Abstract
A visible light-mediated radical Smiles rearrangement has been developed to address the challenging synthesis of a gem-difluoro group present in an opioid receptor-like 1 (ORL-1) antagonist currently in development. This method led to the direct and efficient introduction of the difluoroethanol motif into a range of aryl- and heteroaryl systems, representing a new disconnection for the synthesis of this versatile functionality. When applied to the target compound, the photochemical step was demonstrated on 15 g scale using industrially relevant catalyst loadings of Ru(bpy)3Cl2 (0.01 mol%). This transformation allowed an overall five-step route that compares favourably to the current synthetic sequence and demonstrates, in this specific case, a clear strategic benefit of photocatalysis.
Keywords: Catalysis, Heterocycles, Photocatalysis, Radical Reactions, Rearrangement
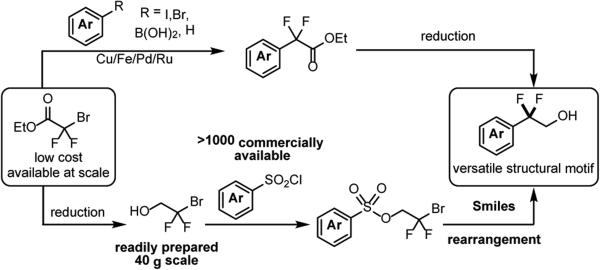
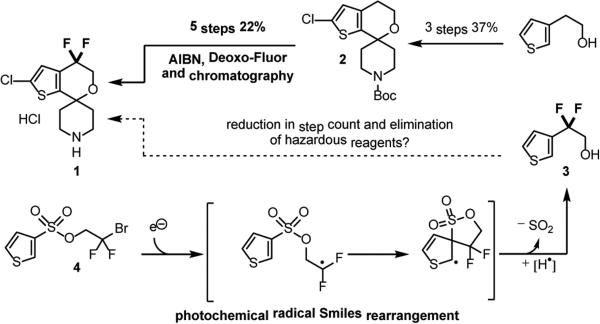
In recent years there has been an exponential increase in the number of transformations mediated by visible light photocatalysis,1 however there remain few reports detailing its application to agrochemical or pharmaceutical synthesis.2 Key to the expansion of this mode of catalysis is the demonstration of a strategic benefit when compared to other routes, most likely showcased via the ability of photocatalysis to provide new synthetic disconnections. We aimed to test the potential advantage of photocatalysis in the synthesis of difluorospirocyclic thiophene 1,3 a building block in the synthesis of an ORL-1 antagonist currently in development for the treatment of depression and/or obesity.4 A production campaign demonstrated rapid access to the carbocyclic framework 2 in good yield and on multi-kg scale. However the benzylic fluorination of 2 proved to be a challenge, requiring four synthetic steps in an overall 25% yield. Furthermore, this sequence included an AIBN initiated radical bromination, utilized 2.6 equivalents of Deoxo-Fluor® as the fluoride source,5 and required chromatographic purification. Benzylic fluorination remains a general problem, with classical methods including nucleophilic or electrophilic fluorination of pre-functionalized substrates.6 Direct benzylic C-H fluorination strategies, typically employing either transition metals,7 or non-metal promoters8 have recently been reported. Chen has shown visible light photocatalysis as a potentially viable method,9 however this protocol requires the use of 3.0 equivalents of SelectFluor II®. An appealing strategy to address the high step count, low yield, and the use of specialized fluorinating reagents for the fluorination sequence would be to start from pre-fluorinated thiophene 3.
This route could make use of ethyl bromodifluoroacetate as a low cost, readily available source of the requisite benzylic gemdifluoro group,10 diverting the key step from C-F to C-C bond formation. C-H,11 or directed coupling strategies,12 as well as radical arylation methods13 are well documented (Scheme 2), however in the context of 1 proved poorly selective. Specifically, employing coupling conditions originally introduced by Kobayashi,14 treatment of 4-bromo-2-chlorothiophene with superstoichiometric copper and ethyl bromodifluoroacetate led primarily to functionalization at the five position.15 Radical arylation is generally limited to the two position of heteroaromatics; in our system with both two and five positions blocked, none of the desired product was obtained.16 Given the need for selectivity and step efficiency, we surveyed methods that would lead directly and regiospecifically to the difluoro functionality. The Smiles rearrangement is an intramolecular nucleophilic substitution that occurs exclusively at the ipso-position of an activated aromatic leaving group, typically a sulfonate.17 The radical Smiles rearrangement has been demonstrated,18 and can proceed without the requirement for a strongly electron withdrawing group adjacent to the sulfonate. Tada et al. have shown an AIBN initiated (50 mol%) rearrangement using stoichiometric Bu3SnH, conditions not deemed applicable in our setting.19 Given the extensive literature detailing visible-light mediated reductive dehalogenation,1a,20 we were confident of developing a radical Smiles rearrangement from a difluorobromo sulfonate such as 4 (Scheme 1). Furthermore, given the wide availability of sulfonyl chlorides (>1000 commercially available) and the ready access to bromodifluoroethanol,16 we envisioned this as ageneral method for the synthesis of the difluoroethanol motif (Scheme 2).
Scheme 2.
Approaches to the difluoroethanol motif.
Scheme 1.
Previously reported route and the photochemical radical Smiles rearrangement design.
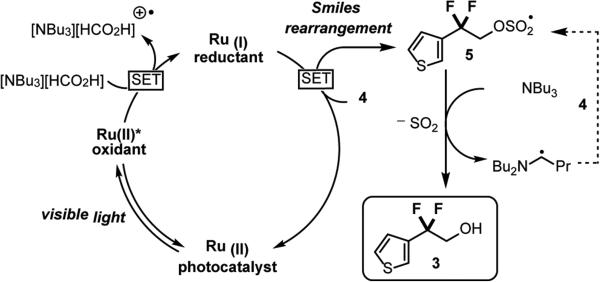
Our mechanistic design plan (Scheme 3) proceeds via quenching of the excited state of a photocatalyst such as Ru(bpy)32+ [E1/2II*/I = +0.77 V vs SCE] by a suitable electron donor (such as [NBu3][HCO2H]) to generate the strongly reducing Ru(I) species[E1/2II/I = −1.33 V vs SCE].21 Single electron reduction of thiophene 4 provides the desired difluoromethyl radical and regenerates the Ru(II) photocatalyst. The newly formed radical may then undergo intramolecular ipso-addition-elimination with the sulfonate functionality (Scheme 1) to give 5, with subsequent H-atom abstraction followed by polar extrusion of SO2 leading to the desired product.
Scheme 3.
Mechanistic proposal for the photocatalyzed radical Smiles rearrangement.
At this juncture, we cannot rule out the possibility of radical chain processes contributing to the formation of product, especially given the low photocatalyst loading and short reactions times for 6 (vide infra).22 These conditions (0.01 mol% initiator, 1 h) are in contrast to the classical chain process using Bu3SnH that requires significantly more initiator (AIBN 50 mol%), longer reaction times (22 h), and elevated temperature (80 °C).19 In our system, propagation may occur following H-atom abstraction from NBu3 by 5 to give an α-aminoalkyl radical which has previously been shown as a competent reductant of C-Br bonds.22b, 23
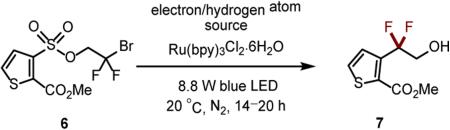
Initial studies employing sulfonate 4 yielded no observable rearrangement product, providing only moderate consumption of starting material amongst a complex mixture.16 Considering the structural features required for an efficient radical Smiles rearrangement,19 as well as both cost and availability of sulfonyl chlorides,24 we continued investigations with thiophene 6.25 Employing conditions similar to those previously developed for the reduction of activated alkyl bromides20(a) gave a promising 36% yield (Table 1, entry 1). Switching to DMSO as the solvent gave an improved yield of 53% without the need for degassing.26 The addition of formic acid—a known H-atom source—20(a) provided full consumption of the starting material (entry 3), and following a switch to NBu3 as the electron/H-atom source,20(b) the desired product 7 was formed in 86% yield (entry 4). Increased dilution gave full starting material consumption within 1 h (entry 5), even with 100-fold reduced catalyst loading (entry 6). A reaction conducted in the absence of photocatalyst returned unreacted 6 (entry 7).
Table 1.
Reaction performed at 0.14 M with 1.0 mol% photocatalyst without degassing unless otherwise stated.
| |||||
|---|---|---|---|---|---|
| electron/H-atom source | solvent | catalyst loading | 6 (%)a | 7 (%)a | |
| 1b | iPr2NEtc | DMA | 1.0 mol% | 43 | 36 |
| 2 | iPr2NEtc | DMSO | 1.0 mol% | 28 | 53 |
| 3 | iPr2NEtc formic acidc | DMSO | 1.0 mol% | <2 | 67 |
| 4 | NBu3 c formic acidc | DMSO | 1.0 mol% | <2 | 86 |
| 5d,e | NBu3 f formic acidf | DMSO | 1.0 mol% | <2 | 95 |
| 6d,e | NBu3f formic acidf | DMSO | 0.01 mol% | <2 | 94 (89)g |
| 7 | NBu3 f formic acidf | DMSO | none | >98 | <2 |
yield calculated via 19F NMR spectroscopic analysis of the crude reaction mixture relative to trifluorotoluene as the internal standard.
reaction degassed via freeze-pump-thaw.
2.5 equiv.
0.07 M.
reaction time 1 h.
1.5 equiv.
isolated yield. DMA (dimethylacetamide), DMSO (dimethyl sulfoxide).
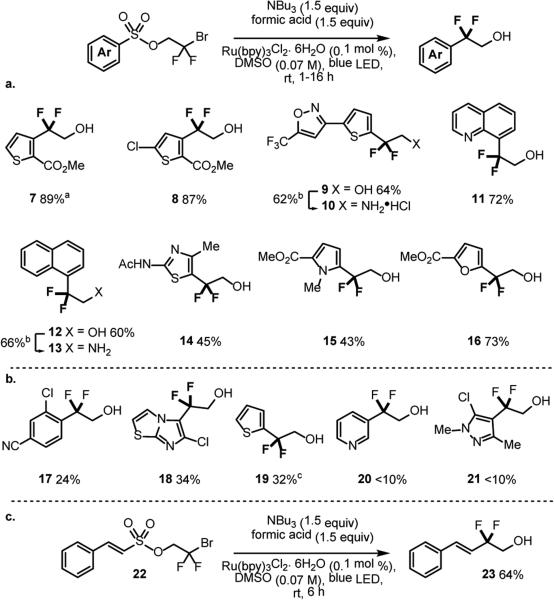
We next applied our optimized protocol to a range of other aromatic and heteroaromatic substrates (Scheme 4). Good yields were obtained for an alternative thiophene with application to the synthesis of 1 (Scheme 4, 8, 87%) as well as a more complex thiophene substrate (9, 64%). Extended aromatic systems proceeded efficiently, presumably due to a lower penalty associated with dearomatization upon radical ipso-addition (11 and 12, 72% and 60%). Other heterocyclic systems typically found in pharmaceutical targets such as thiazole 14, pyrrole 15, and furan 16, could be obtained in moderate to good yield (45%, 43%, and 73% respectively). We also explored the limits of this methodology and found that benzene derived substrates or heterocycles lacking radical stabilizing groups provided low to moderate yields (Scheme 4b, 17—21, <10%—34%).27 While the difluoroethanol motif is featured in a number of biologically active compounds such as Abediterol,28 it is also used as a precursor to the more common difluoroethylamine functionality.29 Access to this motif via a two-step trifylationamination sequence was demonstarted on representative Smiles products 9 and 12, providing the corresponding amines in good yield. Finally, we also applied the Smiles rearrangement to a single example of a vinyl sulfonate (Scheme 4c)18b in 64% yield to afford the vinyl difluoroethanol motif30 featured in prostaglandin analogue, Tafluprost.
Scheme 4.
Substrate Scope. [a] 0.01 mol% Ru(bpy)3Cl2 [b] Tf2O (1.1 equiv), Pyridine (1.6 equiv), MeCN 0 °C to rt then NH4OH (10 equiv), 50 °C. [c] yield calculated via 19F NMR spectroscopic analysis of the crude reaction mixture relative to trifluorotoluene as the internal standard.
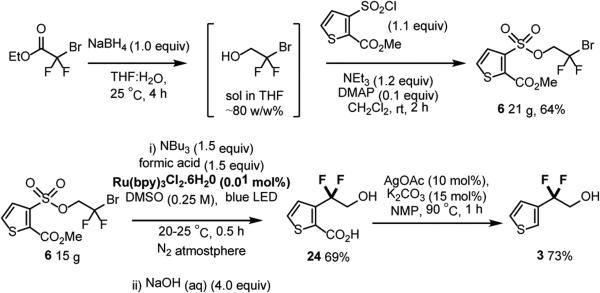
Following the establishment of this protocol as a general method, we returned to target compound 1. The starting material 6 could be readily obtained on >20 g scale, isolated via crystallization in 64% yield (Scheme 5). While the low catalyst loading and fast reaction time associated with the radical Smiles rearrangement of 6 are ideal from a synthetic perspective, the dilute reaction conditions limited the assessment of this reaction at larger scale. Conducting the reaction at increased concentrations (0.26 M) led to competitive formation of the sulfonic acid, resulting in consistently lower yield (70—75% by 19F NMR). This issue could be mitigated via a two-step, one-pot, photocatalyzed Smiles rearrangement ester hydrolysis on a 15 g scale, employing industrially relevant catalyst loadings (0.01 mol%), and yielding 69% of the thiophene carboxylic acid 24 without purification. The silver catalyzed decarboxylation31 proceeded to give the thiophene difluoroethanol 3 in 73% yield following distillation (Scheme 5).
Scheme 5.
Application to the synthesis of 3.
Efforts to realize the subsequent transformation of 3 to the spirocyclic thiophene 1 proved challenging, potentially due to the decreased nucleophilic character of the hydroxyl that is now proximal to a difluoro substituent.
We progressed by investigating the possibility of eliminating the downstream chlorination step by repeating the three-step sequence with the corresponding chlorinated sulfonyl chloride providing the target thiophene 26 in 28% overall yield.32 26 could be converted to the required spirocyclic material 1 via lithiation, addition to N-Boc-4-piperidinone 27 in 60% yield, followed by concurrent spirocyclization/Boc deprotection in 59% yield (Scheme 6). This unoptimized 5-step sequence addresses many of the undesirable features of the current synthetic route. Most importantly, the challenging benzylic fluorination can be accomplished by switching this key transformation to a C-C rather than C-F bond formation. This allows the use of ethyl bromodifluoroacetate to introduce the gem-difluoro, directly and regiospecifically accessing the difluoroethanol motif under mild conditions using low catalyst loadings.
Scheme 6.
Synthesis of spirocyclic thiophene 1.
Conclusion
In conclusion, we have developed a visible light-mediated radical Smiles rearrangement that addresses a current and pressing challenge associated with the synthesis of a key starting material for an ORL-1 antagonist. The method is general for other aromatic and heteroaromatic substrates, allowing rapid and efficient access to the benzylic difluoroethanol motif from widely available sulfonyl chlorides. Preliminary assessment of this methodology with regards to the target compound has been demonstrated on significant scale (15 g) and proceeds with short reaction times (as low as 0.5 h), with industrially relevant catalyst loadings (0.01 mol%). Furthermore, employing ethyl bromodifluoroacetate as a source of the benzylic difluoro group rather than the use of AIBN and Deoxo-Fluor® is highly desirable. Importantly, this study demonstrates the viability of photocatalysis to provide a strategic advantage in process development by enabling a new retrosynthetic disconnection and greater route flexibility. Ongoing research is aimed at assessing the wider utility of the photochemical Smiles rearrangement and application to the target on greater scale.
Supplementary Material
Footnotes
Research was supported by a Lilly Innovation Fellowship Award to J.J.D. from Eli Lilly and Company. Financial support for this research from the NIH-NIGMS (R01-GM096129), the Camille Dreyfus Teacher-Scholar Award Program, Eli Lilly, and the University of Michigan is gratefully acknowledged. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program to H. A. under Grant No. DGE 1256260. We thank Prof. D. Pratt (University of Ottawa) for helpful mechanistic discussion.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx.
References
- 1.For selected reviews see: Tucker JW, Stephenson CRJ. J. Org. Chem. 2012;77:1617–1622. doi: 10.1021/jo202538x.Prier CK, Rankic DA, MacMillan DWC. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r.Douglas JJ, Nguyen JD, Cole KP, Stephenson CRJ. Aldrichim. Acta. 2014;47:16–27.
- 2.a Shih H-W, VanderWal MN, Grange RL, MacMillan DWC. J. Am. Chem. Soc. 2010;132:13600–13603. doi: 10.1021/ja106593m. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chu L, Ohta C, Zuo Z, MacMillan DWC. J. Am. Chem. Soc. 2014;136:11602–11605. doi: 10.1021/ja505964r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Douglas JJ, Cole KP, Stephenson CRJ. J. Org. Chem. 2014;79:11631–11643. doi: 10.1021/jo502288q. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Miyazawa K, Koike T, Akita M. Adv. Synth. Catal. 2014;13:2749–2755. [Google Scholar]; e Chu L, Lipshultz JM, MacMillan DWC. Angew. Chem., Int. Ed. 2015;54:7929–7933. doi: 10.1002/anie.201501908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeBaillie AC, Jones CD, Magnus NA, Mateos C, Torrado A, Wepsiec JP, Tokala R, Raje P. Org. Process Res. Dev. 2014 DOI: 10.1021/op5000094. [Google Scholar]
- 4.Collado ABB, Buezo ND, Jimenez-Aguado AM, Blanco CL, Martinez-Grau MA, Pedregal-Tercero C, Escribano MAT. 2012 Jul 31; U.S. Patent 8,232,289 B2.
- 5.Bis(2-methoxyethyl)aminosulfur trifluoride (Deoxo-Fluor®) is a fuming liquid that reacts violently with water to generate HF.
- 6.Champagne PA, Desroches J, Hamel J-D, Vandamme M, Paquin J-F. Chem. Rev. 2015 doi: 10.1021/cr500706a. DOI: 10.1021/cr500706a. [DOI] [PubMed] [Google Scholar]
- 7.a Hull KL, Anani WQ, Sanford MS. J. Am. Chem. Soc. 2006;128:7134–7135. doi: 10.1021/ja061943k. [DOI] [PubMed] [Google Scholar]; b Bloom S, Pitts CR, Miller DC, Haselton N, Holl MG, Urheim E, Lectka T. Angew. Chem., Int. Ed. 2012;51:10580–10583. doi: 10.1002/anie.201203642. [DOI] [PubMed] [Google Scholar]; c Bloom S, Pitts CR, Woltornist R, Griswold A, Holl MG, Lectka T. Org. Lett. 2013;15:1722–1724. doi: 10.1021/ol400424s. [DOI] [PubMed] [Google Scholar]; d Liu W, Groves JT. Angew. Chem., Int. Ed. 2013;52:6024–6027. doi: 10.1002/anie.201301097. [DOI] [PubMed] [Google Scholar]
- 8.a Amaoka Y, Nagatomo M, Inoue M. Org. Lett. 2013;15:2160–2163. doi: 10.1021/ol4006757. [DOI] [PubMed] [Google Scholar]; b Xu P, Guo S, Wang L, Tang P. Angew. Chem., Int. Ed. 2014;53:5955–5958. doi: 10.1002/anie.201400225. [DOI] [PubMed] [Google Scholar]; c Bloom S, McCann M, Lectka T. Org. Lett. 2014;16:6338–6341. doi: 10.1021/ol503094m. [DOI] [PubMed] [Google Scholar]
- 9.a Xia J-B, Zhu C, Chen C. J. Am. Chem. Soc. 2013;135:17494–17500. doi: 10.1021/ja410815u. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cantillo D, de Frutos O, Rincón JA, Mateos C, Kappe CO. J. Org. Chem. 2014;79:8486–8490. doi: 10.1021/jo5016757. [DOI] [PubMed] [Google Scholar]
- 10.Ethyl bromodifluoroacetate CAS [667-27-6] is available at approximately $100/kg.
- 11.a Belhomme M-C, Poisson T, Pannecoucke X. J. Org. Chem. 2014;79:7205–7211. doi: 10.1021/jo5010907. [DOI] [PubMed] [Google Scholar]; b Shao C, Shi G, Zhang Y, Pan S, Guan X. Org. Lett. 2015;17:2652–2655. doi: 10.1021/acs.orglett.5b01024. [DOI] [PubMed] [Google Scholar]
- 12.a Fujikawa K, Fujioka Y, Kobayashi A, Amii H. Org. Lett. 2011;13:5560–5563. doi: 10.1021/ol202289z. [DOI] [PubMed] [Google Scholar]; b Feng Z, Min Q-Q, Xiao Y-L, Zhang B, Zhang X. Angew. Chem., Int. Ed. 2014;53:1669–1673. doi: 10.1002/anie.201309535. [DOI] [PubMed] [Google Scholar]
- 13.a Murakami S, Ishii H, Tajima T, Fuchigami T. Tetrahedron. 2006;62:3761–3769. [Google Scholar]; b Ohtsuka Y, Yamakawa T. Tetrahedron. 2011;67:2323–2331. [Google Scholar]; c Lin Q, Chu,1 L, Qing F-L. Chin. J. Chem. 2013;31:885–891. [Google Scholar]; d Jung J, Kim E, You Y, Cho EJ. Adv. Synth. Catal. 2014;356:2741–2748. [Google Scholar]
- 14.a Taguchi T, Kitagawa O, Morikawa T, Nishiwaki T, Uehara H, Endo H, Kobayashi Y. Tetrahedron Lett. 1986;27:6103–6106. [Google Scholar]; b Sato K, Kawata R, Ama F, Omote M, Ando A, Kumadaki I. Chem. Pharm. Bull. 1999;47:1013–1016. doi: 10.1248/cpb.47.1326. [DOI] [PubMed] [Google Scholar]
- 15.Presumably this occurred through the migration of an aryl copper intermediate.
- 16.See SI for further information.
- 17.a Levy AA, Rains HC, Smiles S. J. Chem. Soc. 1931:3264–3269. [Google Scholar]; b Matsui K, Maeno N, Suzuki S, Shizuka H, Morita T. Tetrahedron Lett. 1970;11:1467–1469. [Google Scholar]; c Wang Z. Comprehensive Organic Name Reactions and Reagents. John Wiley & Sons, Inc.; p. 2010. [Google Scholar]
- 18.Loven R, Speckamp WN. Tetrahedron Lett. 1972;13:1567–1570.Motherwell WB, Pennell AMK. J. Chem. Soc., Chem. Commun. 1991:877–879.Studer A, Bossart M. Tetrahedron. 2001;57:9649–9667.Chen Z-M, Zhang X-M, Tu Y-Q. Chem. Soc. Rev. 2015;44:5220–5245. doi: 10.1039/c4cs00467a. More recent reports from Nevado have utilized radical formation, aryl migration, desulfonylation as a versatile sequence in array of cascade processes. For lead references showing trifluoromethylation see Kong W, Casimiro M, Merino E, Nevado C. J. Am. Chem. Soc. 2013;135:14480–14483. doi: 10.1021/ja403954g.Kong W, Casimiro M, Fuentes N, Merino E, Nevado C. Angew. Chem., Int. Ed. 2013;52:13086–13090. doi: 10.1002/anie.201307377.
- 19.Tada M, Shijima H, Nakamura M. Org. Biomol. Chem. 2003;1:2499–2505. doi: 10.1039/b303728b. [DOI] [PubMed] [Google Scholar]
- 20.a Narayanam JMR, Tucker JW, Stephenson CRJ. J. Am. Chem. Soc. 2009;131:8756–8757. doi: 10.1021/ja9033582. [DOI] [PubMed] [Google Scholar]; b Nguyen JD, D'Amato EM, Narayanam JMR, Stephenson CRJ. Nat. Chem. 2012;4:854–859. doi: 10.1038/nchem.1452. [DOI] [PubMed] [Google Scholar]
- 21.Kalyanasundaram K. Coord. Chem. Rev. 1982;46:159–244.. The catalytic cycle may also proceed via oxidative quenching of Ru(bpy) 2+*3 by 4.
- 22.For an in-depth analysis of chain process in visible light photocatalysis see Cismesia MA, Yoon TP. Chem. Sci. DOI:10.1039/x0xx00000x.McTiernan CD, Pitre S,P, Scaiano JC. ACS Catal. 2014;4:4034–4039.
- 23.a Ismaili H, Pitre S,P, Scaiano JC. Catal. Sci. Technol. 2013;3:935–937. [Google Scholar]; b Pitre S,P, McTiernan CD, Ismaili H, Scaiano JC. ACS Catal. 2014;4:2530–2535. [Google Scholar]
- 24.2-carbomethoxy-3-thiophenesulfonyl chloride is available at approximately $250-300/Kg.
- 25.We anticipated that the unwanted methylester at the two-position could be readily hydrolyzed and decarboxylated.
- 26.A reaction left open to air gave 24% of the desired product after a prolonged (20 h) reaction time.
- 27.In cases where the Smiles rearrangement did not proceed efficiently, unproductive loss of starting material was observed as well as premature H-atom abstraction yielding the reduced product.
- 28.Aparici M, Gómez-Angelats M, Vilella D, Otal R, Carcasona C, Viñals M, Ramos I, Gavaldà A, De Alba J, Gras J, Cortijo J, Morcillo E, Puig C, Ryder H, Beleta J, Miralpeix M. J. Pharmacol. Exp. Ther. 2012;342:497–509. doi: 10.1124/jpet.112.193284. [DOI] [PubMed] [Google Scholar]
- 29.This strategy was employed by Merck and Co. on multi Kg scale in the synthesis of a thrombin inhibitor Org. Process Res. Dev. 2004;8:192–200.
- 30.For a recent approach to this motif via Pd catalysis see Feng Z, Min Q-Q, Zhao H-Y, Gu J-W, Zhang X. Angew. Chem., Int. Ed. 2015;54:1270–1274. doi: 10.1002/anie.201409617.
- 31.Gooßen LJ, Rodríguez N, Linder C, Lange PP, Fromm A. ChemCatChem. 2010;2:430–442. [Google Scholar]
- 32.Although the current yield through these steps is low, it is largely a result of the Smiles rearrangement hydrolysis step (48% on a single attempt at 5 g scale at 0.5 M)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.