Abstract
Development of an effective vaccine targeting tumor associated carbohydrate antigens (TACAs) is an appealing approach toward tumor immunotherapy. While much emphasis has been typically placed on generating high antibody titers against the immunizing antigen, the impact of immunogen design on the diversity of TACA-specific antibodies elicited has been overlooked. Herein, we report that the immunogen structure can significantly impact the breadth and the magnitude of humoral responses. Vaccine constructs that induced diverse TACA-binding antibodies provided much stronger recognition of a variety of Tn positive tumor cells. Optimization of the breadth of the antibody response led to a vaccine construct that demonstrated long lasting effcacy in a mouse tumor model. After challenged with the highly aggressive TA3Ha cells, mice immunized with the new construct exhibited a statistically significant improvement in survival relative to controls (0% vs 50% survival; p < 0.0001). Furthermore, the surviving mice developed long-term immunity against TA3Ha. Thus, both the magnitude and the breadth of antibody reactivity should be considered when designing TACA-based antitumor vaccines.
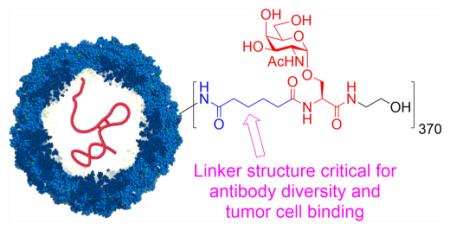
Cancer cells often express high levels of characteristic glycan structures, which are referred to as tumor associated carbohydrate antigens (TACAs).1 An example of TACA is the Tn antigen,2 which has been found in 70–90% of breast, lung, prostate, and pancreatic tumors but is rarely expressed in healthy tissues. High levels of Tn antigen expression correlate significantly with shortened disease-free interval and increased metastasis.3–5 As a result, Tn antigen has sequence, 17,18 lipid structures of glycolipids, 22 19–21 or neighbor-been ranked among the top 50 tumor-associated antigens,6 and ing carbohydrates of the TACAs. For instance, the Tn antigen innovative studies have been performed on Tn-based vaccines either alone or as part of a multiantigen construct.
Development of anti-TACA vaccines is still very challenging due to their low immunogenicity and T-cell independent nature. Despite much effort dedicated to improve anti-TACA antibody responses,11–14 no TACA-based vaccines have been approved by FDA yet. Phase III studies of GM2–KLH and STn–KLH have failed to show therapeutic benefits even though significant antibody titers were stimulated in cancer patients.15,16 Thus, there is still much yet to be learned about what constitutes effective ant-tumor responses.
In the development of carbohydrate based anticancer vaccines, much emphasis has been placed on the investigation of carrier and modification of antigen structures to enhance humoral responses.11–14 One potential complexity in TACAbased vaccine design is the heterogeneities of local environments of TACAs on tumor cell surfaces. Antibody recognition of the TACA epitope can be influenced by the glycoprotein sequence,17,18 lipid structures of glycolipids,19–21 or neighboring carbohydrates of the TACAs.22 For instance, the Tn antigen can be found in a variety of glycoproteins including epiglycanin23 and mucin-1 (MUC1).24 Even in a protein such as MUC1, because it can contain hundreds of tandem repeats and each repeat region bears five potential glycosylation sites, there are many possible Tn containing structures.25 As a result, a specific antibody generated against the immunizing TACA structure may not recognize the same TACA displayed on tumor cells due to differential conformations. As an example, anti-Tn mAbs MLS128 and 83D4 only interact with clusters of two or three neighboring Tns in glycopeptides but fail to recognize two Tns separated by an unglycosylated amino acid.26 Several anti-Tn IgG mAbs raised by Jurkat cells only recognized Tn antigen in the context of unique peptide motifs.17 A reinvestigation of the STn–KLH vaccine suggested that induction of anti-STn antibodies targeting a wide range of STn-carrying glycoproteins rather than a single one is critical in controlling tumor growth, suggesting the significance of eliciting diverse TACA-specific antibodies.27 Unfortunately, current vaccination approaches mostly focused on the magnitude of antibody responses against the immunizing antigen, with little attention paid to the breadth of antibody repertoire. Therefore, strategies that can elicit a diverse range of antibodies capable of binding the target antigen within a variety of contexts are highly desirable to enhance immune recognition and reduce immune escape of cancer cells.
The breadth of antibody response depends on the activation of naive germline B cell pool and subsequent somatic hypermutation in germinal centers, although the exact regulatory mechanism is not well understood.28 Recent studies have revealed the impact of adjuvants or hapten density on the spectrum of antibody responses,29–31 while the role of other factors remain to be fully elucidated. Herein we report that the immunogen structure can have a profound effect on the diversity of antibodies. A well-designed Tn immunogen on the virus-like particle (VLP) bacteriophage Qβ scaffold improved the antibody titers but perhaps more importantly greatly expanded the diversity of the antibodies induced. As a result, the recognition of Tn-positive tumor cell lines was much enhanced leading to effective protection of mice from tumor development. The results presented provide important design considerations for the development of carbohydrate-based anticancer vaccine.
RESULTS AND DISCUSSION
First Generation Qβ–Tn Conjugates Failed To Elicit TA3Ha Reactive Antibodies
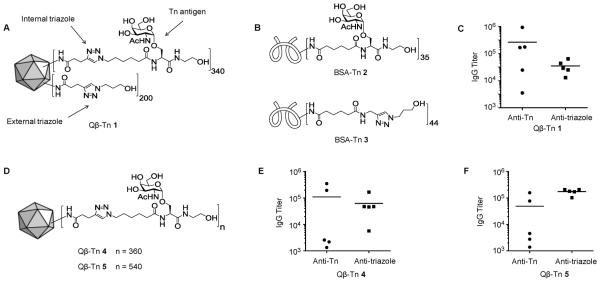
To overcome the low immunogenicity of Tn and elicit a powerful humoral response, it is critical to conjugate Tn with a carrier moiety that can potentially induce the activation of CD4+ helper T cells and antibody isotype switching to IgG. We became interested in investigating the utility of VLPs such as bacteriophage Qβ as a carrier for Tn. Our first generation construct Qβ–Tn 1 was synthesized using the copper-catalyzed alkyne–azide cyclo-addition (CuAAC) click reaction,32 which attached an average of 340 copies of Tn per Qβ capsid through a triazole linker (termed internal triazole), as well as 200 copies of triazole without Tn (external triazole) (Figure 1A). Mice were immunized with Qβ–Tn 1 with two booster injections on days 14 and 28. On day 35, sera were collected and titrated against BSA–Tn conjugate 2 and BSA–triazole 3 (Figure 1B) to measure the overall antibody responses against the carbohydrate and triazole linker, respectively, in enzyme-linked immunosorbent assays (ELISAs, Figure 1C). The results showed an average Tn titer of 263 600 with a moderate average anti-triazole titer of 35 300. The postimmune sera reacted with Tn-expressing human lymphoma Jurkat cells through FACS analysis. In order to demonstrate the effcacy of antitumor activities, a mouse tumor model using the Tnexpressing murine TA3Ha cells has been established.33 However, despite binding with Jurkat cells and the high IgG titers against BSA–Tn 2, none of the sera from mice immunized with Qβ–Tn 1 showed significant reactivity with TA3Ha cells even at a relatively high concentration (1:10 dilution). These results suggest immunization of Qβ–Tn 1 would be ineffective toward protecting the host from TA3Ha tumor growth.
Figure 1.
Schematic representations of (A) vaccine construct Qβ–Tn 1 and (B) BSA–Tn 2 and BSA–triazole 3. (C) Day 35 IgG titers from mice vaccinated with Qβ–Tn 1. (D) Schematic representation of vaccine constructs Qβ–Tn 3 and 4. (E, F) IgG titers elicited by Qβ–Tn 4 and 5 against Tn and triazole, respectively.
In order to improve tumor cell recognition, Qβ–Tn construct 4 (Figure 1D) was prepared, which contained 360 copies of Tn without any external triazoles to test whether the removal of external triazoles could boost the anti-Tn titers. However, upon immunizing mice following the same protocol as Qβ–Tn 1, lower levels of Tn-specific IgG antibodies (average titer of 111 000) were found and the titers of antitriazole antibodies increased (average titer of 63 000) (Figure 1E). Next, the density of Tn on Qβ was increased from 340 to 540 (Qβ–Tn 5, Figure 1D), since higher local antigen density was previously shown to enhance anti-Tn antibody titers.32 of 49 100), while the average triazole titer increased to 175 000 (Figure 1F). The postimmune sera from mice immunized with Qβ–Tn 4 or Qβ–Tn 5 again failed to bind with TA3Ha cells.
Alkyl Amide-Linked Qβ–Tn 6 Generates TA3Ha Reactive Antibodies
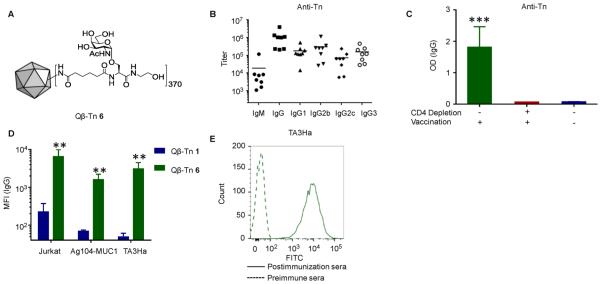
The ineffectiveness of the triazole-linked Qβ–Tn constructs prompted us to synthesize a new vaccine (Qβ–Tn 6) where an alkyl amide linker was utilized to couple Tn antigen with Qβ (Figure 2A and Scheme S1). The average number of Tn per capsid was 370, similar to that of Qβ–Tn 1. Mice were immunized with Qβ–Tn 6 following the same immunization protocol as Qβ–Tn 1. ELISA analysis showed that these mice produced primarily an IgG humoral response with exceptionally high average titers (~1 461 000) of anti-Tn IgG (Figure 2B). Analysis of the IgG subtypes demonstrated balanced Th1/Th2 responses with significant titers of all major IgG subclasses (Figure 2B). When the CD4+ helper T cells from the mice were depleted using an anti-CD4 mAb prior to vaccination with Qβ–Tn 6, the anti-Tn IgG responses decreased significantly suggesting that CD4+ helper T cells play important roles in regulating class switching of antiglycan responses (Figure 2C).
Figure 2.
(A) Schematic representation of vaccine Qβ–Tn 6. (B) Anti-Tn titers from mice vaccinated with Qβ–Tn 6. (C) Mice were depleted of CD4+ T cells prior to vaccination, and anti-Tn IgG responses were compared with control mice without depletion or vaccination. The results show that CD4+ T cells are critical for high antibody responses. (D) Sera from mice immunized with Qβ–Tn 6 showed significantly higher binding with multiple Tn positive tumor cells than those with Qβ–Tn 1. Results are expressed as the mean of individual ± SEM; **p < 0.01 and ***p < 0.001 using Student’s t test. (E) Recognition of TA3Ha cells by serum from mice immunized with Qβ–Tn 6 (For clarity, only one representative example of binding by postimmune serum is shown).
With the superior anti-Tn titers, the binding of the antibodies with multiple tumor cell lines was analyzed by flow cytometry. Although the average anti-Tn IgG titers from Qβ–Tn 6 and Qβ–Tn 1 differed only by 4-fold, the average mean fluorescence intensities (MFI) of sera binding to Jurkat cells increased more than 50 times with Qβ–Tn 6 (Figure 2D and Figure S5). Importantly, the postimmune sera from Qβ–Tn 6 immunized mice bound strongly with TA3Ha cells (Figure 2D,E). The breath of tumor cell recognition by Qβ–Tn 6 induced antibodies was further demonstrated through their binding with Ag104-MUC1 cells (Figure 2D and Figure S5).
The generation of a strong anti-Tn humoral response has been very challenging. To induce anti-Tn antibodies, a variety of delivery platforms have been investigated including synthetic multiple antigenic glycopeptide and protein carriers such as keyhole limpet hemocyanin (KLH) and desialylated ovine submaxillary mucin.7–10,34–36 It has been found that conjugates bearing the synthetically more accessible monomeric Tn elicited little anti-Tn antibodies presumably due to the small size of the antigen.9,36 To boost the antibody levels, Tn clusters have been investigated. However, even with these types of immunogens, most of the anti-Tn titers were still modest9,36 with only one study reporting IgG titers over 100 000.10 Therefore, with its ability to induce super high anti-Tn IgG titers (~1 461 000) using monomeric Tn antigen and the strong binding by the antibodies induced to a wide range of tumor cells, the Qβ–Tn 6 construct represents a significant advance in anti-Tn vaccine design.
Qβ–Tn 6 Vaccine Greatly Enhanced the Breadth of Antibody Repertoire
The drastic enhancement of tumor cell recognition by Qβ–Tn 6 induced antibodies could be due to higher antibody avidity to Tn antigen. To evaluate this, a chaotropic ELISA procedure was developed. The postimmune sera from Qβ–Tn 1 and Qβ–Tn 6 immunized mice were incubated with BSA–Tn 2 immobilized in ELISA plates. After removal of unbound antibodies, increasing concentrations of aqueous ammonium thiocynate solution were added. The concentration of ammonium thiocynate needed to remove 50% of the antibodies bound to BSA–Tn (IC50) was determined as an avidity measure. Both groups were eluted from the plate with similar concentrations of ammonium thiocynate indicating that there were no substantial differences in avidities of antibodies generated (Figure S6).
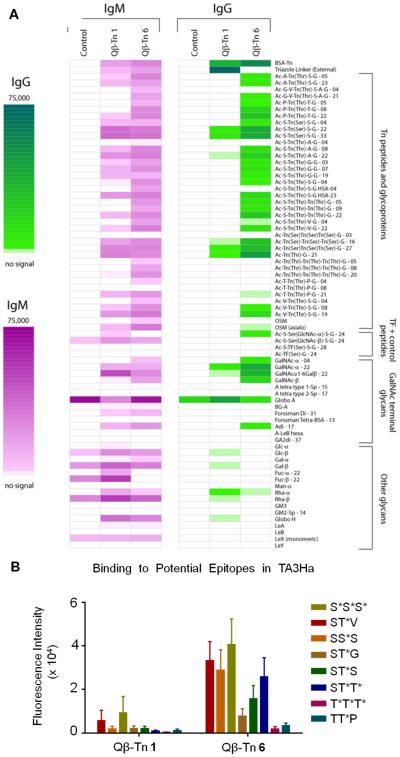
Another factor that can impact tumor cell binding is the diversity of antibodies generated. The epitope profiles were analyzed at a molecular level using a glycan microarray,37 which contains a panel of 328 glycoconjugates derived from glycoproteins or glycolipids with 39 Tn peptides and 44 other GalNAc terminal glycans (for a full list of array components, see Table S1). Sera from mice immunized with Qβ–Tn 1 or Qβ–Tn 6 were incubated on the microarray and then detected by a fluorescently labeled secondary antibody (Figure 3A). In agreement with the ELISA results, IgG bearing components. In contrast, sera from mice immunized with Qβ–Tn 6 exhibited much broader binding. For example, although the immunizing Tn antigen contains serine only, both serine and threonine linked Tn monomers and dimers were recognized well. Tn trimer binding revealed interesting dependence on backbone sequences. The IgG antibodies showed strong reactivity toward the Tn cluster of Tn(Ser)–Tn(Ser)–Tn(Ser) but not its threonine analog. This is consistent with several observations that the recognition of cluster Tn is strongly modulated by the peptide backbone17,26 The abilities of Qβ–Tn 6 to elicit antibodies capable of binding cluster Tn despite the monomeric Tn utilized for synthesis of Qβ–Tn 6 were presumably due to the high density organized display of Tn on Qβ mimicking Tn clusters.
Figure 3.
(A) Glyco-microarray profiles comparing pre- and postimmune sera from control mice and mice immunized with Qβ–Tn 1 and Qβ–Tn 6. The average fluorescence signals from at least four mice per group are presented (IgM data shown in magenta and IgG data shown in green). Glycans are attached to BSA prior to printing on the array surface. The numbers after the glycan abbreviation correspond to the average number of glycans per molecule of albumin. (B) Sera from mice immunized with vaccine Qβ–Tn 1 and Qβ–Tn 6 was analyzed against potential epitopes present in epiglycanin (TA3Ha cell) by microarray.
Tn antigens are present on tumor cells in a variety of configurations including both monomeric and cluster forms. With a murine Lewis lung cancer model, Matsumoto et al. recently reported that an increased level of Tn clusters in syndecan-1 was closely correlated with enhanced invasion and metastasis.38 On the other hand, overexpression of hypoglycosylated MUC1 is a hallmark in a large variety of epithelial cancers, and the immunodominant epitopes of MUC1 do not contain three consecutive glycosylation sites. In a histochemical study with 322 cases of invasive breast ductal carcinomas, nonconsecutive Tn in MUC1 was implicated in aggressive growth and lymphatic metastasis of breast cancer cells.39 The antibodies elicited by Qβ–Tn 6 were capable of recognizing both Tn monomer and clusters (Figure 3), rending it attractive as a vaccine candidate.
Among all the Tn-containing glycopeptides on the microarray, multiple peptide sequences can be found in glycoprotein epiglycanin, CD43, and MUC1, which are the major Tnbearing proteins in TA3Ha, Jurkat, and Ag104-MUC1, respectively. Antibodies generated by Qβ–Tn 6 but not Qβ–Tn 1 showed strong signals to these epitopes (Figures 3B and S7), which may account for their dramatic difference in reactivity with these tumor cells. In addition, antibodies generated from Qβ–Tn 6 exhibited little bindings to non-Tn carbohydrate epitopes, which highlights their Tn specificity (Figure 3B).
Relative antibody avidity can also be established from the microarray by comparing the intensities of array components due to antibody binding. Consistent with the chaotropic ELISA result, sera generated by Qβ–Tn 1 and Qβ–Tn 6 showed similar ratios of binding to Tn conjugates arrayed at low and high densities (Figure S8) supporting the aforementioned conclusion that antibodies generated by these two constructs have comparable avidities.
Qβ–Tn 1 Rapidly Elicited Antitriazole Antibodies, Which Could Hinder Tn Binding
In order to shine light on the role of immunogen structure on antibody responses, the binding of Qβ–Tn 1 and Qβ–Tn 6 by soybean agglutinin (SBA, a Tn selective lectin), an anti-Tn mAb Bric 111, or polyclonal antibodies40 was compared. The recognition of Tn on both constructs by all these Tn receptors were similar (Figure S9). This suggests that the cyclic triazole moiety in Qβ–Tn 1 most likely did not restrict the conformations of Tn for recognition by B cell receptors in vivo.
Another possibility examined is that the anti-triazole antibodies induced by Qβ–Tn 1 can bind with the triazoles on the vaccine construct, potentially shielding Tn from interacting with B cell receptors. To test this, the binding of Qβ–Tn 1 and Qβ–Tn 6 by SBA was measured in the presence of serum enriched with polyclonal anti-triazole antibodies. While this serum reduced SBA binding to Qβ–Tn 6 by 15%, its effect on Qβ–Tn 1 was much more prominent, resulting in 50% inhibition of SBA binding (Figure 4A). Furthermore, we found that in Qβ–Tn 1 immunized mice, anti-triazole antibodies were generated much faster than those against Tn (Figure 4B). These results support the possibility that anti-triazole antibodies can partially block the binding of Qβ–Tn 1 to Tn-specific B cells in vivo, thus potentially reducing the number and types of Tn-specific B cells that can get in contact with the Tn antigen and decreasing the diversity of anti-Tn antibodies.
Figure 4.
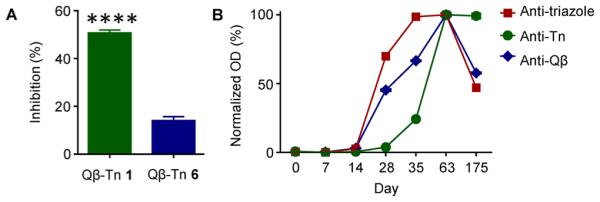
(A) Inhibitory effect of anti-triazole antibodies on Tn recognition by SBA. Vaccines were coated on ELISA plates, then treated with anti-triazole antibodies, which was followed by detection by SBA. The inhibitory effect was calculated as the percentage of signal intensity decrease for each vaccine. (B) Kinetics of the generation of anti-triazole, anti-Qβ, and anti-Tn antibodies in Qβ–Tn 1 immunized mice. The OD values of each group were normalized.
Vaccination with Qβ–Tn 6 in Combination with Chemotherapy Confers effective and Long Lasting Protection against Cancer
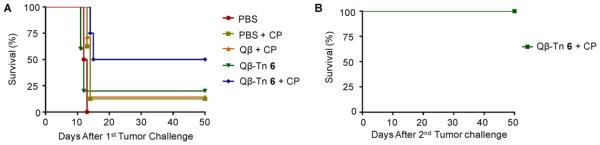
With the strength and diversity of anti-Tn responses elicited by Qβ–Tn 6 established, the ability of the vaccine to protect mice from tumor development was evaluated in a therapeutic model. TA3Ha cells (5000 cells) were injected into the mice intraperitoneally on day 0. The mice were then treated with a low dose of a chemotherapeutic drug, cyclophosphamide (CP), administered 1 day prior to vaccination with Qβ–Tn 6. Control groups received PBS, CP, Qβ–Tn 6, and CP/Qβ. As shown in Figure 5A, all mice in the PBS group died within 12 days. Compared with all other treatments, the combination of Qβ–Tn 6 and CP provided significantly higher protection against tumor development with 50% of the mice surviving tumor challenge after 50 days. Furthermore, the surviving mice from the CP/Qβ–Tn 6 group were challenged with another dose of 5000 TA3Ha cells on day 50. Without any additional treatment, all of these mice rejected the tumor. These results suggest that these CP/Qβ–Tn 6 treated mice gained long-lasting immunity against TA3Ha cells.
Figure 5.
Active vaccination with Qβ–Tn 6 in combination of chemotherapy improved the survival of mice. (A) Groups of mice were intraperitoneally injected with 5000 TA3Ha cells on day 0, with or without intraperitoneal treatment of cyclophosphamide (50 mg kg−1). PBS buffer, Qβ particle plus MPLA, or Qβ–Tn 6 plus MPLA was administrated intravenously. The survival of mice was followed for 50 days. PBS group (n = 8), PBS+CP group (n = 8), Qβ + CP group (n = 7), Qβ–Tn 6 group (n = 5), Qβ–Tn 6 + CP group (n = 8). There is significant difference between Qβ–Tn 6 + CP group and PBS group (p < 0.0001) or all other treatment group (p < 0.05). Statistical analysis of survival was performed with GraphPad Prism using log-rank test. (B) Mice surviving tumor challenge from CP/Qβ–Tn 6 group were rechallenged with 5000 TA3Ha cells. All mice survived without any further treatment.
The observations that the combination of CP and Qβ–Tn 6 vaccination provided much higher protection than vaccination or CP treatment alone suggest strong anti-Tn humoral responses were necessary but not suficient to protect most animals. This is consistent with the observation that direct intraperitoneal injection of an anti-Tn mAb into mice bearing TA3Ha in the peritoneal cavity did not provide much protective benefit.41 At the dose administered, CP does not impact much the proliferation of TA3Ha cells.41 It is known that in C57BL6 mice, the antitumor activities of effector cells are generally weak.42 Although the anticancer mechanisms of CP are complex,43,44 it can potentially abrogate the immunosuppression imposed by myeloid-derived suppressor cells and activate the effector cells such as natural killer cells to induce cytotoxicity to tumor cells that are bound by Tn-specific antibodies.45
Mice Protected by Qβ–Tn/CP from Tumor Challenges Have Elevated Levels of Anti-Tn IgG and IgM Antibodies That Can Recognize TA3Ha Cells
To better understand the immunoprotection, sera from mice surviving the tumor challenge were collected and compared with those from dead mice. Although sera from unprotected mice showed binding to TA3Ha cells, tumor cell binding by sera from protected mice was much higher (Figure 6A,B). Glyco-microarray analysis also demonstrated that sera from live mice bound to many more Tn-serine-containing peptides (Figure S10).
Figure 6.
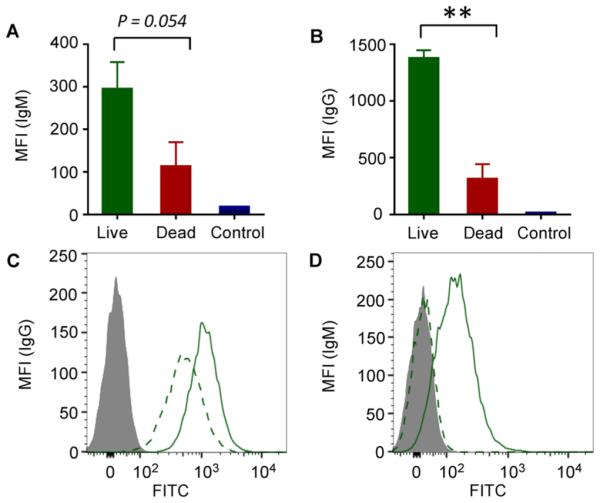
Analysis of anti-Tn responses in live or dead mice immunized with Qβ–Tn 6 plus cyclophosphamide. For live mice, sera were bled on day 23. Sera were collected from dead mice right after mice were sacrificed due to the heavy tumor burden. IgG and IgM binding with TA3Ha tumor cells were analyzed at 1:20 dilution, and mean fluorescence intensities for (A) IgG and (B) IgM binding were shown. Preimmune sera were used as control. Competitions of IgG and IgM antibodies binding with TA3Ha by ascites fluid were shown in panels C and D, respectively. Solid curve, without ascites incubation; dashed curve, with ascites co-incubation; gray filled, preimmune serum binding; **p < 0.01 using Student’s t test.
To test the specificity of antibodies elicited, we performed competitive flow cytometry analysis. The Tn bearing glycoprotein epiglycanin on TA3Ha cells46 is continuously shed and released into ascites.47 TA3Ha cells were co-incubated with sera from protected mice and the ascites. The presence of ascites led to an average of 50% reduction in IgG binding to TA3Ha (Figure 6C). Similar phenomena were observed with IgM, with IgM binding to TA3Ha completely inhibited by ascites (Figure 6D). These results indicate that epiglycanin contains the major epitopes recognized by the antibodies.
In conclusion, we present for the first time that immunogen design in a carbohydrate based conjugate vaccine can profoundly alter the breadth of antibody repertoire. Despite the prevalence of the triazole linker in conjugate vaccines,32,48–54 our data showed that the inclusion of such a linker could dramatically reduce the diversity of anti-TACA humoral responses. The ability to increase the repertoire of anti-Tn antibodies by removing the triazole linker correlated with substantial enhancement of reactivity with Tn-bearing tumor cells, which ultimately enabled the long lasting protection against tumor challenge in a mouse model. Our findings highlight the importance of immunogen design in eliciting broad anti-TACA responses for the development of effective anticancer vaccines.
MATERIALS AND METHODS
Synthesis and Characterization of Qβ Conjugates
Wild-type Qβ particles and Qβ–Tn 1, 4, and 5 were prepared as described previously,32 except that no extra triazole moiety was introduced in Qβ–Tn 4 and 5 by controlling the amount of reagent. Qβ–Tn 6 was synthesized by mixing wild-type Qβ particles and Tn-NHS32 in a mixture of DMSO and PBS buffer (0.1 M, pH = 7) overnight at RT. All the conjugates were combined and purified by repeated filtration using Millipore 100 000 MWCO filter units against PBS buffer. Total protein concentration was measured using the Coomassie Plus protein reagent (Pierce) with bovine serum albumin as standard. Antigen loading per Qβ was determined using electrophoretic analysis and MALDI-TOF. Particle stability after conjugation was shown by size exclusion FPLC on a Superose-6 column.
Immunizations of Mice
Pathogen-free C57BL/6 female mice age 6–10 weeks were obtained from Charles River and maintained in the University Laboratory Animal Resources facility of Michigan State University. All animal care procedures and experimental protocols have been approved by the Institutional Animal Care and Use Committee (IACUC) of Michigan State University. Groups of C57BL/6 mice were injected subcutaneously under the scruff on day 0 with 0.1 mL of various Qβ constructs as emulsions in complete Freund’s adjuvant (Sigma-Aldrich, F5881), and boosters were given subcutaneously under the scruff on days 14 and 28 with 0.1 mL of various Qβ constructs as emulsions in incomplete Freund’s adjuvant (Sigma-Aldrich, F5506). All Tn vaccine constructs administered have the same amounts of Tn antigen (4 μg). Serum samples were collected on day 0 (before immunization), 7, and 35. The final bleeding was done by cardiac bleed.
Antibody Detection by ELISA and Flow Cytometry
Sera were tested as described previously for anti-Tn and anti-triazole antibodies by ELISA using BSA–Tn 2 or BSA–triazole 3.32 The titer was determined by regression analysis with log 10 dilution plotted with optical density.
Sera were tested by flow cytometry on Tn-bearing tumor cell lines Jurkat (kindly provided by Profs. Barbara Kaplan and Norbert Kaminski, Michigan State University) and fresh TA3Ha cells (kindly provided by Prof. John Hilkens, The Netherlands Cancer Institute) isolated from ascites of A/J mice. Cells were incubated with 1:20 diluted mice sera on ice for 30 min and then labeled with goat anti-mouse IgG conjugated with FITC (BioLegend, 405305) for 30 min. Acquisition of cells was performed with LSR II (BD), and data was analyzed with FlowJo software (Tree Star Inc.).
Antitumor Immunotherapy
Five thousand TA3Ha cells were intraperitoneally injected into eight-week old C57BL6 mice on day 0. Mice were injected with PBS buffer or cyclophosphamide (50 mg/kg) intraperitoneally on day 1. Qβ–Tn 6 or Qβ particle was intravenously injected with 20 μg of MPLA on days 1, 4, and 8, while only PBS buffer was injected for PBS group. Survival of mice was monitored for 50 days. Mice free of tumor in Qβ–Tn 6 plus cyclophosphamide group were injected with 5000 TA3Ha cells intraperitoneally, and survival of mice was monitored for another 50 days. As a control, a group of four eight-week old C57BL6 mice received same dose of TA3Ha cells at the same time. Statistical analysis of survival was performed with GraphPad Prism using log-rank test.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the National Cancer Institute (Grant R01CA149451-01A1) for financial support of our work. This work was also supported in part by the intramural research program of the National Institutes of Health, NCI. We thank the Consortium for Functional Glycomics (Grant GM62116; The Scripps Research Institute), T. Tolbert (University of Kansas), L.-X. Wang (University of Maryland), and J. Barchi (National Cancer Institute) for contributing glycans for the microarray.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschem-bio.5b00406.
Information on synthesis and characterization of various Qβ conjugates. Procedures for CD4 lymphocyte depletion experiment, kinetics study of antibody generation, inhibition of Tn binding experiments and procedures for carbohydrate microarray studies. Supplementary Figures S1–S10. Full microarray data (Table S1)(PDF)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv. Exp. Med. Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- (2).Ju TZ, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angew. Chem., Int. Ed. 2011;50:1770–1791. doi: 10.1002/anie.201002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- (4).Freire T, Osinaga E. Immunological and biomedical relevance of Tn antigen. Inmunologia. 2003;22:27–38. [Google Scholar]
- (5).Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- (6).Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Sabbatini PJ, Ragupathi G, Hood C, Aghajanian CA, Juretzka M, Iasonos A, Hensley ML, Spassova MK, Ouerfelli O, Spriggs DR, Tew WP, Konner J, Clausen H, Abu Rustum N, Dansihefsky SJ, Livingston PO. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin. Cancer Res. 2007;13:4170–4177. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- (8).O’Boyle KP, Coatsworth S, Anthony G, Ramirez M, Greenwald E, Kaleya R, Steinberg JJ, Dutcher JP, Wiernik PH. Effects of desialylation of ovine submaxillary gland mucin (OSM) on humoral and cellular immune responses to Tn and sialylated Tn. Cancer Immun. 2006;6:5. [PubMed] [Google Scholar]
- (9).Kagan E, Ragupathi G, Yi SS, Reis CA, Gildersleeve J, Kahne D, Clausen H, Danishefsky SJ, Livingston PO. Comparison of antigen constructs and carrier molecules for augmenting the immunogenicity of the monosaccharide epithelial cancer antigen Tn. Cancer Immunol. Immunother. 2005;54:424–430. doi: 10.1007/s00262-004-0584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Lo-Man R, Vichier-Guerre S, Perraut R, Deriaud E, Huteau V, BenMohamed L, Diop OM, Livingston PO, Bay S, Leclerc C. A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res. 2004;64:4987–4994. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- (11).Buskas T, Thompson P, Boons GJ. Immunotherapy for cancer: synthetic carbohydrate-based vaccines. Chem. Commun. 2009:5335–5349. doi: 10.1039/b908664c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Guo ZW, Wang QL. Recent development in carbohydrate-based cancer vaccines. Curr. Opin. Chem. Biol. 2009;13:608–617. doi: 10.1016/j.cbpa.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Yin Z, Huang X. Recent development in carbohydrate based anticancer vaccines. J. Carbohydr. Chem. 2012;31:143–186. doi: 10.1080/07328303.2012.659364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Monzavi-Karbassi B, Pashov A, Kieber-Emmons T. Tumor-associated glycans and immune surveillance. Vaccines. 2013;1:174–203. doi: 10.3390/vaccines1020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Miles D, Roche H, Martin M, Perren TJ, Cameron DA, Glaspy J, Dodwell D, Parker J, Mayordomo J, Tres A, Murray JL, Ibrahim NK, Theratope Study Group Phase III multicenter clinical trial of the sialyl-Tn (STn)-Keyhole Limpet Hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist. 2011;16:1092–1100. doi: 10.1634/theoncologist.2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Eggermont AMM, Suciu S, Rutkowski P, Marsden J, Santinami M, Corrie P, Aamdal S, Ascierto PA, Patel PM, Kruit WH, Bastholt L, Borgognoni L, Bernengo MG, Davidson N, Polders L, Praet M, Spatz A. Adjuvant ganglioside GM2-KLH/QS-21 vaccination versus observation after resection of primary tumor > 1.5 mm in patients with stage II melanoma: results of the EORTC 18961 randomized phase III trial. J. Clin. Oncol. 2013;31:3831–3837. doi: 10.1200/JCO.2012.47.9303. [DOI] [PubMed] [Google Scholar]
- (17).Blixt O, Lavrova OI, Mazurov DV, Clo E, Kracun SK, Bovin NV, Filatov AV. Analysis of Tn antigenicity with a panel of new IgM and IgG1 monoclonal antibodies raised against leukemic cells. Glycobiology. 2012;22:529–542. doi: 10.1093/glycob/cwr178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Mazal D, Lo-Man R, Bay S, Pritsch O, Deriaud E, Ganneau C, Medeiros A, Ubillos L, Obal G, Berois N, Bollati-Fogolin M, Leclerc C, Osinaga E. Monoclonal antibodies toward different Tn-amino acid backbones display distinct recognition patterns on human cancer cells. Implications for effective immuno-targeting of cancer. Cancer Immunol. Immunother. 2013;62:1107–1122. doi: 10.1007/s00262-013-1425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Itonori S, Hidari K, Sanai Y, Taniguchi M, Nagai Y. Involvement of the acyl chain of ceramide in carbohydrate recognition by an anti-glycolipid monoclonal antibody. - The case of an anti-melanoma antibody, M2590, to GM3-ganglioside. Glycoconju-gate J. 1989;6:551–560. doi: 10.1007/BF01053777. [DOI] [PubMed] [Google Scholar]
- (20).Tagawa Y, Laroy W, Nimrichter L, Fromholt SE, Moser AB, Moser HW, Schnaar RL. Anti-ganglioside antibodies bind with enhanced affinity to gangliosides containing very long chain fatty acids. Neurochem. Res. 2002;27:847–855. doi: 10.1023/a:1020221410895. [DOI] [PubMed] [Google Scholar]
- (21).Galban-Horcajo F, Halstead SK, McGonigal R, Willison HJ. The application of glycosphingolipid arrays to autoantibody detection in neuroimmunological disorders. Curr. Opin. Chem. Biol. 2014;18:78–86. doi: 10.1016/j.cbpa.2014.01.008. [DOI] [PubMed] [Google Scholar]
- (22).Liang CH, Wang SK, Lin CW, Wang CC, Wong CH, Wu CY. Effects of neighboring glycans on antibody-carbohydrate interaction. Angew. Chem., Int. Ed. 2011;50:1608–1612. doi: 10.1002/anie.201003482. [DOI] [PubMed] [Google Scholar]
- (23).Fung PYS, Longenecker BM. Specific immunosuppressive activity of epiglycanin, a mucin-like glycoprotein secreted by a murine mammary adenocarcinoma (TA3-HA) Cancer Res. 1991;51:1170–1176. [PubMed] [Google Scholar]
- (24).Lloyd KO, Burchell J, Kudryashov V, Yin BW, Taylor-Papadimitriou J. Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J. Biol. Chem. 1996;271:33325–33334. doi: 10.1074/jbc.271.52.33325. [DOI] [PubMed] [Google Scholar]
- (25).Hanisch FA. O-glycosylation of the mucin type. Biol. Chem. 2001;382:143–149. doi: 10.1515/BC.2001.022. [DOI] [PubMed] [Google Scholar]
- (26).Osinaga E, Bay S, Tello D, Babino A, Pritsch O, Assemat K, Cantacuzene D, Nakada H, Alzari P. Analysis of the fine specificity of Tn-binding proteins using synthetic glycopeptide epitopes and a biosensor based on surface plasmon resonance spectroscopy. FEBS Lett. 2000;469:24–28. doi: 10.1016/s0014-5793(00)01248-5. [DOI] [PubMed] [Google Scholar]
- (27).Julien S, Picco G, Sewell R, Vercoutter-Edouart A, Tarp M, Miles D, Clausen H, Taylor-Papadimitriou J, Burchell JM. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br. J. Cancer. 2009;100:1746–1754. doi: 10.1038/sj.bjc.6605083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Pollard AJ, Hill AVS. Antibody repertoire: embracing diversity. Sci. Transl. Med. 2011;3:93ps32. doi: 10.1126/scitranslmed.3002694. [DOI] [PubMed] [Google Scholar]
- (29).Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci. Transl. Med. 2011;3:85ra48. doi: 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Wiley SR, Raman VS, Desbien A, Bailor HR, Bhardwaj R, Shakri AR, Reed SG, Chitnis CE, Carter D. Targeting TLRs expands the antibody repertoire in response to a malaria vaccine. Sci. Transl. Med. 2011;3:93ra69. doi: 10.1126/scitranslmed.3002135. [DOI] [PubMed] [Google Scholar]
- (31).Li Q, Rodriguez LG, Farnsworth DF, Gildersleeve JC. Effects of hapten density on the induced antibody repertoire. ChemBioChem. 2010;11:1686–1691. doi: 10.1002/cbic.201000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Yin Z, Comellas-Aragones M, Chowdhury S, Bentley P, Kaczanowska K, BenMohamed L, Gildersleeve JC, Finn MG, Huang X. Boosting immunity to small tumor-associated carbohydrates with bacteriophage Qb capsids. ACS Chem. Biol. 2013;8:1253–1262. doi: 10.1021/cb400060x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Lippman MM, Venditti JM, Kline I, Elam DL. Immunity to a TA3 tumor subline that grows in allogeneic hosts elicited by strain-specific TA3 tumor cells. Cancer Res. 1973;33:679–384. [PubMed] [Google Scholar]
- (34).De Silva RA, Wang Q, Chidley T, Appulage DK, Andreana PR. Immunological response from an entirely carbohydrate antigen: design of synthetic vaccines based on Tn-PS A1 conjugates. J. Am. Chem. Soc. 2009;131:9622–9623. doi: 10.1021/ja902607a. [DOI] [PubMed] [Google Scholar]
- (35).Kuduk SD, Schwarz JB, Chen X-T, Glunz PW, Sames D, Ragupathi G, Livingston PO, Danishefsky SJ. Synthetic and immunological studies on clustered modes of mucinrelated Tn and TF O-linked antigens: the preparation of a glycopeptide-based vaccine for clinical trials against prostate cancer. J. Am. Chem. Soc. 1998;120:12474–12485. [Google Scholar]
- (36).Toyokuni T, Dean B, Cai S, Boivin D, Hakomori S, Singhal AK. Synthetic vaccines: synthesis of a dimeric Tn antigen-lipopeptide conjugate that elicits immune responses against Tn-expressing glycoproteins. J. Am. Chem. Soc. 1994;116:395–396. [Google Scholar]
- (37).Campbell CT, Gulley JL, Oyelaran OO, Hodge JW, Schlom J, Gildersleeve JC. Glyco-immunomics links survival outcomes for PROSTVAC-VF with humoral response to xenoantigen on viral vectors. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E1749–1758. doi: 10.1073/pnas.1314722111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Matsumoto Y, Zhang Q, Akita K, Nakada H, Hamamura K, Tokuda N, Tsuchida A, Matsubara T, Hori T, Okajima T, Furukawa K, Urano T, Furukawa K. pp-GalNAc-T13 induces high metastatic potential of murine Lewis lung cancer by generating trimeric Tn antigen. Biochem. Biophys. Res. Commun. 2012;419:7–13. doi: 10.1016/j.bbrc.2012.01.086. [DOI] [PubMed] [Google Scholar]
- (39).Kawaguchi T, Takazawa H, Imai S, Morimoto J, Watanabe T, Kanno M, Igarashi S. Expression of Vicia villosa agglutinin (VVA)-binding glycoprotein in primary breast cancer cells in relation to lymphatic metastasis: is atypical MUC1 bearing Tn antigen a receptor of VVA? Breast Cancer Res. Treat. 2006;98:31–43. doi: 10.1007/s10549-005-9115-6. [DOI] [PubMed] [Google Scholar]
- (40).Miermont A, Barnhill H, Strable E, Lu XW, Wall KA, Wang Q, Finn MG, Huang X. Cowpea mosaic virus capsid: a promising carrier for the development of carbohydrate based antitumor vaccines. Chem. - Eur. J. 2008;14:4939–4947. doi: 10.1002/chem.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Hubert P, Heitzmann A, Viel S, Nicolas A, Sastre-Garau X, Oppezzo P, Pritsch O, Osinaga E, Amigorena S. Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res. 2011;71:5134–5143. doi: 10.1158/0008-5472.CAN-10-4222. [DOI] [PubMed] [Google Scholar]
- (42).Eisenthal A, Cameron RB, Rosenberg SA. Induction of antibody-dependent cellular cytotoxicity in vivo by IFN-a and its antitumor efficacy against established B16 melanoma liver metastases when combined with specific anti-B16 monoclonal antibody. J. Immunol. 1990;144:4463–4471. [PubMed] [Google Scholar]
- (43).Brode S, Cooke A. Immune-potentiating effects chemotherapeutic drug of the cyclophosphamide. Crit. Rev. Immunol. 2008;28:109–126. doi: 10.1615/critrevimmunol.v28.i2.20. [DOI] [PubMed] [Google Scholar]
- (44).Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- (45).Liu P, Jaffar J, Hellstrom I, Hellstrom KE. Administration of cyclophosphamide changes the immune profile of tumor-bearing mice. J. Immunother. 2010;33:53–59. doi: 10.1097/CJI.0b013e3181b56af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Vandeneijnden DH, Evans NA, Codington JF, Reinhold V, Silber C, Jeanloz RW. Chemical-structure of epiglycanin, the major glycoprotein of the TA3Ha ascites cell - carbohydrate chains. J. Biol. Chem. 1979;254:2153–2159. [PubMed] [Google Scholar]
- (47).Cooper AG, Codington JF, Brown MC. In vivo release of glycoprotein I from the Ha subline of TA3 murine tumor into ascites fluid and serum. Proc. Natl. Acad. Sci. U. S. A. 1974;71:1224–1228. doi: 10.1073/pnas.71.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Lipinski T, Luu T, Kitov PI, Szpacenko A, Bundle DR. A structurally diversified linker enhances the immune response to a small carbohydrate hapten. Glycoconjugate J. 2011;28:149–164. doi: 10.1007/s10719-011-9331-8. [DOI] [PubMed] [Google Scholar]
- (49).Wang QL, Zhou ZF, Tang SC, Guo ZW. Carbohydrate-monophosphoryl lipid A conjugates are fully synthetic self-adjuvanting cancer vaccines eliciting robust immune responses in the mouse. ACS Chem. Biol. 2012;7:235–240. doi: 10.1021/cb200358r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Kaltgrad E, Sen Gupta S, Punna S, Huang CY, Chang A, Wong CH, Finn MG, Blixt O. Anti-carbohydrate antibodies elicited by polyvalent display on a viral scaffold. ChemBioChem. 2007;8:1455–1462. doi: 10.1002/cbic.200700225. [DOI] [PubMed] [Google Scholar]
- (51).Astronomo RD, Kaltgrad E, Udit A, Wang S-K, Doores KJ, Huang C-Y, Pantophlet R, Paulson JC, Wong CH, Finn MG, Burton DR. Defining criteria for oligomannose immunogens for HIV using icosahedral virus capsid scaffolds. Chem. Biol. 2010;17:357–370. doi: 10.1016/j.chembiol.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Cai H, Sun ZY, Chen MS, Zhao YF, Kunz H, Li YM. Synthetic multivalent glycopeptide-lipopeptide antitumor vaccines: impact of the cluster effect on the killing of tumor cells. Angew. Chem., Int. Ed. 2014;53:1699–1703. doi: 10.1002/anie.201308875. [DOI] [PubMed] [Google Scholar]
- (53).Hu QY, Allan M, Adamo R, Quinn D, Zhai HL, Wu GX, Clark K, Zhou J, Ortiz S, Wang B, Danieli E, Crotti S, Tontini M, Brogioni G, Berti F. Synthesis of a well-defined glycoconjugate vaccine by a tyrosine-selective conjugation strategy. Chem. Sci. 2013;4:3827–3832. [Google Scholar]
- (54).Yin Z, Nguyen HG, Chowdhury S, Bentley P, Bruckman MA, Miermont A, Gildersleeve JC, Wang Q, Huang X. Tobacco mosaic virus as a new carrier for tumor associated carbogydrate antigens. Bioconjugate Chem. 2012;23:1694–1703. doi: 10.1021/bc300244a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.