Abstract
The increased summertime prevalence of cattle carriage of enterohemorrhagic Shiga toxin-producing Escherichia coli O157:H7 (STEC O157) is associated with the increased summertime incidence of human infection. The mechanism driving the seasonality of STEC O157 carriage among cattle is unknown. We conducted experimental challenge trials to distinguish whether factors extrinsic or intrinsic to cattle underlie the seasonality of STEC O157 colonization. Holstein steers (n = 20) exposed to ambient environmental conditions were challenged with a standardized pool of STEC O157 strains four times at 6-month intervals. The densities and durations of rectoanal junction mucosa (RAJ) colonization with STEC O157 were compared by season (winter versus summer), dose (109 CFU versus 107 CFU), and route of challenge (oral versus rectal). Following summer challenges, the RAJ STEC O157 colonization density was significantly lower (P = 0.016) and the duration was shorter (P = 0.052) than for winter challenges, a seasonal pattern opposite to that observed naturally. Colonization was unaffected by the challenge route, indicating that passage through the gastrointestinal microbiome did not significantly affect the infectious dose to the RAJ. A 2-log reduction of the challenge doses in the second-year trials was accompanied by similarly reduced RAJ colonization in both seasons (P < 0.001). These results refute the hypothesis that cattle are predisposed to STEC O157 colonization during the summer months, either due to intrinsic factors or indirectly due to gastrointestinal tract microbiome effects. Instead, the data support the hypothesis that the increased summertime STEC O157 colonization results from increased seasonal oral exposure to this pathogen.
INTRODUCTION
Shiga toxin-producing Escherichia coli serotype O157:H7 (STEC O157) is an important zoonotic pathogen estimated to cause >70,000 cases of human infection annually in the United States (1, 2). Human disease is characterized by mild to severe, frequently hemorrhagic diarrhea, and the development of a severe sequela, the hemolytic-uremic syndrome, in approximately 5 to 10% of patients (3). Cattle are an important asymptomatic reservoir host of STEC O157, which may be transmitted to humans by ingestion of contaminated meat, produce, or water or by direct contact with cattle carrying the pathogen (4–6). The seasonal variation in STEC O157 fecal shedding by cattle, specifically the higher summertime prevalence, occurs in diverse regions, including Canada, England, Italy, South Korea, the Netherlands, Turkey, and the United States (7–20). Similar summertime peak shedding is also reported in sheep (11, 15, 21). Contrary patterns have been occasionally reported, including a higher wintertime prevalence (22, 23) or no seasonal variation (24–26); however, these are unusual and sometimes are confounded by other factors such as a change to indoor housing (22).
The seasonal summertime shedding of STEC O157 in cattle parallels both seasonal increases in carcass contamination at abattoirs and the increased summertime incidence of human STEC O157 infection (26–28). Given the evidence linking human infection to the bovine reservoir, it is likely that the reduction of STEC O157 shedding in cattle during summertime might result in reduced carcass contamination rates at the abattoir and, ultimately, reduced human disease burden (29, 30).
Understanding the mechanism of the seasonal variation in STEC O157 colonization of cattle may provide insights for the design of practical, effective interventions to mitigate the risk this pathogen poses to humans. Researchers have suggested that the seasonality of STEC O157 shedding by cattle might be influenced by extrinsic factors such as ambient temperature through effects on environmental proliferation of STEC O157, seasonal variation in protozoal predation or intermicrobial competition affecting STEC O157 in environmental reservoirs, and/or seasonal variation in feed components affecting STEC O157 replication (29). Alternatively or in addition, intrinsic factors such as host gastrointestinal microbiome effects on STEC O157 infectivity and/or day length-associated endocrine effects (31, 32) may mediate the seasonality of STEC O157 colonization of cattle. In this study, we repeatedly challenged a cohort of cattle in the summer and winter months over a 2-year period. The magnitude and duration of STEC O157 colonization at the rectoanal junction mucosa (RAJ) following these seasonal experimental challenges provided data to help distinguish among the alternative hypothesized mechanisms underlying the seasonal variation in STEC O157 colonization of cattle.
MATERIALS AND METHODS
Bacterial strains.
Four STEC O157 strains were used in this study, including two clinical isolates (C1, lab reference number WSU11763, isolated from diarrheic human feces, provided by the Washington Department of Health; C2, ATCC strain 43894, isolated from diarrheic human feces during an outbreak of hemorrhagic colitis in Michigan) and two isolates obtained from healthy cattle (B1, lab reference number WSU5880; B2, lab reference number WSU6996). Strains C1 and C2 represent two of the most common STEC O157 genotypes isolated from human disease, with Shiga toxin (Stx)-associated bacteriophage insertion site (SBI) profiles ASY22c (SNP lineage IIa) and WY12 (SNP lineage Ib), respectively (33, 34). The cattle strains B1 and B2 represent two genotypes rarely isolated from clinical human disease but common in cattle, with SBI profiles SY2c and ASY12c, respectively (both single-nucleotide-polymorphism [SNP] lineage Vc) (33, 34). Cultures were grown in Trypticase soy broth (TSB) (Difco, USA) and incubated with aeration at 37°C for 24 h. Equal volumes of broth cultures of each strain were combined, and 10% (vol/vol) glycerol was added, producing a challenge mixture containing 2.25 × 109 CFU/ml with equal numbers of each strain. This mixture was dispensed in 1-ml aliquots and stored at −80°C. The numbers and the equal representation of the four STEC O157 strains were reconfirmed between the second and third challenge studies and again following the final challenge study to demonstrate that the challenge dose was stable.
Animals.
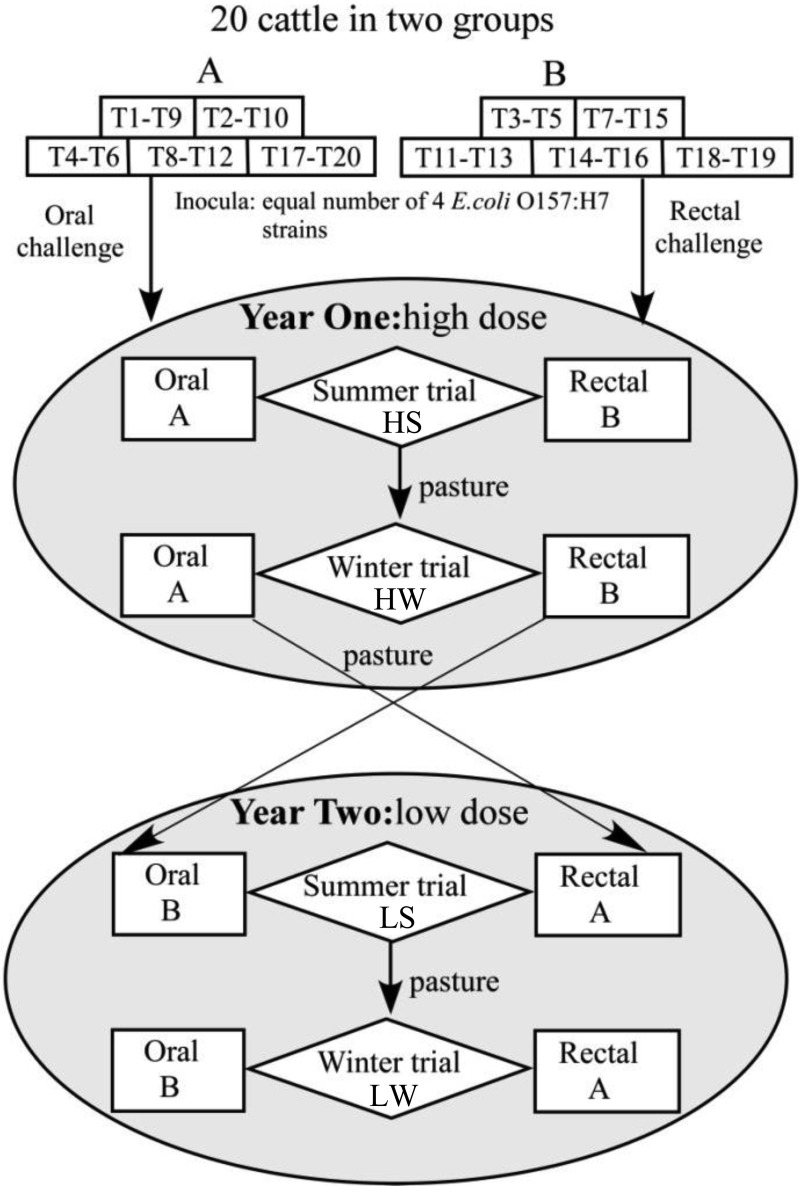
Twenty 5-month-old Holstein steers were obtained from a commercial dairy in central Washington State, ear tagged (T1 through T20), and randomly allocated to two equal-sized groups (A and B) (Fig. 1). Cattle within each group were housed in pairs, with randomly assigned pen mates from the same group. All pens were roofed but lacked side walls and so were open to elements of the outdoor environment, including temperature, daylight, and humidity fluctuations. Each pen contained a water source heated to maintain a minimum temperature of 4°C during the winter. The pens were cleaned daily by removal of manure followed by a water flush over the floors. Throughout the study, all steers were provided a diet composed of 90% alfalfa hay and 10% grain pellets designed to meet the National Research Council growth and maintenance requirements. The feed components were purchased in quantities sufficient to feed the cattle for each entire calendar year, including both the summer and winter challenge trials. Steers had free access to trace-mineral-supplemented salt blocks. The cattle were acclimated to these conditions for 4 weeks before the bacterial challenge. Environmental factors such as temperature and precipitation were recorded in each trial. All personnel followed strict biosafety procedures for the handling and management of cattle, and all procedures were approved by the University of Idaho Institutional Animal Care and Use and Biosafety Committee.
FIG 1.
Experimental design. Twenty 5-month-old Holstein steers (T1 to T20) were randomly assigned to groups (A and B) and pens for four trials conducted at 6-month intervals (HS [high dose, summer], HW [high dose, winter], LS [low dose, summer], and LW [low dose, winter]). Steers within the groups were randomly assigned to pens of two, indicated by boxes. In both years 1 and 2, the summer trials began in August and the winter trials began in February. In the HS and HW trials, group A cattle were challenged orally and group B cattle were challenged rectally with 109 CFU (high dose) STEC O157. In the LS and LW trials, group A cattle were challenged rectally and group B cattle were challenged orally with 107 CFU (low dose) STEC O157. Cattle were pastured following clearance of STEC O157 in each trial until 1 week prior to the start of the next trial.
Challenge trials.
In each of the four experimental trials (Fig. 1), steers in groups A and B were given single doses of the 4-strain STEC O157 mixture by either oral or rectal instillation. The high-dose (2.3 × 109 CFU) summer and winter trials were initiated in August and February, respectively, during year 1 of the study, and the low-dose (1.1 × 107 CFU, produced by a 1:100 dilution of the standardized aliquots) summer and winter trials were initiated in the same months during year 2 of the study. Group A steers were challenged orally and group B rectally in the year 1 trials, and these challenge routes were reversed in the year 2 trials. The oral challenges were delivered to the caudal oral cavity of each steer by disposable syringes, followed immediately by oral instillation of 60 ml of water. The rectal challenges were applied as previously described (35). Briefly, feces were manually removed from the terminal rectum, and the STEC O157 challenge dose was placed directly into the rectum approximately 5 cm anterior to the anus by a disposable syringe. A sterile foam-tipped swab (catalog no. 14-960-3H; Thermo Fisher Scientific, Waltham, MA) was immediately inserted 5 cm into the anus and rubbed vigorously on the RAJ. After swabbing, defecation was inhibited for 10 min by holding the steer's tail firmly against the anus.
During each trial, the steers that were culture negative for STEC O157 on two consecutive sampling days were considered to have cleared the STEC O157 and were moved to pasture until the beginning of the next trial.
Sampling, STEC O157 enumeration, and genotype analysis.
RAJ swab (RAMS) samples were obtained from each steer on days 0 (prior to the challenge inoculation), 1, 3, and 7 and weekly thereafter through 56 days postchallenge using a sterile foam-tipped swab (Thermo Fisher Scientific) and processed for bacterial isolation as previously described (35, 36). Briefly, the RAMS samples were vigorously mixed in 3 ml of ice-cold sterile TSB and kept on ice until further processing. Both direct and enrichment bacterial culture procedures were used. For direct cultures, serial 10-fold dilutions of the RAMS homogenates were plated onto sorbitol MacConkey agar supplemented with cefixime (50 ng/ml), potassium tellurite (2.5 μg/ml), vancomycin (40 mg/liter), and 4-methylumbelliferyl-β-d-glucuronide (MUG) (0.1 mg/ml) (SMAC-CTVM). The plates were incubated overnight at 37°C, and the numbers of colorless (sorbitol-nonfermenting) colonies that did not fluoresce when illuminated by 363-nm light (β-d-glucuronidase negative) were recorded. These were further confirmed to be STEC O157 by O157 latex agglutination testing (up to 20 colonies per sample) (Pro-Lab Diagnostics, Canada), and the proportion of O157 colonies was multiplied by the total sorbitol and β-d-glucuronidase colony counts to estimate the CFU density of STEC O157. The RAMS samples negative by direct culture were enriched by overnight incubation (37°C, aerated), prior to plating on SMAC-CTVM and screening for STEC O157, as described above. The RAMS samples negative on direct culture but positive by enrichment culture were arbitrarily assigned a value of 15 CFU/swab (equivalent to 50% of the minimum detectable direct plating value).
Pen floor and water trough samples were collected weekly and tested for the presence of STEC O157. On each sampling day, a 0.25-m2 area of pen floor was swabbed before cleaning, and the swab was vigorously mixed in 3 ml of TSB. Mixed water and sediment samples (50 ml) from the bottoms of water troughs were placed into sterile conical centrifuge tubes and centrifuged (3,000 × g, 10 min, 4°C). The pellets (1.5 ml) were mixed with 1.5 ml of double-strength TSB, incubated, and processed to detect STEC O157 as described for the RAMS samples above.
Representative STEC O157 colonies were saved (12.5% glycerol in LB, −80°C) from all samples with positive O157 latex agglutination tests both from direct cultures (12 colonies or all present if fewer than 12) and from enrichment cultures (3 colonies or all present if fewer than 3). These STEC O157 isolates were genotyped by multiplex PCR for SBI sites to distinguish the four different challenge strains (34, 37).
Statistical analysis.
NCSS 2007 version 07.1.19 (NCSS, LLC, Kaysville, UT) was used for the statistical analyses. The experimental unit for each trial was the animal, since the challenges and colonizations were measured individually. Since the year 2 challenge doses were reduced from 109 CFU to 107 CFU and also the routes of challenge were switched between groups A and B cattle, these two factors could not be simultaneously analyzed. Therefore, we first performed separate analyses of year 1 and year 2 trial data, neither of which detected any tendency for an effect of the route of the challenge on the magnitude or duration of STEC O157 RAJ colonization. Therefore, in the final analysis, the data from year 1 and 2 trials were combined, and the challenge route variable was excluded from the models tested. For all trials, animals were considered colonized following a challenge dose until negative cultures were obtained on two consecutive sampling dates.
The RAJ colonization data were initially analyzed using repeated-measures analysis of variance (ANOVA) models to determine the effects of the independent variables dose and season on log10(CFU + 1) STEC O157 per RAJ swab as the dependent variable. The RAJ colonization data failed the Anderson-Darling and the Shapiro-Wilk W tests for normal distribution following log transformation, and attempts to find an alternative transformation that normalized the data were unsuccessful. Therefore, the RAMS STEC O157 CFU data for days 1 through 56 in each trial were used to calculate an area under the curve (AUC) value for the RAMS colonization for each animal, using a trapezoidal rule method. Following log10 transformation, the AUC data were normally distributed and were used as the dependent variable to evaluate the effects of the independent factors dose and season using a mixed-model ANOVA. Both of these approaches (repeated-measures ANOVA applied to the log10-transformed RAJ STEC O157 CFU and mixed-model ANOVA applied to the log10 AUC-transformed fecal shedding data) detected similar associations between independent and dependent variables.
The effects of the dose, season, and challenge route on the duration of STEC O157 RAJ colonization were analyzed using Kaplan-Meier survival analyses with log rank tests and Cox-Mantel hazard ratios for multiple comparisons. The individual animal effects on the density and duration of RAJ colonization were analyzed using general linear models with the log10 AUC data and Kaplan-Meier survival analyses and Cox-Mantel hazard ratios for multiple comparisons, respectively. All statistical tests were interpreted using a significance level of ≤0.05.
RESULTS
We compared the seasonal trends of STEC O157 RAJ colonization of a cohort of cattle following four experimental challenge trials conducted in the summer and winter seasons (August and February) over a 2-year period (Fig. 1). The steers were housed in an isolation barn with open sides exposed to ambient temperatures and other weather variables. The weather conditions during the study were typical of the inland Pacific Northwest (Table 1): cold, moist winters and warm, very dry summers. Throughout the study, all cattle remained asymptomatic during STEC O157 carriage. Animal T4 was removed during the last low-dose winter trial due to behavioral problems, and as a result, data from that animal were not available for that trial.
TABLE 1.
Temperatures and cumulative precipitation during each of the four trial periods
| Trial | Days | Mean temp ± SD (°C) |
Precipitation (in.) | |
|---|---|---|---|---|
| High | Low | |||
| High dose, summer (HS) | 1–28 | 28.6 ± 5.1 | 7.8 ± 4.3 | 0.28 |
| 29–56 | 17.0 ± 5.1 | 4.5 ± 2.8 | 2.06 | |
| High dose, winter (HW) | 1–28 | 7.7 ± 4.5 | −1.3 ± 4.4 | 6.45 |
| 29–56 | 14.5 ± 5.3 | 2.0 ± 3.7 | 4.01 | |
| Low dose, summer (LS) | 1–28 | 24.5 ± 4.5 | 2.7 ± 4.4 | 0 |
| 29–56 | 14.4 ± 5.6 | 3.3 ± 4.2 | 4.07 | |
| Low dose, winter (LW) | 1–28 | 8.9 ± 3.1 | −1.0 ± 3.3 | 1.81 |
| 29–56 | 13.0 ± 3.9 | 0.6 ± 3.4 | 3.2 | |
Density of STEC O157 RAJ colonization.
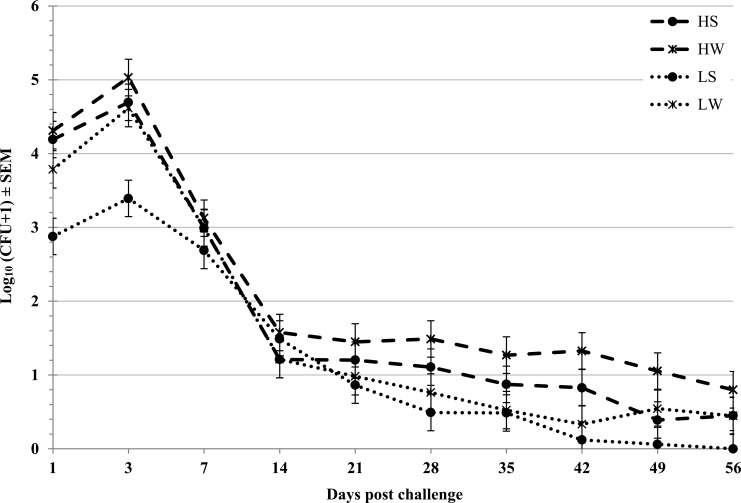
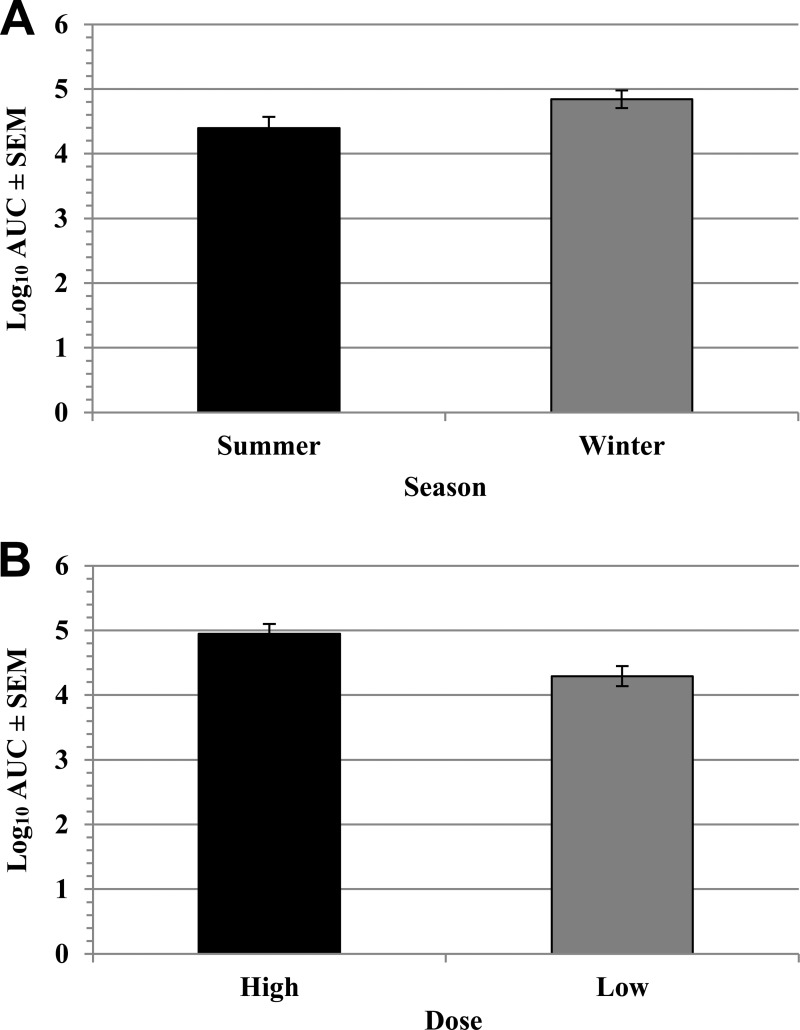
Similar temporal trends in STEC O157 density on the RAMS were observed during each trial, with a peak density (∼104 CFU/swab) on days 1 to 3 postchallenge followed by a general downward trend to ∼10 CFU/swab on day 56 (Fig. 2; see also Table S1 in the supplemental material). To normalize data for analysis, STEC O157 detection on the RAMS on the multiple sampling days was transformed by the calculation of the AUC for each animal for each trial. The STEC O157 shedding AUC values were significantly lower following the summer trials (P = 0.016) than following the winter trials (Table 2; Fig. 3A). The STEC O157 AUC values were significantly higher (P < 0.001) following the higher-dose challenges conducted during year 1 trials than those after the lower-dose (107-CFU) challenges conducted in year 2 (Table 2; Fig. 3B). The STEC O157 shedding AUC values were not significantly affected by the route of challenge (oral versus rectal) at either high or low challenge doses (Table 2).
FIG 2.
Density of STEC O157 carriage by cattle. Enumeration of STEC O157 CFU on RAJ swabs (least square means ± SEM) from 20 steers during 56 days postchallenge for four trials (HS [high dose, summer], HW [high dose, winter], LS [low dose, summer], and LW [low dose, winter]).
TABLE 2.
Comparison of the densities of RAJ STEC O157 colonization represented as log10 transformations of the area under the shedding curve and mean CFU per sampling date
| Yr | Dose | Route | Season | AUC (LSM ± SEM)a | AUC P value | CFU (LSM ± SEM) | CFU P value |
|---|---|---|---|---|---|---|---|
| 1 | High | Oral | Both | 4.91 ± 0.21 | 0.81 | 1.87 ± 0.24 | 0.57 |
| High | Rectal | Both | 4.98 ± 0.21 | 2.07 ± 0.24 | |||
| High | Both | Summer | 4.87 ± 0.21 | 0.57 | 1.79 ± 0.24 | 0.33 | |
| High | Both | Winter | 5.02 ± 0.19 | 2.14 ± 0.24 | |||
| 2 | Low | Oral | Both | 4.12 ± 0.22 | 0.27 | 1.30 ± 0.14 | 0.22 |
| Low | Rectal | Both | 4.47 ± 0.22 | 1.57 ± 0.15 | |||
| Low | Both | Summer | 3.93 ± 0.24 | 0.06 | 1.25 ± 0.14 | 0.08 | |
| Low | Both | Winter | 4.66 ± 0.14 | 1.63 ± 0.15 | |||
| Both | High | Both | Both | 4.95 ± 0.16 | 0.0006 | 1.97 ± 0.13 | 0.009 |
| Low | Both | Both | 4.29 ± 0.16 | 1.43 ± 0.13 | |||
| Both | Both | Summer | 4.40 ± 0.17 | 0.016 | 1.52 ± 0.13 | 0.06 | |
| Both | Both | Winter | 4.84 ± 0.14 | 1.88 ± 0.13 |
AUC, area under the shedding curve; LSM, least-squares means.
FIG 3.
Comparison of the effects of season and challenge dose on STEC O157 carriage. The numbers of STEC O157 CFU per RAJ swab during 56 days postchallenge were converted to a single area under the curve (AUC) value for each animal for each trial. Bars represent least-squares means, and error bars indicate standard errors of the mean (SEM). (A) Least-squares mean log10 AUC values by season. AUC values following the summer and winter challenges differed significantly (P = 0.0157). (B) Least-squares mean log10 AUC values by dose. AUC values after high-dose (109 CFU) and low-dose (107 CFU) challenges differed significantly (P = 0.0005).
We also evaluated the STEC O157 density detected on the RAMS on each sampling day following challenges using repeated-measures ANOVA (Table 2; Fig. 2). These data failed normality tests despite multiple attempted transformations, but nevertheless the resulting analyses were broadly consistent with those determined using the AUC approach: the STEC O157 density on the RAMS was lower following the summer challenges than following the winter challenges, although this trend was not statistically significant (P = 0.06). The STEC O157 density on the RAMS was significantly (P = 0.009) higher following the higher-dose challenges of the year 1 trials than following the lower-dose year 2 trials. The STEC O157 density on the RAMS following the challenge did not differ significantly by the route of challenge (oral versus rectal) in either year 1 or year 2 challenge trials (Table 2).
Duration of STEC O157 RAJ colonization.
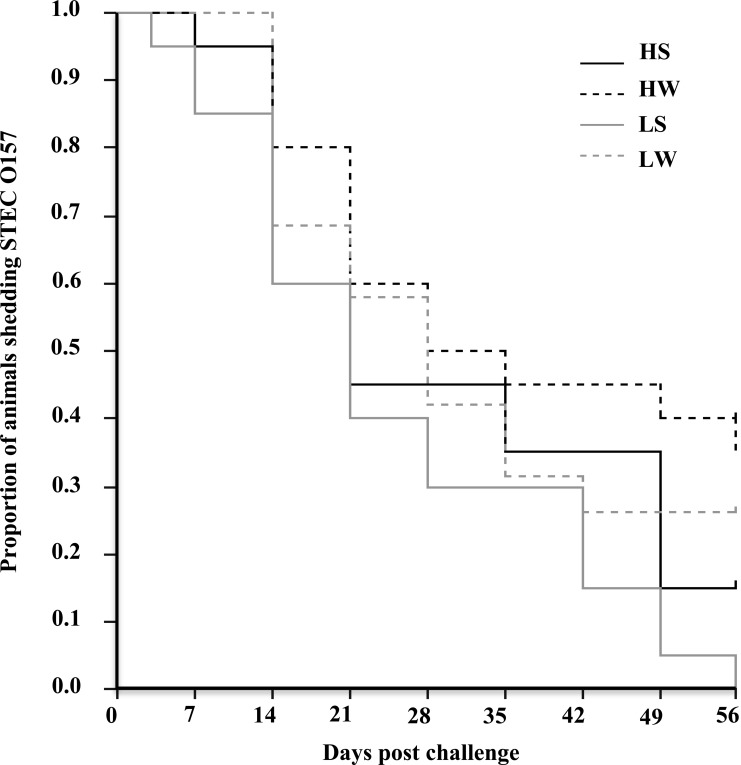
STEC O157 was cultured from the RAMS of all animals in all four trials on day 1 following the challenge, and only a single animal (T5) cleared STEC O157 by day 3 and that occurred in only one (low dose, summer) of the four trials. Beginning on day 7, a clear decrease in the numbers of cattle that were culture positive for STEC O157 was observed during both summer and winter following both high- and low-dose challenges (Fig. 4; see also Table S2 in the supplemental material). By day 56 following challenge, the proportions of STEC O157 culture-positive cattle were 0 (low dose, summer), 0.15 (high dose, summer), 0.26 (low dose, winter), and 0.35 (high dose, winter).
FIG 4.
Kaplan-Meier survival curves for STEC O157 carriage by cattle. Colonization was determined by RAJ swab culture for each of four seasonal trials (HS [high dose, summer], HW [high dose, winter], LS [low dose, summer], and LW [low dose, winter]). Animals were considered colonized until they were culture negative for STEC O157 on two sequential sampling dates. A trend toward more prolonged shedding during the winter months at each dose level was not statistically significant (P = 0.058).
The duration of the detection of STEC O157 on the RAMS was longer following the winter challenges than following the summer challenges, regardless of the challenge dose, and was longer following higher-dose challenges than following lower-dose challenges, although these differences were not statistically significant (log rank tests and Kaplan-Meier survival curve, P = 0.052) (Fig. 4; see also Table S2 in the supplemental material).
Individual animal variations in density and duration of STEC O157 RAJ colonization.
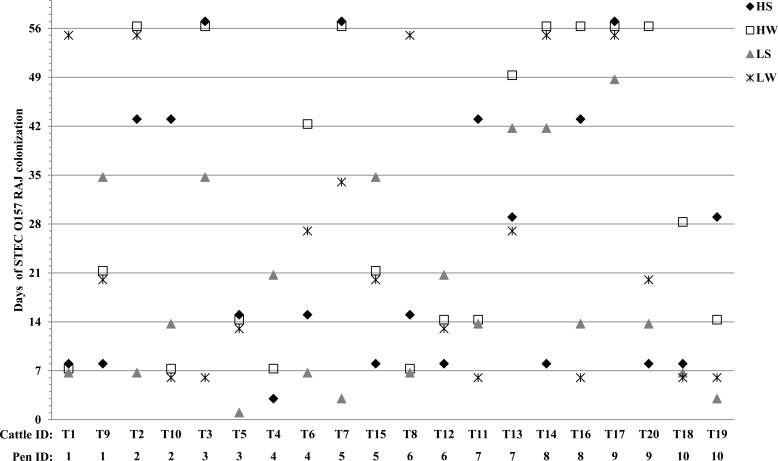
The magnitudes of the STEC O157 AUCs across all four trials did not differ significantly among the 20 cattle (P = 0.40) (see Fig. S1 in the supplemental material). However, the durations of the RAJ colonization did differ among the 20 cattle (log rank test and Kaplan-Meier survival curve, P = 0.02). Specifically, across the four experimental challenges, five animals were STEC O157 culture positive significantly longer than one or more of the other animals (P ≤ 0.05) (Fig. 5): steer T17 was colonized significantly longer than steers T4, T5, T6, T9, T10, T11, T12, T13, T15, T18, and T19; steer T13 was colonized longer than steers T4, T5, T12, T18, and T19; and steers T2, T3, and T14 were colonized longer than steer T18. The duration of the colonization of individual steers was not demonstrably related to the colonization status of the other steers housed in the same pen (Fig. 5).
FIG 5.
Duration of colonization of STEC O157 by individual cattle in each of the four seasonal trials (HS [high dose, summer], HW [high dose, winter], LS [low dose, summer], and LW [low dose, winter]).
Effect of bacterial strain in STEC O157 RAJ colonization.
The four different genotypes were nearly equally represented in stored (−80°C) aliquots of the challenge mixture measured before the first trial (12 to 33%), between the second and third trials (21 to 28%), and after the fourth trial (20 to 28%). Within each trial, the proportions of each strain detected on the RAMS remained roughly similar for the first week following the challenge, but differences in the proportional representation of the strains developed in the second week and persisted through day 56 postchallenge (see Table S3 in the supplemental material). There was no significant difference in the colonization by the clinical and bovine genotypes (see Fig. S2). Within individual animals, detection of a single STEC O157 genotype became more common with increasing time after the challenge, but no consistent genotype was dominant among the steers that remained STEC O157 colonized for 21 or more days postchallenge. In each trial, the predominant colonizing genotypes after the second week postchallenge frequently differed between colonized pen mates (see Table S3).
Environmental STEC O157 on pen floors and in water troughs.
No seasonal differences in STEC O157 contamination were measured among the environmental samples. All trough water/sediment samples were culture negative for STEC O157 through all four challenge trials. However, 47% of floor samples from pens containing one or more STEC O157 culture-positive steers were culture positive for STEC O157, and 13% of the floor samples from pens containing animals with STEC O157 detectable only by enrichment culture (<30 CFU/swab) were culture positive for STEC O157.
DISCUSSION
The mechanism underlying the seasonal variation in cattle STEC O157 colonization is an important question because the summertime increases in the prevalence may overwhelm postharvest food safety measures (28, 38–40), increasing the risk of food contamination, human infection, and disease. Furthermore, identification of the source(s) of seasonal variation in cattle colonization may lead to identification of novel control measures that might significantly reduce the public health burden associated with this pathogen. The key finding of this study was that the cattle colonization resulting from standardized exposure doses given either orally or rectally, at two different dose levels, did not exhibit seasonal variation in a direction that might help explain the seasonal variation repeatedly observed in field studies. In fact, we documented a small but statistically significant seasonal variation with a winter peak, opposite to the summer peak colonization that has been repeatedly documented on farms. This finding suggests that the seasonal variation is not due to factors intrinsic to cattle (such as endocrine factors or other factors mediated by the season or day length). Also, the lack of observed seasonal colonization differences between cattle after oral or RAJ challenge suggests little or no seasonal difference in the ability of STEC O157 to transit the gastrointestinal tract to colonize the RAJ. Therefore, our results are most consistent with the hypothesis that the seasonal variation observed in cattle on farms results from seasonal differences in the exposure dose, rather than intrinsic or gut microbiome factors. The high degree of consistency in fecal shedding observed in this study contrasts markedly with the heterogeneous shedding patterns observed on farms (41); this contrast also supports the hypothesis that the heterogeneous shedding patterns observed on farms result at least in part from heterogeneous oral exposures of cattle to environmental STEC O157.
This study has several limitations, including the relatively small sample size, the physical environment and diet of the cattle that may not be broadly representative of cattle management systems, and the possibility that the challenge doses of STEC O157 were not representative of those resulting in natural cattle infection. A cohort of 20 steers is quite small, given the variability in STEC O157 colonization and fecal shedding that has been reported from large commercial cattle operations (10, 42, 43). However, our study design included elements designed to reduce several sources of variability. First, we used highly consistent challenge doses for all steers for both seasons within each year of our study, whereas oral exposures are likely to be widely variable among cattle within pens on farms. In addition, we maintained a high degree of pen sanitation and water hygiene to minimize the occurrence of transmission resulting from pen and water contamination with STEC O157. The success of these efforts was confirmed by microbiologic monitoring. Also, pen mates carried different predominant genotypes, and culture-negative animals were not recolonized by culture-positive pen mates. In the end, the detection of a statistically significant effect of season (with increased colonization following winter challenges) shows that the cohort size was sufficient to detect seasonal factors affecting bovine colonization.
No single study environment could be considered representative of the overall cattle industry, but our study facility provided cattle with exposure to most environmental variables experienced by cattle naturally, including day length, temperature, and humidity. Our cattle facility was roofed, and so the animals were not directly exposed to precipitation and were only exposed to direct sunlight at dawn and dusk, both factors that have been identified as potential drivers of STEC O157 colonization of cattle (44, 45). We cannot exclude the possibility that direct exposure to precipitation may have changed our results. However, the typical pattern of precipitation in our region is for minimal or no precipitation in August, a time when we observe seasonally increased colonization of cattle, indicating that exposure to rainfall is not required for the seasonal variation in STEC O157 colonization. Similarly, our strict controls on pen and water trough hygiene and our use of the same feed sources and harvest years for both the August and February challenge studies are not representative of routine industry practices but were necessary to minimize the exposure of the study cattle to these (extrinsic to cattle) factors. Therefore, feed and water hygiene and environmental sanitation along with the noncattle reservoir of STEC O157 affecting the fecal-oral transmission remain plausible mechanisms for the seasonal variation observed on farms (46).
Finally, the challenge doses of STEC O157 we used may be larger than those that result in natural infection of cattle (47). While we cannot eliminate this possibility entirely, the observation of similar trends with 107-CFU exposure doses (relatively low compared to those in previous experimental cattle challenges with STEC O157) in the second year of the study shows that these trends are observed under a fairly wide range of challenge exposures. The STEC O157 colonization patterns observed here following both low- and high-dose challenges were not dissimilar to those we have reported following even lower-dose (104 CFU) challenges or natural contact transmission (47), suggesting that the doses used resulted in reasonably normal colonization events.
There were three reasons to anticipate increased summertime colonization in the year 1 trials: in addition to the unknown mechanism underlying the seasonal variation in bovine STEC O157 colonization generally, both increasing age postweaning (41) and the effect of previous exposure to STEC O157 (48, 49) were expected to reduce colonization in February compared to that in the preceding August. Therefore, based on the lack of observed increased summertime colonization in the year 1 trials despite the combined expected effects of season, age, and immunity, we elected to reduce the challenge doses used in the year 2 trials to determine whether the lack of increased summertime colonization might be an artifact of a nonphysiologically high challenge dose. It is also noteworthy that, although we did not analyze specific immune responses, the STEC O157 colonization of our study cohort did not appreciably decrease even after four challenges, suggesting that protective immunity was either not elicited or was not strong enough or of sufficient duration to provide protection.
Subject to the limitations of this study described above, our findings support the hypothesis that the seasonal variation in bovine colonization with STEC O157 observed in the field results from seasonal differences in oral exposures of cattle rather than from seasonal differences in systemic or upper gastrointestinal physiologic factors. The increased oral exposures of cattle might be simply due to increased summertime fecal-oral transmission within cattle operations mediated by proliferation of the pathogen in bedding, feeds, or water troughs, resulting in increased exposure doses (29, 50). Alternatively, given the generally transient nature of bovine colonization, heightened carriage could be due to increased summertime exposure of cattle to another noncattle animal or environmental reservoir. Efforts to identify the source of the increased summer oral exposure of cattle to STEC O157 are ongoing.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lonie Austin, Claudia Deobald, and Harold Rohde for expert animal handling and technical assistance.
Funding Statement
The project was supported, in part, by the University of Idaho Agricultural Experiment Station and by the National Institute for Food and Agriculture, Hatch project numbers IDAO1467 (Carolyn J. Hovde) and IDA01406 (Scott A. Minnich). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02839-15.
REFERENCES
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scallan E, Hoekstra RM, Mahon BE, Jones TF, Griffin PM. 2015. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol Infect 143:2795−2804. doi: 10.1017/S0950268814003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. 1999. Food-related illness and death in the United States. Emerg Infect Dis 5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caprioli A, Morabito S, Brugere H, Oswald E. 2005. Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet Res 36:289–311. doi: 10.1051/vetres:2005002. [DOI] [PubMed] [Google Scholar]
- 5.Mainil JG, Daube G. 2005. Verotoxigenic Escherichia coli from animals, humans and foods: who's who? J Appl Microbiol 98:1332–1344. doi: 10.1111/j.1365-2672.2005.02653.x. [DOI] [PubMed] [Google Scholar]
- 6.Rangel JM, Sparling PH, Crowe C, Griffin PM, Swerdlow DL. 2005. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982−2002. Emerg Infect Dis 11:603–609. doi: 10.3201/eid1104.040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Donkersgoed J, Graham T, Gannon V. 1999. The prevalence of verotoxins, Escherichia coli O157:H7, and Salmonella in the feces and rumen of cattle at processing. Can Vet J 40:332–338. [PMC free article] [PubMed] [Google Scholar]
- 8.Hancock DD, Besser TE, Kinsel ML, Tarr PI, Rice DH, Paros MG. 1994. The prevalence of Escherichia coli O157.H7 in dairy and beef cattle in Washington State. Epidemiol Infect 113:199–207. doi: 10.1017/S0950268800051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mechie SC, Chapman PA, Siddons CA. 1997. A fifteen month study of Escherichia coli O157:H7 in a dairy herd. Epidemiol Infect 118:17–25. doi: 10.1017/S0950268896007194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock DD, Besser TE, Rice DH, Herriott DE, Tarr PI. 1997. A longitudinal study of Escherichia coli O157 in fourteen cattle herds. Epidemiol Infect 118:193–195. doi: 10.1017/S0950268896007212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman PA, Siddons CA, Gerdan Malo AT, Harkin MA. 1997. A 1-year study of Escherichia coli O157 in cattle, sheep, pigs and poultry. Epidemiol Infect 119:245–250. doi: 10.1017/S0950268897007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heuvelink AE, Bleumink B, van den Biggelaar FL, Te Giffel MC, Beumer RR, de Boer E. 1998. Occurrence and survival of verocytotoxin-producing Escherichia coli O157 in raw cow's milk in The Netherlands. J Food Prot 61:1597–1601. [DOI] [PubMed] [Google Scholar]
- 13.Bonardi S, Maggi E, Bottarelli A, Pacciarini ML, Ansuini A, Vellini G, Morabito S, Caprioli A. 1999. Isolation of verocytotoxin-producing Escherichia coli O157:H7 from cattle at slaughter in Italy. Vet Microbiol 67:203–211. doi: 10.1016/S0378-1135(99)00039-5. [DOI] [PubMed] [Google Scholar]
- 14.Karch H, Kohler B. 1999. New knowledge of the molecular biology of enterohemorrhagic Escherichia coli (EHEC) O157. Gesundheitswesen 61(Spec No 1):S46–S51. (In German.) [PubMed] [Google Scholar]
- 15.Paiba GA, Gibbens JC, Pascoe SJ, Wilesmith JW, Kidd SA, Byrne C, Ryan JB, Smith RP, McLaren M, Futter RJ, Kay AC, Jones YE, Chappell SA, Willshaw GA, Cheasty T. 2002. Faecal carriage of verocytotoxin-producing Escherichia coli O157 in cattle and sheep at slaughter in Great Britain. Vet Rec 150:593–598. doi: 10.1136/vr.150.19.593. [DOI] [PubMed] [Google Scholar]
- 16.Schouten JM, Bouwknegt M, van de Giessen AW, Frankena K, De Jong MC, Graat EA. 2004. Prevalence estimation and risk factors for Escherichia coli O157 on Dutch dairy farms. Prev Vet Med 64:49–61. doi: 10.1016/j.prevetmed.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Schouten JM, Graat EA, Frankena K, van de Giessen AW, van der Zwaluw WK, de Jong MC. 2005. A longitudinal study of Escherichia coli O157 in cattle of a Dutch dairy farm and in the farm environment. Vet Microbiol 107:193–204. doi: 10.1016/j.vetmic.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Aslantaş O, Erdogan S, Cantekin Z, Gulacti I, Evrendilek GA. 2006. Isolation and characterization of verocytotoxin-producing Escherichia coli O157 from Turkish cattle. Int J Food Microbiol 106:338–342. doi: 10.1016/j.ijfoodmicro.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Stanford K, Croy D, Bach SJ, Wallins GL, Zahiroddini H, McAllister TA. 2005. Ecology of Escherichia coli O157:H7 in commercial dairies in southern Alberta. J Dairy Sci 88:4441–4451. doi: 10.3168/jds.S0022-0302(05)73131-3. [DOI] [PubMed] [Google Scholar]
- 20.Jo MY, Kim JH, Lim JH, Kang MY, Koh HB, Park YH, Yoon DY, Chae JS, Eo SK, Lee JH. 2004. Prevalence and characteristics of Escherichia coli O157 from major food animals in Korea. Int J Food Microbiol 95:41–49. doi: 10.1016/j.ijfoodmicro.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Kudva IT, Hatfield PG, Hovde CJ. 1996. Escherichia coli O157:H7 in microbial flora of sheep. J Clin Microbiol 34:431–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Synge BA, Chase-Topping ME, Hopkins GF, McKendrick IJ, Thomson-Carter F, Gray D, Rusbridge SM, Munro FI, Foster G, Gunn GJ. 2003. Factors influencing the shedding of verocytotoxin-producing Escherichia coli O157 by beef suckler cows. Epidemiol Infect 130:301–312. doi: 10.1017/S0950268802008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogden ID, MacRae M, Strachan NJ. 2004. Is the prevalence and shedding concentrations of E. coli O157 in beef cattle in Scotland seasonal? FEMS Microbiol Lett 233:297–300. doi: 10.1111/j.1574-6968.2004.tb09495.x. [DOI] [PubMed] [Google Scholar]
- 24.Alam MJ, Zurek L. 2006. Seasonal prevalence of Escherichia coli O157:H7 in beef cattle feces. J Food Prot 69:3018–3020. [DOI] [PubMed] [Google Scholar]
- 25.Richards MS, Corkish JD, Sayers AR, McLaren IM, Evans SJ, Wray C. 1998. Studies of the presence of verocytotoxic Escherichia coli O157 in bovine faeces submitted for diagnostic purposes in England and Wales and on beef carcases in abattoirs in the United Kingdom. Epidemiol Infect 120:187–192. doi: 10.1017/S0950268897008583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brichta-Harhay DM, Guerini MN, Arthur TM, Bosilevac JM, Kalchayanand N, Shackelford SD, Wheeler TL, Koohmaraie M. 2008. Salmonella and Escherichia coli O157:H7 contamination on hides and carcasses of cull cattle presented for slaughter in the United States: an evaluation of prevalence and bacterial loads by immunomagnetic separation and direct plating methods. Appl Environ Microbiol 74:6289–6297. doi: 10.1128/AEM.00700-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barkocy-Gallagher GA, Arthur TM, Rivera-Betancourt M, Nou X, Shackelford SD, Wheeler TL, Koohmaraie M. 2003. Seasonal prevalence of Shiga toxin-producing Escherichia coli, including O157:H7 and non-O157 serotypes, and Salmonella in commercial beef processing plants. J Food Prot 66:1978–1986. [DOI] [PubMed] [Google Scholar]
- 28.Elder RO, Keen JE, Siragusa GR, Barkocy-Gallagher GA, Koohmaraie M, Laegreid WW. 2000. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc Natl Acad Sci U S A 97:2999–3003. doi: 10.1073/pnas.97.7.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hancock D, Besser T, Lejeune J, Davis M, Rice D. 2001. The control of VTEC in the animal reservoir. Int J Food Microbiol 66:71–78. doi: 10.1016/S0168-1605(00)00487-6. [DOI] [PubMed] [Google Scholar]
- 30.Williams MS, Withee JL, Ebel ED, Bauer NE, Schlosser WD, Disney WT, Smith DR, Moxley RA. 2010. Determining relationships between the seasonal occurrence of Escherichia coli O157:H7 in live cattle, ground beef, and humans. Foodborne Pathog Dis 7:1247–1254. doi: 10.1089/fpd.2010.0576. [DOI] [PubMed] [Google Scholar]
- 31.Edrington TS, Callaway TR, Ives SE, Engler MJ, Looper ML, Anderson RC, Nisbet DJ. 2006. Seasonal shedding of Escherichia coli O157:H7 in ruminants: a new hypothesis. Foodborne Pathog Dis 3:413–421. doi: 10.1089/fpd.2006.3.413. [DOI] [PubMed] [Google Scholar]
- 32.Edrington TS, Callaway TR, Hallford DM, Chen L, Anderson RC, Nisbet DJ. 2008. Effects of exogenous melatonin and tryptophan on fecal shedding of E. coli O157:H7 in cattle. Microb Ecol 55:553–560. doi: 10.1007/s00248-007-9300-8. [DOI] [PubMed] [Google Scholar]
- 33.Jung WK, Bono JL, Clawson ML, Leopold SR, Shringi S, Besser TE. 2013. Lineage and genogroup-defining single nucleotide polymorphisms of Escherichia coli O157:H7. Appl Environ Microbiol 79:7036–7041. doi: 10.1128/AEM.02173-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shringi S, Schmidt C, Katherine K, Brayton KA, Hancock DD, Besser TE. 2012. Carriage of stx2a differentiates clinical and bovine-biased strains of Escherichia coli O157. PLoS One 7:e51572. doi: 10.1371/journal.pone.0051572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheng H, Davis MA, Knecht HJ, Hovde CJ. 2004. Rectal administration of Escherichia coli O157:H7: novel model for colonization of ruminants. Appl Environ Microbiol 70:4588–4595. doi: 10.1128/AEM.70.8.4588-4595.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice DH, Sheng HQ, Wynia SA, Hovde CJ. 2003. Rectoanal mucosal swab culture is more sensitive than fecal culture and distinguishes Escherichia coli O157:H7-colonized cattle and those transiently shedding the same organism. J Clin Microbiol 41:4924–4929. doi: 10.1128/JCM.41.11.4924-4929.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Besser TE, Shaikh N, Holt NJ, Tarr PI, Konkel ME, Malik-Kale P, Walsh CW, Whittam TS, Bono JL. 2007. Greater diversity of Shiga toxin-encoding bacteriophage insertion sites among Escherichia coli O157:H7 isolates from cattle than in those from humans. Appl Environ Microbiol 73:671–679. doi: 10.1128/AEM.01035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arthur TM, Keen JE, Bosilevac JM, Brichta-Harhay DM, Kalchayanand N, Shackelford SD, Wheeler TL, Nou X, Koohmaraie M. 2009. Longitudinal study of Escherichia coli O157:H7 in a beef cattle feedlot and role of high-level shedders in hide contamination. Appl Environ Microbiol 75:6515–6523. doi: 10.1128/AEM.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacob ME, Renter DG, Nagaraja TG. 2010. Animal- and truckload-level associations between Escherichia coli O157:H7 in feces and on hides at harvest and contamination of preevisceration beef carcasses. J Food Prot 73:1030–1037. [DOI] [PubMed] [Google Scholar]
- 40.Dodd CC, Sanderson MW, Jacob ME, Renter DG. 2011. Modeling preharvest and harvest interventions for Escherichia coli O157 contamination of beef cattle carcasses. J Food Prot 74:1422–1433. doi: 10.4315/0362-028X.JFP-10-516. [DOI] [PubMed] [Google Scholar]
- 41.Renter DG, Sargeant JM. 2002. Enterohemorrhagic Escherichia coli O157: epidemiology and ecology in bovine production environments. Anim Health Res Rev 3:83–94. doi: 10.1079/AHRR200245. [DOI] [PubMed] [Google Scholar]
- 42.Sargeant JM, Gillespie JR, Oberst RD, Phebus RK, Hyatt DR, Bohra LK, Galland JC. 2000. Results of a longitudinal study of the prevalence of Escherichia coli O157:H7 on cow-calf farms. Am J Vet Res 61:1375–1379. doi: 10.2460/ajvr.2000.61.1375. [DOI] [PubMed] [Google Scholar]
- 43.Sargeant JM, Sanderson MW, Smith RA, Griffin DD. 2003. Escherichia coli O157 in feedlot cattle feces and water in four major feeder-cattle states in the USA. Prev Vet Med 61:127–135. doi: 10.1016/S0167-5877(03)00166-1. [DOI] [PubMed] [Google Scholar]
- 44.Williams KJ, Ward MP, Dhungyel OP, Hall EJ. 2015. Risk factors for Escherichia coli O157 shedding and super-shedding by dairy heifers at pasture. Epidemiol Infect 143:1004–1015. doi: 10.1017/S0950268814001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LeJeune JT, Besser TE, Merrill NL, Rice DH, Hancock DD. 2001. Livestock drinking water microbiology and the factors influencing the quality of drinking water offered to cattle. J Dairy Sci 84:1856–1862. doi: 10.3168/jds.S0022-0302(01)74626-7. [DOI] [PubMed] [Google Scholar]
- 46.Besser TE, Schmidt CE, Shah DH, Shringi S. 2015. “Preharvest” food safety for Escherichia coli O157 and other pathogenic Shiga toxin-producing strains, p 421− 436 In Sperandio V, Hovde CJ (ed), Enterohemorrhagic Escherichia coli and other Shiga toxin-producing E. coli. ASM Press, Washington, DC. [DOI] [PubMed] [Google Scholar]
- 47.Besser TE, Richards BL, Rice DH, Hancock DD. 2001. Escherichia coli O157:H7 infection of calves: infectious dose and direct contact transmission. Epidemiol Infect 127:555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cray WC Jr, Moon HW. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl Environ Microbiol 61:1586–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bretschneider G, Berberov EM, Moxley RA. 2007. Reduced intestinal colonization of adult beef cattle by Escherichia coli O157:H7 tir deletion and nalidixic-acid-resistant mutants lacking flagellar expression. Vet Microbiol 125:381–386. doi: 10.1016/j.vetmic.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Smith DR, Moxley RA, Clowser SL, Folmer JD, Hinkley S, Erickson GE, Klopfenstein TJ. 2005. Use of rope devices to describe and explain the feedlot ecology of Escherichia coli O157:H7 by time and place. Foodborne Pathog Dis 2:50–60. doi: 10.1089/fpd.2005.2.50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.