Abstract
Objectives
Although ergogenic, acute caffeine ingestion may increase urine volume, prompting concerns about fluid balance during exercise and sport events. This meta-analysis evaluated caffeine induced diuresis in adults during rest and exercise.
Design
Meta-analysis.
Methods
A search of three databases was completed on November 1, 2013. Only studies that involved healthy adults and provided sufficient information concerning the effect size (ES) of caffeine ingestion on urine volume were included. Sixteen studies met the inclusion criteria, providing a total of 28 ESs for the meta-analysis. Heterogeneity was assessed using a random-effects model.
Results
The median caffeine dosage was 300 mg. The overall ES of 0.29 (95% confidence interval (CI) = 0.11-0.48, p = 0.001) corresponds to an increase in urine volume of 109 ± 195 mL or 16.0 ± 19.2% for caffeine ingestion vs. non-caffeine conditions. Subgroup meta-analysis confirmed exercise as a strong moderator: active ES = 0.10, 95% CI = −0.07 to 0.27, p = 0.248 vs. resting ES = 0.54, 95% CI = 0.22–0.85, p = 0.001 (Cochran's Q, p = 0.019). Females (ES = 0.75,95% CI = 0.38–1.13, p< 0.001) were more susceptible to diuretic effects than males (ES = 0.13,95% CI = −0.05 to 0.31, p = 0.158) (Cochran's Q, p = 0.003).
Conclusions
Caffeine exerted a minor diuretic effect which was negated by exercise. Concerns regarding unwanted fluid loss associated with caffeine consumption are unwarranted particularly when ingestion precedes exercise.
Keywords: Methylxanthine, Coffee, Diuretic, Fluid balance, Dehydration
1. Introduction
Despite its popularity and ergogenic properties during sports performance,1,2 caffeine is generally recognized as having a mild diuretic effect.3,4 The U.S. Food and Drug Administration purports caffeine has diuretic properties and advises its users to drink extra water to avoid dehydration during exercise in the heat.4 Considering caffeine is often used to enhance endurance performance5 and endurance events sometimes occur in high ambient conditions, these situations prompt concerns about excessive fluid loss and impaired endurance performance. During post-exercise rehydration, ingestion of caffeinated beverage has also been shown to increase urine volume and thus the fluid requirement.6 In certain occupations where maintaining fluid balance is essential, extra cautions have been suggested regarding drinking coffee. Industrial workers performing prolonged labor in hot climates are advised to avoid caffeinated beverages in the workplace.7 Military personnel often engage in sustained operations with limited fluid supply; therefore, current military doctrine recommends closely monitoring caffeine ingestion.8
The underlining mechanism of caffeine induced diuresis is not yet clear. It has been postulated, methylxanthines such as caffeine can inhibit phosphodiesterases in the proximal tubule of the kidneys, which may contribute to the diuretic effect.9 Antagonism of adenosine receptors may also mediate caffeine induced diuresis and natriuresis.10 Because caffeine does not increase the kidneys' glomerular filtration rate,11 the diuretic effect is more likely to be related to its natriuretic effect following adenosine receptor blockade. Evidence shows that caffeine acts on the kidneys by inhibiting sodium reabsorption in the proximal and distal tubules,11 thus increases the solute excretion and consequently free water excretion.
Concerns about fluid deficit associated with caffeine ingestion is highly relevant to sports, health and fitness, industry, and military, where exercise is often accompanied with caffeine ingestion. Caffeine is commonly used as an ergogenic aid in ultra-endurance and multi-day sports events.5 Likewise, coffee and energy drinks are popular beverages for health and fitness, the military, as well as for workplace productivity.8 Whether performing prolonged labor or exercising in hot climates with limited access to fluid replacement, hydration is a challenging issue. Consuming caffeine potentially increases the risks of fluid deficits for athletes, fitness enthusiasts, industrial workers, and military personnel if a diuretic effect exists.
Several studies have challenged the assertion that caffeine could contribute to a severe fluid deficit.12,13 The question still remains concerning the magnitude, significance, and moderators of the diuretic effect. Therefore, the objective of this meta-analysis was to quantify caffeine induced diuresis in adults during rest and exercise. The results can be used to guide caffeine use in sports and exercise in hot conditions during which fluid balance is always a concern for optimal health and performance.
2. Methods
A literature search was performed using the PubMed, Web of Science, and ProQuest Dissertation and Theses. Only English literatures and full length publications were considered. No publication date restriction was imposed. Using a Boolean search, the keywords used were: “caffeine”, “coffee”, “tea”, and “cola”, in conjunction with, “fluid balance”, “diuresis”, “diuretic”, “hydration”, “rehydration”, “dehydration”, and “urine volume”. All of the studies that were located during online searches were then manually cross-referenced for additional studies. Moreover, searches were refined by cross-referencing relevant reviews14-16 to supply studies missed during the online searches. After these processes no longer yielded new citations, a list of potential studies was summarized.
Studies which involved healthy adults regardless of their level of participation in exercise and sports were included. This review was limited to studies identifying urine volume as the primary outcome variable following caffeine ingestion. The sources of caffeine included pills, tea, coffee, and caffeinated beverages. Studies must have included sufficient information for calculating the effect sizes (ESs). If it was not possible to accurately estimate the ES, we contacted the listed corresponding author, or the study was excluded. The final list of studies meeting the above criteria was assessed for risk of bias using the Physiotherapy Evidenced-Based Database Scale (PEDro).17
Data were extracted to a spreadsheet by one investigator initially and were cross-compared by another investigator independently to avoid errors. The extracted data were sample size, mean, and standard deviation (SD) of urine volume for caffeine ingestion and non-caffeine conditions. SD was converted by multiplying SE by the square root of the sample size in those reporting SE only. A number of studies employed multiple treatment conditions, therefore the extracted data were treated as “independent” investigations for the meta-analysis. Extracted data were subsequently coded based on potential moderators. Moderators were categorized: continuous moderators included caffeine dosages and investigation durations; discrete moderators included activity state at time of observation and sex. The coding procedure was performed independently by two investigators and any differences were resolved before the meta-analysis.
Caffeine induced diuresis was quantified by calculating the ES, as well as the absolute and relative change in urine volume between caffeine ingestion and non-caffeine conditions. ES was calculated as the standardized mean difference. There was one study18 that reported a pooled SE only; the ES in this case was calculated by subtracting the mean of non-caffeine conditions from the mean of caffeine ingestion, divided by the pooled SD.19 The SE, variance, and weighting (inverse of the variance of ES) of each investigation were calculated along with the ES. Absolute mean change was calculated by subtracting the mean of non-caffeine conditions from that of caffeine ingestion; relative mean change was calculated as absolute mean change divided by the mean of non-caffeine conditions, multiplied by 100. Obligatory urine volume was not considered, as it arguably introduced minimal differences between caffeine ingestion and non-caffeine conditions particularly in crossover designs.
Both Cochran's Q statistics and measure I2 were calculated to assess the heterogeneity.20 I2 of 25%, 50%, and 75% were interpreted as low, moderate, and high levels of heterogeneity, respectively.21 A random-effects model was chosen for the meta-analysis to compensate for methodological and biological diversities in studies.21 Subgroup meta-analyses and meta-regressions using method-of-moments were performed to reveal moderators. Orwin's fail-safe N22 was used to determine the effect of publication bias on the primary meta-analysis. This meta-analysis included many crossover studies, therefore a sensitivity analysis23 was introduced to assess whether the primary result would have changed due to missing information about the inter-trial correlations in crossover studies.
Data were analyzed using the Comprehensive Meta-Analysis Software (Version 2.2; Biostat, Inc., USA). ES was interpreted as follows: <0.2 as a trivial effect, 0.2–0.49 as a small effect, 0.50–0.79 as a moderate effect, and >0.80 as a large effect.24 Subgroup metaanalysis with more than 2 levels, was adjusted by the Bonferroni correction for the alpha inflation. An alpha < 0.05 was considered to be significant.
3. Results
The literature search was completed on November 1,2013 with a total of 78 initial studies being identified via titles found in the databases. Following review of the titles and abstracts, twenty five studies were retrieved as full text and assessed for eligibility. Of those, nine were excluded on the basis of failure to satisfy the pre-established inclusion criteria. Sixteen studies providing a total of 28 usable investigations were included for the quantitative synthesis. The PEDro quality scores for the studies ranged from 7 to 11.
This meta-analysis represented data from 379 participants, of which 246 were males and 133 were females. Ten studies recruited male participants exclusively, with the remaining recruiting either female participants exclusively (n = 2) or mixed groups (n = 4). Except for one study,18 most studies recruited young participants with a median age of 27 yr (min–max: 22–36). Most studies (n = 13) employed crossover designs. Median caffeine dosage was 300 mg (min–max: 114–741). Half of the studies monitored treatment effect over 12h, and the remaining half explored acute treatment effect (mode = 3h). Participants were either in free living,12,25–27 free living plus exercise,28 exercise,29,30 or rest6,13,18,31–36 conditions for the duration of the main data collection period.
A summary of the 28 investigations from the 16 studies is presented in Fig. 1. Considerable dispersion in the ESs was observed. Of the 28 investigations, six ESs were negative, four ESs were trivial (<0.2), ten ESs were small (0.2–0.49), three ESs were moderate (0.50–0.79), and five ESs were large (>0.80). Primary meta-analysis yielded a small but significant overall ES: ES = 0.29, 95% CI = 0.11–0.48, p = 0.001. This overall ES represented an increase in urine volume of 109 ± 195 mL or 16.0 ± 19.2% for caffeine ingestion vs. non-caffeine conditions.
Fig. 1.
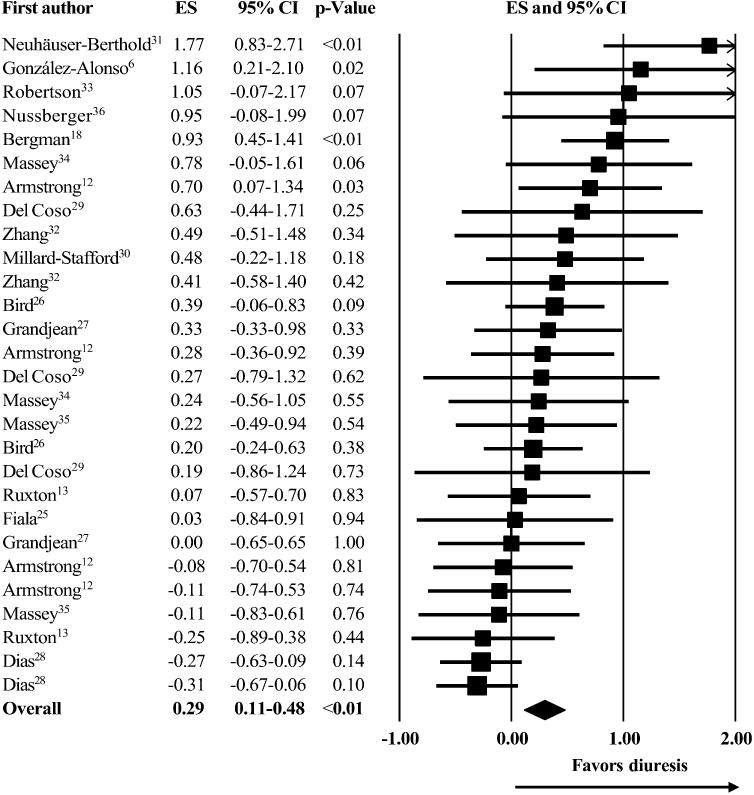
Meta-analysis of caffeine induced diuresis. Each square represents an effect size (ES) for a corresponding investigation with proportional square size according to its weighting. Each horizontal line represents the 95% confidence interval (CI) for an ES. The diamond represents the overall ES with its width according to the 95% CI for the overall ES.
Significant heterogeneity was detected: Q=53.5, df =27, p = 0.002. The measure I2 was in agreement with Cochran's Q detecting a 49.5% moderate level of inter-investigation variation. A summary of the subgroup meta-analyses is presented in Table 1. The diuretic effect of caffeine was significantly modified during exercise and in females. The meta-regressions (Fig. 2) showed neither caffeine dosages nor investigation durations explained the observed heterogeneity. Regarding the publication bias, 32 unpublished investigations would be needed to reduce the current overall ES to a trivial ES of 0.10. The sensitivity analysis did not reveal any change in the overall ES.
Table.
Subgroup meta-analyses exploring moderators that influence caffeine induced diuresis.
| Moderators | n | ES | 95% CI | p | Δ (ml) | Δ (%) | Q | I2 (%) | Between group p (Cochran's Q) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Activity state | Active | 15 | 0.10 | −0.07 to 0.27 | 0.248 | 100 | 11.1 | 17.7 | 21.0 | 0.019 |
| Resting | 13 | 0.54 | 0.22 to 0.85 | 0.001 | 120 | 21.0 | 24.9 | 51.7 | ||
| Sex | Male | 20 | 0.13 | −0.05 to 0.31 | 0.158 | 47 | 13.6 | 25.8 | 26.4 | 0.006 (0.003a) |
| Female | 3 | 0.75 | 0.38 to 1.13 | <0.001 | 113 | 27.5 | 2.0 | 2.3 | ||
| Mixture | 5 | 0.58 | 0.07 to 1.08 | 0.025 | 358 | 17.0 | 10.8 | 63.1 |
ES, effect size; CI, confidence interval;
change in urine volume.
Male vs. female.
Fig. 2.
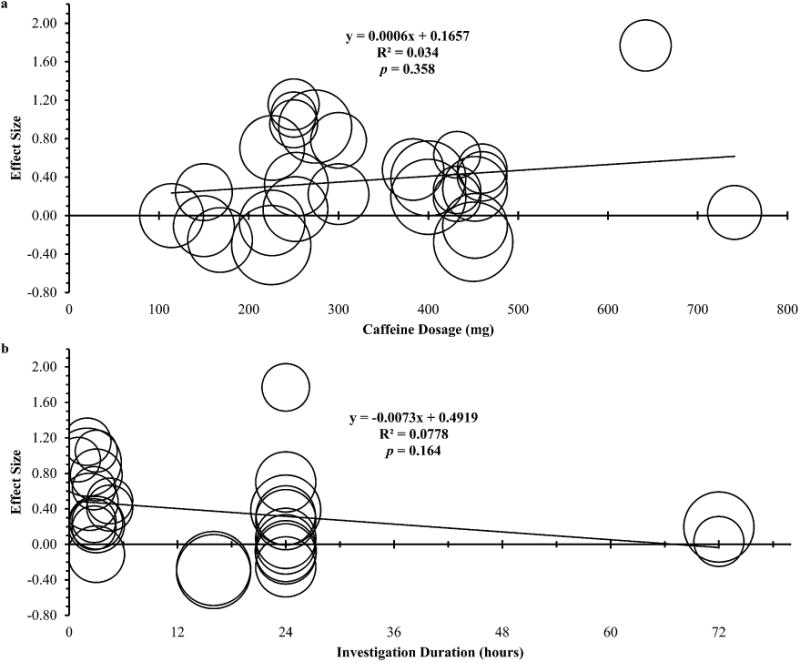
Meta-regressions of caffeine induced diuresis over different caffeine dosages (a) and investigation durations (b). Each circle represents one investigation with the circle size varying with its weighting.
4. Discussion
The major findings of this meta-analysis were three-fold: first, caffeine induced diuresis was small (ES = 0.29) in magnitude, albeit higher in females (ES = 0.75) than males (ES = 0.13); second, the diuretic effect did not exist with exercise (ES = 0.10); third, neither caffeine dosages nor intervention durations influenced the diuresis. Concerns regarding fluid loss and potential adverse effects on fluid balance associated with caffeine ingestion are unfounded.
It has been suggested caffeine ingestion (>300 mg) could induce an acute increase in urine volume.14 The median caffeine dosage in this meta-analysis was 300 mg. The meta-regression (Fig. 2a) revealed, caffeine dosages could not explain the dispersion in the ESs and the suggested influential dosage did not trigger a significant diuretic effect. Most obviously, most of the data clustering around the 300–500 mg range (Fig. 2a) were smaller than a moderate effect. Dosage alone was not an exclusive predictor of caffeine induced diuresis.
The pattern of the data presented in Fig. 2a provides additional information on how other moderators influenced the diuretic effect. Particular interests arise from two data on the far right side. Obviously, both studies25,31 used high dosages of caffeine, however the results were distinctively different. This difference could possibly reside in the investigation durations but most likely in differences in activity state.
An issue for many ultra-endurance athletes, industrial workers, and military personnel would be their fluid balance over several days. Competitive athletes competing in multi-day, ultra-endurance events have been observed with over 2.5% body mass loss per stage of road race.37 Likewise, it has been reported industrial workers are not only subject to dehydration on the job, but could also start the workday with a fluid deficit.38 Therefore, a particular concern would be the cumulative effect of caffeine ingestion on fluid balance during multi-day periods. Generally, the meta-regression (Fig. 2b) revealed the intervention durations could not exclusively explain the dispersion in the ESs. The interpretation (Fig. 2b) should be twofold. First, an overview of the data could imply that caffeine could exert a small but acute diuretic effect, and such effect became less influential when tracking the fluid loss over a full day span. Second, the data were not clustered closely, but rather were dispersed over a wide range. The regression toward the mean suggests the acute diuretic effect of caffeine was small; however, this could be case to case varying in degrees of effect.
The findings in the present analyses are particularly revealing when activity state is taken into account. The subgroup metaanalysis clearly suggests exercise could strongly influence caffeine induced diuresis. Exercise shifted the ES from moderate (0.54) at rest to trivial (0.1) during exercise (Table 1). It is likely exercise exerts an anti-diuretic effect via sympathoadrenal activation. Exercise increases the sympathoadrenal activity which stimulates the release of catecholamines; the catecholamines produce a constriction of the renal arterioles thus lowering glomerular filtration rate.39 Evidence has shown that caffeine induced diuresis disappeared under exercise conditions, during which increased circulation of plasma catecholamine levels was observed.40 Considering the greater the release of catecholamines which is strongly mediated by exercise,41 and the higher intensity and the longer duration of exercise, the less likelihood of caffeine induced diuresis would be. In addition, as environmental heat loads augment the sympathoadrenal activity,42 sports, exercise, and work occurring in hot climates could further reduce the renal plasma flow and glomerular filtration rate,43 and accordingly even less diuretic effect of caffeine could be expected.32
The subgroup meta-analysis further revealed females are more susceptible to any diuretic effects of caffeine. The ES of females was almost 6-fold higher than that of males (Table 1). This difference is likely attributed to the metabolism of caffeine, which is mediated by the activity level of cytochrome P450 1A2 (CYP1A2). In the liver, caffeine is demethylated to 3 dimethylxanthines,1 and the CYP1A2, an active hepatic enzyme, is the key component of this process.44 Considerable variance in the CYP1A2 activity has been documented45 and its variance across individuals is linked to the variability in the ergogenic effects of caffeine.44 Many environmental and lifestyle factors can also influence the CYP1A2 activity.46 Males are more likely to drink caffeinated beverages and often ingest higher dosages of caffeine on a daily basis.47 Because coffee consumption modifies the CYP1A2 activity,46 caffeine is likely to be metabolized slower in females than males and thus exerting any diuretic effects longer in females. The rate of metabolism of caffeine in females could be lower compared to that in males, which may explain in part the greater effect on urine volume.
Finally, two methodological limitations shall be noted. First, the inter-trial correlations in crossover studies were not reported, which could have exerted a biasing effect on the ESs. The sensitivity analysis was thus completed to assess the effects of imputed inter-trial correlations on the result. No change in the overall ES was observed, therefore the crossover designs plausibly resulted in minimal impact on the quantitative assessment. Second, the information on the influential dosage could be expanded greatly if relative caffeine dosages were available. In contexts of high performance sports, caffeine is often prescribed based on athletes' body weights. Nonetheless, the current results can still be generalized to real-life situations where caffeine ingestion is generally below 600 mg.47
5. Conclusion
Caffeine ingestion did not lead to excessive fluid loss in healthy adults and the diuretic effect does not exist with exercise. However, a sex difference was apparent with the diuretic effect greater in females than males. This meta-analysis thus discredits the notion that caffeine ingestion leads to excessive fluid loss via diuresis in healthy, and individuals exercising or working. Caffeine is a safe ergogenic aid that can be used by athletes, fitness enthusiasts, industrial workers, and military personnel without concerns for any negative impact on fluid balance. Competitive athletes should practice caution to not exceed limits enforced by governing bodies of their sport.
Practical implications.
Most of the included studies used dosage (300 mg) comparable to the amounts of caffeine in a normal drinking pattern from coffee and caffeinated beverages. This dosage can produce many positive effects on exercise and sports performance. In industry, this dosage could represent beneficial effects over a 10-h work period. Similarly, this dosage is effective to enhance both cognitive and physical performance during sustained military operations.
• During sports, participation in health and fitness activities, industrial duties, or military operations that require intense exercise or physical labor over a number of hours and possibly done in hot climates, caffeine ingestion would not exaggerate overall fluid loss.
Acknowledgments
No funding was received.
We thank Prof. Gorden L Warren of Georgia State University and Prof. José González-Alonso of Brunel University for assistance during preparing the manuscript.
Footnotes
Conflict of interest: Jose Antonio PhD is a sports science consultant for a commercial gum company (Oomph Energy Gum) that produces caffeinated chewing gum. For the remaining authors none were declared. The views herein are the authors' personal opinions and should not be viewed as the official views of the Association or the Government or the Universities.
References
- 1.Graham TE. Caffeine and exercise: metabolism, endurance and performance. Sports Med. 2001;31(11):785–807. doi: 10.2165/00007256-200131110-00002. [DOI] [PubMed] [Google Scholar]
- 2.Davis JK, Green JM. Caffeine and anaerobic performance: ergogenic value and mechanisms of action. Sports Med. 2009;39(10):813–832. doi: 10.2165/11317770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Food Standards Agency. Healthy, happy eating for exams and the summer. [Accessed April 29, 2009]; Available at: http://food.gov.uk/scotland/news-updates/news/pressreleases/2009/apr/healthyeatingexamssummer.
- 4.U.S. Food and Drug Administration. Medicines in my home: caffeine and your body. [Accessed September 20, 2013]; Available at: http://www.fda.gov/downloads/drugs/resourcesforyou/consumers/buyingusingmedicinesafely/understandingover-the-countermedicines/ucm205286.pdf.
- 5.Garth AK, Burke LM. What do athletes drink during competitive sporting activities? Sports Med. 2013;43(7):539–564. doi: 10.1007/s40279-013-0028-y. [DOI] [PubMed] [Google Scholar]
- 6.González-Alonso J, Heaps CL, Coyle EF. Rehydration after exercise with common beverages and water. Int J Sports Med. 1992;13(5):399–406. doi: 10.1055/s-2007-1021288. [DOI] [PubMed] [Google Scholar]
- 7.Workplace Health and Safety Queensland. Heat stress. [Accessed October 24, 2013]; Available at: http://www.deir.qld.gov.au/workplace/hazards/dangers/heat-stress/index.htm.
- 8.Committee on Military Nutrition Research, Food and Nutrition Board, Institute of Medicine. Caffeine for the sustainment of mental task performance: formulations for military operations. Washington, DC: National Academy Press; 2001. Response to military questions, conclusions, and recommendations, Chapter 7. [Google Scholar]
- 9.Coulson R, Scheinman SJ. Xanthine effects on renal proximal tubular function and cyclic AMP metabolism. J Pharmacol Exp Ther. 1989;248(2):589–595. [PubMed] [Google Scholar]
- 10.Rieg T, Steigele H, Schnermann J, et al. Requirement of intact adenosine A1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J Pharmacol Exp Ther. 2005;313(1):403–409. doi: 10.1124/jpet.104.080432. [DOI] [PubMed] [Google Scholar]
- 11.Shirley DG, Walter SJ, Noormohamed FH. Natriuretic effect of caffeine: assessment of segmental sodium reabsorption in humans. Clin Sci (Lond) 2002;103(5):461–466. doi: 10.1042/cs1030461. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong LE, Pumerantz AC, Roti MW, et al. Fluid, electrolyte, and renal indices of hydration during 11 days of controlled caffeine consumption. Int J Sport Nutr ExercMetab. 2005;15(3):252–265. doi: 10.1123/ijsnem.15.3.252. [DOI] [PubMed] [Google Scholar]
- 13.Ruxton CH, Hart VA. Black tea is not significantly different from water in the maintenance of normal hydration in human subjects: results from a randomised controlled trial. Br J Nutr. 2011;106(4):588–595. doi: 10.1017/S0007114511000456. [DOI] [PubMed] [Google Scholar]
- 14.Maughan RJ, Griffin J. Caffeine ingestion and fluid balance: a review. J Hum Nutr Diet. 2003;16(6):411–420. doi: 10.1046/j.1365-277x.2003.00477.x. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong LE, Casa DJ, Maresh CM, et al. Caffeine, fluid-electrolyte balance, temperature regulation, and exercise-heat tolerance. Exerc Sport Sci Rev. 2007;35(3):135–140. doi: 10.1097/jes.0b013e3180a02cc1. [DOI] [PubMed] [Google Scholar]
- 16.Lopez RM, Casa DJ. The influence of nutritional ergogenic aids on exercise heat tolerance and hydration status. Curr Sports Med Rep. 2009;8(4):192–199. doi: 10.1249/JSR.0b013e3181ae4f66. [DOI] [PubMed] [Google Scholar]
- 17.Moseley AM, Herbert RD, Sherrington C, et al. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro) Aust J Physiother. 2002;48(1):43–49. doi: 10.1016/s0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- 18.Bergman EA, Massey LK, Wise KJ, et al. Effects of dietary caffeine on renal handling of minerals in adult women. Life Sci. 1990;47(6):557–564. doi: 10.1016/0024-3205(90)90616-y. [DOI] [PubMed] [Google Scholar]
- 19.Thomas JR, Nelson JK, Silverman SJ. Research methods in physical activity. 6th. Champaign, Human Kinetics; 2011. Statistical issues in research planning and evaluation, Chapter 7. [Google Scholar]
- 20.Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and Forest plots using a Microsoft Excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5(1):52. doi: 10.1186/1756-0500-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in metaanalyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orwin RG. A fail-safe N for effect size in meta-analysis. J Educ Behav Stat. 1983;8(2):157–159. [Google Scholar]
- 23.Borenstein M, Hedges LV, Higgins JP, et al. Introduction to meta-analysis. 1st. West Sussex, Wiley; 2009. Software, Chapter 44. [Google Scholar]
- 24.Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, Lawrence Erlbaum Associates; 1988. The t test for means, Chapter 2. [Google Scholar]
- 25.Fiala KA, Casa DJ, Roti MW. Rehydration with a caffeinated beverage during the nonexercise periods of 3 consecutive days of 2-a-day practices. Int J Sport Nutr Exerc Metab. 2004;14(4):419–429. doi: 10.1123/ijsnem.14.4.419. [DOI] [PubMed] [Google Scholar]
- 26.Bird ET, Parker BD, Kim HS, et al. Caffeine ingestion and lower urinary tract symptoms in healthy volunteers. Neurourol Urodyn. 2005;24(7):611–615. doi: 10.1002/nau.20179. [DOI] [PubMed] [Google Scholar]
- 27.Grandjean AC, Reimers KJ, Bannick KE, et al. The effect of caffeinated, non-caffeinated, caloric and non-caloric beverages on hydration. J Am Coll Nutr. 2000;19(5):591–600. doi: 10.1080/07315724.2000.10718956. [DOI] [PubMed] [Google Scholar]
- 28.Dias JC, Roti MW, Pumerantz AC, et al. Rehydration after exercise dehydration in heat: effects of caffeine intake. J Sport Rehabil. 2005;14(4):294–300. [Google Scholar]
- 29.Del Coso J, Estevez E, Mora-Rodriguez R. Caffeine during exercise in the heat: thermoregulation and fluid-electrolyte balance. Med Sci Sports Exerc. 2009;41(1):164–173. doi: 10.1249/MSS.0b013e318184f45e. [DOI] [PubMed] [Google Scholar]
- 30.Millard-Stafford ML, Cureton KJ, Wingo JE, et al. Hydration during exercise in warm, humid conditions: effect of a caffeinated sports drink. Int J Sport Nutr Exerc Metab. 2007;17(2):163–177. doi: 10.1123/ijsnem.17.2.163. [DOI] [PubMed] [Google Scholar]
- 31.Neuhäuser-Berthold M, Beine S, Verwied SC, et al. Coffee consumption and total body water homeostasis as measured by fluid balance and bioelectrical impedance analysis. Ann Nutr Metab. 1997;41(1):29–36. doi: 10.1159/000177975. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Carter SJ, Schumacker RE, et al. Effect of caffeine ingestion on fluid balance during exercise in the heat and during recovery. SAfrJ Sports Med. 2014;26(2):43–47. http://dx.doi.org/10.7196/SAJSM.513. [Google Scholar]
- 33.Robertson D, Frolich JC, Carr RK, et al. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. N Engl J Med. 1978;298(4):181–186. doi: 10.1056/NEJM197801262980403. [DOI] [PubMed] [Google Scholar]
- 34.Massey LK, Wise KJ. The effect of dietary caffeine on urinary excretion of calcium, magnesium, sodium and potassium in healthy young females. Nutr Res. 1984;4(1):43–50. [Google Scholar]
- 35.Massey LK, Berg TA. The effect of dietary caffeine on urinary excretion of calcium, magnesium, phosphorus, sodium, potassium, chloride and zinc in healthy males. Nutr Res. 1985;5(11):1281–1284. [Google Scholar]
- 36.Nussberger J, Mooser V, Maridor G, et al. Caffeine-induced diuresis and atrial natriuretic peptides. J Cardiovasc Pharmacol. 1990;15(5):685–691. doi: 10.1097/00005344-199005000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Ebert TR, Martin DT, Stephens B, et al. Fluid and food intake during professional men's and women's road-cycling tours. Int J Sports Physiol Perform. 2007;2(1):58–71. doi: 10.1123/ijspp.2.1.58. [DOI] [PubMed] [Google Scholar]
- 38.Brake D, Bates G. Fluid losses and hydration status of industrial workers under thermal stress working extended shifts. Occup Environ Med. 2003;60(2):90–96. doi: 10.1136/oem.60.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clausen JP, Trap-Jensen J. Arteriohepatic venous oxygen difference and heart rate during initial phases of exercise. J Appl Physiol. 1974;37(5):716–719. doi: 10.1152/jappl.1974.37.5.716. [DOI] [PubMed] [Google Scholar]
- 40.Wemple R, Lamb D, McKeever K. Caffeine vs caffeine-free sports drinks: effects on urine production at rest and during prolonged exercise. Int J Sports Med. 1997;18(1):40–46. doi: 10.1055/s-2007-972593. [DOI] [PubMed] [Google Scholar]
- 41.Zouhal H, Jacob C, Delamarche P, et al. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008;38(5):401–423. doi: 10.2165/00007256-200838050-00004. [DOI] [PubMed] [Google Scholar]
- 42.Powers SK, Howley ET, Cox R. A differential catecholamine response during prolonged exercise and passive heating. Med Sci Sports Exerc. 1982;14(6):435–439. doi: 10.1249/00005768-198206000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Smith JH, Robinson S, Pearcy M. Renal responses to exercise, heat and dehydration. J Appl Physiol. 1952;4(8):659–665. doi: 10.1152/jappl.1952.4.8.659. [DOI] [PubMed] [Google Scholar]
- 44.Womack CJ, Saunders MJ, Bechtel MK, et al. The influence of a CYP1A2 polymorphism on the ergogenic effects of caffeine. J IntSoc Sports Nutr. 2012;9(1):7. doi: 10.1186/1550-2783-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics. 2008;9(5):625–637. doi: 10.2217/14622416.9.5.625. [DOI] [PubMed] [Google Scholar]
- 46.Magkos F, Kavouras SA. Caffeine use in sports, pharmacokinetics in man, and cellular mechanisms of action. Crit Rev Food Sci Nutr. 2005;45(7–8):535–562. doi: 10.1080/1040-830491379245. [DOI] [PubMed] [Google Scholar]
- 47.U.S. Food and Drug Administration. Caffeine intake by the U.S. population. [Accessed October 24, 2013]; Available at: http://www.fda.gov/downloads/aboutfda/centersoffices/officeoffoods/cfsan/cfsanfoiaelectronicreadingroom/ucm333191.pdf.


