Abstract
After invasion of red blood cells, malaria matures within the cell by degrading hemoglobin avidly. For enormous protein breakdown in trophozoite stage, many efficient and ordered proteolysis networks have been postulated and exploited. In this study, a potential interaction of a 60-kDa Plasmodium falciparum (Pf)-heat shock protein (Hsp60) and Pf-calpain, a cysteine protease, was explored. Pf-infected RBC was isolated and the endogenous Pf-Hsp60 and Pf-calpain were determined by western blot analysis and similar antigenicity of GroEL and Pf-Hsp60 was determined with anti-Pf-Hsp60. Potential interaction of Pf-calpain and Pf-Hsp60 was determined by immunoprecipitation and immunofluorescence assay. Mizoribine, a well-known inhibitor of Hsp60, attenuated both Pf-calpain enzyme activity as well as P. falciparum growth. The presented data suggest that the Pf-Hsp60 may function on Pf-calpain in a part of networks during malaria growth.
Keywords: Plasmodium falciparum, heat shock protein 60, calpain, mizoribine
INTRODUCTION
Malaria is a highly prevalent disease in the developing countries; annually there are about 300 million clinical cases and 1 million deaths worldwide [1]. Molecular heat shock protein (Hsp) has been highlighted as a novel anti-malarial target [2]. About 2% of Plasmodium falciparum encodes the genes for Hsp and a total of 95 genes coding for P. falciparum Hsp have been listed. However, only a small number of them have been characterized biochemically and functionally, and the roles of most Hsp are still uncovered in malaria [3,4]. To date, Hsp40, Hsp70, and Hsp90 have been known to play essential roles in the development of P. falciparum parasites [5,6]. Amongst these Hsps, Hsp90 has been well exploited as a potential target of anti-malarial drugs [7].
For many years, P. falciparum Hsp60 has been known to possess sequence similarity with that of the human or bacterial Hsp60 [8]. Typically, Hsp60 belongs to the family of type I chaperones in mitochondria and binds to various polypeptides, preventing their non-specific association for acquisition of native conformation [9]. However, the Hsp60 function as a molecular chaperone is still questionable in P. falciparum. A few of Hsp60 genes have been identified from protozoan parasites such as Entamoeba histolytica [10], Brugia malayi [11], and Trichomonas vaginalis [12]. The high level of transcript Hsp60 from P. falciparum was identified in blood stage parasites, and the localization of Plasmodium Hsp60 was identified in the mitochondrion and apical complex in P. falciparum [3,13]. Apart from functioning as a typical mitochondrial molecular chaperone, Hsp60 has also been detected in the cytosol, cell surface, extracellular space, and biological fluids, indicating the potential extramitochondrial activities of mitochondrial Hsp60 [14-17]. According to the system analysis of Hsp networks in P. falciparum based on PlasmoDB, the hypothetical associative proteases were predicted as cysteine proteases which are involved in hemoglobin metabolism [3,18]. Therefore, it would be an imperative study to investigate the correlation between Hsprelated networks with focusing on proteases.
Plasmodium actively expresses about 100 proteases during the intra-erythrocytic stages of parasite development [19,20]. Among various malaria proteases, cysteine protease falcipain and aspartic protease plasmepsin catalyze hemoglobin as nutrient in the parasite food vacuole (FV) [21]. Plasmepsin IV is localized in FV and plasmepsin V is excluded from the FV in malaria [22]. Endogenous expression of Plasmodium calpain has been profiled along the asexual cycle [23,24]. In our previous study, Plasmodium calpain was confirmed to play a role in degradation of hemoglobin and activate plasmepsin IV [25]. However, the role of Plasmodium calpain networks is still questionable. Despite a system analysis of the potential networks between P. falciparum Hsp and cysteine proteases [18], relevance of Plasmodium calpain and Hsps has not been understood.
Mammalian Hsp60 has been reported to be inhibited by mizoribine [26]. However, there is no report of anti-malarial activity of mizoribine yet. In this study, we extended the studies with activity of P. falciparum Hsp60 on P. falciparum calpain, using mizoribine as an Hsp60 inhibitor. To reveal the activity of P. falciparum Hsp60 on P. falciparum calpain, in vitro interaction and subcellular co-location of P. falciparum Hsp60 and P. falciparum calpain were determined, and the inhibitory effect of Hsp60 inhibitor on Pf-calpain activity and P. falciparum growth was determined.
MATERIALS AND METHODS
Antibodies
The rabbit polyclonal P. falciparum (Pf)-Hsp60 antibody was purchased from LSBio (Seattle, Washington, USA). The rabbit polyclonal anti-Pf-calpain was generated as described in a previous study [27]. Antisera against GroEL raised in mice were generated in this study. The secondary goat-anti-rabbit IgG conjugated with horseradish peroxidase (HRP) was purchased from Bio-Rad (Hercules, California, USA). Cy5-conjugated goat-anti-rabbit IgG, FITC-conjugated rabbit-anti-mouse IgG, and anti-GAPDH antibody produced in rabbits were purchased from Sigma-Aldrich (St. Louis, Missouri, USA).
Reagents
Percoll was purchased from Amersham Pharmacia Biotech (Uppsala, Sweden). Mizoribine and N-Acetyl-L-leucyl-L-leucyl-L-norleucinal (ALLN) were purchased from Sigma-Aldrich. SYTOX® Green Nucleic Acid Stain was purchased from Life Technologies (Carlsbad, California, USA).
In vitro culture of P. falciparum
P. falciparum 3D7 (ATCC PRA-405D) strain was purchased from ATCC (Manassas, Virginia, USA) and kept at -80˚C as frozen stocks. This 3D7 strain was grown in human erythrocytes, as described previously [28]. Briefly, parasites were maintained in continuous culture with 5% hematocrit of type O human red blood cells suspended in RPMI 1640 medium supplemented with 24 mM sodium bicarbonate, 25 mM HEPES, 0.8% hypoxanthine, 0.9% albumax, and 25 µg/ml gentamycin. The 6-well plates were placed in an incubator (5% CO2, 5% O2, and 90% N2 atmosphere) at 37˚C, and the medium was changed daily when parasitemia reached at least 5%. The parasite density was determined by Giemsa staining of thin smears as a percentage of infected erythrocytes in fields of total 500 erythrocytes.
Isolation of the infected RBC
To obtain the parasite-infected RBC, P. falciparum cultures were centrifuged, and each 1 ml of iRBC pellet in PBS was carefully placed on top of 3 ml of 70% Percoll gradient (v/v) [29]. Each tube was centrifuged at 800 g for 10 min and stopped with deceleration 0. The interface layer containing the infected RBCs was washed 2 times with complete media to make a pellet.
Western blot analysis
The identification of Pf-Hsp60 and Pf-calpains was confirmed by western analysis. The pellet of the infected RBCs were resuspended with RIPA lysis buffer (150 mM NaCl, 50 mM Tris–HCl pH 7.6, 0.1% NP-40, and protease inhibitor cocktail) (Pierce, Waltham, Massachusetts, USA). To detect the existence of Pf-Hsp60 and Pf-calpains, 500 µg proteins were used for SDS-PAGE. Besides, GroEL protein was electrophoresed with polyacrylamide gel and transferred onto a PVDF membrane. The membrane was blocked with 5% skim milk and incubated with either anti-Pf-Hsp60 (1:4,000 dilution) or anti-Pf-calpain (1:2,000 dilution). The blots were further incubated with goat anti-rabbit IgG conjugated-HRP antibodies for 1 hr (1:5,000 dilution).
Immunoprecipitation analysis
The eluted proteins and parasite-infected RBCs were resuspended in native lysis buffer, i.e., 20 mM Tris HCl pH 8.0, 137 mM NaCl, 10% glycerol, 1% Nonidet P-40 (NP-40), 2 mM EDTA, and a 500 µg protein was mixed with 10 µg of anti-Pf-Hsp60 for overnight at 4˚C on a rocking platform. Next day, 50 µl of Dynabead Protein A (Invitrogen, Carlsbad, California, USA) were added to antigen-antibody complex, and this binding was allowed to proceed for 4 hr. Finally, the immunoprecipitate was then collected by adding 0.1 M citrate (pH 2.5) to antigen-antibody bead complex for 2 min. SDS-PAGE was performed, and the protein was transferred onto a PVDF membrane. The blot was analysed with anti-Pf-calpain (1:100 dilution) and anti-Pf-Hsp60 (1:2,000 dilution). Negative control sample was prepared with anti-rabbit IgG for specificity of immunoprecipitation.
Immunofluorescence assay
To investigate the localization of Pf-calpain and GroEL-like protein, immunofluorescence assay (IFA) was carried out as previously described [24]. As co-localization requires 2 antibodies generated in different species, anti-GroEL generated in mice was used for IFA with anti-Pf-calpain produced in rabbits instead of anti-Pf-Hsp60 produced in rabbits. The infected RBCs were smeared on a coverslip and dried completely overnight. Following fixation of parasite-infected red blood cells by 1% paraformaldehyde, the presence of each protein was examined using anti-Pf-calpain and anti-GroEL as the primary antibody (1:200 dilution). Subsequent to washing, the erythrocytes were incubated with Cy5-conjugated goat anti-rabbit IgG secondary antibodies and FITC-conjugated rabbit anti-mouse IgG secondary antibodies (1:400 dilution) for 1 hr at room temperature. After washing with PBS, a cover slip was mounted on the slide with mounting media including 4', 6-diamidino-2-phenylindole (DAPI) (Vector, Burlingame, California, USA). The images of fluorescent erythrocytes were visualized by 510 Meta Confocal Laser Scanning Microscope (LSM) (Carl Zeiss, Jena, Germany) using a 1,000× oil immersion objective. The images were processed using LSM viewer. As negative control, rabbit IgG and antisera of mice were used to validate the specificity of the fluorescence signal.
Measurement of calpain activity using fluorogenic substrate assay
To determine the effect of mizoribine on malarial calpain activity, the infected RBCs were lysed in 0.01% saponin/PBS solution, and the proteolytic activity was assessed employing fluorogenic substrate, Succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (Suc-LLVY-AMC) (Bachem, Torrance, California, USA) as described previously [27,28]. Briefly, the isolated RBC lysates were incubated with the serially diluted inhibitors for 1 hr at 37˚C in the presence of MOPs buffer (116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 5.5 mM D-glucose, 50 mM MOPS, and 1 mM CaCl2, pH 7.2). The proteolytic activity was monitored at 30 min after addition of the substrate. The wavelengths of the Infinite F200 fluorometer (Tecan, Männedorf, Switzerland) were set to excitation at 360 nm and emission at 460 nm. The percentage was plotted against the log of the inhibitor concentration using GraphPad Prism 5.0.
In vitro antimalarial activity of mizoribine
To access the effect of mizoribine on malarial growth, 0.5% parasite density in 2% hemocirt was seeded onto a 48-well plate, and mizoribine was serially diluted in media for 48 hr without any medium change. At last, 100 µl of P. falciparum cultures were taken out from each well of 48-well plate, and 1 µl of blood pellet was mixed with 5 mM of SYTOX green solution to obtain a final volume of 1.5 ml. The mixture solution was left in dark for 30 min at room temperature, and the anti-malarial activity of mizoribine was analyzed by FACSTM Calibur Flow cytometer (BD Biosciences, Carlsbad, California, USA).
RESULTS
Sequence analysis of P. falciparum Hsp60
As Hsp families are conserved in most organisms from bacteria to higher eukaryotes, the sequences of Hsp60 is conserved among bacteria, parasites, plants, and humans [8]. As shown in Fig. 1A, full-length sequences of Plasmodium Hsp60 (1-577 amino acids) were aligned in BLAST, using BLASTP 2.2.29+ program. It shows 93-100% identities among 5 Plasmodium species, i.e., P. falciparum (GenBank accession no. AAC47716.1), P. knowlesi (no. XP_002258797.1), P. berghei (no. XP_677968.1), P. yoelii (no. AAC78151.1), and P. reichenowi (no. CDO64587.1)]. In contrast, Pf-Hsp60 shows 72%/88%, 51%/70%, 55%/75%, 54%/72%, 59%/79%, 54%/73% identities/positives for Toxoplasma gondii Hsp60 (no. XP_002367122.1), Trichomonas vaginalis Hsp60 (no. AAB39487.1), Legionella pneumophila Hsp60 (no. ACI47229.1), Arabidopsis thaliana Hsp60 (no. AEE76842.1), and Homo sapiens Hsp60 (no. NP_955472.1). Based on domain analysis tool of European Bioinformatics Institute, GroEL-like equatorial domains (30-216 and 397-546 amino acids) (red squares) and GroEL-like apical domains (214-403 amino acids) (blue squares) were highly conserved among 5 Plasmodium species Hsp60. In this study, Oleispira antarctica GroEL (UniProtKB accession n. Q8KM30) was extracted from Escherichia coli strain Arctic Express, and the antigenicity of anti-Pf-Hsp60 against GroEL was confirmed by western blot analysis. GroEL was detected by anti-Pf-Hsp60 in a dose-dependent matter, supporting the high homology (54%/72% identities/positives) between GroEL and Pf-Hsp60 (Fig. 1B). Alignment results among different species Hsp60 were summarized in Table 1. Because of 54%/73% identities/positives between human -and Pf-Hsp60, it was indispensable to separate the infected RBCs in our study. To verify the purities of iRBC after purification by Percoll gradient, the smeared P. falciparum was examined by microscopy after Giemsa staining. As shown in Fig. 2, RBCs obtained from the interface layer was confirmed to include only the infected RBCs, and the pellet included the uninfected RBCs, indicating that the Percoll isolation method was suitable for separating the infected RBCs from the total RBC population.
Fig. 1.

Multiple sequence alignment of Hsp60s derived from different species. (A) Five Hsp60s derived from different Plasmodium species were aligned with those of 6 non-Plasmodium species. Identical residues are shaded in black while similar amino acids are shown in gray background. GroEL-like equatorial domains (red square) and GroEL-like apical domain (blue square) are shown. (B) Immunoblotting (IB) was performed to detect GroEL with anti-Pf-Hsp60.
Table 1.
Analysis of Hsp60 identities derived from different species
| Species | Identities (%) | Positives (%) | Gaps (%) | GenBank accession no. |
|---|---|---|---|---|
| P. falciparum | 577/577 (100.0) | 577/577 (100.0) | 0/577 (0.0) | AAC47716.1 |
| P. knowlesi | 545/580 (94.0) | 564/580 (97.2) | 3/580 (0.5) | XP_002258797.1 |
| P. berghei | 541/580 (93.3) | 558/580 (96.2) | 4/580 (0.7) | XP_677968.1 |
| P. yoelii | 539/580 (92.9) | 557/580 (96.0) | 4/580 (0.7) | AAC78151.1 |
| P. reichenowi | 574/580 (99.0) | 576/580 (99.3) | 3/580 (0.5) | CDO64587.1 |
| T. gondii | 383/532 (72.0) | 469/532 (88.2) | 4/532 (0.8) | XP_002367122.1 |
| T. vaginalis | 280/549 (51.0) | 387/549 (70.5) | 11/549 (2.0) | AAB39487.1 |
| L. pneumophila | 293/532 (55.1) | 400/532 (75.2) | 7/532 (1.3) | ACI47229.1 |
| O. antarctica | 283/525 (53.9) | 381/525 (72.6) | 10/525 (1.9) | Q8KM30 |
| A. thaliana | 313/530 (59.1) | 422/530 (79.6) | 4/530 (0.0) | AEE76842.1 |
| H. sapiens | 290/535 (54.2) | 395/535 (73.8) | 6/535 (1.1) | NP_955472.1 |
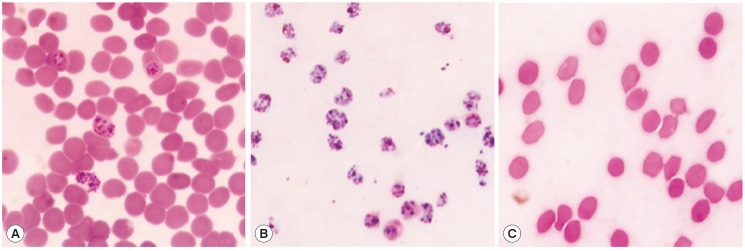
Fig. 2.
Purification of P. falciparum. Total RBC (A) was purified using 70% Percoll and separated into only infected RBCs (iRBC) (B) at interface layer and pellet (C). Giemsa stain was performed and interface cells were confirmed as the schizonts under a microscope.
Expression of Pf-Hsp60 and Pf-calpain in the infected RBCs
To address the endogenous Pf-Hsp60 and Pf-calpain, immunoblotting analysis was performed. The parasite density was about 13% within 6 days. After isolation of the infected RBCs, 500 µg of lysates were electrophoresed on SDS-PAGE. The expression of Pf-Hsp60 (60 kDa), GroEL like protein (60 kDa), and Pf-calpain (240 kDa) in the infected RBC lysates were shown as dominant protein molecular weight (Fig. 3A). Plasmodium Hsp60 was confirmed in cell lysates of P. falciparum 3D7. Besides, anti-GroEL reacted with 60 kDa of the protein, and anti-Pf-Hsp60 reacted to the same size in the infected RBC lysates, indicating the possibility that anti-GroEL could be useful for detection of Pf-Hsp60 in malaria. Finally, the potential complex between Plasmodium Hsp60 and Plasmodium calpain was confirmed by immunoprecipitation using anti-Pf-calpain in cell lysates, and the eluent showed the presence of Pf-Hsp60 by anti-Hsp60 (Fig. 3B). Subcellular localization of Pf-calpain, Pf-Hsp60 was determined by IFA. GroEL-like protein and Pf-calpain appeared in both trophozoites (Fig. 4A) and schizonts (Fig. 4B). The potential co-localization of GroEL-like protein and Pf-calpain were analyzed by merging 2 images, and those signals appeared to be partially co-localized in both trophozoites and schizonts.
Fig. 3.
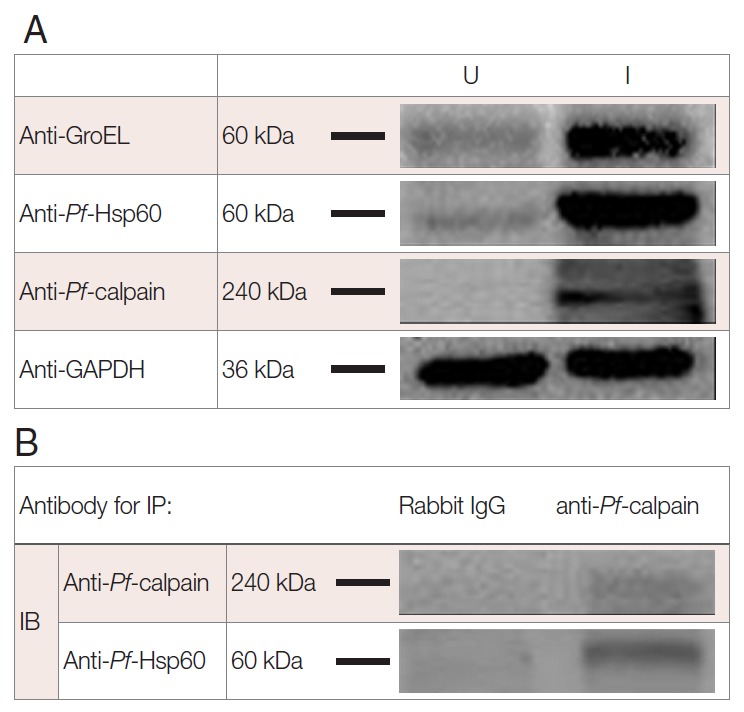
Interaction of Plasmodium Hsp60 and Plasmodium calpain in the infected RBCs. After isolation of P. falciparum (Pf)-infected RBC (iRBC), the presence of endogenous Pf-Hsp60, GroEL like protein, and Pf-calpain was confirmed by immunoblotting (IB) analysis in uninfected (U) and infected (I) RBC (A). Each GroEL like protein, Pf-Hsp60, and Pf-calpain in iRBC were detected dominantly. Immunoprecipitation (IP) was performed to determine the interaction between Plasmodium Hsp60 and Plasmodium calpain. Lysate (500 μg) of iRBC was immunoprecipitated with anti-Pf-calpin and the presence of Pf-Hsp60 in the eluent was confirmed by IB, using anti-Pf-Hsp60 (B).
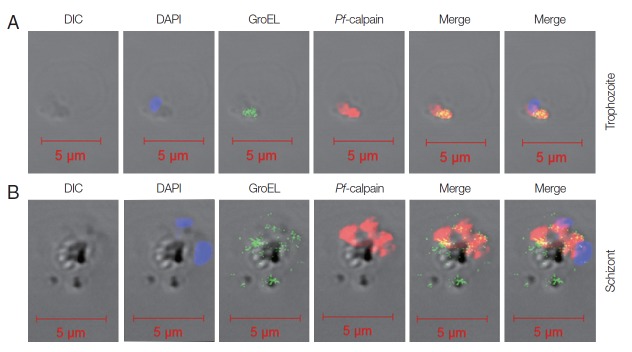
Fig. 4.
Localization of Pf-Hsp60 and Pf-calpain in the infected RBCs. Co-localization of Pf-Hsp60 and Pf-calpain was observed by IFA. Anti-GroEL-antibody was used for detection of Pf-Hsp60 using FITC-conjugated rabbit-anti-mouse IgG (green). Pf-calpain was visualized by Cy5-conjugated goat-anti-rabbit IgG (red). Pf-Hsp60 was partially co-localized (yellow) with Pf-calpan in both trophozoites (A) and schizonts (B).
Interference of mizoribine on calpain activity in P. falciparum
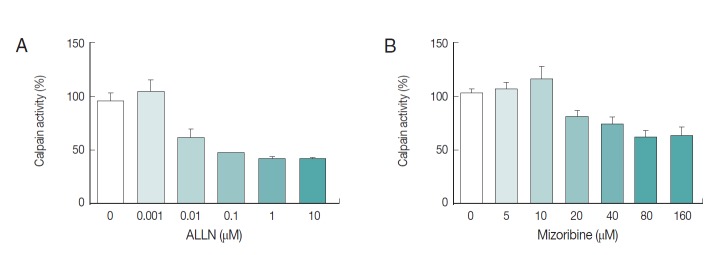
We also explored the inhibition rate of endogenous Pf-calpain activity by mizoribine. Typically, 10 µg of lysate was reacted with serially diluted mizoribine at 37˚C for 60 min. Consecutively, 10 µM of substrate was added to the mixture of lysate and the serially diluted mizoribine for 37˚C for 30 min further. As seen in Fig. 5, mizoribine suppressed the calpain enzyme activity up to 40% at a concentration of more than 80 µM. ALLN was used as the positive control of anti-calpain reagent.
Fig. 5.
Interference of mizoribine on Pf-calpain activity. After isolation of the infected RBCs. the proteolytic activity of Pf-calpain was assessed by fluorogenic substrate, Suc-LLVY-AMC in the presence of ALLN (A) or Mizoribine (B). ALLN and Mizoribine suppressed the calpain enzyme activity in dose-dependent matter.
In vitro inhibition of mizoribine on malarial growth
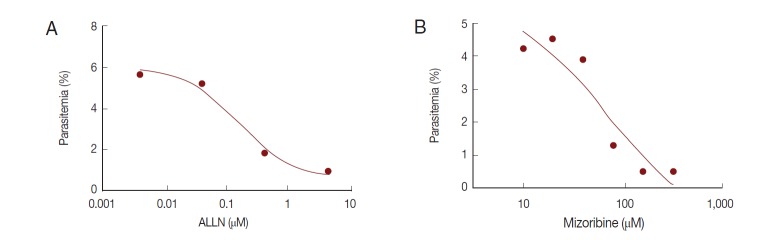
The in vitro efficacy of mizoribine on malarial growth was evaluated. After treatment with mizoribine for 2 days, the growth of P. falciparum was evaluated by flow cytometry. IC50 (growth) of mizoribine was 68.13 µM. IC50 (growth) of ALLN was 0.16 µM which is a 455-fold potent reagent in comparison to mizoribine (Fig. 6).
Fig. 6.
Anti-malarial effect of mizoribine. P. falciparum was cultured in the presence of ALLN or mizoribine in vitro. After treatment of inhibitors for 2 days, the parasite density of P. falciparum was analyzed by flow cytometer. Each IC50 (growth) of ALLN (A) and mizoribine (B) was determined by plotting in Graphpad Prism software.
DISCUSSION
The sudden changes in environment including nutrient deprivation, elevated temperature, low pH, increase in heavy metal ions, and increased parasitic infections are related to Hsp expression [6]. In addition to typical function of Hsps such as folding/unfolding/assembly of protein, transporting protein to adequate subcellular compartment, and signaling, Pf-Hsp has played a pivotal role in modulating the activity of antioxidant enzymes in parasites [13]. An increase in mRNA level of Plasmodium Hsps at the ring stage was noted, which continued up to the trophozoite stage and declined at schizonts [23]. This observation possibly indicates massive Hsp may participate in orchestration of malarial protein networks during its maturation [18]. In our IFA study, the fluorescence level of Hsp60 confirmed by anti-GroEL showed that it was potentially co-localized with Pf-calpain at the trophozoite stage but detected widely at the schizont stage.
Malarial parasites should possess an efficient and highly specific protease system for massive hemoglobin proteolysis [30]. As one of the efficient malarial proteases, malarial calpain was found to activate plasmepsin IV and degrade hemoglobin in our previous study [25]. However, the Pf-calpain-related pathway was uncertain in the infected RBCs. In this study, the role of Hsp60 on Pf-calpain was evaluated to determine the relevance of Hsp60 on cysteine protease activity, leading to impairment of malarial growth. First, Pf-calpain protein level in the infected RBC was determined by western blot analysis but during immunoprecipitation Pf-calpain protein was diffused in a relatively low amount of lysate, indicating an easy degradation of Pf-calpain protein. Because subcellular locations of Hsps have been implicating a Hsp-associated event, these IFA findings allowed us to test the influence of Hsp on Pf-calpain activity.
Currently, mizoribine is known as an effective drug in antitumor therapy as it targets Hsp60 [26]; however, there are no challenging reports on the inhibitor of Hsp60 as novel anti-malarial candidate yet. As a result of testing mizoribine, the inhibitory concentrations of mizoribine for Pf-calpain activity and malarial growth were higher than those of ALLN, a typical calpain inhibitor, supporting the above data of the partial relevance of Hsp60 on Pf-calpain activity. Hsp60 is known to form various complexes [31]. Therefore, the multiple-Hsp complex may be implicated in the Pf-calpain activity. A novel Hsp (28-kDa) has been reported to belong to a malarial metalloprotease in P. vivax [32] but there is no report on the relevance of any Pf-Hsp on Pf-calpain activity. In the present study, a potential network between Pf-Hsp60 and malaria Pf-calpain was revealed, and the combined inhibitors of multiple Hsps would elucidate further Hsp networks of P. falciparum maturation and survival. Although Pf-Hsp60 could be associated with Pf-calpain activity, mizoribine was less effective than ALLN on Pf-calpain and malaria growth. These results indicate a potential relevance between Pf-Hsp60 and Pf-calpain network, and other factors could be involved in this association. We believe in that highlighting the further networks of Pf-Hsps and cysteine proteases would be one of better understandings to develop anti-malarial candidate.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (No. NRF-2015R1D1A4A01020393).
Footnotes
We have no conflict of interest related to this work.
REFERENCES
- 1.World Malaria Report. [ http://www.who.int/campaigns/malariaday/2013/en/index.html]
- 2.Pallavi R, Roy N, Nageshan RK, Talukdar P, Pavithra SR, Reddy R, Venketesh S, Kumar R, Gupta AK, Singh RK, Yadav SC, Tatu U. Heat shock protein 90 as a drug target against protozoan infections: Biochemical characterization of hsp90 from plasmodium falciparum and trypanosoma evansi and evaluation of its inhibitor as a candidate drug. J Biol Chem. 2010;285:37964–37975. doi: 10.1074/jbc.M110.155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acharya P, Kumar R, Tatu U. Chaperoning a cellular upheaval in malaria: heat shock proteins in Plasmodium falciparum. Mol Biochem Parasitol. 2007;153:85–94. doi: 10.1016/j.molbiopara.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Pavithra SR, Kumar R, Tatu U. Systems analysis of chaperone networks in the malarial parasite Plasmodium falciparum. PLoS Comput Biol. 2007;3:1701–1715. doi: 10.1371/journal.pcbi.0030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rug M, Maier AG. The heat shock protein 40 family of the malaria parasite Plasmodium falciparum. IUBMB Life. 2011;63:1081–1086. doi: 10.1002/iub.525. [DOI] [PubMed] [Google Scholar]
- 6.Gitau GW, Mandal P, Blatch GL, Przyborski J, Shonhai A. Characterisation of the Plasmodium falciparum Hsp70-Hsp90 organising protein (PfHop) Cell Stress Chaperone. 2012;17:191–202. doi: 10.1007/s12192-011-0299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayih AG, Pillai DR. Mouse studies on inhibitors of Plasmodium falciparum Hsp90: progress and challenges. Parasitology. 2014;141:1216–1222. doi: 10.1017/S0031182014000754. [DOI] [PubMed] [Google Scholar]
- 8.Syin C, Goldman ND. Cloning of a Plasmodium falciparum gene related to the human 60-kDa heat shock protein. Mol Biochem Parasitol. 1996;79:13–19. doi: 10.1016/0166-6851(96)02633-3. [DOI] [PubMed] [Google Scholar]
- 9.Hill JE, Penny SL, Crowell KG, Goh SH, Hemmingsen SM. cpnDB: a chaperonin sequence database. Genome Res. 2004;14:1669–1675. doi: 10.1101/gr.2649204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mai Z, Ghosh S, Frisardi M, Rosenthal B, Rogers R, Samuelson J. Hsp60 is targeted to a cryptic mitochondrion-derived organelle ("crypton") in the microaerophilic protozoan parasite Entamoeba histolytica. Mol Cell Biol. 1999;19:2198–2205. doi: 10.1128/mcb.19.3.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Misra RC, Verma AK, Verma SK, Kumar V, Siddiqui WA, Siddiqi MI, Murthy PK. Heat shock protein 60 of filarial parasite Brugia malayi: cDNA cloning, expression, purification and in silico modeling and analysis of its ATP binding site. Exp Parasitol. 2012;132:257–266. doi: 10.1016/j.exppara.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Plümper E, Bradley PJ, Johnson PJ. Competition and protease sensitivity assays provide evidence for the existence of a hydrogenosomal protein import machinery in Trichomonas vaginalis. Mol Biochem Parasitol. 2000;106:11–20. doi: 10.1016/s0166-6851(99)00196-6. [DOI] [PubMed] [Google Scholar]
- 13.Akide-Ndunge OB, Tambini E, Giribaldi G, McMillan PJ, Müller S, Arese P, Turrini F. Co-ordinated stage-dependent enhancement of Plasmodium falciparum antioxidant enzymes and heat shock protein expression in parasites growing in oxidatively stressed or G6PD-deficient red blood cells. Malar J. 2009;8:113. doi: 10.1186/1475-2875-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rappa F, Unti E, Baiamonte P, Cappello F, Scibetta N. Different immunohistochemical levels of Hsp60 and Hsp70 in a subset of brain tumors and putative role of Hsp60 in neuroepithelial tumorigenesis. Eur J Histochem. 2013;57: doi: 10.4081/ejh.2013.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun JN, Choi B, Lee KW, Lee DJ, Kang DH, Lee JY, Song IS, Kim HI, Lee SH, Kim HS, Lee NK, Lee SY, Lee KJ, Kim J, Kang SW. Cytosolic Hsp60 is involved in the NF-kappaB-dependent survival of cancer cells via IKK regulation. PLoS One. 2010;5: doi: 10.1371/journal.pone.0009422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaźmierczuk A, Kiliańska ZM. The pleiotropic activity of heatshock proteins. Postepy Hig Med Dosw (Online) 2009;63:502–521. [PubMed] [Google Scholar]
- 17.Fenton WA, Horwich AL. Chaperonin-mediated protein folding: fate of substrate polypeptide. Q Rev Biophys. 2003;36:229–256. doi: 10.1017/s0033583503003883. [DOI] [PubMed] [Google Scholar]
- 18.Pavithra SR, Kumar R, Tatu U. Systems analysis of chaperone networks in themalarial parasite Plasmodium falciparum. PLoS Comput Biol. 2007;3:1701–1715. doi: 10.1371/journal.pcbi.0030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenthal PJ. Proteases of malaria parasites: new targets for chemotherapy. Emerg Infect Dis. 1998;4:49–57. doi: 10.3201/eid0401.980107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Wang X, Liu X, Wang Y. Data-mining approaches reveal hidden families of proteases in the genome of malaria parasite. Genome Res. 2003;13:601–616. doi: 10.1101/gr.913403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drew ME, Banerjee R, Uffman EW, Gilbertson S, Rosenthal PJ, Goldberg DE. Plasmodium food vacuole plasmepsins are activated by falcipains. J Biol Chem. 2008;283:12870–12876. doi: 10.1074/jbc.M708949200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee R, Liu J, Beatty W, Pelosof L, Klemba M, Goldberg DE. Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine. Proc Natl Acad Sci USA. 2002;99:990–995. doi: 10.1073/pnas.022630099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo I, Oksman A, Vaupel B, Goldberg DE. A calpain unique to alveolates is essential in Plasmodium falciparum and its knock-down reveals an involvement in pre-S-phase development. Proc Natl Acad Sci USA. 2009;106:1554–1559. doi: 10.1073/pnas.0806926106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi YY, Jung SY, Cho PY, Soh BY, Zheng B, Kim SY, Park KI, Park H. Confocal microscopic findings of cysteine protease calpain in Plasmodium falciparum. Exp Parasitol. 2010;124:341–345. doi: 10.1016/j.exppara.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Kim YM, Lee MH, Piao TG, Lee JW, Kim JH, Lee S, Choi KM, Jiang JH, Kim TU, Park H. Prodomain processing of recombinant plasmepsin II and IV, the aspartic proteases of Plasmodium falciparum, is auto- and trans-catalytic. J Biochem. 2006;139:189–195. doi: 10.1093/jb/mvj018. [DOI] [PubMed] [Google Scholar]
- 26.Itoh H, Komatsuda A, Wakui H, Miura AB, Tashima Y. Mammalian HSP60 is a major target for an immunosuppressant mizoribine. J Biol Chem. 1999;274:35147–35151. doi: 10.1074/jbc.274.49.35147. [DOI] [PubMed] [Google Scholar]
- 27.Soh BY, Song HO, Lee Y, Lee J, Kaewintajuk K, Lee B, Choi YY, Cho JH, Choi S, Park H. Identification of active Plasmodium falciparum calpain to establish screening system for Pf-calpain-based drug development. Malar J. 2013;12:47. doi: 10.1186/1475-2875-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung SY, Zheng B, Choi YY, Soh BY, Kim SY, Park KI, Park H. Antimalarial effect of N-acetyl-L-Leucyl-L-leucyl-L-norleucinal by the inhibition of Plasmodium falciparum Calpain. Arch Pharm Res. 2009;32:899–906. doi: 10.1007/s12272-009-1612-4. [DOI] [PubMed] [Google Scholar]
- 29.Gomes MM, Budu A, Ventura PD, Bagnaresi P, Cotrin SS, Cunha RL, Carmona AK, Juliano L, Gazarini ML. Specific calpain activity evaluation in Plasmodium parasites. Anal Biochem. 2014;468C:22–27. doi: 10.1016/j.ab.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg DE, Slater AF, Cerami A, Henderson GB. Hemoglobin degradation in the malaria parasite Plasmodium falciparum: an ordered process in a unique organelle. Proc Natl Acad Sci USA. 1990;87:2931–2935. doi: 10.1073/pnas.87.8.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deocaris CC, Kaul SC, Wadhwa R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones. 2006;11:116–128. doi: 10.1379/CSC-144R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fakruddin JM, Biswas S, Sharma YD. Metalloprotease activity in a small heat shock protein of the human malaria parasite Plasmodium vivax. Infect Immun. 2000;68:1202–1206. doi: 10.1128/iai.68.3.1202-1206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]