Abstract
Vertebrate body axis formation depends on a population of bipotential neuromesodermal cells along the posterior wall of the tailbud that make a germ layer decision after gastrulation to form spinal cord and mesoderm. Despite exhibiting germ layer plasticity, these cells never give rise to midline tissues of the notochord, floor plate and dorsal endoderm, raising the question of whether midline tissues also arise from basal posterior progenitors after gastrulation. We show in zebrafish that local posterior signals specify germ layer fate in two basal tailbud midline progenitor populations. Wnt signaling induces notochord within a population of notochord/floor plate bipotential cells through negative transcriptional regulation of sox2. Notch signaling, required for hypochord induction during gastrulation, continues to act in the tailbud to specify hypochord from a notochord/hypochord bipotential cell population. Our results lend strong support to a continuous allocation model of midline tissue formation in zebrafish, and provide an embryological basis for zebrafish and mouse bifurcated notochord phenotypes as well as the rare human congenital split notochord syndrome. We demonstrate developmental equivalency between the tailbud progenitor cell populations. Midline progenitors can be transfated from notochord to somite fate after gastrulation by ectopic expression of msgn1, a master regulator of paraxial mesoderm fate, or if transplanted into the bipotential progenitors that normally give rise to somites. Our results indicate that the entire non-epidermal posterior body is derived from discrete, basal tailbud cell populations. These cells remain receptive to extracellular cues after gastrulation and continue to make basic germ layer decisions.
KEY WORDS: Midline progenitor cells, Tailbud, Canonical Wnt, Notch, Posterior growth, Notochord, Floor plate, Hypochord, Mesogenin 1, MPC, PWPC, Neuromesodermal progenitors
Summary: Progenitor cells that generate the midline tissues of the zebrafish floor plate, notochord, and hypochord make germ layer decisions after gastrulation based on local canonical Wnt and Notch signaling.
INTRODUCTION
A major shift in the view of vertebrate germ layer induction is underway, with evidence from several species indicating that germ layer induction continues after gastrulation (Henrique et al., 2015; Kondoh and Takemoto, 2012). During body axis extension, the post-gastrula vertebrate embryo increases in length in large part due to the formation of new tissues at the posterior (or caudal) end (Benazeraf and Pourquie, 2013). Progenitor cells in a region called the tailbud, which is the posteriormost anatomical structure of all vertebrate embryos that forms after the completion of gastrulation, drive this process (Beck, 2015). Tailbud progenitor cells continuously exit and differentiate into elements of the growing body. Recent work demonstrated that the caudalmost progenitors within the tailbud, the posterior wall progenitor cells (PWPCs; sometimes referred to as neuromesodermal progenitors), are bipotential and continuously make neural/mesodermal fate decisions during embryo elongation. (Freese et al., 2014; Garriock et al., 2015; Gentsch et al., 2013; Gouti et al., 2014; Kondoh and Takemoto, 2012; Martin and Kimelman, 2012; Tzouanacou et al., 2009). These cells give rise to the growing spinal cord, somites and vasculature. Despite the basal plasticity of PWPCs, they never give rise to midline structures of the neural floor plate, mesodermal notochord and endodermal hypochord (Martin and Kimelman, 2012; Tzouanacou et al., 2009). Midline structures instead originate after gastrulation from a population of midline progenitor cells (MPCs), which are specified during gastrulation (Catala et al., 1996, 1995; Kinder et al., 2001; Melby et al., 1996; Schoenwolf and Sheard, 1990; Selleck and Stern, 1991; Shih and Fraser, 1996). After being specified these cells migrate with the organizer and eventually populate a region of the tailbud called the chordoneural hinge (CNH), immediately posterior to the end of the notochord (Wilson et al., 2009). During embryo elongation, MPCs continuously join growing tissues of the midline. In chicken embryos it is clear that different germ layer derivatives form from a single pool of MPCs, whereas in mouse embryos the notochord and floor plate progenitors are spatially distinct (Catala et al., 1996; Jeong and Epstein, 2003; Teillet et al., 1998). Whether MPC fate decisions are made solely during gastrulation or continuously during embryo elongation after gastrulation is unknown. The recent evidence of bipotential progenitors in the tailbud raised the possibility that MPCs could also maintain post-gastrula germ layer plasticity, similar to PWPCs (Beck, 2015; Wilson et al., 2009).
Midline tissues play essential patterning roles during development. The floor plate and notochord provide dorsal-ventral pattern to the spinal cord as well as mediolateral pattern to the adjacent somites, whereas the hypochord organizes the formation of midline blood vessels (Cleaver and Krieg, 1998; Placzek and Briscoe, 2005; Stemple, 2005). The notochord patterns adjacent tissues in part through the secretion of Hedgehog signals, which provide medial character to somites and ventral character to the spinal cord (Stemple, 2005). Floor plate forms just dorsal to the notochord in the ventralmost region of the spinal cord. It initially forms as a single row of cells of triangular cross-section (the medial floor plate) and expands to include adjacent cells of the ventral neural tube (lateral floor plate) (Placzek and Briscoe, 2005). The timing and location of floor plate induction vary across vertebrate species. Cell lineage analyses in chick embryos show that notochord and medial floor plate arise from a single pool of progenitors, whereas in mouse embryos sonic hedgehog-expressing notochord-fated cells are separated from future floor plate cells in the node after gastrulation (Catala et al., 1996; Jeong and Epstein, 2003; Schoenwolf and Sheard, 1990; Selleck and Stern, 1991). In the mouse, Hedgehog signaling from the notochord induces both the medial and lateral floor plate, whereas in zebrafish medial floor plate formation is independent of Hedgehog signaling (Chen et al., 2001; Chiang et al., 1996; Ding et al., 1998; Matise et al., 1998).
The least studied of the midline tissues is the hypochord, which is only present in anamniotes and serves to pattern nearby blood vessels by secreting VEGF (Cleaver and Krieg, 1998). The mesodermal or endodermal origin of the hypochord has been a matter of debate, but a consensus view between disparate anamniote species (including Xenopus laevis, axolotl and zebrafish) has emerged ascribing an endodermal character to this tissue (Cleaver et al., 2000; Eriksson and Lofberg, 2000; Lofberg and Collazo, 1997). Amniotes lack a hypochord but its function in patterning blood vessels may have been taken over by dorsal gut endoderm. This tissue forms adjacent to the same blood vessels and expresses VEGF (Dumont et al., 1995).
The common origin of midline tissues from MPCs in several vertebrate species, in addition to genetic and embryological data, has led to the allocation model of midline development, whereby local signals specify fate within MPCs (reviewed by Le Douarin and Halpern, 2000; Strahle et al., 2004). There is strong evidence for a conserved role of Notch signaling in regulating hypochord allocation during gastrulation, but the signals regulating medial floor plate allocation are less clear (Appel et al., 1999; Latimer and Appel, 2006; Latimer et al., 2002; Peyrot et al., 2011). Species-specific roles for Notch and Midkine signaling have been identified, but a conserved pathway regulating this process across all species has yet to be identified (Gray and Dale, 2010; Lopez et al., 2003, 2005; Peyrot et al., 2011; Schafer et al., 2005).
Zebrafish provide an ideal model with which to study the post-gastrulation regulation of cell fate. The ability to create genetic mosaic animals, along with the use of heat shock-inducible transgenes, allows for the precise manipulation of genes and signaling pathways specifically in individual MPCs after the completion of gastrulation. Using these methods we show that MPCs maintain germ layer plasticity during post-gastrulation stages of body axis formation. We present a model of the tailbud that is a hybrid of the historically opposed fixed fate and blastema models (Holmdahl, 1925; Pasteels, 1943), in which the tailbud consists of neither a uniform population of undifferentiated cells, nor small groups of fixed-lineage cells, but rather independent populations of multipotent progenitors that are held in specific signaling environments that dictate their cell fate.
RESULTS
The possibility that MPCs remain multipotent after gastrulation has been suspected for some time (Cambray and Wilson, 2002; Catala et al., 1995; Davis and Kirschner, 2000; Teillet et al., 1998). New reagents for inducible activation and repression of signaling pathways, combined with targeted cell transplantation, allowed us to study this question directly. We took advantage of a heterochronic cell transplantation strategy that maximizes the contribution of transplanted cells to the MPC pool (Fig. 1A) (Halpern et al., 1995). In most cases, control transplants contribute exclusively to notochord, medial floor plate and hypochord (Fig. 1B,B′). Transplanted cells remain undifferentiated after gastrulation for an extended period, as seen in Fig. 1C, where the indicated cell waits at the caudal end of the notochord for 4-5 h before contributing to the notochord (see also Movie 1).
Fig. 1.
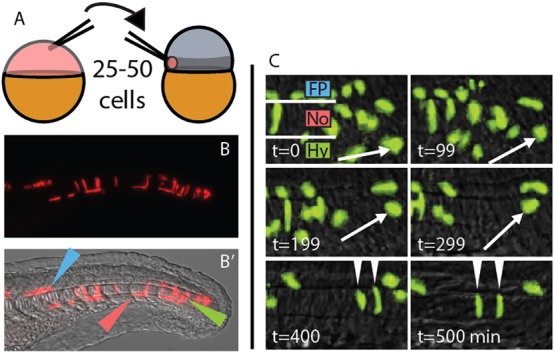
A midline-directed transplant technique reveals long-lived MPCs. (A) A heterochronic cell transplantation scheme, from transgenic or transiently transgenic donors to wild-type zebrafish hosts, maximizes contribution to the MPCs. 25-50 cells are taken from a dye-injected sphere stage donor and placed in the prospective mesendoderm (darker gray band) of a 1000-cell stage host. (B,B′) At 24 h post fertilization (hpf), transplanted cells are observed exclusively in the derivatives of the MPCs. Green, red and blue arrowheads indicate notochord, hypochord and floor plate, respectively. (C) Transplanted cells can remain undifferentiated for an extended time, and can join the notochord, hypochord and floor plate of the neural tube. Arrow marks one cell that divides and differentiates into two notochord cells (arrowheads). t=0 is ∼18 hpf. Fp, floor plate; No, notochord; Hy, hypochord.
Canonical Wnt signaling and sox2 manipulations affect notochord progenitors after gastrulation
In the posterior wall of the tailbud and in cell culture, Wnt signaling induces new mesoderm from PWPCs (Bouldin et al., 2015; Garriock et al., 2015; Gouti et al., 2014; Henrique et al., 2015; Jurberg et al., 2014; Martin and Kimelman, 2012; Tsakiridis et al., 2014). To determine whether Wnt signaling also induces new mesoderm formation within the tailbud MPCs, we used heat shock-inducible transgenic lines to temporally inhibit (hsp70l:TCFΔC-GFP) or activate (hsp70l:β-Catenin-TFP) Wnt signaling (Martin and Kimelman, 2012; Veldman et al., 2013). The T-box gene ntla (also known as T, brachyury homolog a) is expressed in differentiated notochord cells, notochord progenitors located just posterior to the differentiated notochord, and in posterior wall mesoderm progenitors (Martin and Kimelman, 2008; Schulte-Merker et al., 1994). Three hours after inducing a block in Wnt signaling there is a rapid loss of ntla expression in the notochord progenitor domain, but not in the differentiated notochord (Fig. 2B, outlined region). In the same time frame, activation of Wnt signaling causes an increase in ntla in the notochord progenitor region (Fig. 2C, outlined region). To confirm changes in notochord progenitors after Wnt manipulation, we examined the expression of floating head (flh, also known as notochord homeobox; a Xnot ortholog), which is expressed exclusively in notochord progenitors at this stage (Talbot et al., 1995). Expression of flh rapidly decreased after Wnt inhibition and increased within the MPCs following Wnt activation (Fig. 2F,G).
Fig. 2.
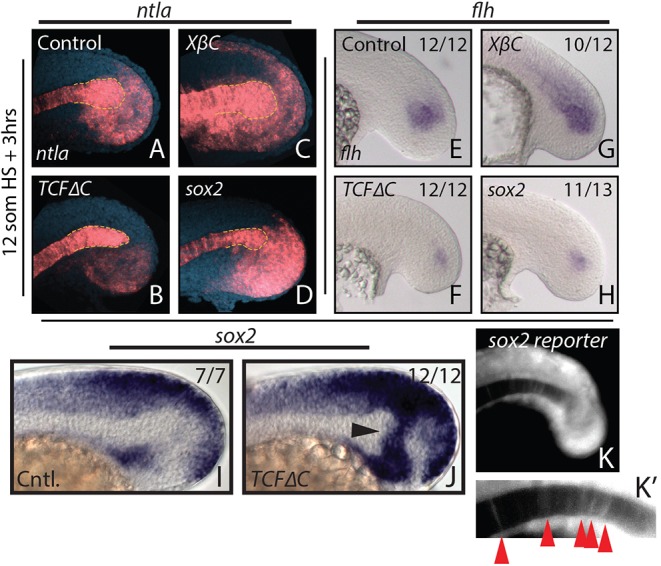
Canonical Wnt signaling affects tailbud notochord progenitor fate through sox2 repression. (A-H) Heat shock-inducible transgenic lines were used to manipulate canonical Wnt signaling or sox2 expression after gastrulation at the 12-somite stage, and stained for ntla or flh expression 3 h after the heat shock. Loss of Wnt signaling causes a reduction in ntla expression specifically in the notochord progenitor domain (A,B, yellow dashed line indicates the progenitor domain), as well as a reduction in the notochord progenitor marker flh (E,F). Activation of Wnt signaling has the opposite effect on notochord progenitors (C,G). (I,J) sox2 is normally expressed in regions directly adjacent to the notochord progenitor domain (I) and expands dramatically into the notochord progenitor domain 2 h after loss of Wnt signaling at the 12-somite stage (J, arrowhead). Heat shock induction of sox2 expression phenocopies Wnt loss of function with respect to ntla (D, dashed yellow line) and flh (H) expression. A sox2 reporter line shows weak fluorescence in notochord cells at the 16-somite stage (K,K′, arrowheads), indicating that notochord cells were once sox2 positive. The number of embryos showing the illustrated phenotype among the total number examined is indicated.
In the mouse tailbud, sustained ectopic expression of the transcription factor Sox2 in tailbud PWPCs is sufficient to cause neural induction at the expense of paraxial mesoderm (Takemoto et al., 2011). In zebrafish, sox2 is expressed in the region of the MPCs (Fig. 2I) and expands dramatically after Wnt signaling inhibition (Fig. 2J, arrowhead). Additionally, an endogenously tagged sox2-p2a-sfGFP reporter line (Shin et al., 2014) exhibits fluorescence in posterior notochord cells, which do not express sox2 transcript or protein, indicating that at least some notochord cells were previously sox2 positive (Fig. 2K,K′, arrowheads). These results suggest that the loss of notochord progenitor markers after Wnt signaling inhibition might be due to a failure to repress sox2 in cells that would otherwise normally become notochord. In order to test this hypothesis directly we created a heat shock-inducible transgenic line to temporally overexpress sox2 (hsp70l:sox2-p2a-NLS kikGR). Heat shock induction of sox2 at the 12-somite stage phenocopied Wnt loss of function with respect to ntla and flh expression (Fig. 2D,H).
Wnt signaling induces notochord in bipotential floor plate/notochord progenitors by repressing sox2 expression
To determine whether cell fate is affected by Wnt manipulations, we transplanted cells from the hsp70l:TCFΔC-GFP or hsp70l:β-Catenin-TFP transgenic lines into wild-type host embryos. This approach tests the ability of Wnt signaling to cell-autonomously specify fate in the MPCs after gastrulation has ended, in the context of an otherwise wild-type embryo. Wild-type cells predominantly join floor plate and notochord in approximately equal measure, with a minority of cells joining hypochord (Fig. 3A). A major advantage of this system is the ability to unambiguously identify cell fate based on position and morphology. We validated the use of widefield microscopy for analysis by using 3D confocal microscopy. The distinctive triangular cross-section of medial floor plate cells and circular cross-section of notochord cells can be seen, as well as their colocalization with expression of the midline marker col2a1 (Fig. 3I,I′). Disruption of Wnt signaling at the end of gastrulation (bud stage) greatly enhanced the contribution of midline progenitors to floor plate and to a lesser extent to hypochord, at the expense of notochord (Fig. 3B,J,J′). Activated Wnt signaling greatly expanded notochord contribution at the expense of floor plate (Fig. 3C).
Fig. 3.

Cell fate distributions are affected by changes in Wnt signaling or sox2 overexpression. (A-H) Cells from stable transgenic donors (A-D) or from transiently transgenic donors (E-H) were transplanted into wild-type hosts and transgene expression induced after the completion of gastrulation (bud stage). (I-J′) In some cases, host embryos were stained by fluorescent in situ hybridization for col2a1 expression (green) and imaged by confocal microscopy. Transplanted cells are in red. A maximum projection image (I,J) and digital transverse section (I′,J′) reveal the precise midline position of transplanted cells from control (I,I′) and hsp70l:TCFΔC-GFP (J,J′) transplanted cells. (K) The contribution of transient transgenic cells to floor plate, notochord and hypochord was quantitated (raw data and statistics are provided in Table 1). Blocking Wnt signaling (B,F,J,J′) expanded the floor plate contribution at the expense of notochord, and activating Wnt had the opposite effect (C,G). Overexpression of sox2 (D,H) produced an effect very similar to blocking Wnt. Cell fate changes are statistically significant (see Table 1). Green, red and blue arrowheads indicate notochord, hypochord and floor plate, respectively.
Initial experiments used donor embryos from stable transgenic lines (Martin and Kimelman, 2012; Veldman et al., 2013). To quantify tissue contribution an alternate transient transgenic approach was employed, using donors injected with plasmid DNA and integrated genomically with the tol2 transposase system, which creates a mosaic scatter labeled embryo (Kikuta and Kawakami, 2009). We used the heat shock vector hsp70l:p2a-NLS kikGR to express our constructs of interest (see Materials and Methods) along with a nuclear label (this method was used for all subsequent cell fate quantitation with the exception of Wnt loss of function, which was performed with a heat shock-inducible TCFΔC-cherry fusion plasmid). Transient transgenic cells acted identically to cells from stable transgenic lines (Fig. 3E-G) and their tissue contribution is shown quantitatively in Fig. 3K and Table 1. Our results indicate that Wnt signaling is necessary and sufficient to induce notochord from MPCs that give rise to the floor plate and notochord.
Table 1.
Raw data of tissue contribution from cell transplants
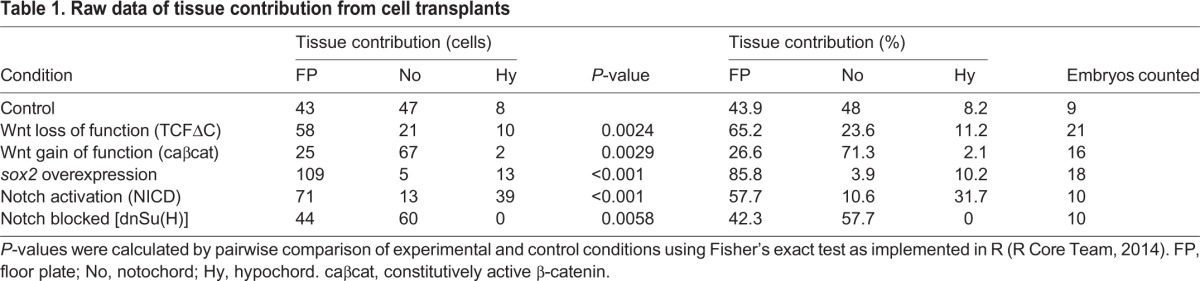
We also performed transplants as before using cells from stable (Fig. 3D) or transient transgenic (Fig. 3H) hsp70l:sox2-p2a-NLS kikGR donor embryos transplanted into wild-type host embryos. After heat shock induction of sox2, almost all transgenic cells joined the floor plate at the expense of notochord (Fig. 3D,H,K, Table 1). These results indicate that a key function of Wnt signaling required for notochord induction is the transcriptional repression of sox2, and provide a mechanistic understanding of how Wnt signaling can induce mesoderm from bipotential neural/mesodermal tailbud progenitor cells. Based on prior studies, we hypothesize that the same mechanism also functions during mesoderm induction in PWPCs (Jurberg et al., 2014; Martin and Kimelman, 2012).
Endoderm induction continues after gastrulation
Although Wnt and Sox2 manipulations clearly showed that germ layer fate decisions continue to be made within the MPCs after gastrulation to generate neural and mesodermal tissues, the effect on endodermal hypochord fate was very subtle. To provide more conclusive evidence that endoderm continues to be induced in the tailbud MPCs after gastrulation ends, we tested for continued responsiveness to Notch signaling, which is required for hypochord induction during gastrulation (Appel et al., 1999; Latimer and Appel, 2006; Latimer et al., 2002; Peyrot et al., 2011). We designed heat shock-inducible vectors in the same manner as the β-catenin and sox2 constructs, to allow inducible expression of the Notch intracellular domain (NICD) or a dominant-negative Suppressor of Hairless [dnSu(H)] (Wettstein et al., 1997), allowing temporal cell-autonomous activation or inhibition of the Notch signaling pathway in transplanted cells, respectively.
Activated Notch signaling resulted in a dramatic increase in the contribution of MPCs to the hypochord at the expense of notochord (Fig. 4A,C, Table 1). Disrupted Notch signaling completely blocked MPC contribution to hypochord, whereas contribution to notochord was increased (Fig. 4B,C, Table 1). These results indicate that Notch signaling acts continuously on MPC fate determination throughout body formation, and provide the first evidence that endoderm continues to be induced from a multipotent progenitor population after gastrulation ends. The inhibition of Notch signaling had no effect on floor plate contribution, whereas Notch activation caused a slight increase in the percentage of floor plate cells. These results are consistent with the previously reported gastrula stage role of Notch signaling in promoting floor plate cell proliferation, but not affecting floor plate specification (Latimer and Appel, 2006).
Fig. 4.
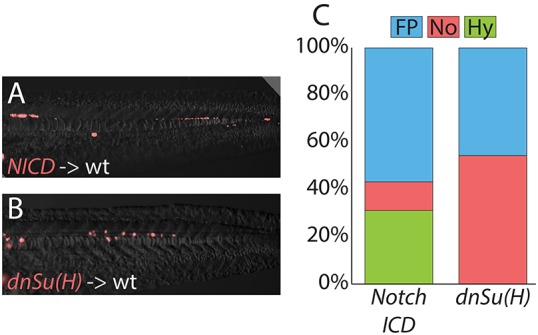
MPCs remain responsive to changes in Notch signals after gastrulation. Transiently transgenic cells were transplanted into wild-type host embryos, and transgene expression activated after gastrulation had completed. Activated Notch signaling cell-autonomously promotes hypochord and floor plate fates at the expense of notochord (A), and Notch activity is required for cells to adopt a hypochord fate (B), with changes quantitated (C; raw data and statistics are provided in Table 1). Cell fate changes are statistically significant (see Table 1).
Two independent populations of MPCs in the zebrafish tailbud
Our results suggest that the generation of notochord through active Wnt signaling and the generation of notochord due to the absence of Notch signaling are independent processes, operating in two separate MPC populations. The first population generates notochord through positive Wnt signaling in bipotential notochord/floor plate progenitors and is independent of Notch signaling, whereas the second population generates notochord through the absence of Notch signaling within hypochord/notochord progenitors, possibly independently of Wnt signaling. An alternative hypothesis is that a single population of pluripotent cells gives rise to all three cell types, and that both the presence of Wnt and the absence of Notch are required in the same cells for notochord induction. Wnt and Notch signaling commonly interact with each other at the molecular level in an antagonistic manner during fate specification, where Wnt inhibits Notch signaling and vice versa (reviewed by Hayward et al., 2008). In this scenario, notochord induction after positive Wnt signaling would require subsequent Notch inhibition, or notochord induction after Notch inhibition would require Wnt activation. To distinguish between these two models, we performed an analysis of combined loss of Wnt and Notch signaling in whole embryos to determine if notochord tissue is rescued.
DAPT, a small-molecule inhibitor of Notch signaling, was used in combination with genetic manipulations of Wnt and Sox2 to test for notochord rescue. To visualize notochord, we used a col2a1 probe, which also labels the floor plate and hypochord (Yan et al., 1995). After inhibition of Wnt signaling or activation of sox2, col2a1 expression is lost from the notochord domain and a thin stripe of expression persists (Fig. 5C,E), which is likely to represent the expanded floor plate based on foxa2 expression (Fig. 5G,I,K). No rescue of col2a1 expression in notochord was observed when DAPT treatment was combined with other manipulations (Fig. 5A-F). However, we found that Notch inhibition rescued the enhanced expression of the floor plate marker foxa2 caused by loss of Wnt or overexpression of sox2 (Fig. 5G-L), consistent with the role of Notch in promoting floor plate proliferation (Latimer and Appel, 2006). Additionally, we found that expanded sox2 expression resulting from Wnt inhibition did not depend on Notch function (Fig. 5M-P). Together, these results suggest that Wnt and Notch function independently during notochord specification from two different progenitor pools.
Fig. 5.
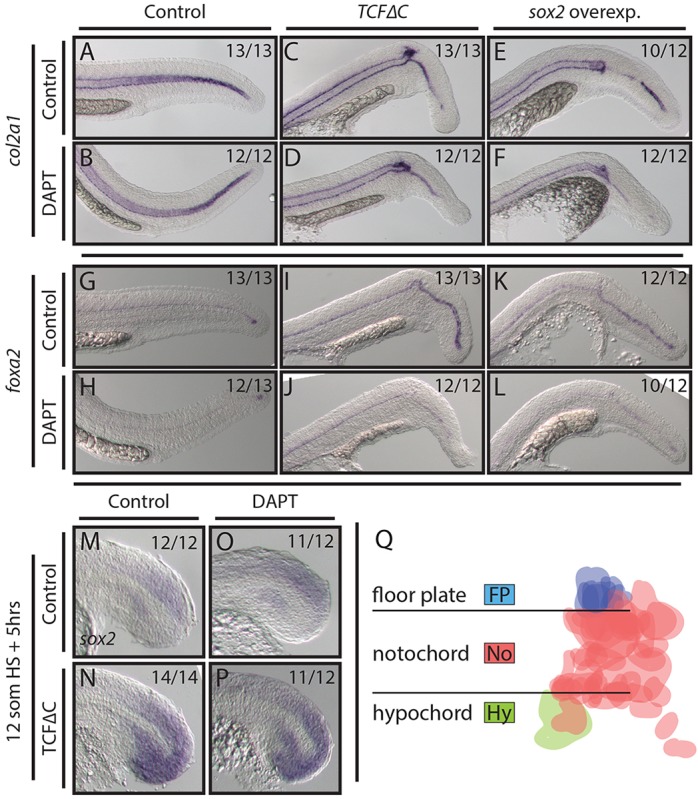
Wnt and Sox2 pattern midline tissues independently of Notch activity. (A-L) Loss of Wnt signaling and overexpression of sox2 using stable transgenic lines causes the notochord domain of col2a1 expression to be lost in the tail (A,C,E), whereas the floor plate marker foxa2 shows enhanced expression (G,I,K). Combining DAPT treatment with these conditions immediately after the heat shock has no effect on notochord patterning (B,D,F) but does reduce foxa2 expression (H,J,L). (M-P) Blocking Wnt signaling results in ectopic sox2 expression throughout the tailbud (M,N). This effect is independent of active Notch signaling (O,P). (Q) A fate map of MPCs (see text for details) indicates that floor plate cells are exclusively derived from a dorsal MPC population (blue), whereas hypochord (green) is derived only from a ventral MPC population. Cells are false colored based on fate; horizontal lines indicate the boundary of the notochord.
To further confirm the existence of separate MPC pools we created a fate map of the MPCs. We marked the starting positions and eventual tissue contribution of clearly distinct MPCs in 18 embryos (75 cells). We found that MPCs initially located dorsal to the notochord contributed only to notochord or floor plate, whereas ventral MPCs contributed to hypochord or notochord (Fig. 5Q). Cells caudal to the posterior end of the notochord were only observed joining the notochord in wild-type embryos. These results provide further evidence of independent MPC populations in the tailbud.
Dorsal and ventral MPCs fail to integrate properly in embryos with ectopic tails
The loss of BMP signaling during late gastrulation stages of zebrafish development results in the formation of a single ectopic tail on the ventral side of the primary tail (Connors et al., 1999; Pyati et al., 2005). The ectopic tail frequently contains notochord cells (Gebruers et al., 2013; Pyati et al., 2005; Yang and Thorpe, 2011). We reasoned that this might represent a splitting of the dorsal and ventral MPC populations, which would provide further evidence for the existence of the two MPC populations that we identified by genetic and lineage tracing-based methods. If ectopic tails represent the failure of the dorsal and ventral MPCs to properly integrate, the primary tail posterior to the ectopic tail should include floor plate and notochord but never hypochord, whereas the ectopic tail should always have notochord and hypochord.
In order to visualize notochord in live embryos, we developed a new transgenic reporter line that expresses the photoconvertible kaede coding sequence under the control of a 1 kb fragment of the ntla promoter. This line exhibits kaede mRNA expression specifically in notochord progenitors, and the Kaede protein perdures in the notochord and is absent from the floor plate and hypochord (Fig. 6A,B). In pntl:kaede embryos treated with the BMP inhibitor DMH1 at 75-85% epiboly (mid- to late gastrulation), we observed that notochord is always present in both the primary and ectopic tail, indicating that the notochord has split when ectopic tails are present (Fig. 6D,D′; 100%, n=24).
Fig. 6.
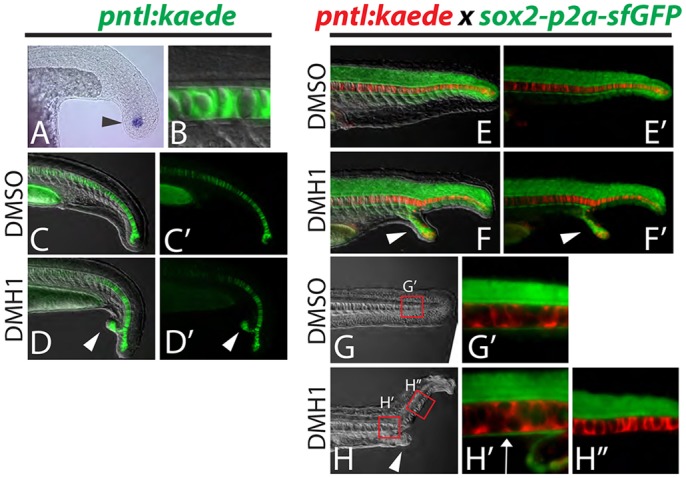
Dorsal and ventral MPCs separate in embryos with ectopic tails. (A-D′) A newly created transgenic reporter line (pntl:kaede) exhibits kaede mRNA expression in notochord progenitor cells (A, arrowhead) and Kaede protein perdures in the notochord only (B). Ectopic tails always contain notochord cells (D,D′, arrowheads). (E-H″) Notochord and hypochord were visualized simultaneously by crossing the pntl:kaede line to the sox2-p2a-sfGFP line and photoconverting the Kaede from green to red. Analysis of ectopic tails at 24 hpf revealed that the hypochord is diverted from the primary midline into the ectopic tail (F,F′, arrowheads). At 36 hpf it is clear that the hypochord is present anterior to the ectopic tail (ectopic tail is pictured in H, arrowhead) as labeled by sfGFP driven by the sox2 locus (H′, arrow), but is absent in the primary midline posterior to the ectopic tail (H″).
To visualize floor plate and hypochord, we used the sox2-p2a-sfGFP reporter line, which expresses sfGFP in the spinal cord including the floor plate, as well as the hypochord. The sox2-p2a-sfGFP reporter line was crossed to the pntl:kaede reporter and the Kaede was photoconverted from green to red to allow simultaneous imaging of the notochord, hypochord and floor plate (Fig. 6E,E′). In embryos with ectopic tails, the hypochord extends into the ectopic tail (along with some notochord), and is completely absent from the midline in the primary tail in regions posterior to the ectopic tail (Fig. 6F,F′,H-H″). By contrast, floor plate is continuous in the primary tail anterior and posterior to the ectopic tail (Fig. S1), and previous work has demonstrated that neural tissue, including floor plate, is never found in the ectopic tail (Gebruers et al., 2013; Pyati et al., 2005; Yang and Thorpe, 2011). These results indicate that embryos with ectopic tails undergo a separation of the dorsal and ventral MPC populations, and that ventral MPC derivatives (notochord and hypochord) populate the ectopic tail and dorsal MPC derivatives (notochord and floor plate) reside in the primary tail.
Tailbud notochord progenitors are competent to become paraxial mesoderm
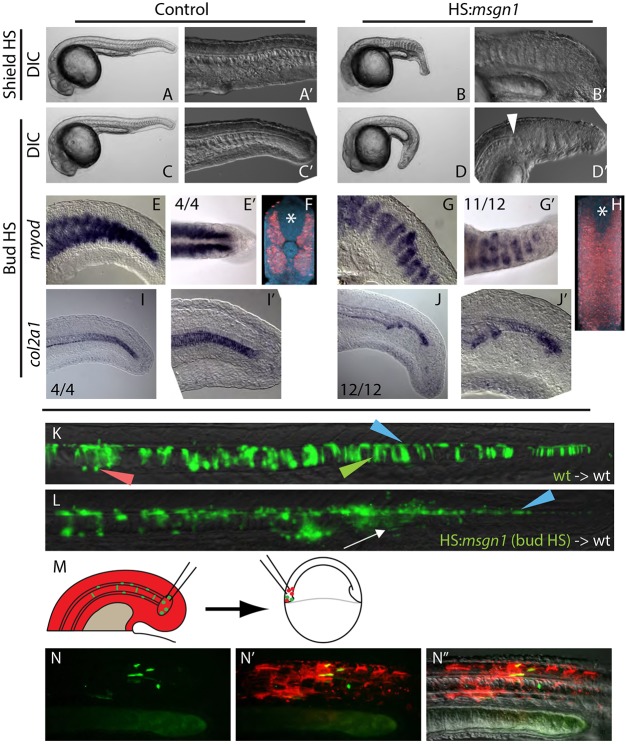
PWPCs give rise to somitic and endothelial mesoderm, but never become notochord (Martin and Kimelman, 2012; Tzouanacou et al., 2009), despite being in close proximity to the MPCs (Kanki and Ho, 1997). Our results demonstrating a Wnt-mediated fate decision between a neural or mesodermal fate for MPCs mirror the results for PWPCs. We hypothesized that MPCs might be at least partially equivalent to PWPCs and adopt different fates in response to slight differences in their locations and environments. To test whether MPCs could be directed to join somites rather than midline tissues, we created a heat shock-inducible msgn1 transgenic line (hsp70l:msgn1-p2a-NLS kikGR). Msgn1 was recently shown in the mouse to be a master regulator of paraxial mesoderm fate, and is normally absent from notochord progenitor cells (Chalamalasetty et al., 2014; Yoo et al., 2003). We asked whether misexpression of msgn1 in notochord progenitors of the zebrafish tailbud is sufficient to cause them to adopt a paraxial mesoderm fate. When msgn1 is misexpressed throughout the whole embryo at the start of gastrulation, embryos fail to form a notochord and somites develop across the midline in its place (Fig. 7B,B′). Embryos also appear shorter than their wild-type siblings, which is likely to be due to the ability of msgn1 to promote the differentiation rather than maintenance of PWPCs, which prematurely exhausts this progenitor pool (Fior et al., 2012; Yabe and Takada, 2012). When msgn1 is induced throughout the embryo at the end of gastrulation (bud stage), the notochord ends abruptly and there is a transition to midline somite formation (Fig. 7D,D′, arrowhead). The change in fate of midline mesoderm was confirmed by examining the expression of myoD (myod1), a marker expressed in skeletal muscle of the somite (Halpern et al., 1995; Weinberg et al., 1996), and col2a1, which is expressed in the notochord (Yan et al., 1995). In embryos in which msgn1 was activated at bud stage, there is an expansion of myoD expression across the midline (Fig. 7G,G′,H) and a loss of midline col2a1 expression (Fig. 7J,J′).
Fig. 7.
Local signals regulate MPC versus PWPC fate. (A-B′) Heat shock induction of msgn1 at the start of gastrulation (shield stage) results in the absence of notochord and the ectopic expansion of somites across the midline at 24 hpf. (C-D′) Heat shock induction of msgn1 after gastrulation (bud stage) causes the notochord to end abruptly, followed posteriorly by the expansion of somites at the midline at 24 hpf. The arrowhead indicates the transition from notochord to somite at the midline. (E-J′) In embryos heat shocked at bud stage, myoD expression at 24 hpf is present in posterior regions of the embryo (E,G) and, when viewed dorsally, can be seen extending across the midline (E′,G′). Fluorescent in situ hybridization of myoD expression reveals (by digital transverse section) the absence of notochord and presence of muscle across the midline (F,H, asterisk marks the position of the spinal cord). Expression of col2a1 indicates that the notochord is truncated (I-J′). (K,L) Ectopic msgn1 expression at bud stage cell-autonomously converts some MPCs to a somitic fate, as seen in transplanted cells. Control donor cells only contribute to floor plate, notochord and hypochord (K); somite contribution was observed in 1/16 embryos. By contrast, HS:msgn1 donor cells transition from notochord contribution to somite contribution (L, white arrow; notochord to somite switch was observed in 12/16 embryos) without affecting floor plate contribution. Green, red and blue arrowheads indicate notochord, hypochord and floor plate, respectively. (M-N″) A serial transplantation scheme (M) was used to target MPCs to the PWPCs. MPCs (green) from the 12-somite stage tailbud are able to contribute to skeletal muscle tissue when analyzed at 24 hpf (N-N″).
In order to determine whether msgn1 acts cell-autonomously to induce a fate change from notochord to paraxial mesoderm, we transplanted hsp70l:msgn1-p2a-NLS kikGR cells into the MPC population of wild-type host embryos and heat shocked the hosts at bud stage. In embryos in which there is only midline contribution before the heat shock, misexpression of msgn1 causes a cell-autonomous fate change from notochord to somite, based on the absence of notochord cells and presence of somite cells, but does not inhibit floor plate formation (Fig. 7L). These results are similar to previous reports of the effects of ectopic msgn1 expression in zebrafish during gastrulation, which is sufficient to repress midline fates and induce presomitic mesoderm fates (Yabe and Takada, 2012).
We performed a novel transplant strategy to further confirm that it is the physical location and local signaling environment that direct MPCs to a notochord instead of a somite fate, rather than an inherent difference in potential. This method was developed to transplant MPCs into the PWPC population in order to determine if the MPCs would contribute to somites if they resided in the PWPC signaling environment (Fig. 7M). We were successful in transplanting cells from the tailbud ten times, with three transplants containing MPCs. Of these, two were able to contribute to somites and form muscle (Fig. 7N-N″). This result further suggests that MPCs are competent to respond to the PWPC signaling environment and become somite tissue.
DISCUSSION
The entire vertebrate posterior body is derived from multipotent progenitor cell populations
The major tissues of the posterior body of vertebrate embryos, including the somites, spinal cord, vasculature, notochord and hypochord (dorsal endoderm), are derived from the tailbud (Beck, 2015; Wilson et al., 2009). Prior work demonstrated that the PWPCs have germ layer plasticity and give rise to the somites, vasculature and spinal cord (Martin and Kimelman, 2012; Takemoto et al., 2011; Tzouanacou et al., 2009). It can also be assumed that in zebrafish the fin mesenchyme, which originates solely from presomitic mesoderm, is derived from PWPCs of the tailbud, as this is the major source of posterior paraxial mesoderm (Lee et al., 2013). Here we show that the remainder of the posterior body is derived from two populations of germ layer plastic MPCs. A dorsal MPC population gives rise to floor plate and notochord, and a ventral MPC population becomes notochord and hypochord (Fig. 8).
Fig. 8.
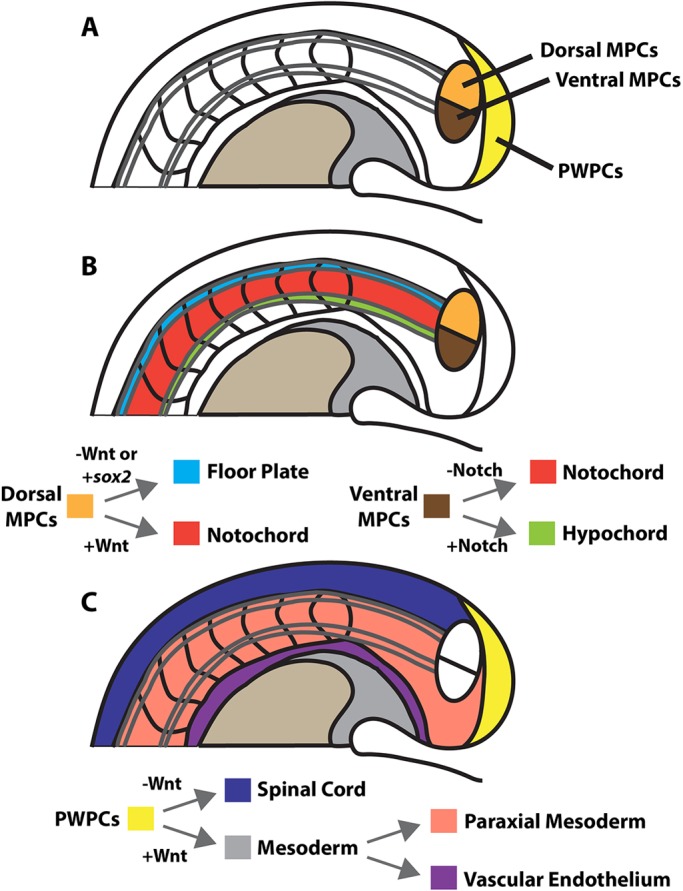
Model of the progenitor populations in the zebrafish tailbud and the signals that allocate cell fate. (A) The entire posterior body is derived from three distinct germ layer plastic progenitor cell populations. Midline tissues of the medial floor plate, notochord and hypochord originate from two MPC populations. (B) The dorsal population (orange) gives rise to notochord and floor plate depending on canonical Wnt signaling-mediated repression of sox2 expression. The ventral population (brown) gives rise to notochord and hypochord depending on the presence or absence of Notch signaling. (C) A model of the PWPCs modified from that of Martin and Kimelman (2012), in which PWPCs (yellow) generate spinal cord and mesoderm, and the mesoderm subsequently gives rise to paraxial mesoderm and vascular endothelium. The neural ectoderm/mesoderm fate decision in both the PWPCs and dorsal MPCs is mediated by canonical Wnt signaling (B,C).
Based on our work, along with the previous analysis of PWPCs, we provide a hybrid view of two historically disparate models of the tailbud. The ‘blastema’ model proposed that the tailbud is a mass of undifferentiated cells that can be induced to form any tissue type of the elongating body axis, whereas the ‘fixed fate’ model suggested that the tailbud is a mosaic of lineage-specific progenitor cells (Holmdahl, 1925; Pasteels, 1943). Our work supports a model situated between the two extremes, in which the zebrafish tailbud is composed entirely of basal progenitor cells capable of giving rise to multiple germ layers, but which reside in specific physical or molecular compartments that prevent cells within a particular compartment from joining all of the tissues of the axis (Fig. 8). For example, we showed that msgn1 must be excluded from MPCs in order for mesodermal descendants to join the notochord rather than somites. Expression of msgn1 in cells that will form somites involves positive inputs from T-box transcription factors, canonical Wnt signaling, and FGF signaling, which may only occur at the correct levels in the region of the PWPCs (Fior et al., 2012; Wittler et al., 2007; Yabe and Takada, 2012). Alternatively, the unique signaling environment of the MPCs can turn on the specific expression of transcriptional repressors such as flh, which may inhibit msgn1 expression, similar to its normal role in inhibiting tbx16 (also known as spadetail) expression (Amacher and Kimmel, 1998). Importantly, the loss of flh function causes a fate change from notochord to somite, suggesting that the MPCs are in a unique environment that prevents them from adopting PWPC fates (Halpern et al., 1995; Talbot et al., 1995).
Two populations of MPCs in the tailbud
A surprising finding of this study is that the tailbud contains not one, but two, MPC populations. The ventral population contributes to hypochord and notochord, and the dorsal population gives rise to notochord and floor plate. Previous studies in zebrafish and mouse have indicated that the notochord may be derived from multiple sources. In zebrafish, cells of Kupffer's vesicle, which is a ciliated organ of asymmetry, originate from dorsal forerunner cells, which are distinct from shield-derived axial mesoderm (Melby et al., 1996). During tailbud stages, Kupffer's vesicle collapses and some of these cells join the posterior notochord but never join the floor plate or other spinal cord fates (Melby et al., 1996). In mouse embryos, a specialized group of cells at the periphery of the node, called the node crown cells, give rise exclusively to tail notochord but not floor plate, and appear to be distinct from other node-derived notochord cells (Cambray and Wilson, 2002; Yamanaka et al., 2007). Therefore, two populations of cells giving rise to posterior notochord might be a common feature of vertebrate development.
The two populations of MPCs that we observed in the tailbud provide an embryological basis for posterior bifurcated notochord phenotypes in zebrafish and mouse, as well as the rare split notochord syndrome (SNS) birth defect observed in humans. In zebrafish, loss of BMP signaling during late gastrulation or early somitogenesis stages results in a partially penetrant phenotype of an ectopic tail on the ventral side of the posterior embryo (Pyati et al., 2005; Stickney et al., 2007; Yang and Thorpe, 2011). The same phenotype occurs after loss of non-canonical Wnt signaling, and in both cases the posterior notochord is bifurcated along the dorsal-ventral axis, with one part in the normal notochord domain and the other extending into the ectopic ventral tail (Gebruers et al., 2013; Pyati et al., 2005; Yang and Thorpe, 2011). This phenotype arises from defective cell migration (Yang and Thorpe, 2011). We showed that embryos with ectopic ventral tails exhibit a separation of the two MPC populations, with the ectopic tail containing descendants of the ventral MPCs, including hypochord and notochord, whereas the primary midline posterior to the ectopic tail contains only descendants of the dorsal MPCs (floor plate and notochord). We hypothesize that BMP and non-canonical Wnt signaling are required for the proper migration and/or coalescence of the two independent MPC populations. Ectopic tails also contain somitic tissue (Pyati et al., 2005; Yang and Thorpe, 2011), which may be due to the organizer activity of the tailbud, as observed in frog embryos (Gont et al., 1993). Alternatively, this might be due to respecification of some of the ventral MPCs as they enter a different signaling environment, similar to that observed upon expanding msgn1 expression into the tailbud MPCs.
During mouse development, loss of EphA2 function causes a very similar dorsal-ventral bifurcation of the posterior notochord (Naruse-Nakajima et al., 2001), indicating that proper morphogenesis in the mammalian tailbud is also likely to be required for the integration of two separate notochord (or possibly MPC) populations. Our work, as well as that of others, provides a molecular and embryological framework with which to investigate the etiology of the rare congenital defect of SNS, in which the posterior notochord is bifurcated, leading to abnormal patterning of the posterior trunk (Yazici et al., 2014).
Canonical Wnt signaling is a conserved regulator of all neural-mesodermal fate decisions in the zebrafish tailbud
Our finding that changes in Wnt signaling after the completion of gastrulation can alter the fate distribution of MPCs provides evidence that these cells continue to make germ layer decisions during post-gastrula stages. Combined with results from PWPCs in zebrafish, mouse and cell culture (Bouldin et al., 2015; Garriock et al., 2015; Gouti et al., 2014; Henrique et al., 2015; Jurberg et al., 2014; Martin and Kimelman, 2012; Tsakiridis et al., 2014), our data indicate that Wnt signaling has a conserved role in the induction of all tailbud mesoderm from discrete bipotential neuromesodermal progenitors. As in PWPCs, Wnt induces mesodermal fate through repression of sox2 expression. In PWPCs, Wnt-mediated repression of sox2 is accomplished by the repressor activity of the Wnt target T-box transcription factors tbx16 in zebrafish and Tbx6 in mouse (Bouldin et al., 2015; Takemoto et al., 2011). The identity of the intermediate effector of Wnt-mediated repression of sox2 in the MPCs is unclear. If it is also a T-box transcription factor, tbx2b is a likely candidate in zebrafish based on its spatiotemporal expression pattern and role in notochord formation (Dheen et al., 1999).
During mammalian development, all floor plate is induced by the underlying notochord through Hedgehog signaling (Chiang et al., 1996; Ding et al., 1998; Matise et al., 1998; Placzek and Briscoe, 2005), making it unclear whether a similar Wnt-mediated allocation mechanism between notochord and floor plate also functions in mammals. Floor plate formation in zebrafish involves both Hedgehog-dependent and -independent processes. The lateral floor plate is induced by notochord-derived Hedgehog signaling, but the medial floor plate originates from a common notochord/floor plate progenitor that does not depend on Hedgehog signaling (Charrier et al., 2002; Chen et al., 2001; Odenthal et al., 2000; Strahle et al., 2004). Medial floor plate formation instead depends on the proper allocation from a common notochord and floor plate progenitor (Halpern et al., 1997). Based on fate-mapping studies and genetic manipulations, the allocation model of medial floor plate formation is predicted to occur in other species as well, such as frog and chick (Le Douarin and Halpern, 2000). Future work is required to determine whether Wnt repression of sox2 is a common mechanism of notochord/floor plate determination from MPCs in other vertebrates.
Conclusions
Our work highlights the strengths of zebrafish as a model organism, including the ability to temporally manipulate signaling in individual cells, allowing for the precise determination of factors regulating cell fate at the cell-autonomous level. These methods allowed us to show that three midline embryonic structures with key patterning roles, namely the hypochord, notochord and floor plate, are continuously generated after gastrulation from basal MPCs located within the tailbud. This indicates, along with prior work, that all non-epidermal tailbud-derived tissues in zebrafish are generated from discrete basal progenitor populations capable of giving rise to at least two different germ layers (Martin and Kimelman, 2012; Tzouanacou et al., 2009). Our results show the presence of two distinct MPC populations in the tailbud: one that contributes to hypochord and notochord and a second that gives rise to notochord and floor plate. This work will provide insight into poorly understood human diseases associated with defective notochord development, including SNS and the rare but highly lethal cancer chordoma (Nibu et al., 2013; Yazici et al., 2014). Chordoma is an aggressive tumor type found along the axis of the body, and is thought to be derived from notochord remnants that persist from embryonic stages into adulthood (Nibu et al., 2013).
MATERIALS AND METHODS
Generation of hsp70l:sox2-p2a-NLS kikGR, hsp70l:msgn1-p2a-NLS kikGR and pntl:kaede transgenic zebrafish
All zebrafish procedures were performed in accordance with and approved by the Stony Brook University Institutional Animal Care and Use Committee (IACUC). sox2 and msgn1 were inserted without their stop codon into the hsp70l:p2a-NLS kikGR vector (Bouldin et al., 2014) to create hsp70l:sox2-p2a-NLS kikGR and hsp70l:msgn1-p2a-NLS kikGR. The plasmid constructs were co-injected with in vitro transcribed tol2 transposase mRNA to create stable transgenic lines Tg(hsp70l:sox2-p2a-NLS kikGR) and Tg(hsp70l:msgn1-p2a-NLS kikGR) with allele designation SB100 and SB101, respectively (Kawakami, 2004). A 1 kb fragment of the ntla promoter (pntl) directly upstream of the start codon was cloned upstream of the kaede coding sequence. A stable transgenic line was made using tol2-based methods.
Induced expression in transgenic and transiently transgenic embryos
Transgenic embryos were incubated for 30 min in prewarmed embryo medium at 40°C (41°C for hsp70l:β-Catenin-TFP embryos) to induce transgene expression. Transient transgenic embryos were created by co-injecting 25 pg tol2 transposase mRNA with 25 pg of one of the following plasmids: hsp70:TCFΔC-cherry; hsp70l:β-Catenin-p2a-NLS kikGR; hsp70l:sox2-p2a-NLS kikGR; hsp70l:NICD-p2a-NLS kikGR; hsp70l:dnSu(H)-p2a-NLS kikGR; or the hsp70l:p2a-NLS kikGR vector without insert. Expression in transient transgenic cells was induced by 39°C heat shock. Heat shocks were performed on bud stage embryos unless otherwise specified.
In situ hybridization and small-molecule treatment
Standard alkaline phosphatase and fluorescent in situ hybridization reactions were performed as previously described (Griffin et al., 1995; Lauter et al., 2011). The small-molecule inhibitors DMH1 or DAPT (both Selleck Chemicals) were used at 10 µM or 100 µM, respectively.
Cell transplantation
Donor embryos were injected with 1% Rhodamine-dextran alone or in combination with plasmid DNA and tol2 transposase mRNA. 25-50 cells from sphere stage (mid-blastula) donor embryos were transplanted into the margin of 1000-cell stage hosts using a CellTram (Eppendorf). Donor cells could be taken from any part of the embryo without affecting the tissue to which they contributed; cells were taken from the animal pole for convenience. This strategy maximizes the contribution of transplanted cells to the midline progenitor zone (Halpern et al., 1995).
MPC to PWPC transplantation
Donor embryos were injected with 1% Fluorescein-dextran and host embryos were injected with 1% Rhodamine-dextran. Transplants to target the MPC population were performed as above. Host embryos containing donor cells only within the midline or MPC populations were isolated at the 12-somite stage and used as MPC donors. Using forceps, the epidermis was stripped from the tailbud to facilitate cell removal. Cells were transplanted from the tailbud to the ventral margin of unlabeled wild-type host embryos, where PWPCs originate.
Microscopy and image analysis
Time-lapse imaging and imaging of fluorescent embryos were performed on either a DMI6000B microscope equipped with a DFC360 FX camera (Leica) or a spinning disk confocal microscope (Zeiss AxioImager with Yokogawa CSU-10 scanner unit). Bright-field images were obtained using a M165FC microscope (Leica) equipped with an Infinity 3 camera (Lumenera). Images were prepared using Photoshop (Adobe) or ImageJ (Schneider et al., 2012). Time-lapse images were aligned using the ImageJ plugin ‘linear stack alignment with SIFT’ (Lowe, 2004) and, in some cases, the ‘straighten’ command was used for ease of analysis.
Acknowledgements
We thank Sarah Malmquist, Ali Guler and Dave Matus for critical reading of the manuscript; John Wallingford for the dnSu(H) plasmid; Jimann Shin and Lilianna Solnica-Krezel for sox2 transgenic reporter zebrafish; and the D. Matus laboratory for use of their spinning disk confocal microscope.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
S.R.T. and H.G. performed experiments; R.H.R. and B.L.M. performed experiments, analyzed the data and wrote the manuscript.
Funding
This work was supported by a National Institutes of Health training grant [T32 GM008468] to H.G., Stony Brook University, and American Heart Association [13SDG14360032] and National Science Foundation [IOS1452928] grants to B.L.M. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.129015/-/DC1
References
- Amacher S. L. and Kimmel C. B. (1998). Promoting notochord fate and repressing muscle development in zebrafish axial mesoderm. Development 125, 1397-1406. [DOI] [PubMed] [Google Scholar]
- Appel B., Fritz A., Westerfield M., Grunwald D. J., Eisen J. S. and Riley B. B. (1999). Delta-mediated specification of midline cell fates in zebrafish embryos. Curr. Biol. 9, 247-257. 10.1016/S0960-9822(99)80113-4 [DOI] [PubMed] [Google Scholar]
- Beck C. W. (2015). Development of the vertebrate tailbud. Wiley Interdiscip. Rev. Dev. Biol. 4, 33-44. 10.1002/wdev.163 [DOI] [PubMed] [Google Scholar]
- Benazeraf B. and Pourquie O. (2013). Formation and segmentation of the vertebrate body axis. Annu. Rev. Cell Dev. Biol. 29, 1-26. 10.1146/annurev-cellbio-101011-155703 [DOI] [PubMed] [Google Scholar]
- Bouldin C. M., Snelson C. D., Farr G. H. III and Kimelman D. (2014). Restricted expression of cdc25a in the tailbud is essential for formation of the zebrafish posterior body. Genes Dev. 28, 384-395. 10.1101/gad.233577.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouldin C. M., Manning A. J., Peng Y.-H., Farr G. H. III, Hung K. L., Dong A. and Kimelman D. (2015). Wnt signaling and tbx16 form a bistable switch to commit bipotential progenitors to mesoderm. Development 142, 2499-2507. 10.1242/dev.124024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambray N. and Wilson V. (2002). Axial progenitors with extensive potency are localised to the mouse chordoneural hinge. Development 129, 4855-4866. [DOI] [PubMed] [Google Scholar]
- Catala M., Teillet M.-A. and Le Douarin N. M. (1995). Organization and development of the tail bud analyzed with the quail-chick chimaera system. Mech. Dev. 51, 51-65. 10.1016/0925-4773(95)00350-A [DOI] [PubMed] [Google Scholar]
- Catala M., Teillet M. A., De Robertis E. M. and Le Douarin M. L. (1996). A spinal cord fate map in the avian embryo: while regressing, Hensen's node lays down the notochord and floor plate thus joining the spinal cord lateral walls. Development 122, 2599-2610. [DOI] [PubMed] [Google Scholar]
- Chalamalasetty R. B., Garriock R. J., Dunty W. C. Jr., Kennedy M. W., Jailwala P., Si H. and Yamaguchi T. P. (2014). Mesogenin 1 is a master regulator of paraxial presomitic mesoderm differentiation. Development 141, 4285-4297. 10.1242/dev.110908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier J. B., Lapointe F., Le Douarin N. M. and Teillet M. A. (2002). Dual origin of the floor plate in the avian embryo. Development 129, 4785-4796. [DOI] [PubMed] [Google Scholar]
- Chen W., Burgess S. and Hopkins N. (2001). Analysis of the zebrafish smoothened mutant reveals conserved and divergent functions of hedgehog activity. Development 128, 2385-2396. [DOI] [PubMed] [Google Scholar]
- Chiang C., Litingtung Y., Lee E., Young K. E., Corden J. L., Westphal H. and Beachy P. A. (1996). Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407-413. 10.1038/383407a0 [DOI] [PubMed] [Google Scholar]
- Cleaver O. and Krieg P. A. (1998). VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development 125, 3905-3914. [DOI] [PubMed] [Google Scholar]
- Cleaver O., Seufert D. W. and Krieg P. A. (2000). Endoderm patterning by the notochord: development of the hypochord in Xenopus. Development 127, 869-879. [DOI] [PubMed] [Google Scholar]
- Connors S. A., Trout J., Ekker M. and Mullins M. C. (1999). The role of tolloid/mini fin in dorsoventral pattern formation of the zebrafish embryo. Development 126, 3119-3130. [DOI] [PubMed] [Google Scholar]
- Davis R. L. and Kirschner M. W. (2000). The fate of cells in the tailbud of Xenopus laevis. Development 127, 255-267. [DOI] [PubMed] [Google Scholar]
- Dheen T., Sleptsova-Friedrich I., Xu Y., Clark M., Lehrach H., Gong Z. and Korzh V. (1999). Zebrafish tbx-c functions during formation of midline structures. Development 126, 2703-2713. [DOI] [PubMed] [Google Scholar]
- Ding Q., Motoyama J., Gasca S., Mo R., Sasaki H., Rossant J. and Hui C. C. (1998). Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development 125, 2533-2543. [DOI] [PubMed] [Google Scholar]
- Dumont D. J., Fong G.-H., Puri M. C., Gradwohl G., Alitalo K. and Breitman M. L. (1995). Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev. Dyn. 203, 80-92. 10.1002/aja.1002030109 [DOI] [PubMed] [Google Scholar]
- Eriksson J. and Lofberg J. (2000). Development of the hypochord and dorsal aorta in the zebrafish embryo (Danio rerio). J. Morphol. 244, 167-176. [DOI] [PubMed] [Google Scholar]
- Fior R., Maxwell A. A., Ma T. P., Vezzaro A., Moens C. B., Amacher S. L., Lewis J. and Saude L. (2012). The differentiation and movement of presomitic mesoderm progenitor cells are controlled by Mesogenin 1. Development 139, 4656-4665. 10.1242/dev.078923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese N. H., Lam B. A., Staton M., Scott A. and Chapman S. C. (2014). A novel gain-of-function mutation of the proneural IRX1 and IRX2 genes disrupts axis elongation in the Araucana rumpless chicken. PLoS ONE 9, e112364 10.1371/journal.pone.0112364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriock R. J., Chalamalasetty R. B., Kennedy M. W., Canizales L. C., Lewandoski M. and Yamaguchi T. P. (2015). Lineage tracing of neuromesodermal progenitors reveals novel Wnt-dependent roles in trunk progenitor cell maintenance and differentiation. Development 142, 1628-1638. 10.1242/dev.111922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebruers E., Cordero-Maldonado M. L., Gray A. I., Clements C., Harvey A. L., Edrada-Ebel R., de Witte P. A. M., Crawford A. D. and Esguerra C. V. (2013). A phenotypic screen in zebrafish identifies a novel small-molecule inducer of ectopic tail formation suggestive of alterations in non-canonical Wnt/PCP signaling. PLoS ONE 8, e83293 10.1371/journal.pone.0083293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch G. E., Owens N. D. L., Martin S. R., Piccinelli P., Faial T., Trotter M. W. B., Gilchrist M. J. and Smith J. C. (2013). In vivo T-box transcription factor profiling reveals joint regulation of embryonic neuromesodermal bipotency. Cell Rep. 4, 1185-1196. 10.1016/j.celrep.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gont L. K., Steinbeisser H., Blumberg B. and de Robertis E. M. (1993). Tail formation as a continuation of gastrulation: the multiple cell populations of the Xenopus tailbud derive from the late blastopore lip. Development 119, 991-1004. [DOI] [PubMed] [Google Scholar]
- Gouti M., Tsakiridis A., Wymeersch F. J., Huang Y., Kleinjung J., Wilson V. and Briscoe J. (2014). In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol. 12, e1001937 10.1371/journal.pbio.1001937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S. D. and Dale J. K. (2010). Notch signalling regulates the contribution of progenitor cells from the chick Hensen's node to the floor plate and notochord. Development 137, 561-568. 10.1242/dev.041608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin K., Patient R. and Holder N. (1995). Analysis of Fgf function in normal and no tail zebrafish embryos reveals separate mechanisms for formation of the trunk and the tail. Development 121, 2983-2994. [DOI] [PubMed] [Google Scholar]
- Halpern M. E., Thisse C., Ho R. K., Thisse B., Riggleman B., Trevarrow B., Weinberg E. S., Postlethwait J. H. and Kimmel C. B. (1995). Cell-autonomous shift from axial to paraxial mesodermal development in zebrafish floating head mutants. Development 121, 4257-4264. [DOI] [PubMed] [Google Scholar]
- Halpern M. E., Hatta K., Amacher S. L., Talbot W. S., Yan Y.-L., Thisse B., Thisse C., Postlethwait J. H. and Kimmel C. B. (1997). Genetic interactions in zebrafish midline development. Dev. Biol. 187, 154-170. 10.1006/dbio.1997.8605 [DOI] [PubMed] [Google Scholar]
- Hayward P., Kalmar T. and Martinez Arias A. (2008). Wnt/Notch signalling and information processing during development. Development 135, 411-424. 10.1242/dev.000505 [DOI] [PubMed] [Google Scholar]
- Henrique D., Abranches E., Verrier L. and Storey K. G. (2015). Neuromesodermal progenitors and the making of the spinal cord. Development 142, 2864-2875. 10.1242/dev.119768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmdahl D. E. (1925). Experimentelle Untersuchungen über die Lage der Grenze zwischen primarer und sekundarer Körperentwicklung beim Hühn. Anat. Anz. 59, 393-396. [Google Scholar]
- Jeong Y. and Epstein D. J. (2003). Distinct regulators of Shh transcription in the floor plate and notochord indicate separate origins for these tissues in the mouse node. Development 130, 3891-3902. 10.1242/dev.00590 [DOI] [PubMed] [Google Scholar]
- Jurberg A. D., Aires R., Novoa A., Rowland J. E. and Mallo M. (2014). Compartment-dependent activities of Wnt3a/beta-catenin signaling during vertebrate axial extension. Dev. Biol. 394, 253-263. 10.1016/j.ydbio.2014.08.012 [DOI] [PubMed] [Google Scholar]
- Kanki J. P. and Ho R. K. (1997). The development of the posterior body in zebrafish. Development 124, 881-893. [DOI] [PubMed] [Google Scholar]
- Kawakami K. (2004). Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods Cell Biol. 77, 201-222. 10.1016/S0091-679X(04)77011-9 [DOI] [PubMed] [Google Scholar]
- Kikuta H. and Kawakami K. (2009). Transient and stable transgenesis using tol2 transposon vectors. Methods Mol. Biol. 546, 69-84. 10.1007/978-1-60327-977-2_5 [DOI] [PubMed] [Google Scholar]
- Kinder S. J., Tsang T. E., Wakamiya M., Sasaki H., Behringer R. R., Nagy A. and Tam P. P. (2001). The organizer of the mouse gastrula is composed of a dynamic population of progenitor cells for the axial mesoderm. Development 128, 3623-3634. [DOI] [PubMed] [Google Scholar]
- Kondoh H. and Takemoto T. (2012). Axial stem cells deriving both posterior neural and mesodermal tissues during gastrulation. Curr. Opin. Genet. Dev. 22, 374-380. 10.1016/j.gde.2012.03.006 [DOI] [PubMed] [Google Scholar]
- Latimer A. J. and Appel B. (2006). Notch signaling regulates midline cell specification and proliferation in zebrafish. Dev. Biol. 298, 392-402. 10.1016/j.ydbio.2006.05.039 [DOI] [PubMed] [Google Scholar]
- Latimer A. J., Dong X., Markov Y. and Appel B. (2002). Delta-Notch signaling induces hypochord development in zebrafish. Development 129, 2555-2563. [DOI] [PubMed] [Google Scholar]
- Lauter G., Soll I. and Hauptmann G. (2011). Multicolor fluorescent in situ hybridization to define abutting and overlapping gene expression in the embryonic zebrafish brain. Neural Dev. 6, 10 10.1186/1749-8104-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M. and Halpern M. E. (2000). Discussion point: origin and specification of the neural tube floor plate: insights from the chick and zebrafish. Curr. Opin. Neurobiol. 10, 23-30. 10.1016/S0959-4388(99)00062-8 [DOI] [PubMed] [Google Scholar]
- Lee R. T. H., Knapik E. W., Thiery J. P. and Carney T. J. (2013). An exclusively mesodermal origin of fin mesenchyme demonstrates that zebrafish trunk neural crest does not generate ectomesenchyme. Development 140, 2923-2932. 10.1242/dev.093534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofberg J. and Collazo A. (1997). Hypochord, an enigmatic embryonic structure: study of the axolotl embryo. J. Morphol. 232, 57-66. [DOI] [PubMed] [Google Scholar]
- Lopez S. L., Paganelli A. R., Rosato Siri M. V., Ocana O. H., Franco P. G. and Carrasco A. E. (2003). Notch activates sonic hedgehog and both are involved in the specification of dorsal midline cell-fates in Xenopus. Development 130, 2225-2238. 10.1242/dev.00443 [DOI] [PubMed] [Google Scholar]
- Lopez S. L., Rosato-Siri M. V., Franco P. G., Paganelli A. R. and Carrasco A. E. (2005). The Notch-target gene hairy2a impedes the involution of notochordal cells by promoting floor plate fates in Xenopus embryos. Development 132, 1035-1046. 10.1242/dev.01659 [DOI] [PubMed] [Google Scholar]
- Lowe D. G. (2004). Distinctive image features from scale-invariant keypoints. Int. J. Comput. Vision 60, 91-110. 10.1023/B:VISI.0000029664.99615.94 [DOI] [Google Scholar]
- Martin B. L. and Kimelman D. (2008). Regulation of canonical Wnt signaling by Brachyury is essential for posterior mesoderm formation. Dev. Cell 15, 121-133. 10.1016/j.devcel.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. L. and Kimelman D. (2012). Canonical Wnt signaling dynamically controls multiple stem cell fate decisions during vertebrate body formation. Dev. Cell 22, 223-232. 10.1016/j.devcel.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matise M. P., Epstein D. J., Park H. L., Platt K. A. and Joyner A. L. (1998). Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development 125, 2759-2770. [DOI] [PubMed] [Google Scholar]
- Melby A. E., Warga R. M. and Kimmel C. B. (1996). Specification of cell fates at the dorsal margin of the zebrafish gastrula. Development 122, 2225-2237. [DOI] [PubMed] [Google Scholar]
- Naruse-Nakajima C., Asano M. and Iwakura Y. (2001). Involvement of EphA2 in the formation of the tail notochord via interaction with ephrinA1. Mech. Dev. 102, 95-105. 10.1016/S0925-4773(01)00290-8 [DOI] [PubMed] [Google Scholar]
- Nibu Y., Jose-Edwards D. S. and Di Gregorio A. (2013). From notochord formation to hereditary chordoma: the many roles of Brachyury. BioMed. Res. Int. 2013, 826435 10.1155/2013/826435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenthal J., van Eeden F. J. M., Haffter P., Ingham P. W. and Nusslein-Volhard C. (2000). Two distinct cell populations in the floor plate of the zebrafish are induced by different pathways. Dev. Biol. 219, 350-363. 10.1006/dbio.1999.9589 [DOI] [PubMed] [Google Scholar]
- Pasteels J. (1943). Proliférations et croissance dans la gastrulation et la formation de la queue des Vertébrés. Arch. Biol. 54, 50. [Google Scholar]
- Peyrot S. M., Wallingford J. B. and Harland R. M. (2011). A revised model of Xenopus dorsal midline development: differential and separable requirements for Notch and Shh signaling. Dev. Biol. 352, 254-266. 10.1016/j.ydbio.2011.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek M. and Briscoe J. (2005). The floor plate: multiple cells, multiple signals. Nat. Rev. Neurosci. 6, 230-240. 10.1038/nrn1628 [DOI] [PubMed] [Google Scholar]
- Pyati U. J., Webb A. E. and Kimelman D. (2005). Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development 132, 2333-2343. 10.1242/dev.01806 [DOI] [PubMed] [Google Scholar]
- R Core Team (2014). R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/. [Google Scholar]
- Schafer M., Rembold M., Wittbrodt J., Schartl M. and Winkler C. (2005). Medial floor plate formation in zebrafish consists of two phases and requires trunk-derived Midkine-a. Genes Dev. 19, 897-902. 10.1101/gad.336305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S. and Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671-675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwolf G. C. and Sheard P. (1990). Fate mapping the avian epiblast with focal injections of a fluorescent-histochemical marker: ectodermal derivatives. J. Exp. Zool. 255, 323-339. 10.1002/jez.1402550309 [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S., van Eeden F. J., Halpern M. E., Kimmel C. B. and Nusslein-Volhard C. (1994). no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development 120, 1009-1015. [DOI] [PubMed] [Google Scholar]
- Selleck M. A. and Stern C. D. (1991). Fate mapping and cell lineage analysis of Hensen's node in the chick embryo. Development 112, 615-626. [DOI] [PubMed] [Google Scholar]
- Shih J. and Fraser S. E. (1996). Characterizing the zebrafish organizer: microsurgical analysis at the early-shield stage. Development 122, 1313-1322. [DOI] [PubMed] [Google Scholar]
- Shin J., Chen J. and Solnica-Krezel L. (2014). Efficient homologous recombination-mediated genome engineering in zebrafish using TALE nucleases. Development 141, 3807-3818. 10.1242/dev.108019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple D. L. (2005). Structure and function of the notochord: an essential organ for chordate development. Development 132, 2503-2512. 10.1242/dev.01812 [DOI] [PubMed] [Google Scholar]
- Stickney H. L., Imai Y., Draper B., Moens C. and Talbot W. S. (2007). Zebrafish bmp4 functions during late gastrulation to specify ventroposterior cell fates. Dev. Biol. 310, 71-84. 10.1016/j.ydbio.2007.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahle U., Lam C. S., Ertzer R. and Rastegar S. (2004). Vertebrate floor-plate specification: variations on common themes. Trends Genet. 20, 155-162. 10.1016/j.tig.2004.01.002 [DOI] [PubMed] [Google Scholar]
- Takemoto T., Uchikawa M., Yoshida M., Bell D. M., Lovell-Badge R., Papaioannou V. E. and Kondoh H. (2011). Tbx6-dependent Sox2 regulation determines neural or mesodermal fate in axial stem cells. Nature 470, 394-398. 10.1038/nature09729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot W. S., Trevarrow B., Halpern M. E., Melby A. E., Farr G., Postlethwait J. H., Jowett T., Kimmel C. B. and Kimelman D. (1995). A homeobox gene essential for zebrafish notochord development. Nature 378, 150-157. 10.1038/378150a0 [DOI] [PubMed] [Google Scholar]
- Teillet M.-A., Lapointe F. and Le Douarin N. M. (1998). The relationships between notochord and floor plate in vertebrate development revisited. Proc. Natl. Acad. Sci. USA 95, 11733-11738. 10.1073/pnas.95.20.11733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiridis A., Huang Y., Blin G., Skylaki S., Wymeersch F., Osorno R., Economou C., Karagianni E., Zhao S., Lowell S. et al. (2014). Distinct Wnt-driven primitive streak-like populations reflect in vivo lineage precursors. Development 141, 1209-1221. 10.1242/dev.101014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzouanacou E., Wegener A., Wymeersch F. J., Wilson V. and Nicolas J.-F. (2009). Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev. Cell 17, 365-376. 10.1016/j.devcel.2009.08.002 [DOI] [PubMed] [Google Scholar]
- Veldman M. B., Zhao C., Gomez G. A., Lindgren A. G., Huang H., Yang H., Yao S., Martin B. L., Kimelman D. and Lin S. (2013). Transdifferentiation of fast skeletal muscle into functional endothelium in vivo by transcription factor Etv2. PLoS Biol. 11, e1001590 10.1371/journal.pbio.1001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. S., Allende M. L., Kelly C. S., Abdelhamid A., Murakami T., Andermann P., Doerre O. G., Grunwald D. J. and Riggleman B. (1996). Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development 122, 271-280. [DOI] [PubMed] [Google Scholar]
- Wettstein D. A., Turner D. L. and Kintner C. (1997). The Xenopus homolog of Drosophila Suppressor of Hairless mediates Notch signaling during primary neurogenesis. Development 124, 693-702. [DOI] [PubMed] [Google Scholar]
- Wilson V., Olivera-Martinez I. and Storey K. G. (2009). Stem cells, signals and vertebrate body axis extension. Development 136, 1591-1604. 10.1242/dev.021246 [DOI] [PubMed] [Google Scholar]
- Wittler L., Shin E.-H., Grote P., Kispert A., Beckers A., Gossler A., Werber M. and Herrmann B. G. (2007). Expression of Msgn1 in the presomitic mesoderm is controlled by synergism of WNT signalling and Tbx6. EMBO Rep. 8, 784-789. 10.1038/sj.embor.7401030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe T. and Takada S. (2012). Mesogenin causes embryonic mesoderm progenitors to differentiate during development of zebrafish tail somites. Dev. Biol. 370, 213-222. 10.1016/j.ydbio.2012.07.029 [DOI] [PubMed] [Google Scholar]
- Yamanaka Y., Tamplin O. J., Beckers A., Gossler A. and Rossant J. (2007). Live imaging and genetic analysis of mouse notochord formation reveals regional morphogenetic mechanisms. Dev. Cell 13, 884-896. 10.1016/j.devcel.2007.10.016 [DOI] [PubMed] [Google Scholar]
- Yan Y.-L., Hatta K., Riggleman B. and Postlethwait J. H. (1995). Expression of a type II collagen gene in the zebrafish embryonic axis. Dev. Dyn. 203, 363-376. 10.1002/aja.1002030308 [DOI] [PubMed] [Google Scholar]
- Yang Y. and Thorpe C. (2011). BMP and non-canonical Wnt signaling are required for inhibition of secondary tail formation in zebrafish. Development 138, 2601-2611. 10.1242/dev.058404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazici M. U., Ekinci S., Turkmen O. K., Yalcin E. G., Ciftci A. O., Gucer S., Orhan D. and Tezcan I. (2014). Recurrent hemoptysis and a mass in the thorax in an infant: the split notochord syndrome. Eur. J. Pediatr. Surg. Rep. 2, 38-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo K. W., Kim C. H., Park H. C., Kim S. H., Kim H. S., Hong S. K., Han S., Rhee M. and Huh T. L. (2003). Characterization and expression of a presomitic mesoderm-specific mespo gene in zebrafish. Dev. Genes Evol. 213, 203-206. [DOI] [PubMed] [Google Scholar]