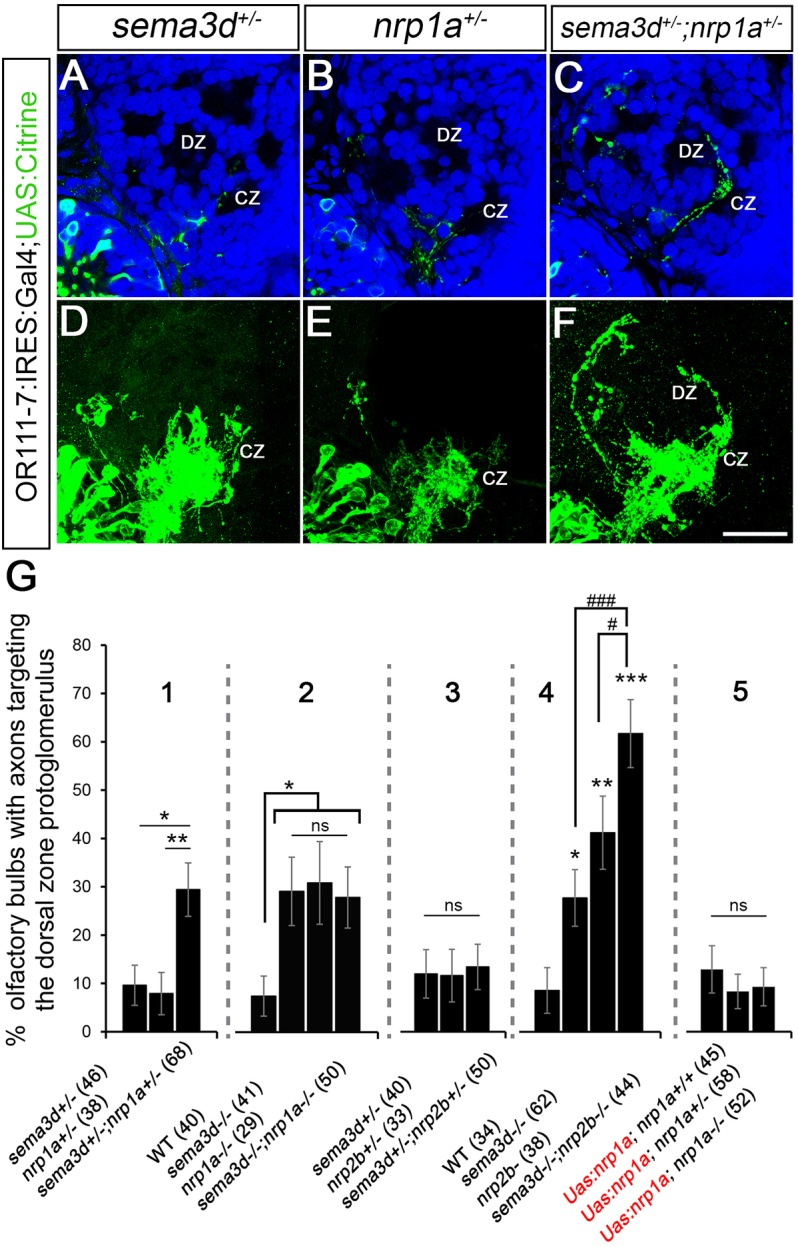
sema3d interacts genetically with nrp1a to promote axon targeting of or111-7 transgene-labeled axons to the CZ. (A-C) Single optical sections through 72 hpf or111-7 transgenic larvae (frontal view). Axons are in green. Dorsal is up and medial is to the right. Propidium iodide (blue) labels cell bodies revealing protoglomeruli as cell-free regions. (D-F) Maximum intensity projections of serial optical sections from the larvae shown in A-C. Scale bar: 25 µm. (G) The percentage of OBs with projections to the DZ protoglomerulus. Statistical significance was estimated using two-tailed (P<0.05*, P<0.01**, P<0.001***) or one-tailed (#P<0.05, ###P<0.001) Fisher's exact tests. Error bars represent s.e. of the sample proportion. sema3d+/−;nrp1a+/− transheterozygotic larvae (C,F; 1 in G) have an increase in projections to the DZ compared with sema3d+/− heterozygotic (A,D) or nrp1a+/− heterozygotic (B,E) siblings. sema3d−/− mutants, nrp1a−/− mutants and sema3d−/−;nrp1a−/− double mutants have a similar increase in projections to the DZ as compared with wild-type siblings (2 in G). By contrast, sema3d+/−;nrp2b+/− transheterozygotes do not have an increase in projections to the DZ when compared with sema3d+/− heterozygotic or nrp2b+/− heterozygotic siblings (3 in G). sema3d−/−;nrp2b−/− double mutants have a greater frequency of projections to the DZ than sema3d−/− or nrp2b−/− siblings (4 in G). nrp1a−/− mutants expressing the UAS:nrp1a:UAS:citrine transgene do not have an increase in misprojections to the DZ or non-protoglomerular regions as compared with wild-type or heterozygotic siblings (5 in G). CZ, central zone; DZ, dorsal zone.
We next examined double-mutant larvae to further probe the relative contributions of Nrp1a or Nrp2b to Sema3D signaling. or111-7 transgene-expressing sensory axons mistargeted the DZ in 29% of sema3d−/− mutants, in 31% of nrp1a−/− siblings, and in 28% of sema3d−/−;nrp1a−/− double-mutant siblings. Thus, the frequency of misprojections is not augmented in double mutants. This is consistent with sema3d and nrp1a working in the same signaling pathway, assuming that both mutants are null, a reasonable assumption given the nature of these mutations. By contrast, or111-7 transgene-expressing axons mistargeted to the DZ in 29% of sema3d−/− mutants, in 43% of nrp2b−/− siblings, and in 64% of sema3d−/−;nrp2b−/− siblings. nrp2b mutants thus have a higher frequency of DZ misprojections than sema3d mutants, and errors increase further in sema3d−/−;nrp2b−/− double mutants (Fig. 5G). This finding is consistent with Sema3D and Nrp2b participating in parallel signaling pathways. Together, these data indicate that Nrp1a is acting as the primary receptor for Sema3D in this context, and that Nrp2b is likely to be mediating the activity of another ligand or ligands.
We examined nrp1a or nrp2b mutants in the Trpc2:venus background and did not observe an increase in projections to the DZ (data not shown). This indicates that, as with sema3d−/− mutants, the or111-7 transgene-labeled axons that misproject to the DZ in nrp1a−/− and nrp2b−/− mutants are likely to be Omp-positive neurons that normally project to the CZ. They are unlikely to be recruited from or111-7 transgene-labeled (and Trpc2-expressing) axons that sometimes terminate in LG1. To further verify that Nrp1a-expressing axons that respond to Sema3D are a subset of Omp-positive neurons rather than Trpc2-positive neurons, we examined sema3d+/−;nrp1a+/− transheterozygotes in which both Omp and or111-7 transgene-expressing neurons are labeled. As expected, axons mistargeting to the DZ in sema3d+/−;nrp1a+/− transheterozygotes are Omp positive (Fig. S1H).
Selective expression of nrp1a in or111-7 transgene-expressing OSNs corrects targeting errors in nrp1a mutants
These results support a model in which Nrp1a serves as an essential receptor component for Sema3D in or111-7 transgene-expressing sensory axons, and that in normal circumstances its presence helps prevent their entry into the DZ protoglomerulus. To test whether nrp1a is required cell-autonomously for normal or111-7 transgene-expressing axon guidance, we generated an nrp1a+/−;UAS:nrp1a;UAS:citrine transgenic line. Three different nrp1a+/;UAS:nrp1a;UAS:citrine founders were mated to nrp1a+/−;or111-7:GAL4 fish. No protoglomerular mistargeting was detected in or111-7 transgene-expressing axons when Nrp1a was overexpressed in otherwise wild-type larvae (Fig. 5G). nrp1a mutants expressing the UAS:nrp1a transgene do not project ectopically to the DZ (Fig. 5G). The ability of Nrp1a expressed in or111-7 transgene-expressing neurons to correct targeting errors seen in nrp1a mutants is consistent with its acting cell-autonomously to guide these axons to their correct target.
nrp1a and dcc are transiently co-expressed in a subset of or111-7 transgene-expressing neurons
Previous work showed that or111-7 transgene-expressing sensory neurons express the netrin receptor Dcc, and that Netrin 1b acts as an attractant that draws this subgroup of axons to the CZ protoglomerulus (Lakhina et al., 2012). We hypothesized that Sema3D/Nrp1a and Netrin 1b/Dcc signaling cooperate to target axons to the CZ. If this were the case, we would predict that nrp1a and dcc should be co-expressed in the majority of or111-7 transgene-expressing neurons and that loss of both receptors might result in increased DZ mistargeting as compared with loss of either receptor alone.
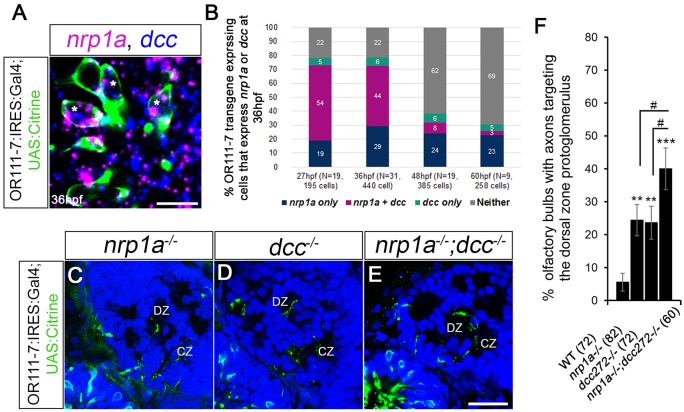
We first asked what proportion of or111-7-expressing axons express nrp1a and/or dcc during early axon targeting. or111-7 transgene expression is first observed at 27 hpf (Lakhina et al., 2012). At this time point we detected ∼10±6 sensory neurons per olfactory pit that express the or111-7 transgene. nrp1a was detected in 73% and dcc was detected in 60% of or111-7 transgene-expressing neurons at 27 hpf (Fig. 6B). At this stage of development, co-expression of nrp1a and dcc was observed in 54% of or111-7 transgene-expressing neurons (Fig. 6B). Only 5% of or111-7 transgene-expressing sensory neurons appeared to express dcc alone, while 19% appeared to express nrp1a alone (Fig. 6B). In cells expressing dcc, 91% also express nrp1a at 27 hpf. There is a decline in the total number and the proportion of colabeled cells from 36 hpf to 48 hpf, whereas the proportion of cells expressing nrp1a or dcc alone remained fairly constant (Fig. 6B). We examined nrp1a−/−;dcc−/− double mutants, expecting to see an increased frequency of DZ misprojections as compared with either mutant alone. or111-7 transgene-expressing axons terminated inappropriately in the DZ in ∼24% of nrp1a−/− or dcc−/− single mutants as compared with 40% in nrp1a−/−;dcc−/− siblings (Fig. 6C-F). We did not observe a genetic interaction between nrp1a and dcc in transheterozygote larvae (data not shown). As DZ misprojections are significantly greater in the double mutant as compared with either single mutant, this result is consistent with Netrin 1b/Dcc and Sema3D/Nrp1a signaling pathways working in parallel to guide or111-7 transgene-expressing cells to the CZ protoglomerulus. The observation that most axons within this subgroup of sensory neurons reach the CZ protoglomerulus even in nrp1a−/−;dcc−/− double mutants indicates that additional guidance pathways cooperate to ensure CZ targeting.
Fig. 6.
Relative contributions of Nrp1a and Dcc to DZ mistargeting. (A) A single optical section through a 36 hpf or111-7 transgenic embryo (frontal view). Cell bodies are in green. Dorsal is up and medial is to the right. nrp1a mRNA is in magenta and dcc mRNA is in blue. (B) The percentages of or111-7-labeled neurons expressing nrp1a, dcc or both at 27, 36, 48 or 60 hpf. (A,B) nrp1a and dcc are co-expressed in a large portion of or111-7-labeled neurons during early development (asterisks in A). (C-E) Single optical sections through 72 hpf or111-7 transgenic larvae (frontal view). Axons are in green. Dorsal is up and medial is to the right. Propidium iodide (blue) labels cell bodies revealing protoglomeruli as cell-free regions. (F) The percentage of OBs with projections to the DZ protoglomerulus. (C-F) nrp1a−/− mutants, dcc−/− mutants and nrp1a−/−;dcc−/− double mutants have an increase in projections to the DZ as compared with wild-type siblings. nrp1a−/−;dcc−/− double mutants have a statistically significant increase in DZ errors when compared with single-mutant siblings. Statistical significance was estimated using two-tailed (**P<0.01, ***P<0.001) or one-tailed (#P<0.05) Fisher's exact tests. Error bars represent s.e. of the sample proportion. CZ, central zone; DZ, dorsal zone. Scale bars: 10 µm in A; 25 µm in C-E.
DISCUSSION
This study identifies Sema3D as a guidance cue that is required for a subset of olfactory sensory axons to target a well-defined intermediate protoglomerular target. or111-7 transgene-expressing axons normally extend just posteriorly to sema3d-expressing cells at the anteriormost margin of the OB. In wild-type larvae these axons congregate in the CZ protoglomerulus, but in sema3d mutants they frequently misproject into the more anterior and dorsal DZ protoglomerulus. These observations can be most simply explained by Sema3D acting as a repellent for or111-7 transgene-expressing axons, directing them posteriorly towards the CZ. We identified the semaphorin receptor component Nrp1a as a likely signaling partner for Sema3D. There are four neuropilins in zebrafish: nrp1a, nrp1b, nrp2a and nrp2b (Bovenkamp et al., 2004; Yu et al., 2004). A large proportion of or111-7 transgene-labeled neurons express nrp1a, nrp1b and nrp2b (Fig. 3). Loss of either nrp1a or nrp2b induces misprojections to the DZ protoglomerulus, making both possible candidate receptors for Sema3D. A genetic interaction can be demonstrated between nrp1a and sema3d in double-heterozygous larvae, but not between sema3d and nrp2b (Fig. 5). The degree of DZ misprojections in sema3d−/−;nrp1a−/− double mutants is similar to that of nrp1a or sema3d single mutants in both penetrance and severity, whereas DZ targeting errors are significantly more prevalent in sema3d−/−;nrp2b−/− double mutants as compared with either single mutant (Fig. 5). Our results support the conclusion that Nrp1a is the key neuropilin mediating Sema3D repulsion in or111-7 transgene-expressing neurons. Additionally, our results strongly suggest that an unidentified ligand – perhaps another one of the many class 3 semaphorins expressed in the zebrafish olfactory system (Table S1) – acts through Nrp2b to promote accurate targeting of or111-7 transgene-expressing axons to the CZ.
Nrp1-mediated signaling has been proposed to affect OSN axon targeting along the anterior-posterior (AP) axis in the main OB of the mouse (Schwarting et al., 2000, 2004; Taniguchi et al., 2003; Imai et al., 2009). Sema3a is thought to be its activating ligand and is expressed in anteromedial and ventral regions of the olfactory nerve layer (Schwarting et al., 2000); it is also expressed in a subset of OSNs (Imai et al., 2009). Selected glomeruli formed by Nrp1-expressing axons are shifted anteriorly in the OBs of Nrp1, Sema3a, or OSN-specific Sema3a mouse mutants (Taniguchi et al., 2003; Imai et al., 2009). Conversely, overexpression of Nrp1 in a subset of OSNs shifts the position of their glomerulus posteriorly (Imai et al., 2009). Our results show that Nrp1a signaling also affects OSN targeting along the AP axis in the zebrafish OB but, at least in the context of our studies, this effect is largely Sema3D dependent. This suggests that Sema3D plays a role in fish reminiscent of the role that Sema3A is proposed to play in mice. We also observed a Sema3D-independent increase in non-protoglomerular dorsally directed errors in nrp1a mutants (Fig. 4), implying that Nrp1a is likely to act as a receptor for additional ligands. Zebrafish sema3aa and sema3ab are expressed in overlapping regions in dorsal and lateral regions of the OB at 36 hpf (Table S1; data not shown). Based on these expression patterns, sema3aa and sema3ab might be candidate guidance cues affecting dorsal-ventral targeting in zebrafish.
The guidance events that we are studying occur early in development, before the emergence of OR-specific glomeruli. During this period, OSNs target a relatively small number (12) of discrete protoglomerular neuropils. A very early requirement for the expression of the guidance receptors that establish the protoglomerular map seems to be emerging. In zebrafish, expression of all of the guidance receptors thus far implicated in early OSN guidance, namely robo2, dcc, and now nrp1a and nrp2b, are all downregulated between 27 hpf and 48 hpf (Miyasaka et al., 2005; Lakhina et al., 2012) (Fig. 6F; data not shown). It has been suggested that immature mouse OSNs express a distinct set of axon guidance genes compared with mature neurons (McIntyre et al., 2010). It is possible that accurate targeting relies upon the early expression of guidance receptors that either pre-sort axons in the olfactory nerve or direct them as they first enter the bulb (Imai et al., 2009; Miller et al., 2010). A different set of receptors might regulate the segregation of OR-specific glomeruli in more mature OSNs.
In this study we show for the first time that sema3d is required for normal OSN targeting in the bulb. Our results support a model in which Sema3D/Nrp1a-mediated repulsion directs a specific subset of sensory axons towards a well-defined intermediate target, the CZ protoglomerulus. This same subset of sensory axons was previously shown to be drawn towards the same intermediate target by Netrin 1b acting through the Dcc receptor (Fig. 7) (Lakhina et al., 2012). Our dcc and nrp1a double-mutant data support a model in which Sema3D/Nrp1a and Netrin 1b/Dcc signal independently and in parallel through competing attractive and repellent mechanisms to direct a subset of axons to the same target. Additionally, Nrp1b, and especially Nrp2b, participate in the normal guidance of this subset of sensory axons though Sema3D-independent signaling pathways. These findings suggest that the first targeting events within the bulb are more complex than previously appreciated, with many different ligand-receptor pairs cooperating to guide sensory axons to particular protoglomeruli. Each cue that we have identified contributes to normal pathfinding, but none is decisive on its own. This should not be surprising. For example, no single mutant was found to be fully penetrant in a collection of mutants affecting axon and neuroblast migration in C. elegans (Hedgecock et al., 1985, 1990). The development of complex neural circuitry originates through an evolutionary process that is likely to add additional guidance cues and receptors on top of those already in use, thereby either increasing the robustness of targeting or altering connectivity. The resulting complexity of cooperating and competing guidance pathways is likely to be a common feature of how neural circuits are assembled.
MATERIALS AND METHODS
Zebrafish maintenance and transgenic lines
Adult zebrafish were raised and maintained according to standard procedures (Mullins, 1994). All experiments were conducted with the approval of the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC). Veterinary care was supervised by University Laboratory Animal Resources (ULAR). Larvae were staged based on hours post fertilization (hpf) and were raised at 28°C. For some experiments, the 36 hpf time point was obtained by incubating for 1 day at 28°C and 1 day at 25°C (Kimmel et al., 1995). Before fixation, larvae were staged based on morphology (Westerfield, 1995). Tg(omp:lyn-RFP) and Tg(trpc2:gap-VENUS) lines were gifts from the Yoshihara laboratory at the RIKEN Brain Science Institute, Saitama, Japan (Sato et al., 2005). The Tg(or111-7:or111-7-IRES:GAL4), Tg(omp:GAL4) and Tg(UAS:gap43-citrine) lines were described by Lakhina et al. (2012). A double-transgenic line, Tg(or111-7:GAL4;UAS:citrine), was generated by crossing Tg(or111-7:or111-7-IRES:GAL4) to Tg(UAS:gap43-citrine). A UAS:nrp1a rescue line was generated by injecting a tol2 UAS:nrp1a;UAS:citrine construct (Dell et al., 2013) into embryos produced from an nrp1asa1485+/− incross using standard procedures (Fisher et al., 2006). These fish were raised to adulthood, screened for germline transmission of the transgene, and genotyped. nrp1asa1485+/−;UAS:nrp1a;UAS:citrine founders were mated with the nrp1asa1485+/−;or111-7:or111-7-IRES:GAL4 line and the resultant larvae were analyzed. Transgenic lines were used alone or crossed into various mutant strains.
Zebrafish mutants
sema3dsa1661 and nrp1asa1485 mutants were generated by the Sanger Centre Zebrafish Mutation Project and obtained from The Zebrafish International Resource Center (ZIRC). These were genotyped using KASPR assays (LGC Genomics; sema3d SNP ID 554-1608.1, nrp1a SNP ID 554-1410.1). The nrp1bfh278 mutant was identified by the Zebrafish Tilling Project and ordered from ZIRC. The nrp2bmn0126GT mutants were a gift from the Ekker laboratory at the Mayo Clinic, Rochester, MN, USA and are available from ZIRC (Clark et al., 2011). Standard PCR-based methods were used to genotype nrp1bfh278 (forward primer, 5′-TCTCTCTTGGGAGGTTCTGC-3′; reverse primer, 5′-TGTCTTTGTGTGTGTGTGCATT-3′; MseI cuts the mutant sequence into 161 bp and 34 bp fragments) and nrp2bmn0126GT (nrp2b forward primer, 5′-GCTGAAGATCGGTATCAGACGAAAAACA-3′; nrp2b reverse primer, 5′-AGACCTGCCATATTGGTGAGTACCGA-3′; RFP reverse primer, 5′-CCTTGAAGCGCATGAACTCCTTGAT-3′) lines. The dcctm272b allele has been described (Lakhina et al., 2012; Jain et al., 2014). Unless otherwise noted, experiments were conducted by mating heterozygous parents. Their fluorescent progeny were collected at 72 hpf and genomic DNA was extracted from tails for genotyping. Matched heads were processed for immunohistochemistry and imaged using confocal microscopy.
Whole-mount immunohistochemistry
Immunohistochemistry was performed as previously described (Lakhina et al., 2012). Larvae were fixed in 4% paraformaldehyde in PBS and dehydrated in methanol. To visualize Citrine-positive axons, larvae were permeabilized in acetone for 20 min at −20°C and stained with goat anti-GFP (1:300; Rockland Immunochemicals, 600-101-215) and donkey anti-goat IgG Alexa Fluor 488 (1:500; Invitrogen) or donkey anti-goat IgG Alexa Fluor 647 (1:500; Invitrogen). To visualize RFP-positive axons, fish were permeabilized for 30 min in 0.1% collagenase at room temperature and stained with rabbit anti-dsRed (1:300; Clontech, 632496) and donkey anti-rabbit IgG Alexa Fluor 647 (1:500; Invitrogen). Propidium iodide staining was performed following secondary antibody treatment as described by Brend and Holley (2009), with the exception that larvae were not treated with RNase. Confocal microscopy was performed on an inverted Leica SP5 using a 40× oil-immersion lens. Stacks were acquired through the entire OB with optical sections taken 1 μm apart.
Whole-mount fluorescent in situ hybridization
Single-label in situ hybridization was performed using antisense digoxigenin (DIG) RNA probes as previously described (Chalasani, 2007). In situ signals were amplified using a cyanine 3-coupled tyramide kit (TSA Plus Cyanine 3; PerkinElmer, NEL744001KT). Double-label in situ hybridization was performed using DIG and fluorescein-labeled probes as previously described (Brend and Holley, 2009), with the exception that embryos were not dehydrated in between detection of the first and second probes and RNase (RNase A, 10 µg/ml; RNase T1, 100 U/ml; Roche, 10109193001) treatment was included after probe removal. The DIG label was amplified using the cyanine 3-coupled tyramide kit and the fluorescein label was amplified using a fluorescein-coupled tyramide kit (PerkinElmer, NEL741001KT). Prior to tyramide amplification, embryos were incubated in either anti-DIG-POD (1:500; Roche, 11207733910) or anti-fluorescein-POD (1:500; Roche, 11426346910). Immunohistochemistry, propidium iodide labeling and imaging were performed following tyramide amplification as described above.
The plasmids used to make probes targeting sema3d, sema3e and nrp1a were as described by Dell et al. (2013). The plasmids used to make probes for sema3aa, sema3ab, sema3fa, sema3fb, sema3ga, sema3gb and nrp2b were gifts from the Moens laboratory at the Fred Hutchinson Cancer Research Center, Seattle, WA, USA (Yu et al., 2004; Yu and Moens, 2005). The plasmid used to make the sema3h probe was a gift from the Halloran laboratory at the University of Wisconsin, Madison, WI, USA (Stevens and Halloran, 2005). For sema3c (refseq accession number XM_687755.5, nucleotides 1539-2400), nrp1b (refseq accession number NM_205674.1, nucleotides 2-972) and nrp2a (refseq accession number NM_212965.1, nucleotides 138-1108) sequences were amplified from cDNA and cloned into pcRII (Invitrogen, K460001) for probe synthesis. Full-length probes were used in all hybridization experiments. The plasmids used to make probes targeting dcc and netrin 1b were as described by Lakhina et al. (2012).
Quantification of targeting errors
The number of OBs containing axons terminating in either individual protoglomeruli or non-protoglomerular regions were counted. The percentage of OBs with axons in particular locations was recorded and Fisher's exact test (two-tailed) was used to determine statistical significance. To determine whether the number of errors was statistically greater in double mutants as compared with single mutants, a one-tailed Fisher's exact test was used (Figs 5 and 6). Error bars represent s.e. of the sample proportion. Axons were scored as projecting to a particular protoglomerulus only if they terminated in that protoglomerulus and not if they passed though it en route to another location.
Acknowledgements
We thank Xiaohe (Diana) Sun for assistance with genotyping.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
A.A.T. and J.A.R. developed concepts and prepared the manuscript. A.A.T., C.L.M., W.Y. and G.J.K. performed experiments and analyzed data.
Funding
This work was supported by a grant from the National Institutes of Health [1R01DC012854]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.127985/-/DC1
References
- Alioto T. S. and Ngai J. (2005). The odorant receptor repertoire of teleost fish. BMC Genomics 6, 173 10.1186/1471-2164-6-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amores A. (1998). Zebrafish hox clusters and vertebrate genome evolution. Science 282, 1711-1714. 10.1126/science.282.5394.1711 [DOI] [PubMed] [Google Scholar]
- Bovenkamp D. E., Goishi K., Bahary N., Davidson A. J., Zhou Y., Becker T., Becker C. G., Zon L. I. and Klagsbrun M. (2004). Expression and mapping of duplicate neuropilin-1 and neuropilin-2 genes in developing zebrafish. Gene Expr. Patterns 4, 361-370. 10.1016/j.modgep.2004.01.014 [DOI] [PubMed] [Google Scholar]
- Bozza T., Feinstein P., Zheng C. and Mombaerts P. (2002). Odorant receptor expression defines functional units in the mouse olfactory system. J. Neurosci. 22, 3033-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brend T. and Holley S. A. (2009). Zebrafish whole mount high-resolution double fluorescent in situ hybridization. J. Vis. Exp. 25 10.3791/1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik A., Fuss S. and Korsching S. I. (2002). Selective targeting of zebrafish olfactory receptor neurons by the endogenous OMP promoter. Eur. J. Neurosci. 15, 798-806. 10.1046/j.1460-9568.2002.01913.x [DOI] [PubMed] [Google Scholar]
- Chalasani S. H., Sabol A., Xu H., Gyda M. A., Rasband K., Granato M., Chien C-. B. and Raper J. A. (2007). Stromal cell-derived factor-1 antagonizes slit/robo signaling in vivo. J. Neurosci. 27, 973-980. 10.1523/JNEUROSCI.4132-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Chédotal A., He Z., Goodman C. S. and Tessier-Lavigne M. (1997). Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Cell 19, 547-559. 10.1016/s0896-6273(00)80371-2 [DOI] [PubMed] [Google Scholar]
- Cho J. H., Lépine M., Andrews W., Parnavelas J. and Cloutier J.-F. (2007). Requirement for Slit-1 and Robo-2 in zonal segregation of olfactory sensory neuron axons in the main olfactory bulb. J. Neurosci. 27, 9094-9104. 10.1523/JNEUROSCI.2217-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K. J., Balciunas D., Pogoda H.-M., Ding Y., Westcot S. E., Bedell V. M., Greenwood T. M., Urban M. D., Skuster K. J., Petzold A. M. et al. (2011). In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nat. Methods 8, 506-512. 10.1038/nmeth.1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier J.-F., Giger R. J., Koentges G., Dulac C., Kolodkin A. L. and Ginty D. D. (2002). Neuropilin-2 mediates axonal fasciculation, zonal segregation, but not axonal convergence. Neuron 33, 877-892. 10.1016/S0896-6273(02)00635-9 [DOI] [PubMed] [Google Scholar]
- Cloutier J.-F., Sahay A., Chang E. C., Tessier-lavigne M., Dulac C., Kolodkin A. L. and Ginty D. D. (2004). Differential requirements for Semaphorin 3F and Slit-1 in axonal targeting, fasciculation, and segregation of olfactory sensory neuron projections. J. Neurosci. 24, 9087-9096. 10.1523/JNEUROSCI.2786-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Col J. A. D., Matsuo T., Storm D. R. and Rodriguez I. (2007). Adenylyl cyclase-dependent axonal targeting in the olfactory system. Development 134, 2481-2489. 10.1242/dev.006346 [DOI] [PubMed] [Google Scholar]
- Conzelmann S., Malun D., Breer H. and Strotmann J. (2001). Brain targeting and glomerulus formation of two olfactory neuron populations expressing related receptor types. Eur. J. Neurosci. 14, 1623-1632. 10.1046/j.0953-816x.2001.01788.x [DOI] [PubMed] [Google Scholar]
- Cutforth T., Moring L., Mendelsohn M., Nemes A., Shah N. M., Kim M. M., Frisén J. and Axel R. (2003). Axonal ephrin-As and odorant receptors: coordinate determination of the olfactory sensory map. Cell 114, 311-322. 10.1016/S0092-8674(03)00568-3 [DOI] [PubMed] [Google Scholar]
- Degenhardt K., Singh M. K., Aghajanian H., Massera D., Wang Q., Li J., Li L., Choi C., Yzaguirre A. D., Francey L. J. et al. (2013). Semaphorin 3d signaling defects are associated with anomalous pulmonary venous connections. Nat. Med. 19, 760-765. 10.1038/nm.3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell A. L., Fried-Cassorla E., Xu H. and Raper J. A. (2013). cAMP-induced expression of Neuropilin1 promotes retinal axon crossing in the zebrafish optic chiasm. J. Neurosci. 33, 11076-11088. 10.1523/JNEUROSCI.0197-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette R. (1989). Development of the nerve fiber layer in the olfactory bulb of mouse embryos. J. Comp. Neurol. 285, 514-527. 10.1002/cne.902850407 [DOI] [PubMed] [Google Scholar]
- Dynes J. L. and Ngai J. (1998). Pathfinding of olfactory neuron axons to stereotyped glomerular targets revealed by dynamic imaging in living zebrafish embryos. Neuron 20, 1081-1091. 10.1016/S0896-6273(00)80490-0 [DOI] [PubMed] [Google Scholar]
- Feiner L., Koppel A. M., Kobayashi H. and Raper J. A. (1997). Secreted chick semaphorins bind recombinant neuropilin with similar affinities but bind different subsets of neurons in situ. Neuron 19, 539-545. 10.1016/S0896-6273(00)80370-0 [DOI] [PubMed] [Google Scholar]
- Fisher S., Grice E. A., Vinton R. M., Bessling S. L., Urasaki A., Kawakami K. and McCallion A. S. (2006). Evaluating the biological relevance of putative enhancers using Tol2 transposon-mediated transgenesis in zebrafish. Nat. Protoc. 1, 1297-1305. 10.1038/nprot.2006.230 [DOI] [PubMed] [Google Scholar]
- Giger R. J., Cloutier J.-F., Sahay A., Prinjha R. K., Levengood D. V., Moore S. E., Pickering S., Simmons D., Rastan S., Walsh F. S. et al. (2000). Neuropilin-2 Is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron 25, 29-41. 10.1016/s0896-6273(00)80869-7 [DOI] [PubMed] [Google Scholar]
- Halloran M. C., Severance S. M., Yee C. S., Gemza D. L., Raper J. A. and Kuwada J. Y. (1999). Analysis of a zebrafish semaphorin reveals potential functions in vivo. Dev. Dyn. 214, 13-25. [DOI] [PubMed] [Google Scholar]
- Hansen A. and Zeiske E. (1993). Development of the olfactory organ in the zebrafish, Brachydanio rerio. J. Comp. Neurol. 333, 289-300. 10.1002/cne.903330213 [DOI] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Thomson J. N. and Perkins L. A. (1985). Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev. Biol. 111, 158-170. 10.1016/0012-1606(85)90443-9 [DOI] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G. and Hall D. H. (1990). The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4, 61-85. 10.1016/0896-6273(90)90444-K [DOI] [PubMed] [Google Scholar]
- Imai T., Suzuki M. and Sakano H. (2006). Odorant receptor-derived cAMP signals direct axonal targeting. Science 314, 657-661. 10.1126/science.1131794 [DOI] [PubMed] [Google Scholar]
- Imai T., Yamazaki T., Kobayakawa R., Kobayakawa K., Abe T., Suzuki M. and Sakano H. (2009). Pre-target axon sorting establishes the neural map topography. Science 325, 585-590. 10.1126/science.1173596 [DOI] [PubMed] [Google Scholar]
- Jain R. A., Bell H., Lim A., Chien C.-B. and Granato M. (2014). Mirror movement-like defects in startle behavior of zebrafish dcc mutants are caused by aberrant midline guidance of identified descending hindbrain neurons. J. Neurosci. 34, 2898-2909. 10.1523/JNEUROSCI.2420-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo W. J., Sweeney L. B., Liang L. and Luo L. (2013). Linking cell fate, trajectory choice, and target selection: genetic analysis of Sema-2b in olfactory axon targeting. Neuron 78, 673-686. 10.1016/j.neuron.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko-Goto T., Yoshihara S.-i., Miyazaki H. and Yoshihara Y. (2008). BIG-2 mediates olfactory axon convergence to target glomeruli. Neuron 57, 834-846. 10.1016/j.neuron.2008.01.023 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B. and Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310. 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Koppel A. M., Feiner L., Kobayashi H. and Raper J. A. (1997). A 70 amino acid region within the semaphorin domain activates specific cellular response of semaphorin family members. Neuron 19, 531-537. 10.1016/S0896-6273(00)80369-4 [DOI] [PubMed] [Google Scholar]
- Lakhina V., Marcaccio C. L., Shao X., Lush M. E., Jain R. A., Fujimoto E., Bonkowsky J. L., Granato M. and Raper J. A. (2012). Netrin/DCC signaling guides olfactory sensory axons to their correct location in the olfactory bulb. J. Neurosci. 32, 4440-4456. 10.1523/JNEUROSCI.4442-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattemann M., Zierau A., Schulte C., Seidl S., Kuhlmann B. and Hummel T. (2007). Semaphorin-1a controls receptor neuron-specific axonal convergence in the primary olfactory center of Drosophila. Neuron 53, 169-184. 10.1016/j.neuron.2006.12.024 [DOI] [PubMed] [Google Scholar]
- Li J., Mack J. A., Souren M., Yaksi E., Higashijima S.-i., Mione M., Fetcho J. R. and Friedrich R. W. (2005). Early development of functional spatial maps in the zebrafish olfactory bulb. J. Neurosci. 25, 5784-5795. 10.1523/JNEUROSCI.0922-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre J. C., Titlow W. B. and McClintock T. S. (2010). Axon growth and guidance genes identify nascent, immature, and mature olfactory sensory neurons. J. Neurosci. Res. 88, 3243-3256. 10.1002/jnr.22497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. M., Maurer L. R., Zou D.-J., Firestein S. and Greer C. A. (2010). Axon fasciculation in the developing olfactory nerve. Neural Dev. 5, 20 10.1186/1749-8104-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka N., Sato Y., Yeo S.-Y., Hutson L. D., Chien C.-B., Okamoto H. and Yoshihara Y. (2005). Robo2 is required for establishment of a precise glomerular map in the zebrafish olfactory system. Development 132, 1283-1293. 10.1242/dev.01698 [DOI] [PubMed] [Google Scholar]
- Miyasaka N., Arganda-Carreras I., Wakisaka N., Masuda M., Sümbül U., Seung H. S. and Yoshihara Y. (2014). Olfactory projectome in the zebrafish forebrain revealed by genetic single-neuron labelling. Nat. Commun. 5, 3639 10.1038/ncomms4639 [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Wang F., Dulac C., Chao S. K., Nemes A., Mendelsohn M., Edmondson J. and Axel R. (1996). Visualizing an olfactory sensory map. Cell 87, 675-686. 10.1016/S0092-8674(00)81387-2 [DOI] [PubMed] [Google Scholar]
- Mori K. and Sakano H. (2011). How is the olfactory map formed and interpreted in the mammalian brain? Annu. Rev. Neurosci. 34, 467-499. 10.1146/annurev-neuro-112210-112917 [DOI] [PubMed] [Google Scholar]
- Mullins M. C.,Hammerschmidt M., Haffter P., and Nüsslein-Volhard C. (1994). Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr. Biol. 4, 189-202. [DOI] [PubMed] [Google Scholar]
- Nguyen-Ba-Charvet K. T., Di Meglio T., Fouquet C. and Chédotal A. (2008). Robos and slits control the pathfinding and targeting of mouse olfactory sensory axons. J. Neurosci. 28, 4244-4249. 10.1523/JNEUROSCI.5671-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S. M., Zheng C., Koos D. S., Feinstein P., Fraser S. E. and Mombaerts P. (2001). Structure and emergence of specific olfactory glomeruli in the mouse. J. Neurosci. 21, 9713-9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S. (1892). Nuevo concepto de la histología de los centros nerviosos: Conferencias Barcelona. [Google Scholar]
- Renzi M. J., Wexler T. L. and Raper J. A. (2000). Olfactory sensory axons expressing a dominant–negative Semaphorin receptor enter the CNS early and overshoot their target. Neuron 28, 437-447. 10.1016/S0896-6273(00)00123-9 [DOI] [PubMed] [Google Scholar]
- Ressler K. J., Sullivan S. L. and Buck L. B. (1994). Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell 79, 1245-1255. 10.1016/0092-8674(94)90015-9 [DOI] [PubMed] [Google Scholar]
- Roos M., Schachner M. and Bernhardt R. R. (1999). Zebrafish semaphorin Z1b inhibits growing motor axons in vivo. Mech. Dev. 87, 103-117. 10.1016/S0925-4773(99)00153-7 [DOI] [PubMed] [Google Scholar]
- Royal S. J. and Key B. (1999). Development of P2 olfactory glomeruli in P2-internal ribosome entry site-tau-LacZ transgenic mice. J. Neurosci. 19, 9856-9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Miyasaka N. and Yoshihara Y. (2005). Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. J. Neurosci. 25, 4889-4897. 10.1523/JNEUROSCI.0679-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting G. A., Kostek C., Ahmad N., Dibble C., Pays L. and Püschel A. (2000). Semaphorin 3A is required for guidance of olfactory axons in mice. J. Neurosci. 20, 7691-7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting G. A., Raitcheva D., Crandall J. E., Burkhardt C. and Puschel A. W. (2004). Semaphorin 3A-mediated axon guidance regulates convergence and targeting of P2 odorant receptor axons. Eur. J. Neurosci. 19, 1800-1810. 10.1111/j.1460-9568.2004.03304.x [DOI] [PubMed] [Google Scholar]
- Serizawa S., Miyamichi K., Takeuchi H., Yamagishi Y., Suzuki M. and Sakano H. (2006). A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell 127, 1057-1069. 10.1016/j.cell.2006.10.031 [DOI] [PubMed] [Google Scholar]
- Sharma A., Verhaagen J. and Harvey A. R. (2012). Receptor complexes for each of the Class 3 Semaphorins. Front. Cell Neurosci. 6, 28 10.3389/fncel.2012.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosulski D. L., Lissitsyna Bloom M., Cutforth T., Axel R. and Datta S. R. (2011). Distinct representations of olfactory information in different cortical centres. Nature 472, 213-216. 10.1038/nature09868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. B. and Halloran M. C. (2005). Developmental expression of sema3G, a novel zebrafish semaphorin. Gene Expr. Patterns 5, 647-653. 10.1016/j.modgep.2005.02.009 [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Inokuchi K., Aoki M., Suto F., Tsuboi A., Matsuda I., Suzuki M., Aiba A., Serizawa S., Yoshihara Y. et al. (2010). Sequential arrival and graded secretion of Sema3F by olfactory neuron axons specify map topography at the bulb. Cell 141, 1056-1067. 10.1016/j.cell.2010.04.041 [DOI] [PubMed] [Google Scholar]
- Taniguchi M., Nagao H., Takahashi Y. K., Yamaguchi M., Mitsui S., Yagi T., Mori K. and Shimizu T. (2003). Distorted odor maps in the olfactory bulb of semaphorin 3A-deficient mice. J. Neurosci. 23, 1390-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar H. B., Purcell A. L. and Greer C. A. (1999). Glomerular formation in the developing rat olfactory bulb. J. Comp. Neurol. 413, 289-304. [DOI] [PubMed] [Google Scholar]
- Vassalli A., Rothman A., Feinstein P., Zapotocky M. and Mombaerts P. (2002). Minigenes impart odorant receptor-specific axon guidance in the olfactory bulb. Neuron 35, 681-696. 10.1016/S0896-6273(02)00793-6 [DOI] [PubMed] [Google Scholar]
- Vassar R., Chao S. K., Sitcheran R., Nuñez M., Vosshall L. B. and Axel R. (1994). Topographic organization of sensory projections to the olfactory bulb. Cell 79, 981-991. 10.1016/0092-8674(94)90029-9 [DOI] [PubMed] [Google Scholar]
- Wang F., Nemes A., Mendelsohn M. and Axel R. (1998). Odorant receptors govern the formation of a precise topographic map. Cell 93, 47-60. 10.1016/S0092-8674(00)81145-9 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (1995). A Guide for the Laboratory Use of Zebrafish (Danio rerio), 3rd edn Eugene: University of Oregon Press. [Google Scholar]
- Whitlock K. E. and Westerfield M. (1998). A transient population of neurons pioneers the olfactory pathway in the zebrafish. J. Neurosci. 18, 8919-8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. W., Ross L. S., Parrett T. and Easter S. S. (1990). The development of a simple scaffold of axon tracts in the brain of the embryonic zebrafish, Brachydanio rerio. Development 108, 121-145. [DOI] [PubMed] [Google Scholar]
- Wolman M. A., Liu Y., Tawarayama H., Shoji W. and Halloran M. C. (2004). Repulsion and attraction of axons by semaphorin3D are mediated by different neuropilins in vivo. J. Neurosci. 24, 8428-8435. 10.1523/JNEUROSCI.2349-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C. S., Chandrasekhar A., Halloran M. C., Shoji W., Warren J. T. and Kuwada J. Y. (1999). Molecular cloning, expression, and activity of zebrafish semaphorin Z1a. Brain Res. Bull. 48, 581-593. 10.1016/S0361-9230(99)00038-6 [DOI] [PubMed] [Google Scholar]
- Yu H.-H. and Moens C. B. (2005). Semaphorin signaling guides cranial neural crest cell migration in zebrafish. Dev. Biol. 280, 373-385. 10.1016/j.ydbio.2005.01.029 [DOI] [PubMed] [Google Scholar]
- Yu H.-H., Houart C. and Moens C. B. (2004). Cloning and embryonic expression of zebrafish neuropilin genes. Gene Expr. Patterns 4, 371-378. 10.1016/j.modgep.2004.01.011 [DOI] [PubMed] [Google Scholar]
- Zhang X. and Firestein S. (2002). The olfactory receptor gene superfamily of the mouse. Nature 5, 124-133. [DOI] [PubMed] [Google Scholar]