Human immunodeficiency virus-discordant couples at risk for transmitting herpes simplex virus type 2 (HSV-2) were selected. Couples recorded sex, condom use, and were followed for HSV-2 transmission. Condoms were 96% effective at preventing HSV-2 transmission from men to women and 65% effective from women to men.
Keywords: HSV-2, transmission, HIV, condom, efficacy
Abstract
Background. The efficacy of condoms for protection against transmission of herpes simplex virus type 2 (HSV-2) has been examined in a variety of populations with different effect measures. Often the efficacy has been assessed as change in hazard of transmission with consistent vs inconsistent use, independent of the number of acts. Condom efficacy has not previously measured on a per-act basis.
Methods. We examined the per-act HSV-2 transmission rates with and without condom use among 911 African HSV-2 and human immunodeficiency virus type 1 (HIV-1) serodiscordant couples followed for an average of 18 months in an HIV prevention study. Infectivity models were used to associate the log10 probability of HSV-2 transmission over monthly risk periods with reported numbers of protected and unprotected sex acts. Condom efficacy was computed as the proportionate reduction in transmission risk for protected relative to unprotected sex acts.
Results. Transmission of HSV-2 occurred in 68 couples, including 17 with susceptible women and 51 with susceptible men. The highest rate of transmission was from men to women: 28.5 transmissions per 1000 unprotected sex acts. We found that condoms were differentially protective against HSV-2 transmission by sex; condom use reduced per-act risk of transmission from men to women by 96% (P < .001) and marginally from women to men by 65% (P = .060).
Conclusions. Condoms are recommended as an effective preventive method for heterosexual transmission of HSV-2.
The prevalence of human simplex virus type 2 (HSV-2) varies globally from about 16% [1] in the United States to over 50% in sub-Saharan Africa [2]. The severity of symptoms varies between persons, but sexual transmission can occur even in the absence of symptoms [3, 4]. Further, HSV-2 seropositivity has been associated with increased risk of human immunodeficiency virus type 1 (HIV-1) acquisition, increasing the importance of this highly prevalent infection in settings with high HIV-1 burden [5]. Suppressive antiherpes therapy reduces but does not eliminate the risk of transmission to sexual partners [3]. No preventive HSV-2 vaccine has been demonstrated effective to date [6, 7].
In the absence of preventive vaccines, barrier methods continue to be a recommended approach for preventing the sexual transmission of HSV-2 [8]. The efficacy of condoms in preventing HSV-2 acquisition and transmission has been previously described, commonly by categorizing condom use (always, sometimes, never) [9–13]. Low (or lack of) efficacy has been attributed to failure of the condom to cover the infectious skin [9, 10], which differs by gender [13]. Using data that include precise numbers of sex acts from a HIV-1 prevention clinical trial [14], we sought to ascertain the per-act efficacy of condoms in preventing HSV-2 transmission within HIV-1/HSV-2 serodiscordant couples.
METHODS
Study Design and Data Collection
The Partners in Prevention HSV-2/HIV-1 Transmission Study, a multisite, placebo-controlled, randomized, clinical trial, was conducted among 3408 HIV-1 discordant couples in which the HIV-1 infected partner was also HSV-2 seropositive. The study assessed whether the risk of HIV-1 transmission could be reduced by daily suppressive HSV-2 antiviral therapy (acyclovir 400 mg twice daily). Study characteristics and main findings have been reported elsewhere [14]. Suppressive acyclovir treatment was not associated with a decreased risk of HIV transmission (HR = 0.92, 95% confidence interval [CI], .60 to 1.41, P = .69).
As noted above, the HIV-infected partner in the HIV-discordant couple (the index participant) was required to be HSV-2 seropositive at enrollment; however, no restrictions were placed on the HSV-2 status of the HIV-1-uninfected partner (the partner participant). Thus, a subset of couples was at risk of transmitting HSV-2. Quarterly sera samples were used to determine the timing of HSV-2 transmission.
We previously reported that the hazard of HSV-2 transmission was not modified by acyclovir suppressive therapy (HR = 1.35, 95% CI, .83 to 2.20, P = .22) [15]. For the present study, we evaluated the same subset of couples at risk of transmitting HSV-2. The number of vaginal sex acts with and without condoms was reported monthly by indexes. Interpolation of sexual behavior was used when the dates of sexual behavior reporting and serologic testing differed. Specifically, we scaled the number of acts, multiplying by the length of time between serologic tests and dividing by the length of time covered by sexual reporting. For example, if 19 acts were reported over 85 days, and the testing interval was 90 days, acts were used. When blood draws occurred at regularly scheduled visits, no interpolation was needed. For this study condoms always refers to male condoms.
Laboratory Methods
HSV-2 serostatus was assessed by HerpeSelect-2 EIA (Focus Technologies), with index ≥3.5 defined as HSV-2 seropositive, and confirmed by the University of Washington HSV Western blot [16].
Statistical Methods
We use the infectivity models of Jewell and Shiboski [17] and recently adapted by Hughes [18] to ascertain the influence of condom use on HSV-2 transmission. The model estimates two per-act infectivity rates, one for protected and one for unprotected acts. The probability of within-couple transmission is:
where X is the vector of person-level predictors of transmission risk (HIV viral load, antiretroviral use, etc.), β is the corresponding vector of coefficients, N0 and N1 are, respectively, the numbers of reported unprotected and protected sex acts, and λ0 and λ1 are per-act risks of transmission for unprotected and protected sex acts.
Of primary interest was the efficacy of condoms to prevent HSV-2 transmission, as computed from the per-act probabilities: . Other measures of interest included plasma HIV RNA and CD4 count, treatment arm, and other factors previously found to be related to HSV-2 transmission: age, number of children, male circumcision status, current or recent reports of genital ulcers by indexes, vaginal drying, the pre-coital practice of removing vaginal lubrication (primarily) with a cloth, and antiretroviral (ARV) drug use by indexes [15]. A single model was fit evaluating whether condom efficacy differed by sex. Subsequently, separate models were evaluated by sex of the susceptible partner. Potential predictors were each included in a model with condom use as the only other predictor (univariate models). For each sex, univariate models with interaction were examined to assess whether condom efficacy differed by the presence of genital ulcers at enrollment in the index participant. Subsequently a multivariate model was built initially using all covariates significant at P < .2 and using backward elimination for final model selection.
The χ2 tests determined associations between HSV-2 transmission and categorical measures. Software included Stata 10.0 and SAS 9.3 for Windows.
Sensitivity Analysis
The number of sexual acts and frequency of condom use as reported by either partner in the couple is not relevant to quantifying the “transmission” risk if the susceptible partner acquires HSV-2 outside the partnership. Ideally, we would have included sex act reporting within the study couple only when the HSV-2 seronegative partner acquired HSV-2 from the study partner. Sequencing of transmitted strains to confirm linked transmission, as was done in the HIV-1 transmission analysis would have been desirable [19]; however, HSV DNA was only obtained from the subset of study participants who had genital ulcers and thus is mostly not available for linkage analysis. Therefore, we examined whether other sexual behavior measures might be useful in identifying couples likely to have transmitted within the partnership; we did this by examining categorical measures associated with confirmed linkage in the HIV-1 transmission analysis.
RESULTS
Nine-hundred eleven couples at risk of transmitting HSV-2 (HIV-1/HSV-2 dually infected index participants and HIV-1 and HSV-2 seronegative partners) were identified from the parent study of 3408 HIV serodiscordant couples, as described previously [15]. In 112 couples the HSV-2 susceptible partner was a woman and in 799 cases the susceptible partner was a man. Couples were followed a median of 18 months, (range 0 to 24). Most were married and had been together a median of 4 years. Of approximately 58 000 sex acts reported by index women, 6% were unprotected; and of approximately 7500 acts reported by index men, 3% were unprotected. Interpolation of sex acts occurred over 47% of quarterly visits, with lengths of reporting periods and serologic testing periods differing by a median of 10 out of about 90 days.
Transmission of HSV-2 occurred in 68 couples, in 17 with susceptible women and 51 with susceptible men. In 16 cases, the index partner reported that no sexual activity occurred within the couple over the quarterly risk period. Since per-act transmission probabilities could not be assessed in these couples, they were censored at the time of last reported sex. We therefore included 38 HSV-2 transmissions from women to men over 973 person-years (p-y) (incidence = 3.9 per 100 p-y, 95% CI, 2.4 to 6.5) and 14 transmissions from men to women over 134 person-years (incidence = 10.4 per 100 p-y, 95% CI, 4.8 to 22.6). As reported previously, 5 couples transmitted both HSV-2 and HIV-1 in this subset at risk for both infections [15].
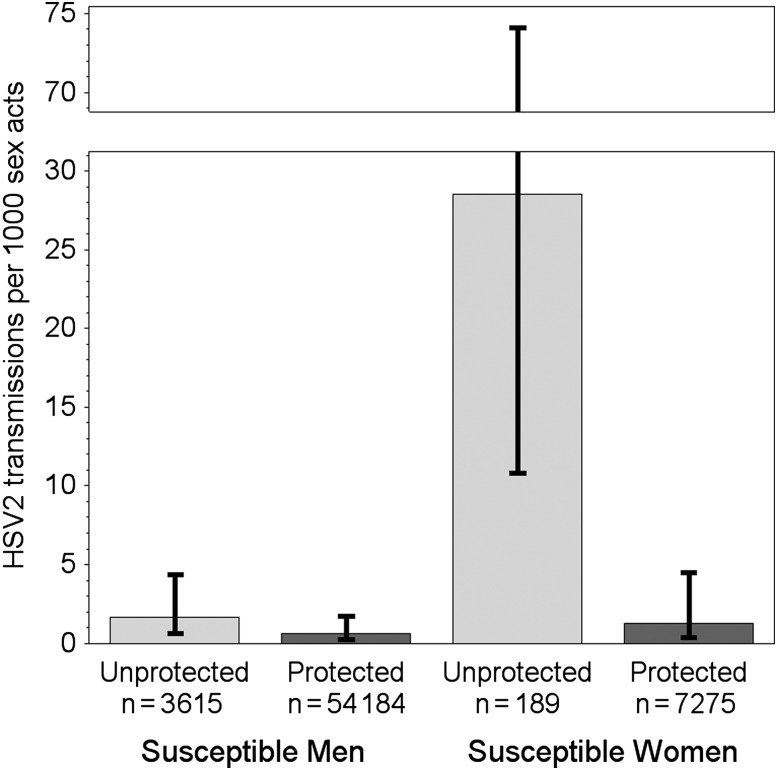
The efficacy of condom use in prevention of HSV-2 transmission differed by sex (Figure 1, P = .014). In separate models by sex, the rate of HSV-2 transmissions from women to men was 1.7 transmissions per 1000 unprotected acts (95% CI, .6 to 4.4) and 0.6 per 1000 protected acts (95% CI, .2 to 1.7). Thus condom use reduced the per-act probability of transmission from women to men by 65% (95% CI, −5% to 88%, P = .060), though the P-value was marginal. From men to women, the rate was 28.5 transmissions per 1000 unprotected acts (95% CI, 10.8 to 74.1) and 1.3 per 1000 protected acts (95% CI, .4 to 4.5) for an estimated condom efficacy of 96% (95% CI, 84% to 99%, P < .001) (Figure 1 and Table 1).
Figure 1.
Herpes simplex virus type 2 (HSV-2) per-act infectivity for protected and unprotected sex acts, by sex of susceptible partner. Numbers shown below each column are the numbers of acts of each type reported over risk periods by source participants. Transmission rates using the infectivity model are 1.7 and 0.6 cases per 1000 unprotected and protected sex acts, respectively, for susceptible men, and 28.5 and 1.3 cases per 1000 unprotected and protected sex acts, respectively, for susceptible women. Condom efficacy, or the proportionate reduction in per-act transmission when using a condom, is therefore estimated at 65% for transmission from women to men and 96% for transmission from men to women. Error bars show 95% confidence intervals for per-act transmission rates.
Table 1.
Univariate and Multivariate Risk Factors for Per-act Probability of HSV-2 Transmission
| Characteristic | Risk Ratio (95% CI) | P Value | Adjusted Risk Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Susceptible men (n = 799) | ||||
| Use of condoms | 0.35 (.12, 1.04) | .060 | 0.35 (.12, 1.04) | .060 |
| Age <30 y | 1.76 (.93, 3.33) | .080 | ||
| Number of children | 0.97 (.81, 1.17) | .76 | ||
| Vaginal drying practice | 0.77 (.30, 1.97) | .58 | ||
| Susceptible is circumcised | 0.97 (.51, 1.87) | .94 | ||
| GUD by self-report | 1.57 (.48, 5.15) | .46 | ||
| GUD on exam | 1.82 (.25, 13.36) | .56 | ||
| HIV VL of source (copies/mL) | ||||
| <10K | Ref | |||
| 10K–50K | 1.07 (.40, 2.90) | .89 | ||
| 50K–100K | 1.42 (.40, 5.10) | .59 | ||
| >100K | 1.15 (.32, 4.15) | .83 | ||
| CD4 of source (cells/µL) | ||||
| >350 | Ref | |||
| 200–350 | 1.02 (.13, 8.19) | .98 | ||
| <200 | 0.95 (.43, 2.10) | .90 | ||
| ARVs taken | 2.15 (.51, 9.00) | .29 | ||
| Acyclovir suppressive therapy | 1.22 (.64, 2.31) | .55 | ||
| Susceptible women (n = 112) | ||||
| Use of condoms | 0.04 (.01, .16) | <.001 | 0.06 (.01, .21) | <.001 |
| Age <30 | 1.79 (.40, 7.99) | .45 | ||
| Number of children | 0.77 (.52, 1.15) | .21 | ||
| Vaginal drying practice | 4.30 (1.46, 12.65) | .008 | 4.30 (1.46, 12.65) | .008 |
| Source is circumcised | 0.19 (.02, 1.48) | .11 | ||
| GUD by report | 0.50 (.06, 3.94) | .51 | ||
| GUD on exam | NC | … | ||
| HIV VL of source (copies/mL) | ||||
| <10K | Ref | |||
| 10 K–50K | 2.63 (.25, 27.8) | .42 | ||
| 50 K–100K | 3.23 (.21, 50.1) | .40 | ||
| >100 K | 5.01 (.54, 46.7) | .16 | ||
| CD4 of source (cells/µL) | ||||
| >350 | Ref | |||
| 200–350 | 4.45 (.46, 43.0) | .20 | ||
| <200 | 0.72 (.20, 2.59) | .62 | ||
| ARVs taken | 2.60 (.33, 20.7) | .37 | ||
| Acyclovir suppressive therapy | 1.50 (.51, 4.42) | .46 | ||
Condom use on a per-act basis is included in all models, even univariate; it is the only predictor in the univariate analysis of condom use. All measures except condom use are assessed either at baseline or per-risk period. NC = model did not converge: only 10 women had GUD on exam, none acquired HSV at that visit.
Abbreviations: ARV, antiretroviral drug; CI, confidence interval; GUD, genital ulcer disease; HIV, human immunodeficiency virus; HSV, herpes simplex virus; Ref, reference; VL, viral load.
In univariate models, age in susceptible men and vaginal drying practices in susceptible women were identified to be (at least marginally) associated with per-act risk of HSV-2 transmission. Suppressive antiviral therapy was not associated with HSV-2 transmission for either sex. Condom efficacy did not differ by whether the index had a history of genital ulcers among susceptible men (P = .98) or women (P = .16). In multivariate analyses, no risk factors besides condom use were significant for susceptible men, but susceptible women were 4.3 times more likely to acquire HSV-2 when vaginal drying was used (95% CI, 1.46 to 12.65). The efficacy estimates for condom use were essentially unchanged in these adjusted models.
Because we anticipated some HSV-2 acquisitions might have occurred from outside the partnership, we limited analysis to those partners most likely to be at risk of transmitting HSV-2. Therefore, we examined the association between confirmed linkage and the reporting of outside partnerships in 127 HIV-1 transmissions with linkage information within the HIV-1 transmission cohort. Among 26 couples in which the susceptible partner ever reported outside sex, 23% of transmissions were confirmed to be linked; whereas among 101 couples never reporting sex outside the partnership, 79% were confirmed to be linked (P < .0001). Therefore, we conducted a sensitivity analysis limited to susceptible persons never reporting outside sex. Among 911 HSV-2 susceptible participants, 678 (74%) never reported an outside partner; and among these persons 47 HSV-2 transmissions occurred (33 to susceptible men and 14 to women). In this subset, condom efficacy was similar to the full cohort for both sexes: 61% among HSV-2 susceptible men, reducing the per-act transmission probability from 1.7 to 0.7 per 1000 p-y (P = .14), and 96% among susceptible women, reducing infectivity from 28.7 to 1.3 transmissions per 1000 p-y (P < .001).
The 16 transmissions that could not be included in evaluation of condom efficacy occurred in couples who reported having no sex during the quarterly period in which HSV-2 transmission occurred. Among those who ever reported having no sex with the study partner, 50% of 242 reported outside partnerships, whereas only 14% of 669 consistently sexually active couples ever reported an outside partnership (χ2 test, P < .0001.) This association between reporting no sex and having outside partnerships may indicate that these 16 transmissions that were not included may have occurred from a partner outside the study.
CONCLUSIONS
In this analysis of HIV-1 and HSV-2 serodiscordant couples from East and Southern Africa, we found condoms reduced the per-act risk of transmission by 65% from women to men and by 96% from men to women; and this finding remained robust in sensitivity analysis. We are not aware of any previously published study providing per-act risk of HSV-2 transmission with and without condom use, for direct computation of per-act efficacy.
These analyses provide an overall estimate of condom efficacy that is similar to the estimated 80% reduction in per-act risk of HIV-1 transmission in this population [18]. The high estimated efficacy of male condoms in reducing HSV-2 transmission has important public health implications for this highly prevalent sexually transmitted infection for which there is not an effective preventive HSV-2 vaccine.
The efficacy of condoms in preventing HSV-2 transmission has been studied in several contexts; condoms are often associated with a decreased risk of HSV-2 transmission. Many of these published studies make two related assumptions: (1) that participants experience the same amount of infectious exposure over time periods of equal length and (2) that condom efficacy can be consistently assessed as a proportion of protected acts regardless of the number of total acts. In a pooled analysis of 6 studies involving nearly 5400 persons at risk of HSV-2 acquisition, largely HSV-2 discordant couples, 100% condom use was associated with a hazard ratio of 0.7 relative to 0% use (95% CI, .40–.94) [10]. Stanaway et al examined the same cohort in a case-crossover design and found an increased odds of HSV-2 acquisition with unprotected (OR = 1.04, 95% CI, 1.02 = 1.05) but not protected sex acts (OR = 1.01, 95% CI, .99–1.03) [12]. In another study of 528 HSV-2-discordant partnerships, condom use during >25% of acts reduced HSV-2 transmission from men to women (HR = 0.085, 95% CI, .01–.67) but not from women to men [13]. In contrast, among 293 women attending sexually transmitted diseases (STD) clinics, no significant efficacy was found for 100% vs partial condom use (HR = 0.8, 95% CI, .2–2.3) [9]. Lastly, in San Francisco, of 1934 women attending STD clinics, the odds of incident HSV-2 infection were higher when condoms were not used at the last sexual intercourse (OR = 2.27, 95% CI, 1.22–4.25). These diverse findings suggest that condom efficacy estimates depend on how condom use is measured [11].
We have previously evaluated this same cohort examining primarily the impact of suppressive acyclovir therapy on HSV-2 transmission, using standard Cox regression models and considering time at risk as the exposure rather than number of acts [15]. In that analysis, we also found both condom use and vaginal drying influenced HSV-2 transmission risk to susceptible women. Other risk factors for transmission present in that analysis which were not present in the current analysis include younger age among susceptible men and having fewer children in susceptible women. Failure to replicate these associations in the per-act analysis may indicate that these characteristics describe higher exposure rather than increased per-act risk.
Although the protection afforded by condom use is greater for susceptible women, the overall transmission risk to women is much higher, such that that even with condoms the rate (1.3 transmissions per 1000 protected acts) is barely below that to susceptible men in the absence of condoms (1.7 of 1000 unprotected acts). Incidence of HSV-2 has been shown previously to vary by sex. Martin et al reported 10.8 cases per 100 p-y in women and 5.8 cases per 100 p-y in men [10]. In the few cases where per-act level transmission probabilities are calculated, sex differences in HSV-2 incidence have also been reported. Wald et al showed that in a group of HIV-1 seronegative, HSV-2 discordant couples, transmission of HSV-2 to men occurred in an average of 0.2/1000 acts and transmission to women occurred in 0.9/1000 acts, when making no distinction between protected and unprotected acts [13]. Our study has a higher per-act rate of transmission, which may be due to lower levels of condom use in the current cohort or to higher HSV-2 infectivity caused by immunosuppression in the HIV-1 infected transmitting partner [20]. As has been shown for HIV transmission, the practice of vaginal drying increased the risk of HSV-2 transmission among African women in this population [21].
Strengths of our study include the large cohort of 911 couples, excellent follow-up, robustness of findings to sensitivity analyses, and use of the highly accurate Western Blot in detection of seroconversion [16]. Limitations of our analysis include using recall over monthly periods to measure sexual behavior, and relying on a single partner's recall. However, this time period of recall has been shown to be similar to the use of diaries [22]. The median (range) time between infection and HSV-2 seroconversion has been estimated at 13 days (3 to 102 days), indicating that perhaps not all seroconversions were captured over the same risk period in which the transmission occurred [23]. An additional limitation is that linkage information is not attainable and reported acts may not be relevant to the transmission. Whether due to 1) recall bias, 2) infection and seroconversion not occurring in the same risk period, or 3) unlinked transmissions, mismeasurement of the relevant sex acts should serve only to reduce the magnitude of observed associations unless there is informative mismeasurement [24, 25]. As it seems highly unlikely that degree of under- or over-reporting would be related to transmission risk, our estimates of condom efficacy are likely conservative. A third limitation is that we were unable to evaluate all HSV-2 transmissions because some occurred when the couple reported having no sex. However, because these susceptible partners were much more likely to report outside partnerships, their omission from evaluation of HSV-2 transmission within the couple seems appropriate.
Our findings suggest that male condoms are very effective in preventing HSV-2 transmission from men to women and are likely to provide some protection for susceptible men as well. The mechanism of this sex difference may be related to the differing ability of the condom to diminish contact with anatomic sites of viral replication, as men tend to shed HSV on the penile shaft, whereas HSV-2 shedding in women occurs on the wider area of the vulva and perineum [26]. In summary, these data support the recommendation of male condoms for the prevention of HSV-2 transmission from HIV-1 seropositive source partners.
Notes
Acknowledgments. The authors wish to thank the study participants for their faithful participation. They also thank Katherine Thomas and Renee Heffron for their thoughtful guidance and for providing statistical code and datasets.
Study team members. Members of the Partners in Prevention Herpes Simplex Virus/Human Immunodeficiency Virus Transmission Study Team are as follows: University of Washington Coordinating Center and Central Laboratories, Seattle, Washington: Connie Celum (principal investigator), Anna Wald (protocol co-chair), Jairam Lingappa (medical director), Jared M. Baeten, Mary Campbell, Lawrence Corey, Robert W. Coombs, James P. Hughes, Amalia Magaret, M. Juliana McElrath, Rhoda Morrow, and James I. Mullins. Study sites and site principal investigators are as follows: Cape Town, South Africa (University of Cape Town): David Coetzee; Eldoret, Kenya (Moi University, Indiana University): Kenneth Fife and Edwin Were; Gaborone, Botswana (Botswana Harvard Partnership): Max Essex and Joseph Makhema; Kampala, Uganda (Infectious Disease Institute, Makerere University): Elly Katabira and Allan Ronald; Kigali, Rwanda (Rwanda Zambia HIV Research Group and Emory University): Susan Allen, Kayitesi Kayitenkore, and Etienne Karita; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi and Craig Cohen; Kitwe, Zambia (Rwanda Zambia HIV Research Group and Emory University): Susan Allen and William Kanweka; Lusaka, Zambia (Rwanda–Zambia HIV Research Group and Emory University): Susan Allen and Bellington Vwalika; Moshi, Tanzania (Kilimanjaro Christian Medical College, Harvard University): Saidi Kapiga and Rachel Manongi; Nairobi, Kenya (University of Nairobi, University of Washington): Carey Farquhar, Grace John-Stewart, and James Kiarie; Ndola, Zambia (Rwanda Zambia HIV Research Group and Emory University): Susan Allen and Mubiana Inambao; Orange Farm, South Africa (Wits Reproductive Health and HIV Institute, University of the Witwatersrand): Sinead Delany-Moretlwe and Helen Rees; Soweto, South Africa (Perinatal HIV Research Unit, University of the Witwatersrand): Guy de Bruyn, Glenda Gray, and James McIntyre; and Thika, Kenya (University of Nairobi, University of Washington): Nelly Rwamba Mugo.
Financial support. This work was supported by a grant (26469) from the Bill and Melinda Gates Foundation and by National Institutes of Health grant AI-030731.
Potential conflicts of interest. A. S. M. reports consultancy fees from AiCuris; G. D-B. is an employee of Sanofi Pasteur and owns Sanofi shares; A. W. received consulting fees from AiCuris, Genocea, and Gilead; K. H. F. has grants through AiCuris, Genocea, and Vical corporations; A. R. served on advisory boards for Novartis and Pfizer. No conflict of interest is declared by the other authors. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the Partners in Prevention HSV/HIV Transmission Study Team, Connie Celum, Anna Wald, Jairam Lingappa, Jared M. Baeten, Mary Campbell, Lawrence Corey, Robert W. Coombs, James P. Hughes, Amalia Magaret, M. Juliana McElrath, Rhoda Morrow, James I. Mullins, David Coetzee, Kenneth Fife, Edwin Were, Max Essex, Joseph Makhema, Elly Katabira, Allan Ronald, Susan Allen, Kayitesi Kayitenkore, Etienne Karita, Elizabeth Bukusi, Craig Cohen, Susan Allen, William Kanweka, Susan Allen, Bellington Vwalika, Saidi Kapiga, Rachel Manongi, Carey Farquhar, Grace John-Stewart, James Kiarie, Susan Allen, Mubiana Inambao, Sinead Delany-Moretlwe, Helen Rees, Guy de Bruyn, Glenda Gray, James McIntyre, and Nelly Rwamba Mugo
References
- 1.Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999–2010. J Infect Dis 2014; 209:325–33. [DOI] [PubMed] [Google Scholar]
- 2.Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ 2008; 86:805–12, A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corey L, Wald A, Patel R et al. . Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med 2004; 350:11–20. [DOI] [PubMed] [Google Scholar]
- 4.Kim HN, Wald A, Harris J, Almekinder J, Heitman C, Corey L. Does frequency of genital herpes recurrences predict risk of transmission? Further analysis of the valacyclovir transmission study. Sex Transm Dis 2008; 35:124–8. [DOI] [PubMed] [Google Scholar]
- 5.Venkatesh KK, van der Straten A, Cheng H et al. . The relative contribution of viral and bacterial sexually transmitted infections on HIV acquisition in southern African women in the Methods for Improving Reproductive Health in Africa study. Int J STD AIDS 2011; 22:218–24. [DOI] [PubMed] [Google Scholar]
- 6.Belshe RB, Leone PA, Bernstein DI et al. . Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 2012; 366:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston C, Koelle DM, Wald A. HSV-2: in pursuit of a vaccine. J Clin Invest 2011; 121:4600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. FDA. Class II Special Controls Guidance Document: Labeling for Natural Rubber Latex Condoms. Guidance Documents (Medical Devices and Radiation-Emitting Products) Vol. 21CFR884.5300, 1998.
- 9.Gallo MF, Warner L, Macaluso M et al. . Risk factors for incident herpes simplex type 2 virus infection among women attending a sexually transmitted disease clinic. Sex Transm Dis 2008; 35:679–85. [DOI] [PubMed] [Google Scholar]
- 10.Martin ET, Krantz E, Gottlieb SL et al. . A pooled analysis of the effect of condoms in preventing HSV-2 acquisition. Arch Intern Med 2009; 169:1233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss NJ, Harper CC, Ahrens K et al. . Predictors of incident herpes simplex virus type 2 infections in young women at risk for unintended pregnancy in San Francisco. BMC Infec Dis 2007; 7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanaway JD, Wald A, Martin ET, Gottlieb SL, Magaret AS. Case-crossover analysis of condom use and herpes simplex virus type 2 acquisition. Sex Transm Dis 2012; 39:388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wald A, Langenberg AG, Link K et al. . Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. JAMA 2001; 285:3100–6. [DOI] [PubMed] [Google Scholar]
- 14.Celum C, Wald A, Lingappa JR et al. . Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010; 362:427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mujugira A, Magaret AS, Celum C et al. . Daily acyclovir to decrease herpes simplex virus type 2 (HSV-2) transmission from HSV-2/HIV-1 coinfected persons: a randomized controlled trial. J Infect Dis 2013; 208:1366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol 1988; 26:662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jewell NP, Shiboski SC. Statistical analysis of HIV infectivity based on partner studies. Biometrics 1990; 46:1133–50. [PubMed] [Google Scholar]
- 18.Hughes JP, Baeten JM, Lingappa JR et al. . Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis 2012; 205:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell MS, Mullins JI, Hughes JP et al. . Viral linkage in HIV-1 seroconverters and their partners in an HIV-1 prevention clinical trial. PLoS One 2011; 6:e16986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schacker T, Zeh J, Hu HL, Hill E, Corey L. Frequency of symptomatic and asymptomatic herpes simplex virus type 2 reactivations among human immunodeficiency virus-infected men. J Infect Dis 1998; 178:1616–22. [DOI] [PubMed] [Google Scholar]
- 21.Low N, Chersich MF, Schmidlin K et al. . Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med 2011; 8:e1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinfurt KP, Lin L, Dombeck CB et al. . Accuracy of 30-day recall for components of sexual function and the moderating effects of gender and mood. J Sex Med 2014; 11:678–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashley RL, Eagleton M, Pfeiffer N. Ability of a rapid serology test to detect seroconversion to herpes simplex virus type 2 glycoprotein G soon after infection. J Clin Microbiol 1999; 37:1632–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little RJA, Rubin DB. Statistical analysis with missing data. 2nd ed. Hoboken, NJ: Wiley, 2002. [Google Scholar]
- 25.Meier AS, Richardson BA, Hughes JP. Discrete proportional hazards models for mismeasured outcomes. Biometrics 2003; 59:947–54. [DOI] [PubMed] [Google Scholar]
- 26.Wald A. Herpes simplex virus type 2 transmission: risk factors and virus shedding. Herpes 2004; 11(suppl 3):130A–7. [PubMed] [Google Scholar]