Abstract
This study examined the types of physical activity (PA) retirement community residents report and the effects of PA and depressive symptoms on functional limitations. Elders (N = 38) enrolled in a 2-year sensor technology study in senior housing completed regular assessments of functional limitations and depressive symptoms with the Short Physical Performance Battery and Geriatric Depression Scale, respectively. Evaluation of reported PA using the Physical Activity Scale for the Elderly coincided with 12-month functional limitation testing. Subjects were 69% female with mean age of 85 years. Individuals reporting greater PA had significantly fewer functional limitations at 12 months. In multiple regression analysis, baseline functional limitations explained 66% of the variance in 12-month functional limitations, while current PA explained an additional 5%. Although PA explained a small amount of variance in 12-month functional limitations, as a modifiable behavior, PA should be championed and supported to help ameliorate functional limitations in older adults.
Keywords: exercise, older adults, physical activity, physical function, retirement community
Extensive epidemiological and intervention research indicate that regular physical activity (PA) helps maintain physical function and avoid disability in later life (Boyle, Buchman, Wilson, Bienias, & Bennett, 2007; McArdle, Katch, & Katch, 2010; Morey et al., 2008; Pahor et al., 2006). A recent meta-analysis of exercise intervention trials in frail older adults found that exercise conferred significant benefits in gait speed, balance, and activities of daily living (ADLs; Chou, Hwang, & Wu, 2012). Despite the compelling evidence for the protective effects of PA on the preservation of functional abilities, national surveys show that the large majority of adults aged 65 and older remain inactive and fail to meet the recommended levels of PA (Centers for Disease Control and Prevention, 2012). National surveys, however, do not represent elders living in retirement communities and other long-term care settings; thus, little is known about the PA behavior of this group of older adults. Among the few studies of PA behavior of retirement community residents, results vary widely. Scores on the Physical Activity Scale for the Elderly (PASE) (Washburn, Smith, Jette, & Janney, 1993) are generally lower than PASE scores for community-dwelling adults (Baker et al., 2007; Harada, Chiu, King, & Stewart, 2001; Schuit, Schouten, Westerterp, & Saris, 1997; Washburn et al., 1993; Zalewski, Smith, Malzahn, VanHart, & O’Connell, 2009). Pedometer and activity-monitor step counts in retirement community residents show great variation, ranging from approximately 3,000 to 8,000 steps per day (Cress, Orini, & Kinsler, 2011; Snyder, Colvin, & Gammack, 2011; Zalewski et al., 2009). Recent research suggests that the extent to which retirement communities encourage and support PA influences residents’ level of PA (Bjornsdottir, Arnadottir, & Halldorsdottir, 2012; Harris-Kojetin, Kiefer, Joseph, Arch, & Zimring, 2005).
This article describes the PA practices of a group of retirement community residents and the relationships among their PA, depressive symptoms, and functional limitations. Strategies to promote PA in older adults are discussed.
Background
PA, defined as bodily movement produced by skeletal muscles that results in energy expenditure, limits the impact of age-related biological changes through its effect on chronic disease development and preservation of functional capacity (Chodzko-Zajko et al., 2009). Although a 40% to 50% loss in muscle mass commonly occurs between the ages of 25 and 80 even among healthy, physically active adults, improvement in muscle composition and strength, gait, and balance have been observed well into the ninth decade of life in response to vigorous resistance exercise training (Fiatarone et al., 1994; Judge, Underwood, & Gennosa, 1993; McArdle et al., 2010; Simons & Andel, 2006). In addition, aerobic exercise has been shown to combat the normal age-related losses in cardiovascular function. Meta-analyses of moderate-intensity endurance-training interventions found significant effects on older adult’s maximal oxygen consumption, resting heart rate, and systolic blood pressure (Huang, Gibson, Tran, & Osness, 2005; Huang, Shi, Davis-Brezette, & Osness, 2005). Vaitkevicius and colleagues (2002) demonstrated that frail elders aged 80 and older can improve aerobic capacity and lower blood pressure following 6 months of regular moderate-intensity aerobic exercise. Thus, although age inevitably leads to decrements in muscle strength, neuromuscular function, and cardiovascular fitness, strong evidence shows that regular PA slows the decline in functional capacity associated with aging and disuse (McArdle et al., 2010).
PA has also been associated with lower risk of depression in older adults, and both aerobic-exercise training and resistance-exercise training have been shown to improve overall psychological well-being (Chodzko-Zajko et al., 2009; Rosenberg, Bombardier, Artherholt, Jensen, & Motl, 2013). When present, depression has both immediate and long-term detrimental effects on physical function and subsequent disability (Russo et al., 2007; van Gool et al., 2005). Moreover, increases in functional limitations over time are associated with concurrent increases in depression, especially among elders of lower socioeconomic status (Schieman & Plickert, 2007). Thus, there appears to be a cyclic relationship between depression and physical function that PA may play a role in moderating.
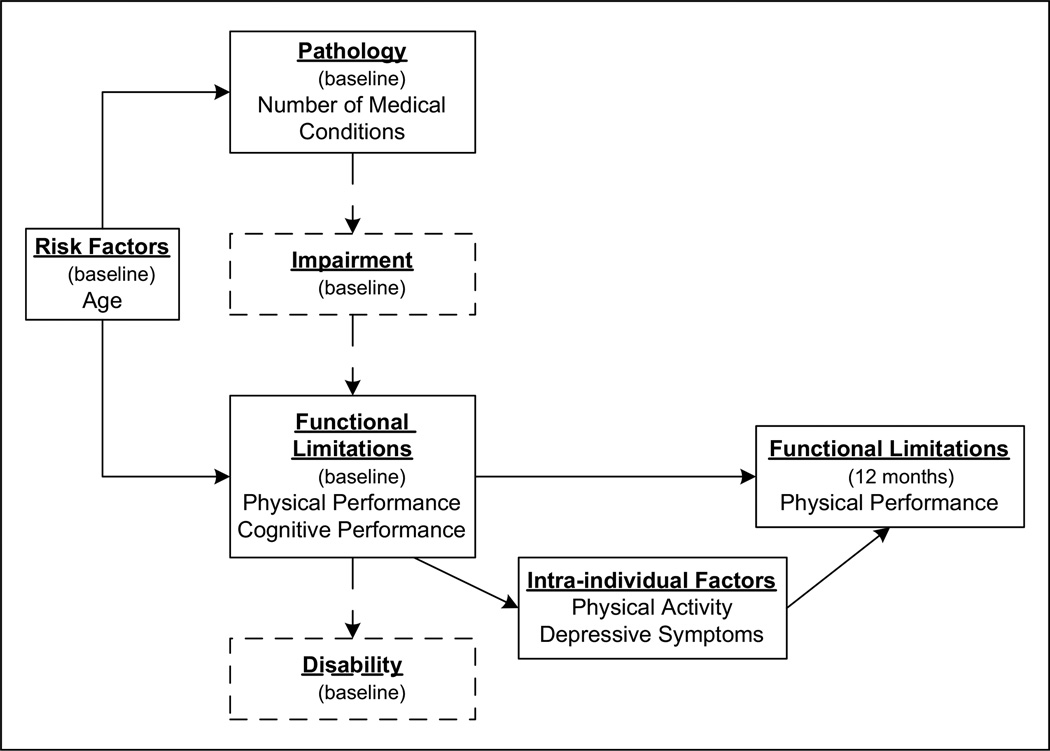
Research on PA in the older adult population frequently uses the disablement process framework as a guiding conceptual model (Hall & McAuley, 2011; Jette, 2003; Motl & McAuley, 2010; Pahor et al., 2006). Originally conceived as a four-stage model in which all disability originates from disease or pathology (with pathology leading to impairment, impairment to functional limitations, and functional limitations to disability), subsequent modifications to the Nagi Disablement Model posit that in addition to pathology, disuse and physiological aging can independently begin the process of decline (Nagi, 1991; Rikli & Jones, 1999; Stewart, 2003). Verbrugge and Jette (1994) extended the four-stage model to include risk factors (e.g., sociodemographic background and biologic factors), intra-individual factors (e.g., lifestyle behaviors and psychosocial attributes), and extra-individual factors (e.g., physical and social environments) that influence the disablement process. PA behavior is hypothesized to work by directly minimizing impairments and functional limitations, thereby reducing or preventing downstream disability (Jette & Keysor, 2003). Positive psychosocial attributes, such as emotional vigor, high self-efficacy, and peer support, may slow the disablement process by beneficial effects on functional limitations and perception of disability, whereas negative attributes, such as depression, may accelerate the progression of disability (van Gool et al., 2005). Finally, because disability is not inherent in the person but rather the product of the interaction of the person with the environment, as an extra-individual factor, the built environment may ameliorate disability via structural accommodations that support performing daily-life activities (e.g., job, household management, and personal care).
Retirement communities represent an environment built specifically to accommodate the needs of older adults with reduced functional capacity. With their smaller living space and closer proximity of services, retirement communities reduce the physical demands of daily life (Cress et al., 2011). Nationally, an estimated 745,000 older adults live in continuing care retirement communities (CCRCs), a popular option for seniors who desire a range of housing levels, supportive services, and health-care options (Kohl, 2010). Although older adults may purposely select retirement-type residences, PA levels may inadvertently decline as a factor of lower environmental demands. On the one hand, retirement-community-built environments may hasten the disablement process by enabling sedentary behavior; on the other hand, retirement communities may delay disablement by designing buildings to facilitate mobility. Furthermore, as a social environment, retirement communities may speed up or slow down disablement through the allocation of resources for PA programs, the structure and types of PA offered, and visible promotion of peer-exercise networks.
Guided by the disablement process framework, the purpose of this study was to examine the types of PA in which retirement community residents engage and to estimate the effect of two intra-individual factors, reported PA and depressive symptoms, on functional limitations, while controlling for age, medical conditions, cognitive status, and past functional limitations. Figure 1 illustrates the framework for the present study. The main disablement pathway was re-oriented from the traditional horizontal axis to the vertical axis so that the change from Baseline to 12-month functional limitations could be depicted horizontally (see Figure 1). Solid lines outline the disablement components included in the present study, whereas components outlined in dashed lines were not evaluated.
Figure 1. Disablement model adapted from a model of the disablement process.
Source. Verbrugge and Jette (1994)
Note. Concepts and relationships analyzed in the present study are represented by solid lines.
Methods
Study Design
The study uses a correlational, repeated-measures design to determine the effect of PA and depressive symptoms on functional limitations. The present study is a substudy of a 2-year longitudinal investigation of motion sensor capacities, Technology to Automatically Detect Early Signs of Illness in Senior Housing. The design of the parent study has been described in detail elsewhere (Rantz et al., 2012). Briefly, the site of the study, TigerPlace, is an independent-living retirement community that supports older adults aging in place, including those with declining health status. In the first year of the study, team members collected and analyzed sensor data from previously installed in-home monitors to develop and refine a web-based system that could alert clinicians to changes in activity patterns. During the second year of the study, the research team received data-driven web-based alerts to validate the accuracy of the alert algorithms and rate the usability of the display interface. A variety of outcomes were measured quarterly for 20 participants who agreed to installation and testing of the sensor network as well as for 22 control participants. The university Institutional Review Board approved the parent study as well as the present sub-study. All participants provided written informed consent.
Sample and Setting
All residents of TigerPlace who were able to participate in quarterly assessments of physical performance were invited to enroll in the parent study. At the time of initial recruitment, 42 of the 64 persons living at TigerPlace agreed to participate. At the 12-month data-collection visit, 4 of the original 42 participants had withdrawn from the study; 1 control participant died immediately after baseline data collection, and 3 participants, 1 control and 2 treatment, moved to other residences. The final sample consisted of 26 women and 12 men. Except for 1 Asian participant, all were non-Hispanic White.
Whether participating in research or not, all residents of TigerPlace undergo a comprehensive health assessment at admission and every 6 months. Residents’ health information—which includes but is not limited to demographic data, medical conditions, medications, vital signs, and assessment of cognitive function, depressive symptoms, quality of life, fall risk, and ADLs—is stored in an electronic health record. In addition, all TigerPlace residents receive care coordination services and have access to a nurse-managed wellness center. Personal-care and medication-management services may be purchased on an as-needed basis. Supervised exercise classes, including Tai Chi, yoga, and therapeutic dance, are offered 5 days a week at no additional charge. Exercise equipment—including a treadmill, recumbent stepper, rowing machine, exercise bicycle, and hand weights—is available for unsupervised use. TigerPlace also has a network of well-maintained sidewalks, gardens, and parking lots that facilitate outdoor walking, environmental features found to be associated with PA behavior among older adults in retirement communities (Booth, Owen, Bauman, Clavisi, & Leslie, 2000; Joseph & Zimring, 2007; Joseph, Zimring, Harris-Kojetin, & Kiefer, 2005). The TigerPlace building is free of potential barriers to PA, such as heavy doors without automation, stairs, and curbs (Pomeroy et al., 2011). A complete description of TigerPlace and the sensor network technology driving the 2-year longitudinal study has been previously published (Rantz et al., 2008; Rantz et al., 2011; Skubic, Alexander, Popescu, Rantz, & Keller, 2009).
Procedures
Participants in the parent study completed baseline and quarterly assessments of physical performance with the Short Physical Performance Battery (SPPB) (Guralnik et al., 1994), the GAITRite (Apparatus and software, Sparta, NJ: CIR Systems) gait analysis mat, and a hydraulic hand dynamometer during the second year of the 2-year study. In the present study, the principal investigator or a research nurse administered the PASE questionnaire in face-to-face interview on the same day as 12-month SPPB testing. Other baseline measures included a health history, medication use, and vital signs. Health-event outcomes, such as emergency-room visits and falls, were also tallied quarterly for the parent study. As standard of care at TigerPlace, the Geriatric Depression Scale (GDS), Mini Mental Status Exam (MMSE), SF12 Health and Mental Health scales, fall risk, and ADL status were assessed bi-annually. Only data relevant to the present study were included in this report.
Data-Collection Instruments
Functional limitations: Cognitive performance
The MMSE is an 11-item measure of global cognitive function, including orientation, attention, immediate and short-term recall, language, and ability to follow simple verbal and written commands (Folstein, Folstein, & McHugh, 1975). Scores can range from 0 to 30; scores below 24 indicate cognitive impairment (Barrie, 2002). Twenty-eight day test-retest reliability was .98 (Folstein et al., 1975). Concurrent validity was established by strong correlations with the Verbal IQ test (r = .776, p < .0001) and the Performance IQ test (r = .660, p < .001; Folstein et al., 1975).
Functional limitations: Physical performance
The SPPB evaluates lower extremity physical function by testing balance (side-by-side, semi-tandem, and tandem), walking speed (4-meter walk), and repeated chair stands (Guralnik et al., 1994). Each test is rated on a 5-point categorical score, with 0 representing inability to perform the test and 4 indicating the highest level of physical performance. Summing the three test scores provides a total performance score ranging from 0 to 12. The SPPB has shown adequate internal consistency (Cronbach’s α was .76) and predictive validity for nursing home admission and mortality outcomes (Guralnik et al., 1994). The SPPB was administered quarterly during the parent study, in accordance with instructions available on the National Institute on Aging website: http://www.grc.nia.nih.gov/branches/ledb/sppb/index.htm (National Institute on Aging and National Institutes of Health, 2012). Cronbach’s alpha in the present study ranged from .76 for baseline SPPB to .78 for 12-month SPPB.
Intra-individual factors: Depressive symptoms
The Geriatric Depression Scale-15 (GDS-15) is a self-report scale that solicits yes/no responses to simple questions about the respondent’s feelings during the past week; scores range from 0 to 15, with higher scores indicating greater depressive symptoms (Sheikh & Yesavage, 1986). GDS-15 scores ≥ 6 indicate the presence of depressive symptoms. The GDS-15 has demonstrated adequate internal consistency reliability (Cronbach’s α of .79) and good sensitivity and specificity in discriminating cases of major depression (area under the curve [AUC] = .896) and minor depression (AUC = .778; Smalbrugge, Jongenelis, Pot, Beekman, & Eefsting, 2008). In the present study, Cronbach’s alpha was .84. Facility nursing staff administered the GDS-15 as standard of care at Tiger-Place. Recent GDS assessment was defined as that obtained within 6 months prior to 12-month SPPB testing.
Intra-individual factors: PA
The PASE is a scale for evaluating the PA level of older adults (Washburn et al., 1993). It relies on subjective recall of PA over the past 7-day period. The PASE can be self-administered, telephone-administered, or interviewer-administered and takes 5 min to 10 min to complete. Items on the scale that address walking outside the home, muscle strengthening, and light, moderate, and vigorous sport and recreation are recorded as never, seldom (1–2 days/week), sometimes (3–4 days/week), and often (5–7 days/week), and duration of participation is estimated at less than 1 hr, 1 hr to 2 hr, 2 hr to 4 hr, and more than 4 hr. Occupational activity is recorded in total hours/week. Housework (light and heavy), home repair, family care, yard work, and gardening are recorded as yes/no. The total PASE score is calculated by (1) multiplying the activity frequency value (i.e., hours/week) for each activity by the respective empirically derived item weight and summing these values; and (2) to this summated score, adding the weighted score for the six household activities if the activity was reported over the past week. Higher scores indicate higher levels of PA. Three- to seven-week test-retest reliability was r = .75 (Washburn et al., 1993). Validity testing with activity monitors and physical performance testing showed significant correlations between PASE scores and activity counts (r = .55, p < .05), SPPB scores (r = .57, p < .01), and the 6-min walk (r = .54, p < .01).
Medical conditions
A summed score was derived from medical conditions recorded in the electronic health record at 12-month data collection (Bennett, Stewart, Kayser-Jones, & Glaser, 2002). Possible scores could range from 0 to 8. Qualifying conditions included (1) arthritis or joint problems; (2) osteoporosis; (3) kidney or liver disease; (4) asthma, chronic bronchitis, or emphysema; (5) hypertension; (6) diabetes; (7) congestive heart failure or heart trouble; and (8) paralysis, stroke, Parkinson’s, or other neurological problems. In the present study, the diagnosis of dementia was considered a neurological problem.
Data Analysis
All data analyses were conducted using SPSS Version 18. The mean of each PASE component and its weighted contribution to the total mean PASE score were computed. Two of the 38 participants were missing a recent GDS assessment and therefore were excluded from analyses involving the GDS score. No other variables had missing data. Bivariate correlational analyses identified variables to retain in subsequent analyses, that is, those whose correlations with PASE or 12-month SPPB score were significant at p < .1. Hierarchical multiple regression analysis with listwise deletion was used to determine the contribution of statistically significant explanatory variables to 12-month SPPB score. A hierarchical approach was chosen to test the effect of PASE score on 12-month SPPB score independent of the influence of other predictor variables. All independent variables were centered at their means for the regression analysis.
Scatterplots and bivariate correlations confirmed linear relationships among the variables included in the regression model. The assumption of normality was verified with histograms of standardized residuals and Q-Q plots of residuals (Norušis, 2005). Skewness, kurtosis, and Shapiro-Wilks tests were all within an acceptable range. A Durbin-Watson statistic of 2.2 verified independence of observations. Plots of residuals versus predicted values were inspected for evidence of heteroscedasticity for each explanatory variable (Keith, 2006). The variance in errors appeared to be consistent across all levels of the dependent variable. Tolerance statistics ranged from .743 to .835, indicating minimal collinearity among the predictor variables (Keith, 2006).
Results
Participant Characteristics
Table 1 provides an overall description of the sample. The most common medical conditions were hypertension and neurological diseases. About half of the sample had hypertension, which is less than in the general population where 72% of men and 80% of women aged 75 and older have this diagnosis (American Heart Association, 2013). Although not captured in the medical conditions list, 3 of the 38 residents had a diagnosis of depressive disorder. A dementia diagnosis accounted for 10 of the 14 participants noted to have a neurological problem, but only 3 of the 38 participants had MMSE scores indicative of moderate dementia (i.e., <20). MMSE scores were not corrected for age or education; thus, the mean MMSE score of 26.45 suggests that the majority of participants were cognitively intact. Nineteen of the 38 participants purchased personal-care and/or medication-management services.
Table 1.
Description of Sample.
| Variables | Total sample (N = 38) |
|---|---|
| Mean age | 85.0 (SD = 7.45) |
| Gender | |
| Male | 12 (31.6%) |
| Female | 26 (68.4%) |
| Race | |
| White | 37 (97.4%) |
| Marital status | |
| Married | 13 (34.2%) |
| Widowed | 25 (65.8%) |
| Mini-mental status exam mean score | 26.45 (SD = 4.3) |
| Mean number of medical conditions | 1.66 (SD = 0.91) |
| Medical conditions distribution | |
| Hypertension | 18 (47.4%) |
| Neurological diseases | 14 (36.8%) |
| Osteoporosis | 9 (23.7%) |
| Cardiac disease | 8 (21.1%) |
| Arthritis | 6 (15.8%) |
| Diabetes | 6 (15.8%) |
| Kidney or liver disease | 1 (2.6%) |
| Asthma/chronic obstructive pulmonary disease | 1 (2.6%) |
Descriptive Statistics of Main Variables
Table 2 presents the total PASE score distribution. The mean PASE score for the entire sample was 42.62 ± 38.4 (range = 0–159), reflecting relatively low levels of PA. Walking (10.6 points) and light housework (15.13 points) contributed the majority (60%) of the points to the total PASE score.
Table 2.
Contribution of PASE Component to Total PASE Score.
| PASE component | Sample means |
Weight | Contribution to total PASE score |
|---|---|---|---|
| Walking (h/d) | .53 | 20 | 10.6 |
| Light recreation/sport (h/d) | .04 | 21 | 0.84 |
| Moderate recreation/sport (h/d) | .02 | 23 | 0.46 |
| Strenuous recreation/sport (h/d) | .02 | 23 | 0.46 |
| Muscular strength/endurance (h/d) | .06 | 30 | 1.8 |
| Job standing or walking (h/d) | .02 | 21 | 0.42 |
| Light housework (% reporting “yes”) | .605 | 25 | 15.125 |
| Heavy housework (% reporting “yes”) | .158 | 25 | 3.95 |
| Home repair (% reporting “yes”) | 0 | 30 | 0 |
| Lawn work/yard care (% reporting “yes”) | .026 | 36 | 0.936 |
| Outdoor gardening (% reporting “yes”) | .263 | 20 | 5.26 |
| Caring for another person (% reporting “yes”) | .079 | 35 | 2.765 |
Note. PASE = Physical Activity Scale for the Elderly.
The total SBBP score declined from 6.16 (3.2) at baseline to 5.71 (3.1) at 12 months, but this change was not statistically significant, t(37) = 1.515, p = .138. Scores on the component chair stand and walking tests had similar declines over the 12-month period. These data are not reported but will be provided on request. The mean GDS score of 3.08 (3.1) indicated minimal depressive symptoms among participants.
Correlational Analyses
Table 3 presents the means, standard deviations, ranges, and Pearson correlations for continuous variables. As expected, 12-month SPPB score was strongly correlated with PASE and baseline SPPB scores. GDS score was significantly correlated only with PASE scores (r = –.43, p < .05). Because age, MMSE, and medical conditions were not significantly associated with either PASE or 12-month SPPB scores, they were not entered in the regression analysis. Therefore, 12-month SPPB score was regressed on baseline SPPB score in Step 1, (recent) GDS score in Step 2, (current) PASE score in Step 3, and GDS-PASE interaction term in Step 4. The order of entry was based on the expectation that PASE score would explain additional variance in 12-month SPPB score beyond that explained by baseline SPPB and GDS scores, and that PASE score may vary according to GDS score. Baseline SPPB and current PASE were significant predictors, explaining 73.5% of the variance in 12-month SPPB score. Table 4 displays the results of this regression analysis and the second model described below.
Table 3.
Summary of Intercorrelations, Means, and Dispersion for Age, Comorbidities, and Scores on the SPPB, MMSE, GDS, and PASE at Baseline and 12 months.
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | M | SD | Range |
|---|---|---|---|---|---|---|---|---|---|
| 1. Age, baseline | — | 85.0 | 7.5 | 64–96 | |||||
| 2. Co-morbidities | .01 | — | 1.66 | .91 | 0–4 | ||||
| 3. SPPB, baseline | −.02 | −.08 | — | 6.18 | 3.2 | 1–11 | |||
| 4. MMSE, 12 month | −.20 | .00 | .09 | — | 26.45 | 4.3 | 15–30 | ||
| 5. GDSa | .20 | −.19 | −.28 | .04 | — | 3.08 | 3.1 | 0–12 | |
| 6. PASE | −.07 | −.19 | .39* | .16 | −.43* | 42.62 | 38.4 | 0–159 | |
| 7. SPPB 12 month | −.05 | −.06 | .81*** | .16 | −.32 | .52** | 5.71 | 3.1 | 0–12 |
Note. For all scales, higher scores are indicative of more extreme responses in the direction of each construct. SPPB = Short Physical Performance Battery; MMSE = Mini Mental State Examination; GDS = Geriatric Depression Scale; PASE = Physical Activity Scale for the Elderly.
n = 36.
p < .05.
p < .01.
p < .001.
Table 4.
Hierarchical Multiple Regression Analyses Predicting 12-Month SPPB From Baseline SPPB, Recent GDS, and Current PASE Scores.
| Predictor | ΔR2 | β |
|---|---|---|
| Model 1 (n = 36) | ||
| Step 1 | .677*** | |
| Baseline SPPB | .823*** | |
| Step 2 | .008 | |
| Baseline SPPB | .796*** | |
| Recent GDS | −.094 | |
| Step 3 | .045* | |
| Baseline SPPB | .725*** | |
| Recent GDS | −.009 | |
| Current PASE | .247* | |
| Step 4 | .004 | |
| Baseline SPPB | .712*** | |
| Recent GDS | .048 | |
| Current PASE | .313* | |
| GDS × PASE | .092 | |
| Total R2 | .735*** | |
| Model 2 (n = 38) | ||
| Step 1 | .660*** | |
| Baseline SPPB | .812*** | |
| Step 2 | .051* | |
| Baseline SPPB | .717*** | |
| Current PASE | .246* | |
| Total R2 | .711*** |
Note. SPPB = Short Physical Performance Battery; GDS = Geriatric Depression Scale; PASE = Physical Activity Scale for the Elderly; β = beta weights.
p < .05.
p < .001.
A second, more parsimonious, hierarchical model that included only the significant predictors from the first regression model was run. The sample size for the second model included the total sample of 38 cases because neither baseline SPPB nor current PASE had missing data. In Step 1, baseline SPPB accounted for 66% of the variance in 12-month SPPB when entered alone into the equation. When current PASE was entered in the second step, the model accounted for 71.1% of the variance in 12-month SPPB. It appears that PASE score explained an additional 5% of the variance in 12-month SPPB scores. Thus, persons with fewer functional limitations at baseline (higher SPPB scores) reported significantly greater PA (higher PASE scores) and demonstrated significantly fewer functional limitations at 12 months.
Discussion
Most elders in the present study reported that walking and light housework were their primary type of PA, a finding consistent with Zalewski and colleagues’ (2009) results. Despite daily opportunities for supervised recreational PA and access to exercise equipment preferred by older adults (e.g., recumbent stepper), participants in the present study chose walking over other types of PA (Looney & Rimmer, 2003). For persons in their mid 80s, walking may represent the most familiar and comfortable type of PA. In addition, the PASE scores of the participants in the present study were low in comparison with published reports of other retirement community residents, including those of similar age (Baker et al., 2007; Harada et al., 2001; Zalewski et al., 2009). Washburn et al. (1993) reported that PASE scores ranged from 0 to 360 in the sample of community-dwelling elders in which the PASE was developed and validated, but mean PASE scores have ranged from 50 to 136 in other studies of retirement home residents (Baker et al., 2007; Harada et al., 2001; Zalewski et al., 2009). Considering that half of the participants in the present study received some type of fee-based service, it is possible that their activity level was lower than is typical for retirement community residents.
The significant associations between the intra-individual factor of PA and baseline and 12-month functional limitations support the relationships hypothesized in Figure 1. PA explained a portion of the variance between baseline and 12-month functional limitations. These findings concur with previous research that demonstrated the beneficial effect of exercise on older adults’ physical function (Buchman et al., 2007; Cress et al., 1999). However, contrary to other studies of retirement community residents, participants in the present study had relatively low mean SPPB scores (i.e., greater functional limitations) at both assessments. In addition, the strong correlation between 12-month and baseline SPPB scores left little variance in 12-month SPPB scores to explain in the regression analysis. Thus, although PASE score accounted for significant variance in 12-month SPPB score, the effect may have been greater if there had been more variation in PASE and SPPB scores.
In contrast, depressive symptoms did not significantly affect functional limitations in the present study. On average, depressive symptoms were low among the participants in this sample, as only 7 of the 36 persons with a recent GDS score had GDS scores of 6 or greater. That the majority of the sample had few to no depressive symptoms aligns with research showing that higher socioeconomic status among White women may mitigate any negative emotional responses to increasing functional limitations (Schieman & Plickert, 2007). Moreover, bi-annual screening for depressive symptoms at TigerPlace may have facilitated early detection and treatment and minimized disabling symptoms.
Although extra-individual factors influencing the disablement process were not evaluated in the present study, the built environment of retirement communities has been shown to influence walking behavior. Cress and colleagues’ (2011) study of retirement community residents found that living-space area and functional ability predicted steps per day, which were on average 3,000 steps less than the community-dwelling sample. Compared with retirement community residents, community dwellers were younger, had two to three times the living space, and significantly better physical function. The authors posited that the reduction in overall PA associated with smaller living quarters and proximity of services in retirement communities may actually accelerate functional decline. Unfortunately, elders may not appreciate the need to modify their PA because the environment may sufficiently accommodate their functional limitations and disability. Thus, older adults relocating to retirement communities may need to offset the loss in habitual daily walking with a structured walking program just to maintain baseline physical function.
Although motivating older adults to exercise is challenging, prior research has identified several successful strategies. Older adult participants of group-based cognitive-behavioral interventions, particularly those who incorporate goal setting and self-monitoring with pedometers, have demonstrated increased PA levels (Brawley, Rejeski, & Lutes, 2000; Gardiner, Eakin, Healy, & Owen, 2011; Snyder et al., 2011; Van Roie et al., 2010). Ecological modifications to cognitive-behavioral interventions, such as using site-specific walking-route maps in addition to individualized goal setting and pedometer self-monitoring, have had remarkable success (Rosenberg et al., 2009). Although improvements to the built environment to facilitate PA are preferable, the expense associated with structural changes to existing buildings and other infrastructures would be prohibitive for most communities. Thus, educating seniors about how to overcome barriers to PA and access environmental resources that support PA may be the most reasonable approach for existing communities.
Prior research suggests that lifestyle activities are just as effective as structured exercise programs at improving health outcomes and may be preferable to some older people (Rejeski & Focht, 2002; Van Roie et al., 2010). Although other factors, for example, age, physical symptoms, exercise self-efficacy, and attitude toward exercise, may influence older adults’ participation in exercise programs, the preference for familiar types of PA, such as recreational walking, may be one of the more important determinants of PA participation for this age group (Crombie et al., 2004; Resnick & D’Adamo, 2011; Stuart, Marret, Kelley, & Nelson, 2002). Initiatives to increase PA in retirement community residents may be more successful if planned with residents’ preferences in mind, but PA programs should incorporate national PA guidelines, which outline the type and duration of exercise needed to derive health benefits (Centers for Disease Control and Prevention, 2011). Muscle-strengthening activities are as equally important as are regular aerobic activities because strengthening exercises help maintain muscle mass, reduce falls, and prevent functional decline. Whereas residents may spontaneously walk for exercise, to effectively promote muscle- strengthening activities, facility management may need to invest in specific equipment, such as free weights and resistance bands, as well as the appropriately trained personnel.
Clinical Implications
Older people may be more inclined to engage in health behaviors that they expect will impact personally relevant outcomes, for example, risk of disability and nursing-home admission. Mihalko, Wickley, and Sharpe (2006) found that residents of independent living communities who underwent SPPB testing followed by individualized feedback about their physical-function evaluation, future risks, and benefits associated with PA were more likely to attend an introductory exercise class than elders who received only the notification that the exercise class would be available. Other research has documented that physician counseling, optimally delivered within the context of medical care for a health problem, can positively influence older adults’ PA behavior (Stuart et al., 2002; Weiss, Wolfson, Yaffe, Shrier, & Puts, 2012). Clinicians could inspire elders’ readiness to engage in PA by providing individualized feedback based on physical assessment of functional capacity and emphasizing the benefits of PA for specific health problems. Requiring little space, time, and equipment, SPPB testing is a useful tool to regularly assess physical performance. Elders with total scores in the 9 to 12 range may need only yearly evaluation because they have a low risk of disability and nursing-home admission. However, elders with SPPB scores below 9 may benefit from bi-annual assessment to detect decline early and intervene promptly. Instructions for administration and scoring of the SPPB are publically available on the National Institute on Aging website. Cress and colleagues (2005) provide a succinct review of recommended practices to initiate and sustain PA behavior in older adults.
The present study has several limitations to discuss. First, the sample was relatively small and homogeneous in terms of race and ethnicity, and both of these factors limit generalizability. Second, the data were collected in late spring to early summer; seasonal variation could have influenced the overall activity level. Third, even in persons with intact cognitive functioning, PA questionnaires are a less valid method of measuring PA than are activity monitors; thus, the extent to which cognitive impairment in some participants further affected the validity of the PASE data remains unknown (Tucker, Welk, & Beyler, 2011). Finally, PASE scores were not collected at baseline; thus, the effects of PA on functional limitations over time cannot be estimated in this study. Future studies of community-dwelling elders planning relocation to a retirement community could help identify whether activity levels and subsequent physical function decline after the move as well as the extent to which characteristics of the built environment affect PA behavior. Ecological models may be more appropriate than the disablement model for future PA research in retirement communities because ecological models account for environmental structures and processes that may influence behavior and functional outcomes (Harris-Kojetin et al., 2005; Rosenberg et al., 2009). In addition, future studies with larger samples could test for potential mediation effects to clarify both direct and indirect effects of PA on functional decline. Furthermore, it would have been illustrative to ask participants their reasons for choosing walking over other opportunities for PA at TigerPlace in an attempt to better understand their personal preferences and overall knowledge of national PA recommendations.
Conclusion
Despite its limitations, this study provides longitudinal data on the functional level of older adults residing in a unique aging-in-place retirement community. Reported PA was associated with physical performance, even after controlling for potentially confounding variables, including age, medical conditions, cognitive performance, and baseline physical performance. The serial measurements of physical performance demonstrated that functional limitations naturally progress over time, especially for older adults with lower levels of PA. Finally, the fact that the participants’ primary types of PA were walking and light housework suggests that muscle- strengthening exercises that could slow the progression of functional limitations are not being done and should be more aggressively promoted in retirement settings.
Acknowledgments
The author would like to thank the residents of TigerPlace who graciously participated in this study. The author acknowledges the statistical consultation of Dr. Gregory F. Petroski.
This article is based on results of National Institute of Nursing Research (NINR)-funded research “Technology to Automatically Detect Early Signs of Illness in Senior Housing.” Results and conclusions are the responsibility of the researcher, and not the opinion of NINR.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Institute of Nursing Research (NINR) Grant 1R21NR011197-02 (Rantz, Marilyn J., PI) and John A. Hartford Foundation Building Academic Geriatric Nursing Capacity Claire M. Fagin Fellowship 2008-2010 (Phillips, Lorraine J., PI).
Biography
Lorraine J. Phillips, PhD, RN, is an associate professor at the University of Missouri Sinclair School of Nursing. Her research focuses on physical activity to optimize functioning and slow disability for older adults in senior housing, features of longterm care environments that facilitate active aging, and the emotional and cognitive health of long-term care residents. She conducts meta-analysis research on physical activity in long-term care populations and is an associate director of the Meta-Analysis Research Center at the Sinclair School of Nursing.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- American Heart Association. Statistical fact sheet 2013 Update: High blood pressure. 2013 Retrieved from http://www.heart.org/idc/groups/heart-public/@wcm/@sop/@smd/documents/downloadable/ucm_319587.pdf. [Google Scholar]
- Baker MK, Kennedy DJ, Bohle PL, Campbell DS, Knapman L, Grady J, Fiatarone Singh MA. Efficacy and feasibility of a novel trimodal robust exercise prescription in a retirement community: A randomized, controlled trial. Journal of the American Geriatrics Society. 2007;55:1–10. doi: 10.1111/j.1532-5415.2006.01031.x. [DOI] [PubMed] [Google Scholar]
- Barrie MA. Objective screening tools to assess cognitive impairment and depression. Topics in Geriatric Rehabilitation. 2002;2:28–46. [Google Scholar]
- Bennett JA, Stewart AL, Kayser-Jones J, Glaser D. The mediating effect of pain and fatigue on level of functioning in older adults. Nursing Research. 2002;51:254–265. doi: 10.1097/00006199-200207000-00006. [DOI] [PubMed] [Google Scholar]
- Bjornsdottir G, Arnadottir SA, Halldorsdottir S. Facilitators of and barriers to physical activity in retirement communities: Experiences of older women in urban areas. Physical Therapy. 2012;92:551–562. doi: 10.2522/ptj.20110149. [DOI] [PubMed] [Google Scholar]
- Booth ML, Owen N, Bauman A, Clavisi O, Leslie E. Social-cognitive and perceived environment influences associated with physical activity in older Australians. Preventive Medicine. 2000;31:15–22. doi: 10.1006/pmed.2000.0661. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Buchman AS, Wilson RS, Bienias JL, Bennett DA. Physical activity is associated with incident disability in community-based older persons. Journal of the American Geriatrics Society. 2007;5:195–201. doi: 10.1111/j.1532-5415.2007.01038.x. [DOI] [PubMed] [Google Scholar]
- Brawley LR, Rejeski WJ, Lutes L. A group-mediated cognitive-behavioral intervention for increasing adherence to physical activity in older adults. Journal of Applied Biobehavioral Research. 2000;5:47–65. [Google Scholar]
- Buchman AS, Wilson RS, Boyle PA, Tang Y, Fleischman DA, Bennett DA. Physical activity and leg strength predict decline in mobility performance in older persons. Journal of the American Geriatrics Society. 2007;55:1618–1623. doi: 10.1111/j.1532-5415.2007.01359.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Physical activity for everyone: Physical activity and health. 2011 Retrieved from http://www.cdc.gov/physicalactivity/everyone/guidelines/olderadults.html.
- Centers for Disease Control and Prevention. Early release of selected estimates based on data from the January – June 2012 National Health Interview Survey. 2012 Retrieved from http://www.cdc.gov/nchs/nhis/released201212.htm#7.
- Chodzko-Zajko WJ, Proctor DN, Singh MAF, Minson CT, Nigg CR, Salem GJ, Skinner JS. Exercise and physical activity for older adults: American College of Sports Medicine position stand. Medicine & Science in Sports & Exercise. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- Chou CH, Hwang CL, Wu YT. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: A meta-analysis. Archives of Physical Medicine and Rehabilitation. 2012;93:237–244. doi: 10.1016/j.apmr.2011.08.042. [DOI] [PubMed] [Google Scholar]
- Cress ME, Buchner DM, Prohaska T, Rimmer J, Brown M, Macera C, Chodzko-Zajko W. Best practices for physical-activity programs and behavior counseling in older adult populations. Journal of Aging and Physical Activity. 2005;13:61–74. doi: 10.1123/japa.13.1.61. [DOI] [PubMed] [Google Scholar]
- Cress ME, Buchner DM, Questad KA, Esselman PC, deLateur BJ, Schwartz RS. Exercise: Effects on physical functional performance in independent older adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1999;54:M242–M248. doi: 10.1093/gerona/54.5.m242. [DOI] [PubMed] [Google Scholar]
- Cress ME, Orini S, Kinsler L. Living environment and mobility of older adults. Gerontology. 2011;57:287–294. doi: 10.1159/000322195. [DOI] [PubMed] [Google Scholar]
- Crombie IK, Irvine L, Williams B, McGinnis AR, Slane PW, Alder EM, McMurdo MET. Why older people do not participate in leisure-time physical activity: A survey of activity levels, beliefs, and deterrents. Age and Ageing. 2004;33:287–292. doi: 10.1093/ageing/afh089. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. New England Journal of Medicine. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gardiner PA, Eakin EG, Healy GN, Owen N. Feasibility of reducing older adults’ sedentary time. American Journal of Preventive Medicine. 2011;41:174–177. doi: 10.1016/j.amepre.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Wallace RB. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing-home admission. Journal of Gerontology. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Hall KS, McAuley E. Examining indirect associations between physical activity, function, and disability in independent- and assisted-living residents. Journal of Physical Activity & Health. 2011;8:716–723. doi: 10.1123/jpah.8.5.716. [DOI] [PubMed] [Google Scholar]
- Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical-activity instruments for older adults. Medicine & Science in Sports & Exercise. 2001;33:962–970. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- Harris-Kojetin L, Kiefer K, Joseph A, Arch M, Zimring C. Encouraging physical activity among retirement-community residents: The role of campus commitment, programming, staffing, promotion, financing, and accreditation. Seniors Housing & Care Journal. 2005;13:3–20. [Google Scholar]
- Huang G, Gibson CA, Tran ZV, Osness WH. Controlled endurance exercise training and VO2max changes in older adults: A meta-analysis. Preventive Cardiology. 2005;8:217–225. doi: 10.1111/j.0197-3118.2005.04324.x. [DOI] [PubMed] [Google Scholar]
- Huang G, Shi X, Davis-Brezette JA, Osness WH. Resting heart rate changes after endurance training in older adults: A meta-analysis. Medicine & Science in Sports & Exercise. 2005;37:1381–1386. doi: 10.1249/01.mss.0000174899.35392.0c. [DOI] [PubMed] [Google Scholar]
- Jette AM. Assessing disability in studies on physical activity. American Journal of Preventive Medicine. 2003;25(3) Suppl. 2:122–128. doi: 10.1016/s0749-3797(03)00175-2. [DOI] [PubMed] [Google Scholar]
- Jette AM, Keysor JJ. Disability models: Implications for arthritis exercise and physical activity interventions. Arthritis & Rheumatism. 2003;49:114–120. doi: 10.1002/art.10909. [DOI] [PubMed] [Google Scholar]
- Joseph A, Zimring C. Where active older adults walk: Understanding the factors related to path choice for walking among active retirement-community residents. Environment & Behavior. 2007;39:75–105. [Google Scholar]
- Joseph A, Zimring C, Harris-Kojetin L, Kiefer K. Presence and visibility of outdoor- and indoor-physical-activity features and participation in physical activity among older adults in retirement communities. Journal of Housing for the Elderly. 2005;19(3–4):141–165. [Google Scholar]
- Judge JO, Underwood M, Gennosa T. Exercise to improve gait velocity in older persons. Archives of Physical Medicine and Rehabilitation. 1993;74:400–406. [PubMed] [Google Scholar]
- Keith TZ. Multiple regression and beyond. Boston, MA: Pearson Education; 2006. [Google Scholar]
- Kohl H. Continuing care retirement communities: Risks to seniors. Report prepared for the United States Senate Special Committee on Aging. 2010 Retrieved from http://www.aging.senate.gov/events/hr224cr.pdf.
- Looney MA, Rimmer JH. Aerobic exercise equipment preferences among older adults: A preliminary investigation. Journal of Applied Measurement. 2003;4:43–58. [PubMed] [Google Scholar]
- McArdle WD, Katch FI, Katch VL. Exercise physiology: Energy, nutrition, and human performance. 7th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- Mihalko SL, Wickley KL, Sharpe BL. Promoting physical activity in independent living communities. Medicine & Science in Sports & Exercise. 2006;38:112–115. doi: 10.1249/01.mss.0000183230.08341.6b. [DOI] [PubMed] [Google Scholar]
- Morey MC, Sloane R, Pieper CF, Peterson MJ, Pearson MP, Ekelund CC, Cohen HJ. Effect of physical activity guidelines on physical function in older adults. Journal of the American Geriatrics Association. 2008;56:1873–1878. doi: 10.1111/j.1532-5415.2008.01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motl RW, McAuley E. Physical activity, disability, and quality of life in older adults. Physical Medicine and Rehabilitation Clinics of North America. 2010;21:299–308. doi: 10.1016/j.pmr.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Nagi SZ. Disability concepts revisited: Implications for prevention. In: Pope AM, Taylov AR, editors. Disability in America: Toward a national agenda for prevention. Washington, DC: National Academy Press; 1991. pp. 309–327. [Google Scholar]
- National Institute on Aging and National Institutes of Health. Assessing physical performance in the older patient. 2012 Feb 13; Retrieved from http://www.grc.nia.nih.gov/branches/ledb/sppb/index.htm.
- Norušis MJ. SPSS 13.0 guide to data analysis. Upper Saddle River, NJ: Prentice Hall; 2005. [Google Scholar]
- Pahor M, Blair S, Espeland M, Fielding R, Gill T, Guralnik J, Maraldi C. Effects of a physical-activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- Pomeroy SH, Scherer Y, Runkawatt V, Iamsumang W, Lindemann J, Resnick B. Person-environment fit and functioning among older adults in a long-term care setting. Geriatric Nursing. 2011;32:368–378. doi: 10.1016/j.gerinurse.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Rantz MJ, Porter R, Cheshier D, Otto D, Servey CH, Johnson RA, Taylor G. TigerPlace, a state-academic-private project to revolutionize traditional long term care. Journal of Housing for the Elderly. 2008;22:66–85. doi: 10.1080/02763890802097045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantz MJ, Skubic M, Koopman RJ, Alexander GL, Phillips L, Musterman K, Miller SJ. Automated technology to speed recognition of signs of illness in older adults. Journal of Gerontological Nursing. 2012;38:18–23. doi: 10.3928/00989134-20120307-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantz MJ, Skubic M, Koopman RJ, Phillips L, Alexander GL, Miller SJ. Using sensor networks to detect urinary tract infections in older adults; In Proceedings, IEEE13th International Conference on e-Health Networking, Application, and Services; 2011. Jun, pp. 142–149. [Google Scholar]
- Rejeski WJ, Focht BC. Aging and physical disability: On integrating group and individual counseling with the promotion of physical activity. Exercise and Sport Sciences Reviews. 2002;30:166–170. doi: 10.1097/00003677-200210000-00005. [DOI] [PubMed] [Google Scholar]
- Resnick B, D’Adamo C. Factors associated with exercise among older adults in a continuing care retirement community. Rehabilitation Nursing Journal. 2011;36:47–53. doi: 10.1002/j.2048-7940.2011.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. Journal of Aging and Physical Activity. 1999;7:129–161. [Google Scholar]
- Rosenberg DE, Bombardier CH, Artherholt S, Jensen MP, Motl RW. Self-reported depression and physical activity in adults with mobility impairments. Archives of Physical Medicine and Rehabilitation. 2013 doi: 10.1016/j.apmr.2012.11.014. Advance online publication. doi: http://dx.doi.org/10.1016/j.apmr.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Rosenberg D, Kerr J, Sallis JF, Patrick K, Moore DJ, King A. Feasibility and outcomes of a multilevel place-based walking intervention for seniors: A pilot study. Health & Place. 2009;15:173–179. doi: 10.1016/j.healthplace.2008.03.010. doi: http://dx.doi. org/10.1016/j.healthplace.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A, Cesari M, Onder G, Zamboni V, Barillaro C, Pahor M, Landi F. Depression and physical function: Results from the aging and longevity study in the Sirente geographic area (ilSIRENTE Study) Journal of Geriatric Psychiatry and Neurology. 2007;20:131–137. doi: 10.1177/0891988707301865. [DOI] [PubMed] [Google Scholar]
- Schieman S, Plickert G. Functional limitations and changes in levels of depression among older adults: A multiple-hierarchy stratification perspective. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62(1):S36–S42. doi: 10.1093/geronb/62.1.s36. [DOI] [PubMed] [Google Scholar]
- Schuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the Physical Activity Scale for the Elderly (PASE): According to energy expenditure assessed by the doubly labeled water method. Journal of Clinical Epidemiology. 1997;50:541–546. doi: 10.1016/s0895-4356(97)00010-3. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontologist: The Journal of Aging and Mental Health. 1986;5:165–173. [Google Scholar]
- Simons R, Andel R. The effects of resistance training and walking on functional fitness in advanced old age. Journal of Aging and Health. 2006;18:91–105. doi: 10.1177/0898264305281102. [DOI] [PubMed] [Google Scholar]
- Skubic M, Alexander G, Popescu M, Rantz M, Keller J. A smart home application to eldercare: Current status and lessons learned. Technology and Health Care. 2009;17:183–201. doi: 10.3233/THC-2009-0551. [DOI] [PubMed] [Google Scholar]
- Smalbrugge M, Jongenelis L, Pot AM, Beekman ATF, Eefsting JA. Screening for depression and assessing change in severity of depression. Is the Geriatric Depression Scale (30-, 15- and 8-item versions) useful for both purposes in nursing home patients? Aging & Mental Health. 2008;12:244–248. doi: 10.1080/13607860801987238. [DOI] [PubMed] [Google Scholar]
- Snyder A, Colvin B, Gammack JK. Pedometer use increases daily steps and functional status in older adults. Journal of the American Medical Directors Association. 2011;12:590–594. doi: 10.1016/j.jamda.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Stewart AL. Conceptual challenges in linking physical activity and disability research. American Journal of Preventive Medicine. 2003;25:137–140. doi: 10.1016/s0749-3797(03)00187-9. [DOI] [PubMed] [Google Scholar]
- Stuart CL, Marret J, Kelley GA, Nelson R. Predictors of physical activity in older adults in an independent living retirement community. American Journal of Geriatric Cardiology. 2002;11:160–162. doi: 10.1111/j.1076-7460.2002.01116.x. [DOI] [PubMed] [Google Scholar]
- Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S. adults: Compliance with the Physical Activity Guidelines for Americans. American Journal of Preventive Medicine. 2011;40:454–461. doi: 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Vaitkevicius PV, Ebersold C, Shah MS, Gill NS, Katz RL, Narrett MJ, Fleg JL. Effects of aerobic exercise training in community-based subjects aged 80 and older: A pilot study. Journal of the American Geriatrics Society. 2002;50:2009–2013. doi: 10.1046/j.1532-5415.2002.50613.x. [DOI] [PubMed] [Google Scholar]
- van Gool CH, Kempen GI, Penninx BW, Deeg DJ, Beekman AT, van Eijk JT. Impact of depression on disablement in late middle-aged and older persons: Results from the Longitudinal Aging Study Amsterdam. Social Science & Medicine. 2005;60:25–36. doi: 10.1016/j.socscimed.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Van Roie E, Delecluse C, Opdenacker J, De Bock K, Kennis E, Boen F. Effectiveness of a lifestyle physical activity versus a structured exercise intervention in older adults. Journal of Aging and Physical Activity. 2010;18:335–352. doi: 10.1123/japa.18.3.335. [DOI] [PubMed] [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Social Science & Medicine. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. Journal of Clinical Epidemiology. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Weiss DR, Wolfson C, Yaffe MJ, Shrier I, Puts MT. Physician counseling of older adults about physical activity: The importance of context. American Journal of Health Promotion. 2012;27:71–74. doi: 10.4278/ajhp.100804-QUAL-263. [DOI] [PubMed] [Google Scholar]
- Zalewski KR, Smith JC, Malzahn J, VanHart M, O’Connell D. Measures of physical ability are unrelated to objectively measured physical-activity behavior in older adults residing in continuing care retirement communities. Archives of Physical Medicine and Rehabilitation. 2009;90:982–986. doi: 10.1016/j.apmr.2008.12.013. [DOI] [PubMed] [Google Scholar]