Abstract
The testis of Drosophila resembles an individual testis tubule of mammals. Both are surrounded by a sheath of smooth muscles, which in Drosophila are multinuclear and originate from a pool of myoblasts that are set aside in the embryo and accumulate on the genital disc later in development. These muscle stem cells start to differentiate early during metamorphosis and give rise to all muscles of the inner male reproductive system. Shortly before the genital disc and the developing testes connect, multinuclear nascent myotubes appear on the anterior tips of the seminal vesicles. Here, we show that adhesion molecules are distinctly localized on the seminal vesicles; founder cell (FC)-like myoblasts express Dumbfounded (Duf) and Roughest (Rst), and fusion-competent myoblast (FCM)-like cells mainly express Sticks and stones (Sns). The smooth but multinuclear myotubes of the testes arose by myoblast fusion. RNAi-mediated attenuation of Sns or both Duf and Rst severely reduced the number of nuclei in the testes muscles. Duf and Rst probably act independently in this context. Despite reduced fusion in all of these RNAi-treated animals, myotubes migrated onto the testes, testes were shaped and coiled, muscle filaments were arranged as in the wild type and spermatogenesis proceeded normally. Hence, the testes muscles compensate for fusion defects so that the myofibres encircling the adult testes are indistinguishable from those of the wild type and male fertility is guaranteed.
KEY WORDS: Male fertility, Dumbfounded, Roughest, Sticks and stones, Hibris
Summary: Drosophila testes muscles arise from stem cells and can compensate for fusion defects to safeguard fertility; this plasticity may compensate for the observed lack of satellite cells in Drosophila.
INTRODUCTION
Function and formation of multinuclear myofibres are largely conserved within the animal kingdom. Numerous studies in vertebrates and Drosophila melanogaster have revealed that multinuclear striated myotubes arise by myoblast fusion (Abmayr and Pavlath, 2012). Despite the similarities, only vertebrates possess muscle stem cells, i.e. satellite cells, that allow growth and regeneration of muscles after injury (Wozniak et al., 2005). In Drosophila, the stem cells most similar to satellite cells are the adult muscle precursor cells (Figeac et al., 2007), which allow modification of larval muscles into templates for dorsal longitudinal indirect flight muscles during metamorphosis (Roy and VijayRaghavan, 1998). These stem cells are set aside during embryogenesis and amplify mitotically in the larvae (Bate et al., 1991). Most of them are associated with imaginal discs as adepithelial cells in late third instar larvae. During metamorphosis, they build the adult musculature of the legs, thorax, and female and male reproductive organs.
Muscle development in the Drosophila embryo has been studied intensively. In the embryo, heterotypic myoblasts recognize and adhere to each other. After signal transduction from the cell surface into the cell via adaptor proteins, F-actin reorganizes at the site of cell-cell contact, the opposing membranes are vesiculated and cytoplasmic continuity is established (Haralalka and Abmayr, 2010; Önel et al., 2014). In this process, several molecular players relevant for the formation of multinuclear myofibres have functional redundancies (Bonn et al., 2013; Duan et al., 2012; Hakeda-Suzuki et al., 2002; Hornbruch-Freitag et al., 2011). Well-studied examples of redundancy during myoblast fusion are cell adhesion molecules of the immunoglobulin superfamily (IgSF), namely Dumbfounded [Duf; also known as Kin of irre (Kirre)], and Roughest (Rst), which are expressed in founder cells (FCs) (Bate, 1990; Ruiz-Gómez et al., 2000). The genes encoding Duf and Rst are localized in the same region on the genome (St Pierre et al., 2014) and only the deletion of both leads to lack of fusion and embryonic lethality before sarcomere formation. Expression of Duf or Rst alone can rescue the deletion phenotype (Ruiz-Gómez et al., 2000; Strünkelnberg et al., 2001). Fusion-competent myoblasts (FCMs) express Sticks and stones (Sns) and Hibris (Hbs). Loss of Sns leads to a nearly complete block of fusion, whereas Hbs seems to be less essential (Bour et al., 2000; Dworak et al., 2001; Shelton et al., 2009).
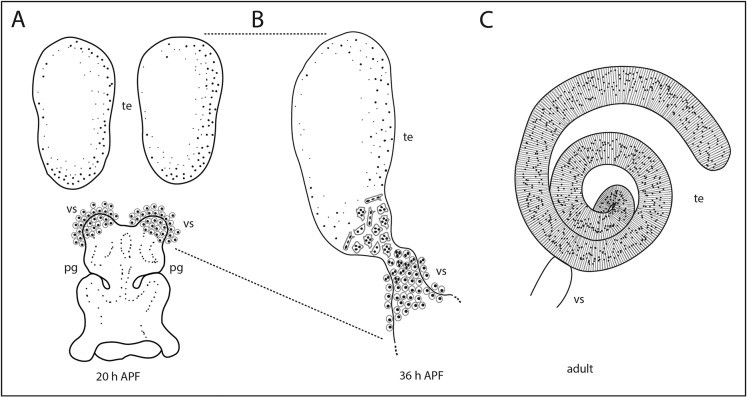
All muscles of the Drosophila male reproductive system originate from adepithelial cells of the sexually dimorphic genital disc (Ahmad and Baker, 2002; Estrada et al., 2003; Kozopas et al., 1998). During metamorphosis, parts of the genital disc differentiate into the prospective seminal vesicle (vs) and the paragonia (pg) (Fig. 1A). The epithelial cells of the seminal vesicle and the developing testes connect to each other so that muscle precursors can migrate from the seminal vesicles onto the testes (Fig. 1B). Evidence from transplantation experiments and cultures of pupal testes indicates that the connection between the seminal vesicles and testes is essential for outgrowth and shaping of the testes (Gärtner et al., 2014; Kozopas et al., 1998; Nanda et al., 2009; Stern, 1941a,b). Different types of muscles can be found around the inner male genitalia, specifically multinuclear smooth-like myofibres surrounding the testes, multinuclear striated muscles of the sperm pump and a number of mononuclear striated muscles (Susic-Jung et al., 2012). In contrast to striated muscles, smooth muscles lack the regular arrangement in a repetitive pattern of sarcomeres with Z-discs and regular pattern of Myosins in the middle and F-actin linked to the Z-disc (Au, 2004). Smooth muscle cells are a heterogeneous group and they are well studied in mammals (Ali et al., 2005; Matsumoto and Nagayama, 2012). By contrast, all muscles of the female reproductive organs are mononuclear and striated (Hudson et al., 2008).
Fig. 1.
Scheme of the origin and development of the testes muscles. (A) Genital disc 20 h after puparium formation (APF) contains a pool of myoblasts on the protruding seminal vesicles (vs). The paired testes (te) are free of myoblasts. (B) By 36 h APF, the testis and the developing seminal vesicle are fused. Multinucleated nascent myotubes migrate onto the testis. (C) The adult testis is surrounded by a sheath of multinuclear smooth-like muscles. Modified after Bodenstein (1950); Susic-Jung et al. (2012).
The molecular players responsible for the development of the muscles of the Drosophila male reproductive system and their mechanisms of action remain mostly unstudied. Likewise, little is known about the origin, development and function of mammalian peritubular myoid cells, i.e. the smooth muscle cells that enclose the seminiferous tubules of the testes where spermatozoa are generated (Svingen and Koopman, 2013). Recent analyses of the development of the different reproductive tract muscles of Drosophila have provided the first evidence that Duf, Sns and Hbs are relevant for arranging the multinuclear smooth-like muscles encircling the testes (Susic-Jung et al., 2012). In the present study, we focused on (1) how the multinuclear state of testes muscles is achieved; (ii) the role in this process of the IgSF proteins known from embryonic myogenesis (in particular, we asked whether these adhesion molecules act also redundantly during the development of the testes muscles); and (3) how loss of the adhesion molecules affects the formation of the male reproductive system and its musculature.
We show that the multinuclear smooth-like myofibres of the testes arise by fusion of two cell types resembling FCMs and FCs with respect to their heterotypic expression of IgSF proteins. All four IgSF proteins were involved in this process, although apparently with a function different to that in embryonic myoblast fusion. Importantly, even when fusion was reduced, the male reproductive tract developed as in the wild type. In this case, the smooth-like muscles displayed organized myofibres. Thus, our results reveal a high plasticity of smooth muscle formation.
RESULTS
Multinuclear myoblasts are found on the developing seminal vesicles of male genital discs
Myoblasts are characterized by the activity of the Mef2 gene, which encodes the highly conserved muscle-specific transcription factor Mef2 (Bour et al., 1995; Lilly et al., 1994; Nguyen et al., 1994; Taylor et al., 1995). We established transgenic fly lines expressing UAS-mCD8-GFP under the control of Mef2-Gal4 allowing ectopic expression in all myoblasts to follow myogenesis during metamorphosis with a live marker.
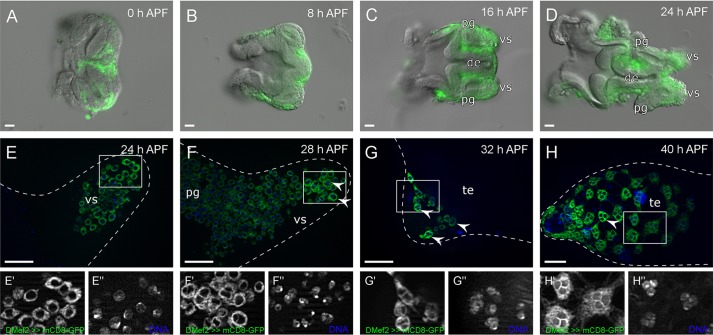
Myoblasts positive for Mef2-driven mCD8-GFP expression were present on the genital disc already at the onset of metamorphosis (Fig. 2A). During the first hours of metamorphosis, these myoblasts proliferated and increased in number [Fig. 1B,C, and monitored by anti-phosphorylated histone H3 (Ser10) mitosis marker staining in Fig. S1]. Myoblasts were found, 16 h after puparium formation (APF), in clusters on the prospective seminal vesicles, paragonia and ejaculatory duct (Fig. 2C). By 24 h APF, the primordia of the paragonia and the seminal vesicles were clearly distinguishable, and mCD8-GFP-positive myoblasts were particularly prominent on the seminal vesicles (Fig. 2D). These myoblasts will mainly give rise to the multinuclear smooth musculature of the testes and were still mononuclear at 24 h APF (Fig. 2E-E″). We first observed binuclear mCD8-GFP-positive myoblasts at 28 h APF, when the seminal vesicles grew closer towards the testes (Fig. 2F-F″). Here, mCD8-GFP expression is visible mainly in the cytoplasm and not efficiently integrated into the plasma membrane. These multinuclear myoblasts were restricted to the myoblast layer over the most anterior part of the prospective seminal vesicle (Fig. 2F, arrowheads); the paragonium still contained only mononuclear myoblasts. Up to this point, genital disc and testes are not attached and Mef2-positive cells cannot be found on the developing testes (Bodenstein, 1950; Susic-Jung et al., 2012). All Mef2-positive cells arriving on the testes at around 32 h APF are already multinuclear (Fig. 2G-G″). Those multinuclear mCD8-GFP-expressing cells cover the testes at 40 h APF (Fig. 2H-H″). Mononuclear Mef2-positive myoblasts were not observed on testes. These results showed that myoblasts amplify during metamorphosis and first become multinuclear on the prospective seminal vesicles of the male genital disc at around 28 h APF. We propose that further fusion leads to three, or rarely more, nuclei of the individual nascent myoblasts. This multinuclearity appears to be completed when the myotubes reach the testes.
Fig. 2.
Myoblasts on the male genital disc become multinuclear right before they migrate onto the testes. (A) Myoblasts labelled by Mef2-driven UAS-mCD8-GFP expression (green) on male genital discs at the onset of puparium formation, (B) 8 h APF, (C) 16 h APF and (D) 24 h APF. (E) Additional Hoechst staining (blue) of DNA in the myoblast nuclei 24 h APF, (F) 28 h APF genital discs, and (G) 32 h and (H) 40 h testes. Single-channel magnifications of the boxed areas are displayed below. E,F,G and H, optical sections. Arrowheads, multinuclear myoblasts on the most anterior tips of the seminal vesicles or the testes. pg, paragonium; vs, seminal vesicle; de, ejaculatory duct; te, testis. Scale bars: 20 µm.
The multinuclear state of testes muscles is probably achieved by cell-cell fusion
To our knowledge, all multinuclear striated muscles in Drosophila arise by fusion of myoblasts. However, the other mechanism of generating multinuclear cells is by cytokinesis failure, i.e. nuclear division without cytokinesis. This process occurs in the early development of the Drosophila embryo and in mammalian heart muscles (Foe et al., 1993; Li et al., 1996; Liu et al., 2010). Thus, we investigated whether syncytial smooth-like muscles of the testes develop by cell cycle arrest after nuclear division. We labelled replicating DNA with the thymidine analogue 5-ethynyl-2′-deoxyuridine (EdU), which can be detected by covalent binding of a fluorescent azide.
Male pupae with Mef2-driven mCD8-GFP were injected with EdU at 24 h APF, shortly before the first binuclear myoblasts formed. After incubation for 8 h, the pupae were dissected and fixed, EdU and GFP fluorescent labels were analysed. During the 8 h incubation, myoblasts became multinuclear and migrated from the male genital disc onto the testis. On the testis, myoblasts expressing mCD8-GFP were detected in the basal area (Fig. 3A, left). At higher magnification, no EdU signal was detected in the nuclei of these nascent myotubes (Fig. 3A′). By contrast, germ cells in the apical part of the testis (Fig. 3A, right) were clearly labelled with EdU (Fig. 3A″, arrow). This demonstrated the progression of development, as well as successful incorporation and detection of EdU in dividing germ cells as an intrinsic positive control. On the basis of these results, we concluded that the multinuclear state of testes muscles arises in a cell-cycle-independent manner.
Fig. 3.
Testes myoblasts fuse to generate multinuclear myotubes. (A-A″) Labelling of replicating DNA with 5-ethynyl-2′-deoxyuridine (EdU, red; arrow in A″) in pupal testis at 24 h APF, followed by incubation for 8 h. Myoblasts were labelled by Mef2-driven UAS-mCD8-GFP expression (green). Asterisk, hub region; magnified areas shown in A′ and A″ are indicated in A. Additional Hoechst staining (blue) of DNA (A′) in the basal area and (A″) in the apical part of the testis. (B-B″) Visualization of rp298-lacZ (red) in nascent myotubes (green) in pupal testes at 40 h APF; B is a merged photo of B′ and B″ with additional Hoechst staining. Arrowhead, rp298-lacZ positive nucleus. A′,A″ and B-B″, optical sections. Scale bars: 20 µm.
To further investigate how the multinuclear testes muscles are formed, we analysed the expression pattern of the rp298-lacZ enhancer trap (Nose et al., 1998). This transgene follows the activity of the duf enhancer and is located to the nucleus by the NLS of the transposase (Mlodzik and Hiromi, 1992). In the Drosophila embryo, rp298-lacZ can be found in nuclei of FCs, but not in FCMs. After fusion, all nuclei in the body wall muscles are positive for rp298-lacZ (Nose et al., 1998). By contrast, multinuclear myotubes of testes at 40 h APF expressed rp298-lacZ predominantly in only one nucleus (Fig. 3B-B″, arrowhead), as has been described for binuclear circular visceral muscles (Klapper et al., 2002). This finding suggested that the nuclei of one testis muscle originate from different myoblasts.
Taken together, these data indicated that nuclear division with cytokinesis failure does not occur during the development of Drosophila testes muscles. We thus concluded that a myoblast fusion process is obligatory in this context and therefore investigated whether testes myoblast fusion is comparable to the FC- and FCM-based mechanism by which striated muscles are established in Drosophila.
Myoblasts on the prospective seminal vesicles show differences in IgSF expression
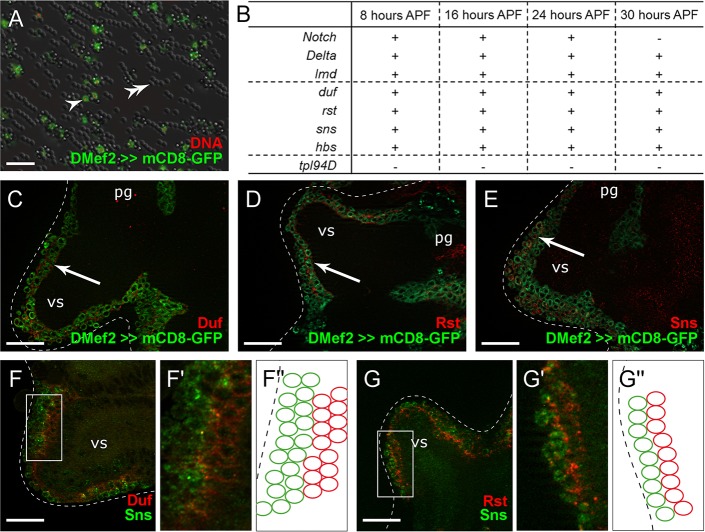
To gain insights into the molecular players involved in testes myoblast fusion, we checked for the presence of transcripts of known myogenesis-relevant genes in the myoblasts of male genital discs at different time points during metamorphosis. We established a protocol to purify myoblasts from male genital discs. Total RNA of age-specific mCD8-GFP-positive myoblasts was isolated (Fig. 4A) and used for RT-PCR analyses (Fig. 4B, Table S1). Hoechst staining of DNA revealed the high purity of isolated myoblasts (Fig. 4A).
Fig. 4.
Adhesion molecules are transcribed and expressed in myoblasts on the male genital disc. (A) Myoblasts expressing Mef2-driven UAS-mCD8-GFP (green, arrowhead) purified using magnetic beads (double arrowhead). Hoechst staining of DNA (red). (B) RT-PCR results using RNA from purified myoblasts at 8 to 24 h APF. (C-E) Staining in male genital discs at 24 h APF in a Mef2-driven UAS-mCD8-GFP background with antibodies against (C) Duf, (D) Rst and (E) Sns. Arrows, myoblasts located on the prospective seminal vesicles. (F-G″) Double staining in male genital discs at 24 h APF with antibodies against (F,F′) Sns and Duf and (G,G′) Sns and Rst. F′ and G′ are enlargements of boxed areas shown in F and G, respectively. F″ and G″ are schematic depictions of the antibody distribution seen in F′ and G′. C-G′, optical sections. vs, seminal vesicle; pg, paragonium. Scale bars: 20 µm.
Initially, we investigated whether distinct myoblasts similar to embryonic FCMs and FCs exist on the genital disc. In the embryo, Notch/Delta-mediated lateral inhibition leads to specification of FCs and FCMs (Baker and Schubiger, 1996; Carmena et al., 1995; Corbin et al., 1991). We confirmed active transcription of the gene encoding the Notch receptor and the gene encoding its ligand Delta in purified myoblasts (Fig. 4B), and lack of transcription of the spermatid specifically expressed negative control Transition protein like94D (Tpl94D) (Rathke et al., 2007). We also demonstrated transcription of the FCM-specific determination factor lame duck (lmd) (Duan et al., 2001) and FC-specific transcription factors (Fig. 4B, Table S1). Several, but not all, genes encoding transcriptional regulators conferring FC or muscle identity in the embryo were transcribed in these myoblasts (Table S1). Thus, we hypothesize that there are FC- and FCM-like myoblasts on male genital discs.
We further investigated whether myoblasts express the genes encoding IgSF proteins that initiate embryonic myoblast fusion. Using gene-specific oligonucleotides, we were able to amplify the adhesion molecule transcripts duf, rst, sns and hbs from myoblast RNA of genital discs 8, 16, 24 and 30 h APF (Fig. 4B), i.e. not only during fusion events, but also earlier. However, this method cannot distinguish between transcripts from testes myoblasts and transcripts from myoblasts that give rise to other muscles of the reproductive tract, such as multinuclear striated muscles of the sperm pump. Therefore, to determine the precise expression pattern and the subcellular localization of these IgSF proteins, we used immunofluorescence staining. In the prospective seminal vesicles of genital discs at 24 h APF, anti-Duf (Fig. 4C), anti-Rst (Fig. 4D) and anti-Sns (Fig. 4E) signals were detected in the membranes of Mef2-positive myoblasts (arrows). The adhesion molecules were distributed evenly over the plasma membrane and not localized to specific regions; as mCD8-GFP expression was localized further into the myoblast than IgSFs, we assume that mCD8 is mainly cytoplasmic in these cells (see also Fig. 2). Only a subset of myoblasts was stained with each antibody; Duf and Rst appeared to be enriched in myoblasts adjacent to the epithelium of the primordial seminal vesicles (Fig. 4C,D), whereas Sns accumulated in myoblasts lying on the periphery of the tissue (Fig. 4E). Furthermore, we did not observe colocalization of Sns and Duf (Fig. 4F,F′) or of Sns and Rst (Fig. 4G,G′) in double staining, which indicated expression of Sns in myoblasts other than those that expressed Duf and Rst. We hypothesize that these different subsets of myoblasts fuse to each other to generate smooth-like testes muscles. Consequently, we refer to them as FC-like myoblasts and FCM-like cells. FC-like myoblasts express Duf and Rst, whereas FCM-like cells mainly express Sns.
Knockdown of Duf, Rst or Sns leads to a lower nuclei number in testes muscles
As the adhesion molecules Duf, Rst and Sns were expressed, we analysed whether their presence is essential for the formation of multinuclear testes muscles. As hbs transcript could be detected in myoblasts isolated from the genital disc, we included hbs in this functional study. We down-regulated duf, rst, sns and hbs specifically in myoblasts using Mef2-Gal4-driven RNA interference (RNAi). The adult-muscle-specific driver line 1151-Gal4 did not reveal any activity in the myoblasts of the male genital disc and was therefore excluded from further studies (data not shown). Because Duf and Rst can act in redundancy to each other, we additionally downregulated both duf and rst in a duf-RNAi;rst-RNAi double knockdown. For comparison, we expressed Dicer-2 (Dcr-2) in the RNAi background, because it enhances the effect of RNAi (Dietzl et al., 2007).
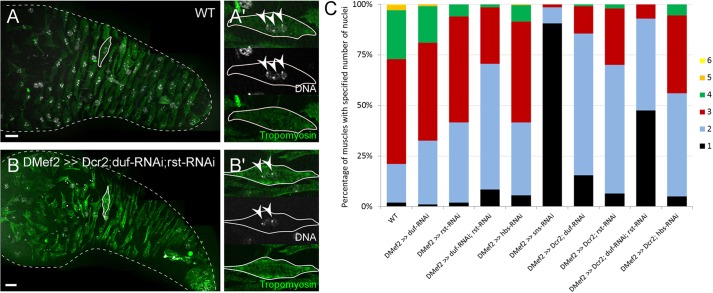
As the muscle sheath tightly encircles the adult testis, single muscles and their cell nuclei are difficult to visualize; we saw only 1-2 nuclei on one side of the testes (Susic-Jung et al., 2012; Fig. 1C). First nascent myotubes reach the testes at 32 h APF (Fig. 2G) and are still individually distinguishable at 48 h APF; they did not surround the testes at this time (Fig. 2H, 40 h APF). At these time points, only multinucleated nascent myotubes but no mononuclear myoblasts were observed on the testes (Fig. 2E,F). We counted the number of nuclei in nascent myotubes on wild-type testes at 36 h, 42 h and 48 h APF (Table S2). The number of nuclei did not increase during ongoing metamorphosis, making further fusion on the testes unlikely. We conclude that fusion mainly takes place on the genital disc. Therefore, we analysed testes from pupae around 42 h APF, because migrating nascent myotubes have not stretched to their full size, which allows individual myotubes to be clearly recognized and the number of nuclei per muscle to be easily determined (Fig. 5A,B).
Fig. 5.
Knockdown of adhesion molecules leads to reduced nuclei number in testes muscles. (A,B) Visualization of muscles and nuclei using anti-Tropomyosin (green) and Hoechst staining (white) of (A) wild-type myotubes and (B) myotubes expressing Mef2-driven Dcr-2, duf-RNAi; rst-RNAi. Images are merged photos of single optical sections. The outlined muscle is displayed in split channels in A′,B′. Arrowheads, muscle nuclei. Scale bars: 20 µm. (C) Determination of nuclei number of nascent myotubes in which duf, rst, duf;rst, hbs and sns were downregulated by Mef2-driven RNAi and in wild-type testes at about 42 h APF. In each case, 200 muscles (100% of the cells) were counted; the length of each colour-coded bar in each stack indicates the percentage of muscles with the specified number of nuclei (see Table S2 for details).
In the wild type, the majority of nascent myotubes comprised 3 nuclei, and some had 2 or 4 nuclei (Fig. 5C); the average was 3.1 nuclei. In rare cases, mononuclear myotubes or myotubes with 5 or 6 nuclei were observed. In myotubes in which duf, rst, hbs or sns were individually downregulated, the number of nuclei was reduced (Fig. 5C, see also Figs S2, S3 and Table S2 for controls and details). Co-expression of Dcr-2 with the RNAi constructs led to a more severe reduction in nuclei number (Fig. 5C); expression of either duf-RNAi or rst-RNAi with Dcr-2 increased the number of mono- and binuclear myotubes from the observed 21% in the wild type to 85% and 70%, respectively. The average number of nuclei declined to 2 and 2.3, respectively. Knockdown of hbs resulted in an average number of 2.4 nuclei, which was slightly lower than that of the controls. When both duf and rst were downregulated, the number of nuclei was reduced further than in the single knockdowns. Again, co-expression of Dcr-2 led to a more severe reduction in the nuclei number; mono- and binuclear muscles were observed in 93% of the myotubes, with an average nuclei count of about 1.6. Expression of a sns-RNAi construct by Mef2-Gal4 led to the most severe reduction in nuclei, with an average of about 1.1; more than 90% of myotubes remained mononuclear. Furthermore, these pupae did not develop into adults. Co-expression of Dcr-2 with sns-RNAi resulted in early pupal lethality and therefore could not be analysed. From these findings, we concluded that the number of nuclei in testis muscles depends on the IgSF proteins that initiate myoblast fusion in the embryo.
Development of the male reproductive tract and spermatogenesis appears normal despite a severe reduction in fusion efficiency
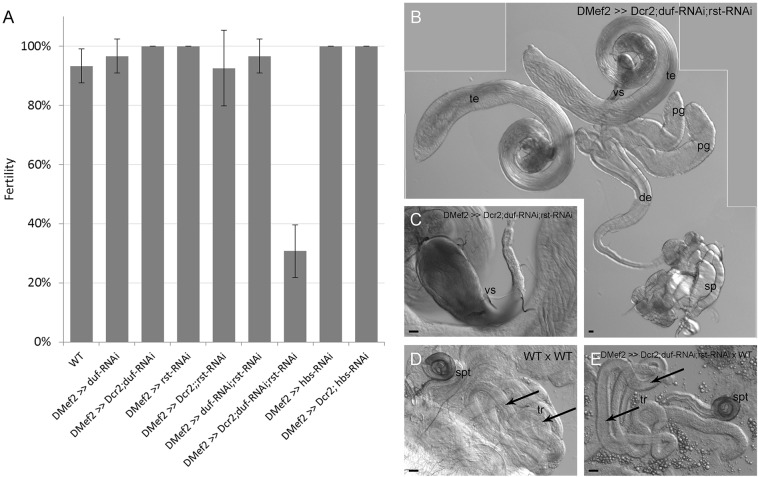
We then analysed whether the nuclei number of testes muscles affects functionality. We tested whether males in which duf, rst or hbs were downregulated were fertile. Three virgin wild-type females were mated with single males for 7 days; we tested up to 30 RNAi knockdown males. Offspring were evaluated 14 days after mating started. sns-RNAi males could not be analysed because depletion of sns led to pupal lethality.
Males with downregulated duf, rst or hbs, alone or in combination with Dcr-2 as well as the duf;rst double knockdown produced offspring, which indicated normal fertility. Only males with Mef2-driven duf;rst double knockdown in the Dcr-2 background had reduced fertility (Fig. 6A). Only 31% of single crossings produced offspring, and the number of offspring was lower than in the other males (Table S2 and Fig. S4); 69% were infertile. The reproductive tract of these infertile males had no visible blockages, constrictions or other defects (Fig. 6B). The seminal vesicles were filled with motile sperm (Fig. 6C, Movie 1); no obvious defects in spermatogenesis were detected. To identify the cause of reduced fertility in these flies, we first checked the mated wild-type females that failed to produce progeny. In wild-type female reproductive organs, sperm are stored in the spermatheca (spt) and the tubular receptacles (tr; Fig. 5D). The spermatheca and tubular receptacles of females mated with RNAi knockdown males were free of sperm (compare Fig. 6D and E). Subsequently, we examined the fertility of Mef2-driven Dcr-2;duf-RNAi;rst-RNAi females by crossing them with wild-type males. Their fertility was also severely reduced, showing that the effect was not sex-specific as would be expected for defects due to muscles of the testes. Furthermore, these males and females were able to crawl and jump but were unable to fly (Table S2). As sperm production in the males is not disturbed and females display reduced fertility, we assume that the cause of reduced fertility in these flies is a general problem in behaviour and/or movement rather than defective testes muscle development. We also conclude that except for Dcr-2;duf-RNAi;rst-RNAi, a reduced number of nuclei in the testes muscles does not necessarily interfere with male fertility.
Fig. 6.
RNAi-mediated knockdown of duf, rst or hbs in the reproductive tract musculature does not influence male fertility. (A) Fertility of duf, rst, duf;rst and hbs knockdown males compared with wild-type males. Error bars represent s.d. (B) Reproductive tracts of infertile males (merged photograph). (C) Seminal vesicles of infertile males are filled with mature sperm. (D) Wild-type females mated with wild-type males contain sperm in their tubular receptacles (arrows) or spermatheca. (E) Reproductive tracts of females of infertile crossings with RNAi knockdown males are free of sperm (arrows). spt, spermatheca; tr, tubular receptacle; te, testis; vs, seminal vesicle; pg, paragonium; de, ejaculatory duct; sp, sperm pump. Scale bars: 10 µm in B, 20 µm in C-E.
Knockdown of duf, rst or hbs leads to a wild-type adult muscle pattern with a normal filament arrangement
As fertility was not affected by RNAi-mediated knockdown of duf, rst or hbs, the question arose whether testes muscles are formed correctly despite a reduced nuclei number. We analysed the overall shape and filament arrangement of adult reproductive tracts of RNAi knockdown males using F-actin and DNA staining. The testes of all RNAi knockdown flies had the characteristic coiled morphology of the wild type, and as already mentioned, the adult reproductive tracts did not display any visible defects, constrictions or other alterations compared with the wild type (Fig. 6B, Dcr-2;duf-RNAi;rst-RNAi; other knockdown flies not shown). Furthermore, the muscles from hbs-RNAi, duf-RNAi, rst-RNAi and duf-;rst-RNAi knockdown males did not display obvious defects. Fibre distribution and filament arrangements of the testes muscle sheaths and the sperm pump musculature were normal when observed under light microscopy (Fig. 7, Dcr-2;duf-RNAi;rst-RNAi; other knockdown flies not shown) independent of the co-expression of Dcr-2. In addition, no defects were found in the muscle sheaths of paragonia, seminal vesicles or ejaculatory ducts of any of the tested RNAi knockdown flies (Fig. S5). Adult males with a reduced Sns level could not be analysed because sns-RNAi is pupal lethal.
Fig. 7.
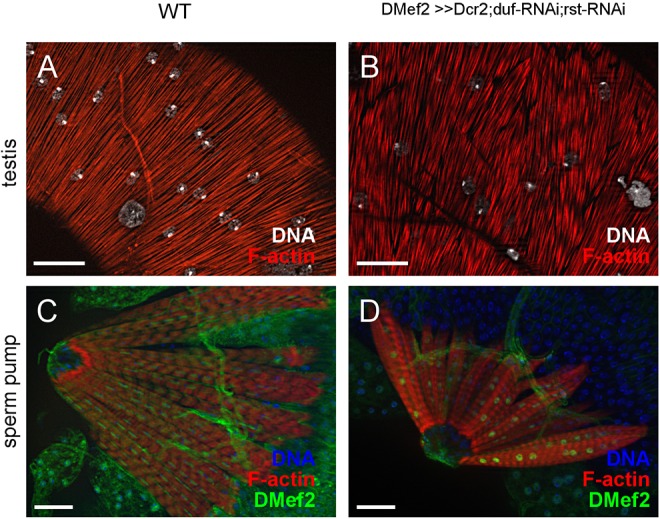
Knockdown of duf and rst during development does not lead to defects in filament organization in adult reproductive muscles. Filament arrangement of (A,C) wild-type and (B,D) duf-RNAi;rst-RNAi double knockdown in (A,B) smooth-like testes muscles and sarcomere pattern of (C,D) multinuclear sperm pump muscles. F-Actin (red) was visualized with Phalloidin; Hoechst dye (white in A and B; blue in C and D) was used to label DNA in nuclei. In C and D, anti-Mef2 antibody (green) was used to detect muscle nuclei. All photographs are optical sections. Scale bars: 20 µm.
Our results show that knockdown of IgSF proteins resulted in a reduced nuclei number in testes muscles. Surprisingly, this did not affect (1) the migration of nascent myotubes from the seminal vesicles onto the testes, (2) the morphogenesis of the testes from the larval to the adult shape, (3) filament arrangement and integrity of the reproductive tract musculature and (4) the fertility of the males. Hence, we propose that testes muscles possess mechanisms to compensate reduced fusion.
DISCUSSION
Despite the differences between the diverse types of striated muscles of Drosophila, they share common mechanisms during development. For example, the expression pattern of Duf suggests that Duf plays a similar role in the development of larval muscles (Ruiz-Gómez et al., 2000), leg muscles (Soler et al., 2004), flight muscles (Gildor et al., 2012) and abdominal muscles (Dutta et al., 2004). The cellular adhesion mediated by the IgSF proteins and their signalling in myoblasts is essential for myoblast fusion and formation of striated muscles.
Smooth-like testes muscles arise from heterotypic fusion of FC-like and FCM-like cells, localized in two distinct myoblast layers
It is unclear how the special smooth-like testes muscles obtain their multinuclear state. In general, cells can fuse or skip cleavage after nuclear division to become multinuclear (Gentric and Desdouets, 2014; Lacroix and Maddox, 2012). It was shown that garland cell nephrocytes can overcome fusion defects by cell division without cytokinesis (Zhuang et al., 2009). In the male reproductive organs, the binuclear epithelial cells of the paragonia arise by cytokinesis skipping (Taniguchi et al., 2014). By contrast, our study demonstrated that multinuclearity in testes muscles is achieved by myoblast fusion. Only one of the nuclei of these small syncytia is positive for rp298-lacZ, as we observed previously for the bi-nucleated circular visceral muscles in the embryo (Klapper et al., 2002). We proposed that rp298-lacZ – reflecting Duf activity – is no longer transcribed after fusion and thus, no new β-galactosidase is synthesized in the cytoplasm and the protein cannot therefore be imported into the other nuclei of these small syncytia. Thus, downregulation of duf transcription could limit the degree of fusion, resulting in small syncytia. By contrast, in the somatic mesoderm, Menon et al. (2005) proposed that reshuffling Duf and its adaptor protein Rols to the membrane provides a mechanism that regulates the rate of fusion to yield larger syncytia in agreement with the observation that all nuclei of the syncytia are positive for rp298 (Menon et al., 2005).
Furthermore, we found that characteristic IgSF molecules of embryonic myoblast fusion were expressed distinctly in these myoblasts. This is in agreement with previous expression patterns of sns and duf reporter constructs (Susic-Jung et al., 2012). The FCM- and FC-like cell status of two different populations of myoblasts is supported by our finding of only one rp298-positive nucleus in the small syncytia, suggesting that this nucleus of one testis muscle cell originates from a different myoblast to the others.
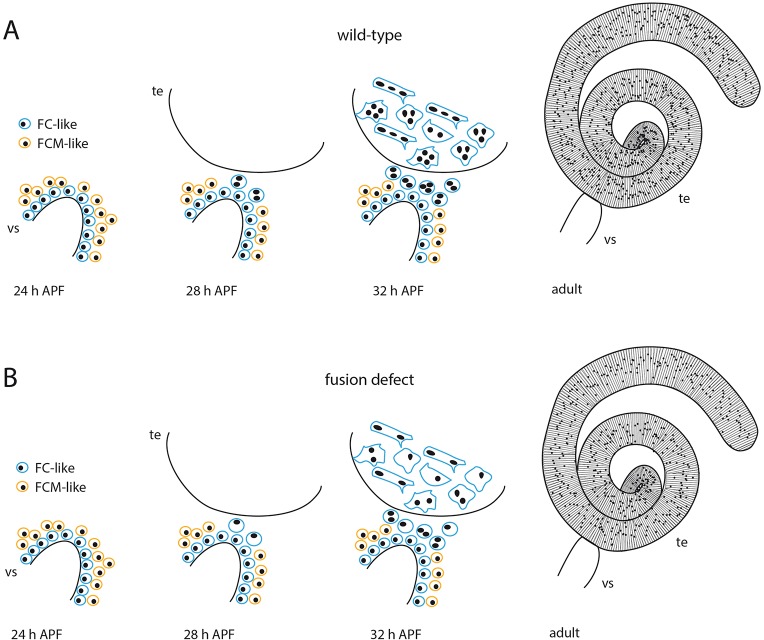
Antibody staining revealed that FC-like myoblasts express Duf and Rst and form a basal layer directly adjacent to the epithelium, whereas the FCM-like cells express Sns and lie more in the periphery (schematized in Fig. 8). In the embryo, adhesion molecules are mainly visible at the opposing membranes of growing myotubes and FCMs (Kesper et al., 2007; Rochlin et al., 2010; Sens et al., 2010; Önel et al., 2011). By contrast, testes myoblasts expressed adhesion molecules along their entire surface, which indicates discernible differences in the formation of the different muscle types.
Fig. 8.
Scheme of the distinct layers of FC-like and FCM-like cells that fuse on the seminal vesicle to generate the testes muscles. (A) Myoblasts on the seminal vesicle (vs) are separated into two layers. FC-like myoblasts (blue) express Duf and Rst and lie adjacent to the epithelium of the seminal vesicle, whereas FCM-like cells (yellow) express Sns and appear to be located on the periphery. In heterotypic fusion events, FC- and FCM-like cells fuse to form multinuclear nascent myotubes. These myotubes then migrate onto the testes (te). The adult testis is tightly encircled by multinuclear muscles. (B) The knockdown of IgSF molecules impairs the fusion process. Nascent myotubes with a reduced nuclei number still migrate from the seminal vesicle onto the testes and are able to enclose the adult testes as in the wild type.
Duf and Rst might act independently, not redundantly, to create smooth-like myofibres
Knockdown of duf, rst or sns during metamorphosis led to reduced fusion processes in each muscle. Thus, we conclude that myoblast fusion during development of multinuclear smooth-like testes muscles shares common molecular players with previously studied myoblast fusion processes of Drosophila and vertebrates. However, we observed a distinct mode of action of Duf and Rst during testes-relevant myoblast fusion. Fusion was also reduced when only duf or rst was knocked down. In the embryo, only a double knockout leads to defects, whereas the reintroduction of either Duf or Rst is sufficient to rescue the double-mutant phenotype (Ruiz-Gómez et al., 2000; Strünkelnberg et al., 2001). In thoracic muscle development during metamorphosis, duf-knockdown myoblasts fuse like the wild type, but duf-RNAi;rst-RNAi double-knockdown flight muscles have severe fusion defects, which also indicates a redundant function of Duf and Rst (Gildor et al., 2012). By contrast, Duf and Rst cannot completely substitute for each other in the arrangement of ommatidia during eye formation (Ramos et al., 1993; Reiter et al., 1996). Our data demonstrate that in testes myoblast fusion, the phenotype of the single RNAi knockdown is enhanced when both genes are knocked down simultaneously, which indicates an additive effect.
Fusion is restricted to the myoblasts at the anterior tip of the seminal vesicles
We observed that testes myoblast fusion was restricted to a small area of the most anterior tip of the prospective seminal vesicles (Figs 2 and 8). Furthermore, our RNAi data indicated that proper expression of Duf, Rst and Sns is crucial for testes myoblast fusion to proceed. As adhesion molecules were expressed in neighbouring FC- and FCM-like cells long before fusion occurs, we conclude that these molecules alone are not sufficient to trigger fusion of adjacent myoblasts. In other fusion processes, key regulators called fusogens are required and are sufficient for fusion to proceed (Aguilar et al., 2013). To date, the fusogen in myoblasts of Drosophila has not been identified. Our results demand an accurate spatially and temporally regulated expression of a potential fusogen within the testes myoblasts. Therefore, there might be an extrinsic signal that triggers fusion solely in the myoblasts over the seminal vesicles. Alternatively, fusion might be repressed in all other myoblasts. Clarification of these possibilities will be a focus of future research.
Compensation of fusion failure leads to correct filament organization of testes muscles, shaping of the testes and male fertility
In Drosophila and vertebrates, reduced fusion of striated muscles can lead to severe defects or embryonic lethality (Abmayr and Pavlath, 2012; Rochlin et al., 2010; Önel and Renkawitz-Pohl, 2009). Furthermore, defective morphogenesis of the reproductive system can induce male sterility (Linnemannstons et al., 2014). However, despite a reduction in fusion in the knockdown testes muscles, no obvious defects in formation of the reproductive tract and progression of spermatogenesis were detected in the resulting adult males. The seminal vesicles were always connected to the testes, and the myotubes migrated onto the testes. Even the filament arrangement in these flies appeared normal in light microscopy, independent of the level of the fusion block (Fig. 7, Fig. 8B). Thus, we assume that testes muscles remain functional even if fusion is reduced. We conclude that progression of myoblast fusion is not required for further development of the testes muscles, including attachment, filament production and arrangement. Kreisköther et al. (2006) showed that sarcomeres of striated larval muscles are established very late in embryogenesis after muscles attached to the epidermis. This process seems to proceed independent of myoblast fusion in some muscles also in fusion mutants (Drysdale et al., 1993). Similar observations have been reported for striated muscles in zebrafish; mutants of the IgSF genes jamb and jamc display fusion defects during fast-twitch muscle development, whereas elongation and sarcomere formation are not disturbed (Powell and Wright, 2011).
In summary, our results indicate that testes muscles compensate for fusion defects in a manner not previously reported. We suggest that testes muscles have an enormous potential for increasing the plasticity of smooth muscles. We propose that this plasticity compensates for the lack of satellite cells in Drosophila and their ability to regenerate muscle defects and thereby safeguards reproductive capability.
MATERIALS AND METHODS
Fly stocks
Flies were maintained and RNAi was performed in Drosophila standard medium at 25°C. w1118 (BL6326) was used as the wild-type control. The following transgenic flies were used: rp298-lacZ (Nose et al., 1998), Mef2-Gal4 (Ranganayakulu et al., 1995), UAS-Dcr-2;Mef2-Gal4 (BL25756), UAS-mCD8-GFP (BL32186), UAS-duf-RNAi (V3111), UAS-rst-RNAi (V951), UAS-hbs-RNAi (V40898) and UAS-sns-RNAi (V108577). It has been shown previously that these RNAi constructs mediate efficient knockdown with other driver lines (Gildor et al., 2012; Machado et al., 2011; Susic-Jung et al., 2012). BL flies were obtained from Bloomington Drosophila Stock Center; V flies were ordered from the Vienna Drosophila RNAi Center.
Tissue preparation and immunofluorescence
To acquire pupae of a defined age, white prepupae were collected at 0 h APF and aged on a moistened filter. Pupae or adult flies were dissected in a drop of phosphate-buffered saline (PBS; 0.13 mM NaCl, 7 mM Na2HPO4, 3 mM NaH2PO4) under a stereomicroscope. For antibody staining, tissues were fixed in 3.7% formaldehyde in PBS for 20 min, primary antibody was incubated overnight at 4°C and secondary antibody was added to the specimens at room temperature for 1 h. Hoechst 33258 (3 µg/ml; Sigma-Aldrich, 94403) was used to label DNA and Atto565-phalloidin (4 nmol/l; Sigma-Aldrich, 94072) was used to stain F-actin.
The following antibodies were used: anti-GFP (1:2000; Abcam, ab6556), anti-tropomyosin (1:1000; Abcam, ab50567), anti-phosphorylated histone H3 (Ser10) mitosis marker (1:500; Millipore, 06-570), anti-Mef2 (1:500; kindly provided by Hanh T. Nguyen, University of Erlangen, Germany), anti-Duf and anti-Rst (1:500 and 1:50, respectively; both gifts from Karl-Friedrich Fischbach, University of Freiburg, Germany), anti-β-Gal (1:1000; Biotrend, RGA1-45A-Z) and anti-Sns (1:250; generated by Pineda Antibody Service, Berlin). For fluorescent immunohistochemistry, the following secondary antibodies were used: anti-rat Alexa Fluor 488 (Jackson ImmunoResearch Laboratories), anti-rabbit DyLight488 (Vector Laboratories) and anti-guinea pig Cy2 (Jackson ImmunoResearch Laboratories).
EdU labelling of pupal tissue
To detect proliferation, Click-iT EdU Alexa Fluor 594 (Molecular Probes, Invitrogen) was used. EdU (0.1 µl of 0.5 mM) with Toluidine Blue (0.25%), for a better visualization in PBS, was injected into the dorso-medial part of the pupal thorax as described elsewhere (Ito and Hotta, 1992) at 24 h APF and incubated for 8 h at 25°C. After dissection and fixation of the testes, anti-GFP staining was performed. EdU was detected following the supplier's instructions.
RNA isolation from myoblasts
Cells were isolated following the protocol of Wang et al. (2006) with modifications. Briefly, 20 UAS-mCD8-GFP;Mef2-Gal4 genital discs of 8, 16, 24 and 30 h APF were dissected in cold dissociation buffer (Sigma, C1544) and kept cold until further processing. Washed genital discs were then dissociated by elastase treatment (5 µg/ml; Sigma, E0127). Myoblasts were purified using magnetic beads [Dynabeads Mouse CD8 (Lyt2), Invitrogen] according to the supplier's instructions. Subsequently, total RNA was isolated from these myoblasts using the RNAqueous-Micro total RNA Isolation Kit (Ambion) following instructions therein. RT-PCR was performed using the OneStep RT-PCR Kit (Qiagen) and oligonucleotides listed in Table S3.
Image acquisition and processing
Conventional fluorescent images and optical sections were gathered with a Zeiss AxioObserver Z.1 inverse microscope with an attached ApoTome that was used for structured illumination microscopy. Images were merged using Adobe Photoshop CS 5.1, plates were arranged in GIMP 2.8 and charts were generated in Microsoft Excel 2010.
Acknowledgements
We thank Susanne Önel and Anja Rudolf for fruitful discussions and critical reading of the manuscript, Katja Gessner for organizational assistance and graphic design expertise, Peer Fender for help with graphics, Ljubinka Cigoja for injecting Drosophila pupae, Hanh Nguyen and Karl-Friedrich Fischbach for antibodies, Xin Chen for methodical support and Karen A. Brune for linguistic revision. We received fly strains from Vienna Drosophila RNAi Center, Austria. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
J.K., D.B. and R.R.-P. conceived and designed the experiments. J.K., K.F., D.B. and S.R.-F. performed the experiments, and J.K. and R.R.-P. wrote the manuscript.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft [Re 628/16-1 and GRK 1216]. Deposited in PMC for immediate release.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.126730/-/DC1
References
- Abmayr S. M. and Pavlath G. K. (2012). Myoblast fusion: lessons from flies and mice. Development 139, 641-656. 10.1242/dev.068353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar P. S., Baylies M. K., Fleissner A., Helming L., Inoue N., Podbilewicz B., Wang H. and Wong M. (2013). Genetic basis of cell-cell fusion mechanisms. Trends Genet. 29, 427-437. 10.1016/j.tig.2013.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S. M. and Baker B. S. (2002). Sex-specific deployment of FGF signaling in Drosophila recruits mesodermal cells into the male genital imaginal disc. Cell 109, 651-661. 10.1016/S0092-8674(02)00744-4 [DOI] [PubMed] [Google Scholar]
- Ali F., Paré P. D. and Seow C. Y. (2005). Models of contractile units and their assembly in smooth muscle. Can. J. Physiol. Pharmacol. 83, 825-831. 10.1139/y05-052 [DOI] [PubMed] [Google Scholar]
- Au Y. (2004). The muscle ultrastructure: a structural perspective of the sarcomere. Cell. Mol. Life Sci. 61, 3016-3033. 10.1007/s00018-004-4282-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. and Schubiger G. (1996). Autonomous and nonautonomous Notch functions for embryonic muscle and epidermis development in Drosophila. Development 122, 617-626. [DOI] [PubMed] [Google Scholar]
- Bate M. (1990). The embryonic development of larval muscles in Drosophila. Development 110, 791-804. [DOI] [PubMed] [Google Scholar]
- Bate M., Rushton E. and Currie D. A. (1991). Cells with persistent twist expression are the embryonic precursors of adult muscles in Drosophila. Development 113, 79-89. [DOI] [PubMed] [Google Scholar]
- Bodenstein D. (1950). The postembryonic development of Drosophila. In Biology of Drosophila (ed. Demerec M.), pp. 275-367. New York: John Wiley & Sons, Inc. [Google Scholar]
- Bonn B. R., Rudolf A., Hornbruch-Freitag C., Daum G., Kuckwa J., Kastl L., Buttgereit D. and Renkawitz-Pohl R. (2013). Myosin heavy chain-like localizes at cell contact sites during Drosophila myoblast fusion and interacts in vitro with Rolling pebbles 7. Exp. Cell Res. 319, 402-416. 10.1016/j.yexcr.2012.12.005 [DOI] [PubMed] [Google Scholar]
- Bour B. A., O'Brien M. A., Lockwood W. L., Goldstein E. S., Bodmer R., Taghert P. H., Abmayr S. M. and Nguyen H. T. (1995). Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 9, 730-741. 10.1101/gad.9.6.730 [DOI] [PubMed] [Google Scholar]
- Bour B., Chakravarti M., West J. and Abmayr S. (2000). Drosophila SNS, a member of the immunoglobulin superfamily that is essential for myoblast fusion. Genes Dev. 14, 1498-1511. [PMC free article] [PubMed] [Google Scholar]
- Carmena A., Bate M. and Jimenez F. (1995). Lethal of scute, a proneural gene, participates in the specification of muscle progenitors during Drosophila embryogenesis. Genes Dev. 9, 2373-2383. 10.1101/gad.9.19.2373 [DOI] [PubMed] [Google Scholar]
- Corbin V., Michelson A. M., Abmayr S. M., Neel V., Alcamo E., Maniatis T. and Young M. W. (1991). A role for the Drosophila neurogenic genes in mesoderm differentiation. Cell 67, 311-323. 10.1016/0092-8674(91)90183-Y [DOI] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K.-C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S. et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151-156. 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- Drysdale R., Rushton E. and Bate M. (1993). Genes required for embryonic muscle development in Drosophila melanogaster A survey of the X chromosome. Dev. Biol. 202, 276-295. 10.1007/bf00363217 [DOI] [PubMed] [Google Scholar]
- Duan H., Skeath J. B. and Nguyen H. T. (2001). Drosophila Lame duck, a novel member of the Gli superfamily, acts as a key regulator of myogenesis by controlling fusion-competent myoblast development. Development 128, 4489-4500. [DOI] [PubMed] [Google Scholar]
- Duan R., Jin P., Luo F., Zhang G., Anderson N. and Chen E. H. (2012). Group I PAKs function downstream of Rac to promote podosome invasion during myoblast fusion in vivo. J. Cell Biol. 199, 169-185. 10.1083/jcb.201204065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D., Anant S., Ruiz-Gomez M., Bate M. and VijayRaghavan K. (2004). Founder myoblasts and fibre number during adult myogenesis in Drosophila. Development 131, 3761-3772. 10.1242/dev.01249 [DOI] [PubMed] [Google Scholar]
- Dworak H. A., Charles M. A., Pellerano L. B. and Sink H. (2001). Characterization of Drosophila hibris, a gene related to human nephrin. Development 128, 4265-4276. [DOI] [PubMed] [Google Scholar]
- Estrada B., Casares F. and Sánchez-Herrero E. (2003). Development of the genitalia in Drosophila melanogaster. Differentiation 71, 299-310. 10.1046/j.1432-0436.2003.03017.x [DOI] [PubMed] [Google Scholar]
- Figeac N., Daczewska M., Marcelle C. and Jagla K. (2007). Muscle stem cells and model systems for their investigation. Dev. Dyn. 236, 3332-3342. 10.1002/dvdy.21345 [DOI] [PubMed] [Google Scholar]
- Foe V. E., Odell G. M. and Edgar B. A. (1993). Mitosis and morphogenesis in the Drosophila embryo: point and counterpoint. In The Development of Drosophila melangaster (ed. Bate M. and Martinez-Arias A.), pp. 149-300. Cold Spring Harbor; New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Gärtner S. M., Rathke C., Renkawitz-Pohl R. and Awe S. (2014). Ex vivo culture of Drosophila pupal testis and single male germ-line cysts: dissection, imaging, and pharmacological treatment. J. Vis. Exp. 91, 51868 10.3791/51868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentric G. and Desdouets C. (2014). Polyploidization in liver tissue. Am. J. Pathol. 184, 322-331. 10.1016/j.ajpath.2013.06.035 [DOI] [PubMed] [Google Scholar]
- Gildor B., Schejter E. D. and Shilo B.-Z. (2012). Bidirectional Notch activation represses fusion competence in swarming adult Drosophila myoblasts. Development 139, 4040-4050. 10.1242/dev.077495 [DOI] [PubMed] [Google Scholar]
- Hakeda-Suzuki S., Ng J., Tzu J., Dietzl G., Sun Y., Harms M., Nardine T., Luo L. and Dickson B. J. (2002). Rac function and regulation during Drosophila development. Nature 416, 438-442. 10.1038/416438a [DOI] [PubMed] [Google Scholar]
- Haralalka S. and Abmayr S. M. (2010). Myoblast fusion in Drosophila. Exp. Cell Res. 316, 3007-3013. 10.1016/j.yexcr.2010.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbruch-Freitag C., Griemert B., Buttgereit D. and Renkawitz-Pohl R. (2011). Drosophila Swiprosin-1/EFHD2 accumulates at the prefusion complex stage during Drosophila myoblast fusion. J. Cell Sci. 124, 3266-3278. 10.1242/jcs.083907 [DOI] [PubMed] [Google Scholar]
- Hudson A. M., Petrella L. N., Tanaka A. J. and Cooley L. (2008). Mononuclear muscle cells in Drosophila ovaries revealed by GFP protein traps. Dev. Biol. 314, 329-340. 10.1016/j.ydbio.2007.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K. and Hotta Y. (1992). Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev. Biol. 149, 134-148. 10.1016/0012-1606(92)90270-Q [DOI] [PubMed] [Google Scholar]
- Kesper D. A., Stute C., Buttgereit D., Kreisköther N., Vishnu S., Fischbach K.-F. and Renkawitz-Pohl R. (2007). Myoblast fusion in Drosophila melanogaster is mediated through a fusion-restricted myogenic-adhesive structure (FuRMAS). Dev. Dyn. 236, 404-415. 10.1002/dvdy.21035 [DOI] [PubMed] [Google Scholar]
- Klapper R., Stute C., Schomaker O., Strasser T., Janning W., Renkawitz-Pohl R. and Holz A. (2002). The formation of syncytia within the visceral musculature of the Drosophila midgut is dependent on duf, sns and mbc. Mech. Dev. 110, 85-96. 10.1016/S0925-4773(01)00567-6 [DOI] [PubMed] [Google Scholar]
- Kozopas K. M., Samos C. H. and Nusse R. (1998). DWnt-2, a Drosophila Wnt gene required for the development of the male reproductive tract, specifies a sexually dimorphic cell fate. Genes Dev. 12, 1155-1165. 10.1101/gad.12.8.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisköther N., Reichert N., Buttgereit D., Hertenstein A., Fischbach K.-F. and Renkawitz-Pohl R. (2006). Drosophila rolling pebbles colocalises and putatively interacts with alpha-Actinin and the Sls isoform Zormin in the Z-discs of the sarcomere and with Dumbfounded/Kirre, alpha-Actinin and Zormin in the terminal Z-discs. J. Muscle Res. Cell Motil. 27, 93-106. 10.1007/s10974-006-9060-y [DOI] [PubMed] [Google Scholar]
- Lacroix B. and Maddox A. S. (2012). Cytokinesis, ploidy and aneuploidy. J. Pathol. 226, 338-351. 10.1002/path.3013 [DOI] [PubMed] [Google Scholar]
- Li F., Wang X., Capasso J. M. and Gerdes A. M. (1996). Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J. Mol. Cell. Cardiol. 28, 1737-1746. 10.1006/jmcc.1996.0163 [DOI] [PubMed] [Google Scholar]
- Lilly B., Galewsky S., Firulli A. B., Schulz R. A. and Olson E. N. (1994). D-MEF2: a MADS box transcription factor expressed in differentiating mesoderm and muscle cell lineages during Drosophila embryogenesis. Proc. Natl. Acad. Sci. USA 91, 5662-5666. 10.1073/pnas.91.12.5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemannstons K., Ripp C., Honemann-Capito M., Brechtel-Curth K., Hedderich M. and Wodarz A. (2014). The PTK7-related transmembrane proteins off-track and off-track 2 are co-receptors for Drosophila Wnt2 required for male fertility. PLoS Genet. 10, e1004443 10.1371/journal.pgen.1004443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Yue S., Chen X., Kubin T. and Braun T. (2010). Regulation of cardiomyocyte polyploidy and multinucleation by CyclinG1. Circ. Res. 106, 1498-1506. 10.1161/CIRCRESAHA.109.211888 [DOI] [PubMed] [Google Scholar]
- Machado M. C. R., Octacilio-Silva S., Costa M. S. A. and Ramos R. G. P. (2011). rst transcriptional activity influences kirre mRNA concentration in the Drosophila pupal retina during the final steps of ommatidial patterning. PLoS ONE 6, e22536 10.1371/journal.pone.0022536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T. and Nagayama K. (2012). Tensile properties of vascular smooth muscle cells: bridging vascular and cellular biomechanics. J. Biomech. 45, 745-755. 10.1016/j.jbiomech.2011.11.014 [DOI] [PubMed] [Google Scholar]
- Menon S. D., Osman Z., Chenchill K. and Chia W. (2005). A positive feedback loop between Dumbfounded and Rolling pebbles leads to myotube enlargement in Drosophila. J. Cell Biol. 169, 909-920. 10.1083/jcb.200501126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M. and Hiromi Y. (1992). Enhancer trap method in Drosophila: its application to neurobiology. In Methods in Neurosciences: Gene Expression in Neural Tissues (ed. Conn P. M.) pp. 397-414. Amsterdam: Elsevier. [Google Scholar]
- Nanda S., DeFalco T. J., Loh S. Y. H., Phochanukul N., Camara N., Van Doren M. and Russell S. (2009). Sox100B, a Drosophila group E Sox-domain gene, is required for somatic testis differentiation. Sex Dev. 3, 26-37. 10.1159/000200079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H. T., Bodmer R., Abmayr S. M., McDermott J. C. and Spoerel N. A. (1994). D-mef2: a Drosophila mesoderm-specific MADS box-containing gene with a biphasic expression profile during embryogenesis. Proc. Natl. Acad. Sci. USA 91, 7520-7524. 10.1073/pnas.91.16.7520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Isshiki T. and Takeichi M. (1998). Regional specification of muscle progenitors in Drosophila: the role of the msh homeobox gene. Development 125, 215-223. [DOI] [PubMed] [Google Scholar]
- Önel S.-F. and Renkawitz-Pohl R. (2009). FuRMAS: triggering myoblast fusion in Drosophila. Dev. Dyn. 238, 1513-1525. 10.1002/dvdy.21961 [DOI] [PubMed] [Google Scholar]
- Önel S.-F., Dottermusch C., Sickmann A., Buttgereit D. and Renkawitz-Pohl R. (2011). Role of the actin cytoskeleton within FuRMAS during Drosophila myoblast fusion and first functionally conserved factors in vertebrates. In Cell Fusions: Regulation and Control (ed. Larsson L.-I.) pp. 139-170 Berlin: Springer. [Google Scholar]
- Önel S.-F., Rust M. B., Jacob R. and Renkawitz-Pohl R. (2014). Tethering membrane fusion: common and different players in myoblasts and at the synapse. J. Neurogenet. 28, 302-315. 10.3109/01677063.2014.936014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell G. T. and Wright G. J. (2011). Jamb and jamc are essential for vertebrate myocyte fusion. PLoS Biol. 9, e1001216 10.1371/journal.pbio.1001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos R. G., Igloi G. L., Lichte B., Baumann U., Maier D., Schneider T., Brandstatter J. H., Frohlich A. and Fischbach K. F. (1993). The irregular chiasm C-roughest locus of Drosophila, which affects axonal projections and programmed cell death, encodes a novel immunoglobulin-like protein. Genes Dev. 7, 2533-2547. 10.1101/gad.7.12b.2533 [DOI] [PubMed] [Google Scholar]
- Ranganayakulu G., Zhao B., Dokidis A., Molkentin J. D., Olson E. N. and Schulz R. A. (1995). A series of mutations in the D-MEF2 transcription factor reveal multiple functions in larval and adult myogenesis in Drosophila. Dev. Biol. 171, 169-181. 10.1006/dbio.1995.1269 [DOI] [PubMed] [Google Scholar]
- Rathke C., Baarends W. M., Jayaramaiah-Raja S., Bartkuhn M., Renkawitz R. and Renkawitz-Pohl R. (2007). Transition from a nucleosome-based to a protamine-based chromatin configuration during spermiogenesis in Drosophila. J. Cell Sci. 120, 1689-1700. 10.1242/jcs.004663 [DOI] [PubMed] [Google Scholar]
- Reiter C., Schimansky T., Nie Z. and Fischbach K. F. (1996). Reorganization of membrane contacts prior to apoptosis in the Drosophila retina: the role of the IrreC-rst protein. Development 122, 1931-1940. [DOI] [PubMed] [Google Scholar]
- Rochlin K., Yu S., Roy S. and Baylies M. K. (2010). Myoblast fusion: when it takes more to make one. Dev. Biol. 341, 66-83. 10.1016/j.ydbio.2009.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. and VijayRaghavan K. (1998). Patterning muscles using organizers: larval muscle templates and adult myoblasts actively interact to pattern the dorsal longitudinal flight muscles of Drosophila. J. Cell Biol. 141, 1135-1145. 10.1083/jcb.141.5.1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Gómez M., Coutts N., Price A., Taylor M. V. and Bate M. (2000). Drosophila dumbfounded: a myoblast attractant essential for fusion. Cell 102, 189-198. 10.1016/S0092-8674(00)00024-6 [DOI] [PubMed] [Google Scholar]
- Sens K. L., Zhang S., Jin P., Duan R., Zhang G., Luo F., Parachini L. and Chen E. H. (2010). An invasive podosome-like structure promotes fusion pore formation during myoblast fusion. J. Cell Biol. 191, 1013-1027. 10.1083/jcb.201006006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton C., Kocherlakota K. S., Zhuang S. and Abmayr S. M. (2009). The immunoglobulin superfamily member Hbs functions redundantly with Sns in interactions between founder and fusion-competent myoblasts. Development 136, 1159-1168. 10.1242/dev.026302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler C., Daczewska M., Da Ponte J. P., Dastugue B. and Jagla K. (2004). Coordinated development of muscles and tendons of the Drosophila leg. Development 131, 6041-6051. 10.1242/dev.01527 [DOI] [PubMed] [Google Scholar]
- St Pierre S. E., Ponting L., Stefancsik R. and McQuilton P. (2014). FlyBase 102-advanced approaches to interrogating FlyBase. Nucleic Acids Res. 42, D780-D788. 10.1093/nar/gkt1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern C. (1941a). The growth of testes in Drosophila. I. The relation between vas deferens and testis within various species. J. Exp. Zool. 87, 113-158. 10.1002/jez.1400870109 [DOI] [Google Scholar]
- Stern C. (1941b). The growth of the testes in Drosophila. II. The nature of interspecific differences. J. Exp. Zool. 87, 159-180. 10.1002/jez.1400870110 [DOI] [Google Scholar]
- Strünkelnberg M., Bonengel B., Moda L., Hertenstein A., de Couet H., Ramos R. and Fischbach K. (2001). rst and its paralogue kirre act redundantly during embryonic muscle development in Drosophila. Development 128, 4229-4239. [DOI] [PubMed] [Google Scholar]
- Susic-Jung L., Hornbruch-Freitag C., Kuckwa J., Rexer K.-H., Lammel U. and Renkawitz-Pohl R. (2012). Multinucleated smooth muscles and mononucleated as well as multinucleated striated muscles develop during establishment of the male reproductive organs of Drosophila melanogaster. Dev. Biol. 370, 86-97. 10.1016/j.ydbio.2012.07.022 [DOI] [PubMed] [Google Scholar]
- Svingen T. and Koopman P. (2013). Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes Dev. 27, 2409-2426. 10.1101/gad.228080.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Kokuryo A., Imano T., Minami R., Nakagoshi H. and Adachi-Yamada T. (2014). Isoform-specific functions of Mud/NuMA mediate binucleation of Drosophila male accessory gland cells. BMC Dev. Biol. 14, 46 10.1186/s12861-014-0046-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. V., Beatty K. E., Hunter H. K. and Baylies M. K. (1995). Drosophila MEF2 is regulated by twist and is expressed in both the primordia and differentiated cells of the embryonic somatic, visceral and heart musculature. Mech. Dev. 50, 29-41. 10.1016/0925-4773(94)00323-F [DOI] [PubMed] [Google Scholar]
- Wang X., Bo J., Bridges T., Dugan K. D., Pan T.-C., Chodosh L. A. and Montell D. J. (2006). Analysis of cell migration using whole-genome expression profiling of migratory cells in the Drosophila ovary. Dev. Cell 10, 483-495. 10.1016/j.devcel.2006.02.003 [DOI] [PubMed] [Google Scholar]
- Wozniak A. C., Kong J., Bock E., Pilipowicz O. and Anderson J. E. (2005). Signaling satellite-cell activation in skeletal muscle: markers, models, stretch, and potential alternate pathways. Muscle Nerve 31, 283-300. 10.1002/mus.20263 [DOI] [PubMed] [Google Scholar]
- Zhuang S., Shao H., Guo F., Trimble R., Pearce E. and Abmayr S. M. (2009). Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 136, 2335-2344. 10.1242/dev.031609 [DOI] [PMC free article] [PubMed] [Google Scholar]