Fifty treatment-naive or treatment-experienced patients with genotype 1 hepatitis C virus infection and advanced fibrosis received ledipasvir, sofosbuvir, and a third directly acting antiviral for 6 weeks, with a 76% response rate. Significant liver fibrosis remains a limitation of such therapy.
Keywords: hepatitis C, advanced fibrosis, short-duration
Abstract
Background. Treatment of genotype 1 hepatitis C virus (HCV) infection with combination directly acting antivirals (DAA) for 8–24 weeks is associated with high rates of sustained virologic response (SVR). We previously demonstrated that adding a third DAA to ledipasvir and sofosbuvir (LDV/SOF) can result in high SVR rates in patients without cirrhosis. In this study, we investigated whether a similar regimen would yield equivalent rates of cure in patients with advanced liver fibrosis.
Methods. Fifty patients were enrolled at the Clinical Research Center of the National Institutes of Health and associated healthcare centers. Enrollment and follow-up data from April 2014 to June 2015 are reported here. Eligible participants were aged ≥18 years, had chronic HCV genotype 1 infection (serum HCV RNA ≥2000 IU/mL), and stage 3–4 liver fibrosis. HCV RNA was measured using a reverse-transcription polymerase chain reaction assay.
Results. Of patients treated with LDV, SOF, and the NS3/4A protease inhibitor GS-9451 for 6 weeks, 76% (38 of 50; 95% confidence interval, 60%–85%) had SVR achieved 12 weeks after the end of treatment. There was no statistically significant difference in treatment efficacy between treatment-naive patients (72%, 18 of 25) and those with treatment experience (80%; 20 of 25) (P = .51). Overall, 11 patients (22%) experienced virologic relapse, and 1 (2%) was lost to follow-up at 4 weeks after treatment. No serious adverse events, discontinuations, or deaths were associated with this regimen.
Conclusions. Adding a third DAA to LDV/SOF may result in a moderate SVR rate, lower than that observed in patients without cirrhosis. Significant liver fibrosis remains an impediment to achieving SVR with short-duration DAA therapy.
Chinese Clinical Trials Registration. CT01805882.
The treatment of genotype 1 chronic hepatitis C virus (HCV) infection has undergone a rapid shift, culminating in safe and effective directly acting antiviral (DAA) therapy. Previous interferon-based regimens resulted in sustained virologic response (SVR) rates ranging from 50% with ribavirin [1, 2] to 80% with telaprevir or boceprevir [3–5]. In 2013, Osinusi et al [6] demonstrated that therapy could be shortened to 24 weeks using sofosbuvir (SOF, an HCV NS5B inhibitor) and ribavirin, with SVR rates of 68%. In 2014, 2 interferon- and ribavirin-free regimens were approved for treatment of patients with HCV genotype 1 infection in the United States, each with response rates of >90% when administered for 12–24 weeks [7–10].
Despite this success, the significant costs associated with second-generation DAA agents [11], along with known decline in medication adherence seen in longer courses of treatment [12], have led to significant interest in short-duration therapy. A combination of the NS5A inhibitor ledipasvir (LDV) with SOF for 8 weeks demonstrated higher rates of relapse compared with 12 weeks in noncirrhotic, treatment-naive (TN) patients [13]. Subsequently, in a proof-of-concept investigation, Kohli and colleagues [14] found that adding a third DAA for 6 weeks of therapy produced SVR rates of 95% in TN patients without cirrhosis. Notably, augmented viral kinetics were seen in the arm using the serine NS3/4A protease inhibitor GS-9451 as the third agent.
However, it remains unclear whether the success of 6-week therapy can be generalized to include patients with advanced fibrosis or cirrhosis, particularly those with prior treatment experience. Determining which specific patient populations respond to short-duration therapy is critical to potentially optimize the cost-effectiveness of HCV therapy.
We conducted a phase IIA clinical trial to evaluate the efficacy, safety, and tolerability of LDV, SOF, and GS-9451 for 6 weeks in TN and treatment-experienced (TE) patients with advanced liver fibrosis and chronic HCV genotype 1 infection, and to explore which host and viral factors mediate response.
METHODS
Patients and Study Design
Fifty patients were enrolled at the Clinical Research Center of the National Institutes of Health (NIH) and associated healthcare centers. Enrollment and follow-up data from April 2014 to June 2015 are reported here. Eligible participants were aged ≥18 years of age and had chronic HCV genotype 1 infection (serum HCV RNA, ≥2000 IU/mL) and stage 3–4 liver fibrosis as demonstrated by liver biopsy or by a combination of FibroSure test and aspartate aminotransferase–platelet ratio index. Written or oral informed consent was obtained from all participants during screening and enrollment. Full eligibility criteria are included in Supplementary Appendix 1.
Study Design
Twenty-five TN and 25 TE patients were sequentially enrolled and treated for 6 weeks with 90 mg of LDV coformulated with 400 mg of SOF as a single tablet, along with a second 80-mg tablet of GS-9451, both taken once daily. Study medications were stopped if HCV RNA had not declined by >2 log10 at week 4, unless such a decline was below the lower limit of quantification (LLOQ). Adherence was assessed by self-report at each visit.
Study Funding and Oversight
The study was approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases (NIAID) and was conducted in compliance with the Good Clinical Practice guidelines, the Declaration of Helsinki, and regulatory requirements. The NIAID Office of Clinical Research Policy and Regulatory Operations served as the study sponsor and medical monitor.
Efficacy Assessments
Serum HCV RNA levels were measured using a reverse-transcription polymerase chain reaction assay (Roche COBAS TaqMan HCV RNA assay version 2.0) with an LLOQ of 25 IU/mL and a lower limit of detection of 15 IU/mL. Plasma HCV RNA levels were also measured at multiple time points using the Abbott RealTime HCV assay, with an LLOQ of 12 IU/mL.
Safety Assessments
Adverse events and clinical laboratory results were recorded throughout the study. Adverse events were graded from 1 (mild) to 4 (severe), according to the NIAID Division of AIDS toxicity table, version 1.0 [15].
IL28B and IFNL4 Genotyping
Whole blood was collected using PAXgene Blood DNA tubes (Qiagen) and stored at −80°C until DNA extraction with the Paxgene Blood DNA Kit (PreAnalytiX, a Qiagen/BD Company) and genotyping was performed as described elsewhere [14].
Clinical End Points
The primary efficacy end point was the proportion of participants with plasma HCV RNA below the LLOQ 12 weeks after treatment completion (SVR12) as shown by the Roche assay. The primary safety end point was the frequency and severity of adverse events. Secondary end points include the proportion of participants with unquantifiable HCV RNA at specified time points, treatment discontinuations due to adverse events and safety laboratory changes, and evaluation of HCV resistance-associated variants (RAVs) at baseline in all patients and at viral relapse for applicable patients. A post hoc comparison of viral kinetics between patient cohorts was also performed.
Deep Sequencing of HCV
Deep sequencing of the HCV NS3/NS4, NS5A and NS5B genes (at ≥5000 readings) to identify RAVs was performed by the DDL Diagnostics Laboratory using Illumina MiSeq technology, as described elsewhere [6]. This sequencing was completed using samples collected at baseline in all patients and at the time of virologic failure in patients with relapse.
Statistical Analysis
The primary efficacy and safety end points were based on an intention-to-treat analysis and included all patients who received ≥1 dose of study medication. Sample size was calculated to provide a sufficiently high probability (≥10%) of observing ≥1 adverse event and with prespecified confidence intervals (CI) for estimates of efficacy assuming 25 patients in each cohort. With 25 patients in each cohort, if the true probability of an adverse event due to a regimen is ≥10%, there exists a 93% chance of observing ≥1 such adverse event. With a sample size of 25, if SVR12 is achieved in all patients, the 95% CI for that estimate is 86%–100%.
Baseline demographics were compared using t tests for continuous outcomes and Fisher and χ2 tests for binary outcomes. The observed declines in HCV load in the 2 arms of this study were compared using t tests. A univariate analysis of predictors of treatment outcome (SVR12) was performed using t tests for continuous variables and χ2 or Fisher exact tests for categorical variables. Univariate logistic regression was used to model the crude association between SVR12 and baseline host and viral factors with odds ratios and 95% CI. Analyses were performed using PRISM 6.0, S-Plus 8.0, and SAS 9.3 software, with differences considered statistically significant at P = .05.
RESULTS
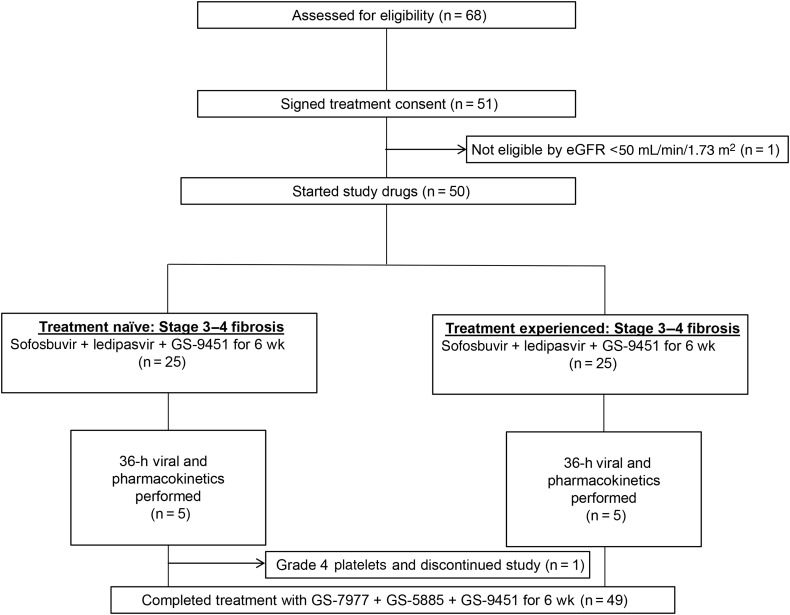
Of 68 participants screened, 50 were enrolled in this study (Figure 1).
Figure 1.
Patient disposition. Abbreviation: eGFR, estimated glomerular filtration rate.
Baseline Characteristics of Participants
Baseline characteristics were largely similar between patient cohorts in this study (Table 1). Participants were predominantly black (60%; 30 of 50) and male (66%; 33 of 50), and most had the IL28B non-CC (70%; 35 of 30) or IFNL4 non-TT/TT genotype (96%; 48 of 50) and were infected with HCV genotype 1a (74%; 37 of 50). Twenty-two percent (11 of 50) had high baseline plasma HCV RNA levels (>6 million IU/mL by Roche assay). Fifty-four percent (27 of 50) had stage 3 liver disease, and 44% (22 of 50) had stage 4 disease. All patients with prior treatment experience (n = 25) had been treated with pegylated interferon plus ribavirin (44% were nonresponders, 36% experienced relapse, and 20% could not tolerate therapy due to adverse events).
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants
| Characteristic | Participants, No. (%)a |
P Value | |
|---|---|---|---|
| Treatment Naive (n = 25) | Treatment Experienced (n = 25) | ||
| Age, mean (SD), y | 57.3 (5.6) | 58.8 (3.3) | .25 |
| Male | 18 (72) | 15 (60) | .37 |
| Raceb | |||
| Black | 20 (80) | 10 (40) | .01 |
| White | 4 (16) | 13 (52) | |
| Other (multiracial or unknown) | 1 (4) | 2 (8) | |
| Ethnicityb | |||
| Hispanic | 0 (0) | 3 (12) | .23 |
| Non-Hispanic | 25 (100) | 22 (88) | |
| BMI, mean (SD) | 29.9 (4.9) | 31.2 (8.1) | .49 |
| HCV genotypec | |||
| 1a | 15 (60) | 23 (92) | .02 |
| 1b | 9 (36) | 2 (8) | |
| 1a or 1b | 1 (4) | 0 (0) | |
| HCV RNA >6 million IU/mL | 6 (24) | 5 (20) | .73 |
| IL28B genotype | |||
| CC | 5 (20) | 10 (40) | .15 |
| CT | 17 (68) | 10 (40) | |
| TT | 3 (12) | 5 (20) | |
| IFNL4 genotype | |||
| TT/TT | 1 (4) | 1 (4) | .85 |
| ΔG/TT | 21 (84) | 19 (76) | |
| ΔG/ΔG | 3 (12) | 5 (20) | |
| Knodell HAI, METAVIR, or FibroSure fibrosis scored | |||
| 1 | 1 (4)e | 0 (0) | .78 |
| 2 | 0 (0) | 0 (0) | |
| 3 | 14 (56) | 13 (52) | |
| 4 | 10 (40) | 12 (48) | |
| Presence of NS3, NS5A, or NS5B RAV with >5-fold resistance | |||
| No | 20 (80) | 19 (76) | .73 |
| Yes | 5 (20) | 6 (24) | |
Abbreviations: BMI, body mass index; HAI, histology activity index; HCV, hepatitis C virus; RAV, resistance-associated variant; SD, standard deviation.
a Data represent No. (%) of participants unless otherwise specified.
b Race and ethnicity were self-reported.
c HCV genotyping was inconclusive in 1 treatment-naive patient, who may have been infected with HCV genotype 1a or 1b/6.
d Liver biopsy was required in patients in whom a FibroSure score of >0.48 and an aspartate aminotransferase–platelet ratio index (APRI) of >1 within 6 months of screening were not available or in whom these results were discordant. In the treatment-naive cohort, 2 patients (8%) met FibroSure/APRI eligibility criteria, 10 (40%) had liver biopsy specimens scored using the Knodell HAI system, and 13 (52%) had specimens scored using the METAVIR scoring system. In the treatment-experienced cohort, liver fibrosis scoring was completed with FibroSure/APRI in 4 patients (16%), with Knodell HAI in 9 (36%), and with METAVIR in 12 (48%). Fibrosis scores reported as 2.5 or 2–3 were categorized as stage 3, and those reported as 3–4 or 3.5–4 were categorized as stage 4.
e One patient was deemed eligible for the study because of stage 3 fibrosis shown by the METAVIR scoring system before enrollment. However, this patient was deemed to have stage fibrosis 1 after the slide was read again at the National Institutes of Health using the Knodell HAI system.
Adherence
By self-report, 18% of patients (5 in the TN cohort and 4 in the TE cohort) missed ≥2 doses of any study medication during the 6-week therapy (range, 2–14 missed doses; median, 2 missed doses). Forty-one patients (82%) had no missed doses. There was no significant difference in adherence between the 2 cohorts (P > .99).
Virologic Response
Of patients treated with LDV, SOF, and GS-9451 for 6 weeks, SVR12 was achieved in 76% (38 of 50; 95% CI, 60%–85%). There was no significant difference in treatment efficacy between TN patients (72%; 18 of 25) and TE patients (80%; 20 of 25) (P = .51). Overall, 11 patients (22%) experienced virologic relapse; in the TN cohort, 1 patient (2%) was lost to follow-up 4 weeks after treatment.
On-Treatment and End-of-Treatment Response
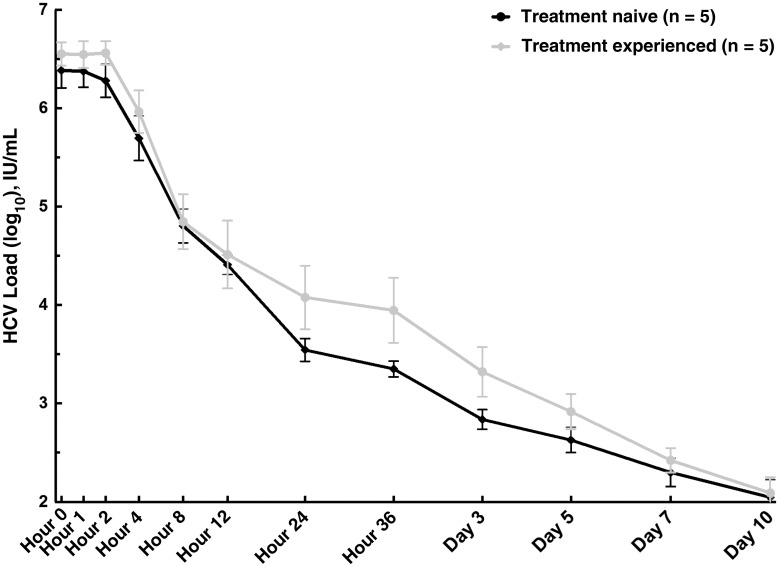
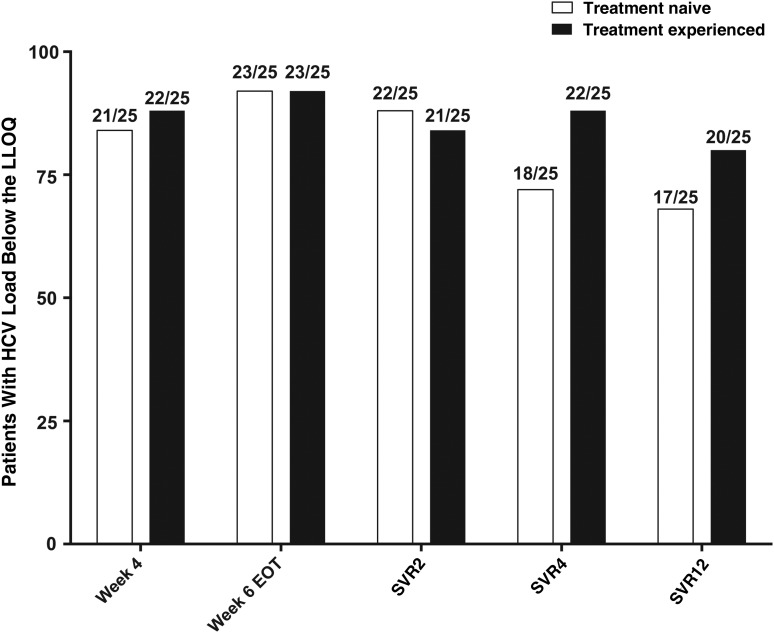
There was no difference in early HCV decline (Figure 3) or by weeks 2 and 4 between the 2 patient cohorts (Supplementary Figure 1). At week 4 of treatment, 84% of TN participants (21 of 25) had HCV RNA levels below the LLOQ, compared with 88% (22 of 25) in the TE cohort. At week 6, the end of treatment, 92% in each group (23 of 25) had unquantifiable HCV RNA (Figure 2). Viral load measurements were not obtained at the week 6 visit in 2 patients in the TN cohort and 1 in the TE cohort. In 1 TE patient with detectable HCV RNA (86.3 IU/mL at the end of treatment), the level continued to decline after treatment (to 16.2 IU/mL and below the LLOQ at weeks 2 and 4 after treatment respectively), and SVR12 was ultimately achieved.
Figure 3.
Early viral kinetics. Abbreviation: HCV, hepatitis C virus.
Figure 2.
Patients with hepatitis C virus (HCV) RNA levels below the lower level of quantification (LLOQ) at various times during treatment and follow-up. Abbreviations: EOT, end of treatment; SVR2, SVR4, and SVR12, sustained virologic response at 2, 4, and 12 weeks after the EOT.
Timing of Viral Relapse
Of the 7 TN patients without SVR, relapse occurred in 1 by week 2 after treatment (4%), in 3 by week 4 after treatment (13%), and in 2 by week 12 after treatment (8%). One patient had undetectable HCV RNA at week 4 after treatment but was then lost to follow-up. Of the 5 TE patients without SVR, relapse occurred in 2 by week 2 after treatment (9%), 1 by week 4 after treatment (4%), and 2 by week 12 after treatment (9%).
Analysis of Viral Mutants Associated With Resistance to DAAs Used in the Study
Baseline analysis of RAVs to the study agents was performed in all 50 patients, with a lower limit of detection of 1% mutant population prevalence. In addition, viral mutants were analyzed in 11 patients with confirmed viral relapse at the time of relapse. RAVs at baseline and at relapse are summarized in Supplementary Tables 1A and 1B.
At baseline, 11 patients (22%; 11 of 50) had NS3/4 or NS5A viral mutants conferring >5-fold resistance to GS-9451 or LDV, respectively. SVR was achieved in 73% of these patients (8 of 11) and 77% of those without high-level RAVs (30 of 39). Of the 11 patients in either arm with viral relapse, 27% had high-level RAVs (3 of 11), and 73% (8 of 11) did not (P = .69). A total of 19 patients had NS3/4 RAVs at baseline, conferring <5-fold resistance to GS-9451. Of the 13 patients with Q80K mutation, all were infected with HCV genotype 1a; 18% (2 of 13) experienced viral relapse, compared with 30% (8 of 27) of patients with genotype 1a without Q80K. There were no identified variants with resistance to SOF in baseline or relapse samples.
At relapse, frequencies of RAVs were also determined and classified as emerged (those not present at baseline) or enriched (those increased in frequency). Among 11 patients in whom relapse occurred after the completion of therapy, 18% (2 of 11) had maintained NS5A or NS3/4 RAVs, 27% (2 of 11) had enriched NS5A mutants, and 27% (2 of 11) had emergence of new NS5A RAVs.
One TE patient (Supplementary Table 1B; patient 25) with baseline R155K and L31M mutants and relapse 2 weeks after treatment completion had emergence of 4 additional NS5A RAVs, together conferring >1000-fold resistance to LDV in vitro. A second TE patient (Supplementary Table 1B; patient 12) took a single dose of study medication but discontinued treatment owing to grade 4 laboratory results at baseline. This patient was monitored with analysis of viral mutants at week 10, which demonstrated emergence of 5 distinct NS5A mutants, together conferring >1000-fold resistance to LDV in vitro.
Predictors of Treatment Outcome by Combined Univariate Analysis
The relationship between SVR12 and various baseline factors was assessed through univariate analysis in both TN and TE patients (Table 2). Patients with SVR12 were slightly younger than those who without SVR12 (mean age, 57.3 vs 60.5 years; P = .02). Host factors, including female sex (P = .18), IL28B genotype (P = 1.0), IFNL4 genotype (P = 1.0), and body mass index (P = .58), self-reported nonadherence of missing ≥2 doses (P = 1.13), and F3 fibrosis (P = 2.05), were not associated with SVR12. Baseline viral factors previously associated with higher response rates [16], including genotype 1b (P = .25), HCV load of <6 million IU/mL (P = 1.0), and lack of high-level RAVs (P = 1.0) were not found to affect rates of SVR12 in this combined cohort.
Table 2.
Baseline Predictors of Treatment Outcome (SVR12)
| Predictors | Treatment-Naive Patients |
Treatment-Experienced Patients |
All Patients |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SVR12 (n = 18) | No SVR12a (n = 7) | P Values | SVR12 (n = 20) | No SVR12 (n = 5) | P Values | SVR12 (n = 38) | No SVR12b (n = 12) | P Values | OR (95% CI) | |
| Host factors | ||||||||||
| Age, mean, y | 55.9 | 61.0 | .02 | 58.6 | 59.8 | .46 | 57.3 | 60.5 | .02 | 0.82 (.68–.99) |
| Female, No. (%) | 7 (39) | 0 (0) | .13 | 8 (40) | 2 (40) | >.99 | 15 (39) | 2 (17) | .18 | 3.26 (.63–17.01) |
| Black, No. (%) | 3 (17) | 1 (14) | >.99 | 8 (40) | 2 (40) | >.99 | 11 (29) | 3 (25) | >.99 | 1.22 (.28–5.38) |
| BMI, mean, kg/m2 | 30.3 | 28.8 | .41 | 31.4 | 30.7 | .80 | 30.9 | 29.6 | .58 | 1.03 (.93–1.15) |
| IL28B CC genotype, No. (%) | 3 (17) | 2 (29) | .60 | 8 (40) | 2 (40) | >.99 | 11 (29) | 4 (33) | >.99 | 0.82 (.20–3.27) |
| IFNL4 TT/TT genotype, No. (%) | 1 (5) | 0 (0) | >.99 | 1 (5) | 0 (0) | >.99 | 2 (5) | 0 (0) | >.99 | ∞ |
| Baseline LDL, mean, mg/dL | 83.4 | 72.6 | .37 | 69.9 | 40.0 | .05 | 76.3 | 60.7 | .12 | 1.02 (.99–1.05) |
| Baseline hemoglobin A1C, mean, mmol/mol, derived (%) | 5.9 | 5.8 | .81 | 5.9 | 6.0 | .84 | 5.9 | 5.9 | .98 | 1.01 (.48–2.12) |
| Liver fibrosis score 3, No. (%) | 10 (59) | 4 (57) | >.99 | 12 (60) | 1 (20) | .16 | 22 (59) | 5 (42) | .28 | 2.05 (.55–7.70) |
| Self-reported nonadherence (≥2 missed doses), No. (%) | 3 (17) | 2 (29) | .60 | 4 (20) | 0 (0) | .55 | 7 (18) | 2 (17) | >.99 | 1.13 (.20–6.34) |
| Viral factors | ||||||||||
| HCV genotype 1b, No. (%) | 9 (50) | 1 (14) | .34 | 2 (10) | 0 (0) | >.99 | 11 (29) | 1 (8) | .25 | 4.48 (.52–39.01) |
| Baseline HCV RNA <6 million IU/mL, No. (%)c | 14 (78) | 5 (71) | >.99 | 16 (80) | 4 (80) | >.99 | 30 (79) | 9 (75) | >.99 | 1.25 (.27–5.73) |
| Absence of NS3, NS5A, or NS5B RAV with >5-fold resistance, No. (%) | 14 (78) | 6 (86) | >.99 | 16 (80) | 3 (60) | .56 | 30 (79) | 9 (75) | >.99 | 1.25 (.27–5.73) |
Abbreviations: BMI, body mass index; CI, confidence interval; HCV, hepatitis C virus; LDL, low-density lipoprotein; OR, odds ratio; RAV, resistance-associated variant; SVR12, sustained virologic response at 12 weeks after treatment completion.
a The no-SVR12 group includes 1 patient lost to follow-up and 6 who experienced virologic relapse.
b The no-SVR12 group includes 1 patient lost to follow-up and 11 who experienced virologic relapse.
c By Roche assay.
Safety
Forty-nine of the 50 patients from both TN and TE arms completed therapy. Adverse events were recorded as any related event after day 0 until 30 days after treatment completion. In the TE arm, one grade 3 adverse event occurred: 1 patient with diabetes mellitus experienced hyperglycemia, which decreased in grade over time. In the TN arm, 1 patient had an asymptomatic increase in alanine aminotransferase level, without other associated abnormalities, and another patient had an elevated serum creatinine level. Both adverse events resolved without intervention.
Table 3.
Adverse Events and Laboratory Abnormalities During Treatment Period
| Adverse Events | Patients, No. (%) |
|
|---|---|---|
| Treatment Naive (n = 25) | Treatment Experienced (n = 25) | |
| Any adverse event | 25 (100) | 25 (100) |
| Grade 3 or 4 | 10 (40) | 10 (40) |
| Grade 2, 3, or 4 | 23 (92) | 25 (100) |
| Treatment related | 14 (56) | 21 (84) |
| Grade 3 or 4 | 2 (8) | 1 (4) |
| Grade 2, 3, or 4 | 6 (24) | 7 (28) |
| Serious adverse event | 0 (0) | 3 (12) |
| Treatment related | 0 (0) | 0 (0) |
| Deaths | 0 (0) | 0 (0) |
Three serious adverse events occurred during the study period, all deemed unrelated to study drugs. One patient with grade 4 platelets on day 0 inadvertently received 1 dose of medication. Study drugs were discontinued with stabilization of platelet count; the patient was followed up monthly until the patient withdrew consent and was treated according to the standard of care in the community. There were 2 serious adverse events, both occurring after completion of drug treatment; 1 patient had an episode of angina pectoris, and another experienced infectious colitis that resolved with antibiotic therapy. No patient discontinued therapy owing to a drug-related adverse event, and no deaths occurred in the course of this study.
DISCUSSION
In this phase II clinical trial, the use of the NS3/4 protease inhibitor GS-9451 in combination with the NS5A inhibitor LDV and NS5B inhibitor SOF administered for 6 weeks was moderately effective, with SVR12 achieved in 76% of patients (38 of 50) and without an observed difference in response rate between TN (72%; 18 of 20) and TE (80%; 20 of 25) patients. Twenty-two percent of patients (11 of 50) experienced viral relapse. The therapy was safe and well tolerated, with no grade 4 events, serious adverse events, or discontinuations related to study drugs.
Our study demonstrated the modest efficacy of combination DAA therapy for 6 weeks in a cohort of predominantly African American patients with advanced liver fibrosis, including those with treatment experience and cirrhosis. These findings support the scientific principle that enhancing the potency of DAA regimens permits the treatment duration to be shortened by eliminating all HCV-infected hepatocytes. However, response rates were lower than those observed elsewhere in patients without cirrhosis [14]. As such, our findings suggest that in the setting of advanced fibrosis, short-duration therapy with current agents will not achieve the high rates of SVR seen in clinical trials with standard treatment durations.
We also investigated potential host and viral factors associated with response. Analysis revealed no clear correlates of response on combined univariate analysis. However, the success of our 6-week trial, in patients with early-stage fibrosis who were demographically comparable to the present cohort, suggests that hepatic fibrosis may be the major factor predicting decreased effectiveness for DAA therapy.
Hepatic fibrosis is known to impair multiple pathways of HCV clearance, including reduced drug delivery due to venous shunting, limited drug uptake secondary to fibrotic changes, decreased drug metabolism from reduced liver function, and impaired immune signaling pathways [17]. Lower rates of SVR have been reported with the use of DAA regimens in the setting of advanced fibrosis [7, 10, 18], and in previous studies of interferon-based therapy, hepatic fibrosis is an independent negative predictor of SVR [19]. Given the high potency of DAAs, it is unclear whether immune response plays a significant role in viral eradication. However, in a recent study of patients treated with SOF and ribavirin, Meissner et al [20] demonstrated that the resetting of intrahepatic type I interferon response was associated with SVR, suggesting a role for healthy liver in aiding HCV clearance, even in the setting of interferon-free therapy.
It is likely that given both compromise in drug delivery and viral kinetics in the setting of advanced fibrosis, short-duration therapy may not achieve high rates of SVR and is therefore unlikely to be pursued as a large-scale treatment strategy in patients with advanced fibrosis. These findings are supported by recent models of HCV viral dynamics [21] and results from 3- and 4-DAA combination therapy [22], which indicate that ultrashort durations of <6 weeks are not effective in the majority of patients.
In this study, we evaluated the influence of RAVs on treatment response. We demonstrated a 22% baseline prevalence of RAVs, without an impact on SVR, consistent with other published trials of longer-duration LDV and SOF therapy [13]. For the 11 patients with viral relapse to 6-week combination DAA therapy, it is unclear how these viral mutants will evolve and what influence, if any, they may have on treatment. We report findings in patient 12, who took only 1 dose of study medications, but after 10 weeks had emergence of 5 NS5A mutants conferring high-level resistance. Despite this, approximately 10 months after initial dose, the patient was treated again in the community with 12 weeks of LDV/SOF, and SVR12 was achieved. Given the unique physiologic characteristics of patients with advanced fibrosis, further research is warranted to identify the ideal retreatment strategy in patients with prior DAA exposure. The remaining 10 patients with viral relapse are receiving or awaiting retreatment.
Our study was limited by small sample size, a nonrandomized, unblinded design, and the specific patient demographics of our cohort; the statistical power of our conclusions is therefore restricted, with unclear applicability to larger-scale settings. In conclusion, our investigation is among the first to demonstrate moderate rates of success with 6-week combination DAA therapy in a cohort of patients with advanced liver fibrosis.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We acknowledge the contributions of the following individuals: Michelle Espinosa (administrative support), Erin Rudzinski, BS, and Susan Vogel, RN, BSN (clinical monitoring support), Judith Starling, PharmD (pharmacy), Jerome Pierson, PhD, and John Tierney, BSN, MPM (regulatory support); Richard Williams, PhD, and Mike Mowatt, PhD (technology transfer support), Marc Teitelbaum, MD, CPI (sponsor medical monitor), Mary Hall (protocol support), Cathy Rehm and Sarah Jones (laboratory support), and Senora Mitchell (clinic support).
Author contributions. S. Kattakuzhy and S. Kottilil had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; S. Kattakuzhy performed the literature search; S. Kottilil, M. A. P., and H. Masur contributed to the study design; S. Kattakuzhy, E. W., S. Sidharthan, Z. S., M. McLaughlin, A. P., R. S., C. G., E. A., and M. McManus collected data; S. Kattakuzhy, S. Sidharthan, Z. S., B. E., S. Shrivastava, and H. Mo analyzed data; S. Kattakuzhy, E. W., H. Masur, and S. Kottilil interpreted data; S. Kattakuzhy, S. Sidharthan, Z. S., B. E., and H. Mo contributed to figure design; S. Kattakuzhy wrote the first draft of the manuscript; and all authors reviewed the manuscript and had the opportunity to revise it.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government. Entities providing financial support did not have a role in the writing of the manuscript or the decision to submit it for publication.
Supplement sponsorship. The Regulatory Compliance and Human Participants Protection Branch of the National Institute of Allergy and Infectious Diseases (NIAID) served as the study sponsor and was involved in the review and approval of the study via the usual peer-review process as well as the study management. It did not play a role in the design of the study; data collection and analysis; interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Financial support. This project has been funded in whole or in part with funds from the National Cancer Institute, National Institutes of Health (NIH) (contract HHSN261200800001E) and the NIAID. It was also supported in part by the German Research Foundation and by the clinical research unit KFO 129. Study medications were provided by Gilead Sciences, and the study was partially funded by a collaborative research and development agreement between NIH and Gilead Sciences.
Potential conflicts of interest. G. T. serves on the Gilead and Merck advisory boards and as a speaker for Gilead Sciences; J. C. is a member of the regional advisory boards for Abbott, Bristol-Myers Squibb, and Gilead Sciences; and H. Mo and A. O. are employees of Gilead Sciences. All other author report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fried MW, Shiffman ML, Reddy KR et al. . Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. New Engl J Med 2002; 347:975–82. [DOI] [PubMed] [Google Scholar]
- 2.Manns MP, McHutchison JG, Gordon SC et al. . Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358:958–65. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson IM, McHutchison JG, Dusheiko G et al. . Telaprevir for previously untreated chronic hepatitis C virus infection. New Engl J Med 2011; 364:2405–16. [DOI] [PubMed] [Google Scholar]
- 4.Kwo PY, Lawitz EJ, McCone J et al. . Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet 2010; 376:705–16. [DOI] [PubMed] [Google Scholar]
- 5.Limaye AR, Draganov PV, Cabrera R. Boceprevir for chronic HCV genotype 1 infection. New Engl J Med 2011; 365:176–8. [DOI] [PubMed] [Google Scholar]
- 6.Osinusi A, Meissner EG, Lee YJ et al. . Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA 2013; 310: 804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afdhal N, Reddy KR, Nelson DR et al. . Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. New Engl J Med 2014; 370:1483–93. [DOI] [PubMed] [Google Scholar]
- 8.Afdhal N, Zeuzem S, Kwo P et al. . Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. New Engl J Med 2014; 370:1889–98. [DOI] [PubMed] [Google Scholar]
- 9.Ferenci P, Bernstein D, Lalezari J et al. . ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. New Engl J Med 2014; 370:1983–92. [DOI] [PubMed] [Google Scholar]
- 10.Poordad F, Hezode C, Trinh R et al. . ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. New Engl J Med 2014; 370:1973–82. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy M. New drug for hepatitis C contributes to 13% rise in spending on prescription drugs in US. Brit Med J 2015; 350:h2055. [DOI] [PubMed] [Google Scholar]
- 12.Peterson T, Gordon LA, Townsend K et al. . Pill burden and treatment length reduce adherence to IFN-free hepatitis C therapy in an urban cohort [abstract 667] In: 21st Conference on Retroviruses and Opportunistic Infections, March 3–6, 2014. [Google Scholar]
- 13.Kowdley KV, Gordon SC, Reddy KR et al. . Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. New Engl J Med 2014; 370:1879–88. [DOI] [PubMed] [Google Scholar]
- 14.Kohli A, Osinusi A, Sims Z et al. . Virological response after 6 week triple-drug regimens for hepatitis C: a proof-of-concept phase 2A cohort study. Lancet 2015; 385:1107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.http://www.niaid.nih.gov/labsandresources/resources/daidsclinrsrch/documents/daidsaegradingtable.pdf. [Google Scholar]
- 16.Cavalcante LN, Lyra AC. Predictive factors associated with hepatitis C antiviral therapy response. World J Hepatol 2015; 7:1617–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al Marzooqi SH, Feld JJ. Sorting out cirrhosis: mechanisms of non-response to hepatitis C therapy. Liver Int 2015; 35:1923–33. [DOI] [PubMed] [Google Scholar]
- 18.Muir AJ, Poordad F, Lalezari J et al. . Daclatasvir in combination with asunaprevir and beclabuvir for hepatitis C virus genotype 1 infection with compensated cirrhosis. JAMA 2015; 313:1736–44. [DOI] [PubMed] [Google Scholar]
- 19.Guedj H, Guedj J, Negro F et al. . The impact of fibrosis and steatosis on early viral kinetics in HCV genotype 1–infected patients treated with Peg-IFN-alfa-2a and ribavirin. J Viral Hepatitis 2012; 19:488–96. [DOI] [PubMed] [Google Scholar]
- 20.Meissner EG, Bon D, Prokunina-Olsson L et al. . IFNL4-ΔG genotype is associated with slower viral clearance in hepatitis C, genotype-1 patients treated with sofosbuvir and ribavirin. J Infect Dis 2014; 209:1700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perelson AS, Guedj J. Modelling hepatitis C therapy-predicting effects of treatment. Nat Rev Gastroenterol Hepatol. 2015; 12:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohli A, Kattakuzhy S, Kottilil S et al. . Four-week directly acting anti-HCV regimens in HCV genotype-1 patients without cirrhosis. Ann Intern Med 2015, publication pending. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.