Abstract
Continuous monitoring of vector species composition, abundance, dynamics, feeding pattern, and host finding strategy is the base to determine when, what, and how control should be implemented. Thus, this study was conducted to assess entomological parameters of anopheline mosquitoes in nine villages in Seka district, southwestern Ethiopia, from June to December 2012. Mosquito collection was carried out from selected households in each of the nine study villages using light trap catches from June to December 2012. Differences in mean mosquito density, parity rates before, and after indoor residual spraying (IRS) operation were compared. In total, 1,136 adult female anopheline mosquitoes were collected during the study period. All anopheline mosquitoes collected belong to three species. Anopheles gambiae senso lato Giles was the most predominant (69.7%) followed by Anopheles coustani s.l. Laveran (22.7%) and Anopheles pharoensis Theobald (7.6%). There was significant variation in mean mosquito density among An. gambiae s.l., An. coustani s.l., and An. pharoensis. Parity rate of An. gambiae s.l. before spray operation was significantly higher than after spray operation. The highest peak biting activity of An. gambiae s.l. was between 1800 and 2100 hours. The longevity of An. gambiae s.l. ranged from 3.4 to 12.5 d. The highest vector abundance and parity rate were recorded in July and August. In conclusion, the behavioral plasticity and early biting activity of An. gambiae s.l. could affect current vector control tools (IRS and long lasting insecticidal nets). Hence, it is imperative to explore intervention tools for outdoor malaria vector control in addition to the existing IRS and long-lasting insecticidal nets.
Keywords: Anopheles mosquitoes, mosquito longevity, parity rate, infectivity rate, malaria
Globally, about half of the world populations (3.3 billion) are at risk of malaria infection (World Health Organization [WHO] 2011). Adult female mosquitoes of the genus Anopheles are vectors for the Plasmodium parasites and are thus responsible for malaria transmission. There are 490 species in the genus Anopheles, and 70 of these are vectors of malaria. In sub-Saharan Africa, there are 140 Anopheles species of which approximately 20 are known to transmit malaria parasites to human beings. Of these, Anopheles gambiae s.s, Anopheles arabiensis Patton, and Anopheles funestus Giles are the most widely distributed and important malaria vector species in tropical Africa (Gillies and Coetzee 1987, Foley et al. 2010).
In Ethiopia, malaria is seasonal in most parts of the country, with unstable transmission that could lead to an outbreak of epidemics. Early studies in Ethiopia indicated that there were 42 Anopheles species (Gebremariam et al. 1988). An. arabiensis, member of the An. gambiae complex, is the principal vector in the country. Other vectors which occur in Ethiopia are An. funestus group, Anopheles pharoensis, and Anopheles nili, An. funestus, and An. pharoensis are considered to be secondary vectors.
In Ethiopia, long-lasting insecticidal nets, indoor residual spraying (IRS), and environmental management are the most widely used tools for malaria vector control. However, it is important to have comprehensive information on the bionomics of mosquitoes in targeted areas in order to assess the technical, operational, and economic implications and to avoid unnecessary wastage of resources.Thus, determining the species composition and distribution of vectors are vital for effective vector control (Coetzee 2004, World Health Organization [WHO] 2008, Ramirez et al. 2009).
To the best of our knowledge, no entomological assessment and monitoring had been conducted in Seka-Chekorsa district before. Therefore, the aim of this study was to assess species composition, abundance, distribution, spatiotemporal dynamics, feeding behavior, peak biting time, longevity, and infection rate of anopheline mosquitoes in Seka-Chekorsa district, Jimma zone, southwestern Ethiopia.
Materials and Methods
Study Setting
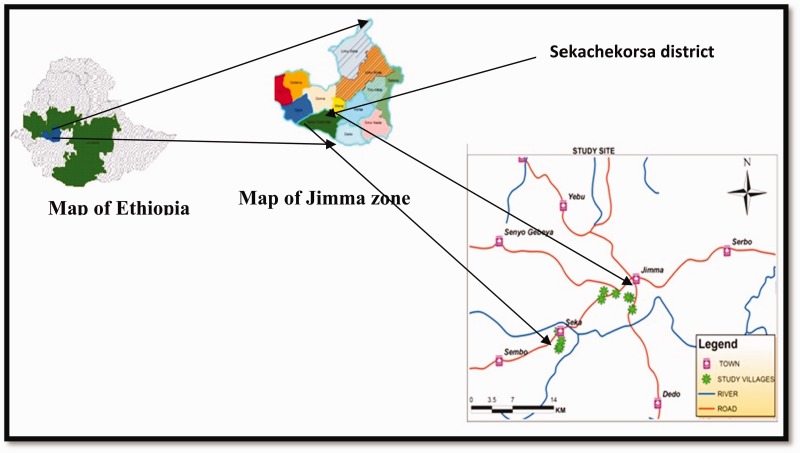
The study was conducted from June to December 2012 in Seka-Chekorsa district, Jimma zone, southwestern Ethiopia. Seka-Chekorsa district is located in Jimma zone, Oromia Regional State, southwestern Ethiopia. The district is about 367 km from the capital, Addis Ababa, and 17 km southwest of Jimma town with an latitude 7° 36′41″ N and longitude 36° 44′12″ E (Fig. 1).
Fig. 1.
Map of the study area.
Altitude ranges from 1,580 to 2,560 masl. Annual minimum and maximum rainfall ranges from 1,400 to 1,601 mm, respectively. The mean maximum and minimum temperatures are 30°C and 16°C, respectively. Seka-Chekorsa district has 38 kebeles (the smallest administrative unit in Ethiopia). Of these, about 14 kebeles are malarious and have potential mosquito breeding sites (Seka-Chekorsa district health office personal communication). Of 14 malarious kebeles of Seka-Chekorsa district, three kebeles (Kofe, Bore, and Ushane Koche) were randomly selected for this study. Mosquito sampling was conducted in nine houses selected from the three kebeles (Three houses from each kebele). The houses within the village were selected considering the flight range of anopheline mosquitoes from potential breeding sites (the marshy wetland, animal foot print, artificial ponds, stream margins, swamps, quarry ditch, rain pools, brick [for pot making or pit making], and other favorable sites) (World Health Organization [WHO] 1975). Mosquitoes were collected at fortnight interval from June to December 2012.
Mosquito Sampling
Adult female Anopheles mosquito collections were carried out in each of the selected houses for 7 mo (from June to December 2012) using Centre for Disease Control and prevention (CDC) light traps (Model 512; John W. Hock Co., Gainesville, FL). Traps were set both indoor and outdoor in each of the selected dwellings (WHO 1975, Mboera 2005). CDC light trap was set to run between 1800 and 0600 hours. Indoor CDC light trap was set inside the house close to the bed room (1.5 m above the bed), whereas outdoor CDC light trap was set at a distance 15–20 m from the same house used for indoor mosquito collection. Mosquitoes were collected from two houses at fortnight interval from June to December 2012.
Hourly Light Trap Catches of Anopheline Mosquitoes and Identification
Adult mosquitoes hourly light trap catches (LTCs) were conducted twice per month per house in each village (Wacho Gono, Malko, and Agalo) from June to December 2012. The collected mosquito samples were kept in labeled paper cups, which were replaced every hour until 0600 hours. Indoor CDC light trap was set close to the bed room at 1.5 m above the bed from desk to down, whereas outdoor CDC light trap was set at a distance 15–20 m away of the same house used for indoor mosquito collection. The collected mosquitoes were identified morphologically to species using standard keys (Gillies and Coetzee 1987), then counted, labeled, and kept in Eppendorf tubes over silica gel for further laboratory processing at Asendabo Vector Biology Laboratory, Jimma University.
Parity Rate Determination
Unfed female An. gambiae s.l. specimens collected from all selected houses at fortnight interval were dissected for parity rate (WHO 1975). Each unfed female mosquito was anesthetized using chloroform. A drop of Phosphate Buffer Solution (PBS) solution was added on a slide and each specimen was kept on a slide. After the thorax of each specimen gripped by forceps, the seventh and eighth abdominal segment was pulled using a needle. Then ovaries were examined under stereo microscope for ovarial tracheoles, and parity was determined following standard method (Detinova 1962).
Sporozoite Rate Determination
The sporozoite rate determination was conducted following the protocol of Wirtz et al. (1987). The head-thorax region of each mosquito was removed with a sharp clean surgical blade on filter paper then transferred to labeled 1.5 ml Eppendorf tube using clean forcipes (three mosquito samples were pooled together). Then 50 µl of BB-IGEPAL CA-630 was added to each tube and ground with pestle and homogenized, Then, 200 µl BB was added in each vial. Each well of micro plates was coated with 50 µl capture MAb, and the plate was covered and incubated for 1 h at room temperature. The capture MAb was aspirated and filled with BB completely and incubated for 1 h at room temperature. After aspiration of BB, 50 µl positive and negative control was added to each well in the first and second column. Thus, 50 µl mosquito sample was loaded to each well except the first and second column of each plate. The plate then was covered and incubated for 2 h at room temperature. Then, it was aspirated and washed two times with PBS-Tween 20. Peroxidase-conjugate MAb (50 µl) was added to each well and incubated for 1 hour at room temperature. Conjugate was aspirated and washed three times using PBS-Tween 20. Then, 100 μl ABTS substrate was added and incubated for 30 min. Finally the result was read visually.
Data Analysis
Data were analyzed using SPSS statistical software package version 16.0 (SPSS Inc, Chicago, IL). Before the analysis was conducted, data were cleaned and normalized after transforming into Log +1 in SPSS. Test of significance was estimated assuming α (two sided) = 0.05. P-value less than 0.05 was considered significant during the analysis. Daily survival rate (S) = , where gc = estimated gonotrophic cycle of An. gambiae s.l. of the population was estimated. Moreover, the gonotrophic cycle was estimated to be 3 d following previous reports by Krafsur (1977) from South West Ethiopia and life expectancy (LE) = 1/-Ln S was estimated following Davidson (1954).
Results
Species Composition, Abundance, and Distribution of Anopheline Mosquitoes
Overall 1,136 adult female anopheline mosquitoes belonging to three species (An. gambiae s.l., Anopheles coustani s.l., and An. pharoensis) were collected during the 7-mo survey period (Table 1). An. gambiae s.l. was the predominant species (69.7%, n = 792) followed by An. coustani s.l. (22.7%, n = 258) and An. pharoensis (7.6%, n = 86). The Kruskal–Wallis test showed that there was significant difference in species co-occurrence among villages (H(8) = 38.776, P < 0.001). Of those villages, the three Anopheles species frequently sampled together were from Delcho Degoye village with a mean of 81.75, whereas the lowest was from Ejersa village with a mean of 29.50.
Table 1.
Mean monthly anopheline mosquitoes density by month of collection in Seka-Chekorsa district, Jimma zone, southwestern Ethiopia (June–December, 2012)
| Months | Mean ± SE |
||
|---|---|---|---|
| An. Gambiae s.l. | An. Coustani complex | An. pharoensis | |
| June | 0.7550 ± 0.08093a,b | 0.2761 ± 0.05986a,b,c | 0.1032 ± 0.04183a,b,c |
| July | 0.8773 ± 0.05813a,b | 0.4342 ± 0.06804a,b | 0.2300 ± 0.05968a,b |
| August | 0.9581 ± 0.05370a | 0.3677 ± 0.07332a,b | 0.0697 ± 0.03870b,c |
| September | 0.6973 ± 0.06537b | 0.4238 ± 0.05269a,b | 0.1852 ± 0.06464a,b,c |
| October | 0.7276 ± 0.04927a,b | 0.5220 ± 0.06201a | 0.2884 ± 0.07387a |
| November | 0.3724 ± 0.04900c | 0.0934 ± 0.03762c | 0.0265 ± 0.02651b,c |
| December | 0.1729 ± 0.04900c | 0.1937 ± 0.05722b,c | 0.0000 ± 0.00000c |
| Total | 0.6515 ± 0.3230 | 0.3301 ± 0.02527 | 0.1290 ± 0.02033 |
*Means with the same letter (s) in the same column are not significantly different from each other at P < 0.05.
Temporal Dynamics of Anopheline Mosquitoes
Over all, the highest 243 (21.40%) and lowest 37 (3.2%) anopheline mosquitoes was observed in August and December, respectively. The abundance of An. gambiae s.l. was peaked during August, while An. coustani complex and An. pharoensis peaked during October. Results of one way ANOVA showed that there was significant difference in mean monthly density of An. gambiae s.l. (F(6,119) = 21.096, P < 0.001), An. coustani complex (F(6,119) = 6.343, P < 0.001), and An. pharoensis (F(6,119) = 4.770, P < 0.001). The mean monthly An. gambiae s.l. density was positively correlated with relative humidity, rain fall, and temperature of the study area. Mean monthly An. gambiae s.l. density showed significant positive correlation with RF (r = 0.68; P = 0.008), RH (r = 0.74; P = 0.002) and minimum temperature (r = 0.67, P = 0.008) (Fig. 2).
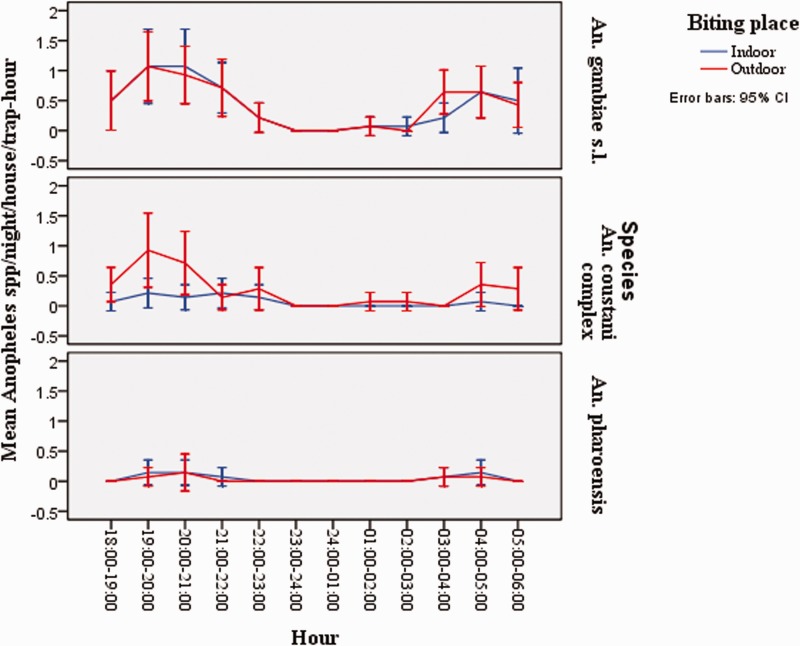
Fig. 2.
Mean hourly indoor and outdoor biting activities of An. gambiae s.l., An. coustani s.l. and An. pharoensis using CDC light trap catch in Seka Chekorsa district, Jimma zone, southwestern Ethiopia (June–December 2012).
Indoor and Outdoor Anopheline Mosquito Density
There was no significant difference in mean indoor and outdoor density of An. gambiae s.l. (t(1, 8) = −0.94, P = 0.129). However, there was significant difference in mean indoor and outdoor density of An. coustani s.l. (t(1, 8) = −6.49, P = 0.002) and An. pharoensis (t(1, 8) = −4.456, P = 0.004) (Table 2). Moreover, mean indoor density of An. gambiae s.l. before spray operation (June to August) was significantly higher (t(1, 124) = 5.66, P < 0.001) than mean indoor density of An. gambiae s.l. after spray operation (September to December). However, the difference in mean indoor density before and after spray operation for An. coustani s.l. (t(1,124) = −1.132, P = 0.260) and An. pharoensis (t(1,124) = −1.579, P = 0.156) was not significant (Table 1).
Table 2.
Mean indoor and outdoor density/trap/night of anopheline mosquitoes of Seka-Chekorsa district, Jimma Zone, southwestern Ethiopia (June–December, 2012)
| Species | Density | Log 10 mean ± SE | CI (95%) | P |
|---|---|---|---|---|
| An. gambiae s.l. | Indoor | 0.5034 ± 0.04152 | −0.14054, 0.05917 | 0.129 |
| Outdoor | 0.5441 ± 0.07060 | |||
| An. coustani complex | Indoor | 0.0186 ± 0.00894 | −0.49239, −0.23434 | 0.002* |
| Outdoor | 0.3820 ± 0.06079 | |||
| An. pharoensis | Indoor | 0.0251 ± 0.01269 | −0.21654, −0.06885 | 0.004* |
| Outdoor | 0.1978 ± 0.04067 |
*Significant at P < 0.05.
Hourly Activity of Anopheline Mosquitoes
The highest peak biting activity both indoor and outdoor for An. gambiae s.l., An. coustani s.l., and An. pharoensis was between 1800 and 2100 hours. Biting activity for An. gambiae s.l. both indoor and outdoor also increased between 0300 and 0600 hours (Fig. 2).
Parity Rates and Probability of Surviving Sporogony of Plasmodium species in An. gambiae s.l.
Of 193 unfed adult female An. gambiae s.l. samples dissected, 111 (57%) were parous (Table 3). The mean life expectancy of An. gambiae s.l. before and after spray operation was 8.4 d and 3.8 d, respectively. Parity rate of An. gambiae s.l. before spray operation (June–August 2012) was significantly (t(1, 10) = 2.32, df = 10, P = 0.043) higher than after spray operation (September–November 2012). The highest probability of surviving sporogony in July for Plasmodium falciparum and Plasmodium vivax was 0.32 and 0.40, respectively, followed by the probability of surviving sporogony in August for Ps. falciparum and Ps. vivax with 0.18 and 0.24, respectively. The overall mean probability of surviving sporogony for Ps. falciparum and Ps. vivax in An. gambiae s.l. was 0.12 and 0.17, respectively (Table 3).
Table 3.
Parity rate and probability of surviving sporogony of Plasmodium species in An. gambiae s.l. by month in Seka-Chekorsa district, Jimma zone, southwestern Ethiopia (June–November 2012)
| Months | No. UF mosq dissected/month/housetrap | PR | S | LE | Temp. (°C) | EIP of Pf (d) | EIP of Pv (d) | PSS of Pf | PSS of Pv |
|---|---|---|---|---|---|---|---|---|---|
| June | 28 | 0.57 | 0.83 | 5.3 | 26.33 | 10.75 | 8.88 | 0.13 | 0.19 |
| July | 37 | 0.78 | 0.92 | 12.5 | 24.1 | 13.7 | 10.94 | 0.32 | 0.40 |
| August | 40 | 0.65 | 0.87 | 7.1 | 24.92 | 12.44 | 10.1 | 0.18 | 0.24 |
| September | 43 | 0.47 | 0.78 | 4 | 26 | 11.1 | 9.13 | 0.06 | 0.10 |
| October | 24 | 0.42 | 0.75 | 3.4 | 27.83 | 9.38 | 7.88 | 0.07 | 0.10 |
| November | 21 | 0.48 | 0.78 | 4 | 27.95 | 9.29 | 7.81 | 0.1 | 0.14 |
| Total | 193 | 0.57 | 0.83 | 5.3 | 26.12 | 10.62 | 8.79 | 0.12 | 0.17 |
PR, parity rate; S, daily survival rate; LE, life expectancy; EIP, extrinsic incubation period; PSS, probability of surviving sporogony; Pf, Plasmodium falciparum; Pv, Plasmodium vivax.
Infection Rates of An. gambiae s.l.
Of 192 An. gambiae s.l. specimens tested for Plasmodium circumsporozoite protein using sand witch ELISA, none were found positive for both species (Ps. falciparum and Ps. vivax).
Discussion
Better understanding of the bio-ecology and spatiotemporal distribution of malaria vectors is essential to design effective strategies for sustaining malaria control and elimination (Moiroux et al. 2014). In this study, key entomological parameters such as species composition, abundance, distribution, spatiotemporal dynamics, feeding behavior, peak biting activity, longevity, and infection rate of anopheline mosquitoes were assessed in an area with seasonal malaria transmission in southwestern Ethiopia.
The distribution of anopheline mosquitoes in the nine study villages revealed that An. gambiae s.l., An. coustani s.l., and An. pharoensis were found in sympathry. An. gambiae s.l. was the predominant malaria vector in the study area, which is consistent with reports from other parts of Ethiopia. This may be a concern as An. gambiae s.l. is the principal vector of malaria in sub-Saharan Africa in general, East Africa and Ethiopia in particular (Gillies and Coetzee 1987, Abose et al. 1998, Seyoum et al. 2002, Shililu et al. 2003, Coetzee 2004). Similarly (Kibret et al. 2009, 2010) collected adult mosquitoes from irrigated and nonirrigated villages around Zeway and in vicinity of Koka dam, central Ethiopia, reported co-occurrence of the three species. An. gambiae s.l. was widely distributed throughout the nine study villages, and it was collected throughout the survey period; this is in agreement with other studies by Gebremariam et al. (1988) and Woyessa et al. (2004) who reported that An. gambiae s.l. are omnipresent in malarious areas including the highlands of Ethiopia.
There was significant difference in mean monthly density of An. gambiae s.l., An. coustani s.l. and An. pharoensis between August and December. The highest abundance of An. gambiae s.l. was recorded in August which is part of the main rainy season in the study area. Relatively higher abundance of An. coustani s.l. and An. pharoensis was recorded in October which is characterized by low rains and relative humidity as compared to August. Similar findings were reported by Kibret et al. (2010) from central Ethiopia.
An. gambiae s.l. was equally exophagic (i.e., outdoor feeding) and endophagic (i.e., indoor feeding) and showing no significant difference in indoor and outdoor biting pattern. In contrast, both An. pharoensis and An. coustani s.l. showed exophagic behavior, with high outdoor density in all study villages. The dual feeding behavior of An. gambiae s.l. is in agreement with the findings by Kibret et al. (2009). However, early reports by Krafsur (1977) showed that An. gambiae s.l. was endophagic in Gambella region, southwestern Ethiopia. Other studies also documented that An. gambiae s.l. to be predominantly exophagic in some areas of Ethiopia (Woyessa et al. 2004) and in Africa (Oyewole et al. 2007, Fornadel et al. 2010b, Reddy et al. 2011). On the other hand, An. pharoensis and An. coustani s.l. are well-known exophagic species in Ethiopia (Adugna and Petros 1996, Abose et al. 1998, Taye et al. 2006), Cameroon (Antonio-Nkondjio et al. 2006), Kenya (Ijuma et al. 2002), and Sudan (El Gaddal et al. 1985).
The even distribution of An. gambiae s.l. in both indoor and outdoor suggests that the adaptive behavior of this species to bite both indoor and outdoor. It is also possible that the outdoor resting tendencies of these mosquito species might have been enhanced by the use of IRS as evidenced by the higher outdoor mosquito density following IRS operation in the study area. Repeated IRS was shown to increase outdoor feeding response of anopheline mosquitoes (Ameneshewa and Service 1996). In addition, the observed feeding pattern in mosquitoes could be a response to the excito-repellent effect of residual insecticides, diverting the endophagic mosquitoes to seek hosts outdoors. However, bendiocarb, which belongs to the carbamate insecticide family and used for IRS in this particular study area, has more of contact irritancy than excito-repellent effect on An. gambiae s.l. (Evans 1993). Hence, An. gambiae s.l. could have developed resistance to the insecticide since there had been strong evidences of resistance by vector mosquitoes from nearby areas around Gilgel Gibe hydroelectric dam (Yewhalaw et al. 2010, 2011).
Overall, hourly biting activity of An. gambiae s.l. in the study area commenced at the early part of the night before the inhabitants retire to bed and decline at mid night then showed a tendency to increase close to dawn. The highest indoor and outdoor peak biting activity of An. gambiae s.l. were observed between 1900 and 2000 hours, respectively. Previous works from Northern part of Ethiopia (Yohannis and Boelee, 2012), Central Ethiopia (Kibret et al. 2010), and from Zambia (Fornadel et al. 2010a) documented similar findings. In contrast, earlier studies from Cameroon (Tanga et al. 2010) reported that peak biting time of this species occurred at mid night between 23.00 and 3.00 hours.
According to Fornadel et al. (2010a), other potential behavioral changes which have been observed in anopheline mosquitoes with the introduction of ITNs and IRS are shifts toward outdoor and/or early biters. Like other aspects of mosquito behavior, the night biting activity of An. gambiae s.l. varies across Africa. Bugoro et al. (2011) suggested that shift to early night outdoor feeding thought to be due to an excito repellent response to the IRS.
Parity rate of An. gambiae s.l. was highest from June to August, which indicates that older mosquitoes were prevalent during these months. This also further suggested that mosquito populations were gonotrophically older during the mentioned months, contributing to their vectorial capacity. However, low parity rate of An. gambiae s.l. was recorded in September, October, November, and December. The low parity rates recorded in mosquito populations after September could be attributed to the IRS as spray operation was conducted during September in the study area. A similar study by Ameneshewa (1995) in Awash, Central Ethiopia, also documented higher parity rates of An. arabiensis during the rainy season than the dry season. In contrast to this, Kibret et al. (2009) reported higher parity rates in An. arabiensis during the dry months of the year, though this study was conducted around a hydropower dam area where mosquito breeding sites could be available throughout the year.
The results of this study also revealed that populations of An. gambiae s.l. in the study area had life expectancy ranging from 3.4 to 12.5 d. The mean monthly probability of An. gambiae s.l. surviving the sporogony was very low (0.1–0.40 and 0.06–0.32 for Ps. vivax and Ps. falciparum, respectively). This indicates that relatively small proportion of the populations of An. gambiae s.l. could survive long for completion of sporogonic cycle for infective bites. Very low surviving sporogony was also documented in several studies that could have otherwise considerable importance with respect to vector efficiency to transmit malaria parasites due to the fact that long-lived mosquitoes maximize the chances of the transmission of Plasmodium species to human hosts as allows the parasite to complete its extrinsic incubation period (Olayemi and Ande 2008, O’Connor et al. 2009, Tanga et al. 2010).
In this study, none of An. gambiae s.l. processed for CSP was found infected by either of the plasmodium parasites. This could be attributed to the low number of mosquito specimens tested for CSP and/or may be due to the observed low longevity of the vector during the survey period, as most of vector may die before the parasites develop to infective stage (Tchuinkam et al. 2010, Bugoro et al. 2011).
In conclusion, of the three anopheline species identified, An. gambiae s.l., the principal vector of malaria parasite, was predominant in the study area. The highest abundance and parity rates of An. gambiae s.l. were recorded in July and August. Hence, this information calls for the control program to implement the IRS intervention in the study area in late July or early August.
The observed behavioral plasticity and early biting activity of An. gambiae s.l. is more challenging to effectively control this important vector using conventional vector control tools such as IRS and long-lasting insecticidal net both of which are indoor vector control interventions. Hence, it is imperative to explore possibilities for developing outdoor vector control intervention tools. Moreover, the early biting activity of the anopheline mosquitoes in the study area indicates that malaria infection could occur before people go to bed.
Acknowledgments
We acknowledge Health Office of Seka-Chekorsa district administration and the study community for providing consent to conduct this study. We would like to thank Jimma University for logistic support and southwestern branch regional office of Ethiopian Meteorological Agency for providing meteorological data. All Entomology field Technicians (AbdoJemal, Mufti A/ Giddi, AberaTesema, Abdo A/ Giddi, Workneh Jaleta, Nasir A/ Raya,) of Asendabo Vector Biology Laboratory, Jimma University, are highly acknowledged for assisting in field mosquito collection. This work was financially supported by Jimma University.
References Cited
- Abose T., Ye-ebiyo Y., Olana D., Alamirew D., Beyene Y., Regassa L., Mengesha A. 1998. Reorientation and definition of the role of malaria vector control in Ethiopia, 38 p. WHO/Mal/1998.1085. World Health Organization, Geneva, Switzerland. [Google Scholar]
- Adugna N, Petros B. 1996. Determination of the human blood index of some anopheline mosquitoes by using ELISA. Ethiop. Med. J. 34: 1–10. [PubMed] [Google Scholar]
- Ameneshewa B. 1995. The behavior and biology of Anopheles arabiensis in relation to epidemiology and control of malaria in Ethiopia, p. 288 Ph.D thesis, University of Liverpool, UK. [Google Scholar]
- Ameneshewa B., Service M. W. 1996. Resting habits of Anopheles arabiensis in the Awash valley of Ethiopia. Ann. Trop. Med. Parasitol. 90: 515–521. [DOI] [PubMed] [Google Scholar]
- Antonio-Nkondjio C., Kerah C. H., Simard F., Awono-Ambene P., Chouaibou M., Tchuinkam T., Fontenille D. 2006. Complexity of malaria vectorial system in Cameroon: contribution of secondary vectors to malaria transmission. J. Med. Entomol. 43: 1215–1221. [DOI] [PubMed] [Google Scholar]
- Bugoro H., Iro’ofa C., Mackenzie D., Apairamo A., Hevalao W., Corcoran S., Bobogare A., Beebe N., Russel T., Chen C., et al. 2011. Changes in vector species composition and current vector biology and behavior will favor malaria elimination in Santa Isabel Province, Solomon Islands. Malar. J. 10: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee M. 2004. Distribution of the African malaria vectors of Anopheles gambiae Complex. Am. J. Trop. Med. Hyg. 70: 103–104. [PubMed] [Google Scholar]
- Davidson G. 1954. Estimation of the survival rate of anopheline mosquitoes in nature. Nature 174: 792–793. [DOI] [PubMed] [Google Scholar]
- Detinova S. 1962. Age grading methods in Diptera of medical importance with special reference to some vectors of malaria. Monogr. Ser. World Health Org. 47: 1–216 [PubMed] [Google Scholar]
- El Gaddal A., Haridi M., Hassan T., Hussein H. 1985. Malaria control in the Gezira-Managil irrigated scheme of the Sudan. J. Trop. Med. Hyg. 88: 153–159. [PubMed] [Google Scholar]
- Evans G. 1993. Laboratory evaluation of the irritancy of bendiocarb, lambdacyhalothrin and DDT to Anopheles gambiae. J. Am. Mosq. Control Assoc. 9: 285–293. [PubMed] [Google Scholar]
- Foley R., Desmond H., Wilkerson W., Birney I., Stanley H., Christensen J., Leopoldo M., Rueda L. 2010. MosquitoMap and the Mal-area calculator: new web tools to relate mosquito species distribution with vector borne disease. Int. J. Health. Geogr. 9: 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornadel C., Norris L., Glass G., Norris D. 2010a. Analysis of Anopheles arabiensis blood feeding behavior in Southern Zambia during the two years after introduction of insecticide-treated bed nets. Am. J. Trop. Med. Hyg. 83: 848–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornadel C., Norris L., Norris D. 2010b. Centers for Disease Control light traps for monitoring Anopheles arabiensis human biting rates in an area with low vector density and high insecticide-treated bed net use. Am. J. Trop. Med. Hyg. 83: 838–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremariam N., Abdulahi Y., Mebrate A. 1988. Malaria, pp. 136–150. In Zein Z. A., Kloos H. (eds.), Ecology and disease in Ethiopia. Ministry of Health, Addis Ababa, Ethiopia. [Google Scholar]
- Gillies M. T., Coetzee M. 1987. A supplement to the anopheline of Africa South of the Sahara (Afrotropical region), Johannesburg, South Africa: the South African Institute for Medical Research No. 55. [Google Scholar]
- Ijuma J., Mosha F., Lindsay S. 2002. Malaria transmission risk variation derived from different agricultural practices in an irrigated area northern Tanzania. Med. Vet. Entomol. 16: 28–38. [DOI] [PubMed] [Google Scholar]
- Kibret S., McCartney M., Lautze J., Jayasinghe G. 2009. Malaria transmission in the vicinity of impounded water: evidence from the Koka Reservoir, Ethiopia, p. 47 Colombo, Sri Lanka: International Water Management Institute. [Google Scholar]
- Kibret S. Y., Alemu E., Boelee H., Tekie D., Alemu B., Petros 2010. The impact of a small-scale irrigation scheme on malaria transmission in Ziway area, Central Ethiopia. Trop. Med. Int. Health 15: 41–50. [DOI] [PubMed] [Google Scholar]
- Krafsur E. 1977. The bionomics and relative prevalence of Anopheles species with respect to the tramsmission of Plasmodium to man in western Ethiopia. J. Med. Entomol. 14: 180–194. [DOI] [PubMed] [Google Scholar]
- Mboera L. 2005. Sampling techniques for adult Afrotropical malaria vectors and their reliability in the estimation of entomological inoculation rate. Tanzan. Health Res. Bull. 7: 117–124. [DOI] [PubMed] [Google Scholar]
- Moiroux N., Armel D., Abdul A. S., Fabrice C., Vincent C., Hélène G. 2014. Spatio-temporal analysis of abundances of three malaria vector species in southern Benin using zero-truncated models. Parasites & Vectors. 7: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor L., Gingrich J., Unnasch T., Hassan H. 2009. Gonotrophic age structure of mosquitoes in the Culex pipiens complex (Diptera: Culicidae) and possible influences on host meal selection. J. Parasitol. Vector Biol. 1: 25–30. [Google Scholar]
- Olayemi K., Ande T. 2008. Survivorship of Anopheles gambiae in relation to malaria transmission in Ilorin, Nigeria. J. Health Allied Sci. 7: 1–5. [Google Scholar]
- Oyewole I., Awololab T., Ibidapo C., Oduola A., Okwac O., Obansa J. 2007. Behavior and population dynamics of the major anopheline vectors in a malaria endemic area in southern Nigeria. J. Vect. Borne. Dis. 44: 56–64. [PubMed] [Google Scholar]
- Ramirez J. L., Garver L. S., Dimopoulos G. 2009. Challenges and approaches for mosquito targeted malaria control. Curr. Mol. Med. 9: 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M., Overgaard H., Abaga S., Reddy V., Caccone A., Kiszewski A., Slotman M. 2011. Outdoor host seeking behavior of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar. J. 10: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyoum A., Balcha F., Balkew M., Ali A., Gebre-Michael T. 2002. Impact of cattle keeping on human biting rate of anopheline mosquitoes and malaria transmission around Ziway, Ethiopia. East Afr. Med. J. 79: 485–490. [DOI] [PubMed] [Google Scholar]
- Shililu J., Ghebremeskel T., Mengistu S., Fekadu H., Zerom M., Mbogo C., Githure J., Gu W., Novak R., Beier J. 2003. Distribution of anopheline mosquitoes in Eritrea. Am. J. Trop. Med. Hyg. 69: 295–302. [PubMed] [Google Scholar]
- Tanga M., Ngunduc W., Juditha N., Mbuha J., Tendongfora N., Simarde F., Wanjia S. 2010. Climate change and altitudinal structuring of malaria vectors in south-western Cameroon: their relation to malaria transmission. Trans. R. Soc. Trop. Med. Hyg. 104: 453–460. [DOI] [PubMed] [Google Scholar]
- Taye A., Haddis M., Adugna N., Tilahun D., Wirtz A. 2006. Biting behavior and Plasmodium infection rates of Anopheles arabiensis from Sille, Ethiopia. Acta Trop. 97: 50–54. [DOI] [PubMed] [Google Scholar]
- Tchuinkam T., Simard F., Lélé-Defo E., Téné-Fossog B., Tateng-Ngouateu A., Antonio-Nkondjio C., Mpoame M., Toto J., Njiné T., Fontenille D., et al. 2010. Bionomics of anopheline species and malaria transmission dynamics along an altitudinal transect in Western Cameroon. BMC Infect. Dis. 10: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (WHO) World Health Organization. 1975. Manual on practical entomology part II method and techniques, pp. 1–186. Geneva, Switzerland. [Google Scholar]
- (WHO) World Health Organization. 2008. Mosquitoes of the genus Anopheles in countries of the WHO European Region having faced a recent resurgence of malaria. (http://www.euro.who.int/__data/assets/pdf_file/0006/98763/E92010.pdf; accessed in May 5, 2015). [Google Scholar]
- (WHO) World Health Organization. 2011. World malaria report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- Wirtz A., Burkot R., Graves M., Andre G. 1987. Field evaluation of enzyme-linked immunosorbent assays for Plasmodium falciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera: Culicidae) from Papua New Guinea. J. Med. Entomol. 24: 433–437. [DOI] [PubMed] [Google Scholar]
- Woyessa A., Gebre-Micheal T., Ali A. 2004. An indigenous malaria transmission in the outskirts of Addis Ababa, Akaki Town and its environments. Ethiop. J. Health Dev. 18: 3–7. [Google Scholar]
- Yewhalaw D., Bortel W., Denis L., Coosemans M., Duchateau L., Speybroeck N. 2010. First evidence of high knockdown resistance frequency in Anopheles arabiensis (Diptera: Culicidae) from Ethiopia. Am. J.Trop. Med. Hyg. 83: 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewhalaw D., Wassie F., Steurbaut W., Spanoghe P., Bortel W. Van, Denis L., Tessema D., Getachew Y., Coosemans M., Duchateau L., et al. 2011. Multiple insecticide resistance: an impediment to insecticide-based malaria vector control program. PLoS One 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohannis M., Boelee E. 2012. Early biting rhythm in the Afro-tropical vector of malaria, Anopheles arabiensis and challenges for its control in Ethiopia. Med.Vet. Entomol. 26: 103–105. [DOI] [PubMed] [Google Scholar]