A dual community-based screening algorithm that uses point-of-care antibody and qualitative nucleic acid amplification tests was cost-effective among at-risk men who have sex with men (MSM) in San Diego, and among similar populations of MSM with human immunodeficiency virus prevalence rates >0.4%.
Keywords: acute HIV, MSM, testing, cost analysis, NAT
Abstract
Background. In nonhealthcare settings, widespread screening for acute human immunodeficiency virus (HIV) infection (AHI) is limited by cost and decision algorithms to better prioritize use of resources. Comparative cost analyses for available strategies are lacking.
Methods. To determine cost-effectiveness of community-based testing strategies, we evaluated annual costs of 3 algorithms that detect AHI based on HIV nucleic acid amplification testing (EarlyTest algorithm) or on HIV p24 antigen (Ag) detection via Architect (Architect algorithm) or Determine (Determine algorithm) as well as 1 algorithm that relies on HIV antibody testing alone (Antibody algorithm). The cost model used data on men who have sex with men (MSM) undergoing community-based AHI screening in San Diego, California. Incremental cost-effectiveness ratios (ICERs) per diagnosis of AHI were calculated for programs with HIV prevalence rates between 0.1% and 2.9%.
Results. Among MSM in San Diego, EarlyTest was cost-savings (ie, ICERs per AHI diagnosis less than $13.000) when compared with the 3 other algorithms. Cost analyses relative to regional HIV prevalence showed that EarlyTest was cost-effective (ie, ICERs less than $69.547) for similar populations of MSM with an HIV prevalence rate >0.4%; Architect was the second best alternative for HIV prevalence rates >0.6%.
Conclusions. Identification of AHI by the dual EarlyTest screening algorithm is likely to be cost-effective not only among at-risk MSM in San Diego but also among similar populations of MSM with HIV prevalence rates >0.4%.
Ambitious recommendations for universal human immunodeficiency virus (HIV) testing in the United States are supported by observations that HIV diagnoses are frequently linked to reduced sexual risk behaviors and earlier uptake of antiretroviral therapy, both of which are expected to result in decreased HIV transmission [1–4]. Diagnosis of HIV infection during the acute stage of infection is especially important since transient levels of extremely high titer HIV RNA during acute HIV infection (AHI) are associated with rapid immune destruction and significantly greater infectivity than during chronic infection [5, 6].
The recognition that persons with AHI contribute disproportionately to population-level HIV transmission supports the updated recommendations for laboratory diagnosis of HIV infection in healthcare settings that use fourth-generation immunoassays to detect HIV-1/HIV-2 antibody (Ab) and HIV-1 p24 antigen (Ag) to detect AHI [7]. Detection of p24 Ag by the ARCHITECT Ag/Ab Combo assay is currently the most widely used approach for detecting AHI [8–13]. Alternative approaches for detecting and differentiating p24 Ag and HIV Ab include the rapid Alere Determine HIV-1/2 Combo assay and more sensitive HIV-1 nucleic acid amplification tests (NATs) [14, 15].
While AHI screening in healthcare settings is now recommended, one might wonder whether routine AHI screening should be the standard of care in community HIV screening programs, where about 40% of new HIV infections are diagnosed in the United States [16]. The main deterrents of widespread use of community HIV screening algorithms to detect AHI appear to be the elevated costs, need for venipuncture, concerns about turnaround time, and lack of laboratory capacity [6, 17–19]. Absence of point-of-care (POC) tests that reliably detect AHI may be the major cost-contributing factor, as AHI screening strategies in community-based settings frequently require second visits or alternative approaches to inform clients of their test results. However, detailed cost analyses to compare testing strategies that identify acutely HIV-infected (ie, before seroconversion) and nonacutely HIV-infected (ie, after seroconversion) persons have not been performed. Therefore, there is uncertainty about the HIV prevalence rate needed to justify the cost of these testing algorithms compared with strategies that rely on POC HIV Ab testing only.
We developed and evaluated the first economic model to estimate the annual cost of a community-based AHI screening algorithm that consists of POC Ab plus individual donation qualitative NAT (ID-NAT; ie, the EarlyTest algorithm, I). We compared EarlyTest to 2 p24 Ag–based algorithms that detect AHI by either using Architect (II) or Determine (III). We further compared costs of these algorithms to an algorithm that relies on Ab testing alone (Antibody, IV).
MATERIAL AND METHODS
The 1-year cost analyses were conducted using an established HIV testing program perspective. The cost model was based on numbers of tests per year in men who have sex with men (MSM) who enrolled in the EarlyTest HIV screening program for the San Diego Primary Infection Resource Consortium (SD PIRC) between 2006 and 2014. There were 3000 HIV tests performed per year, resulting in an overall prevalence of 2.9% (n = 87) newly diagnosed HIV infections (0.7% [n = 21] with AHI and 2.2% (n = 66) with established infection). Participants were MSM who reported sexual contact with men during the 12 months prior to testing. Basic demographics, risk behaviors, and frequency of repeat HIV testing from this cohort have been published previously [20–22]. An estimated date of infection was calculated for all recently infected persons using previously published criteria for serologic and virologic test results [23]. AHI was defined as having a negative HIV Ab test in the presence of detectable HIV-1 RNA (ie, Fiebig stages I–II), with an estimated date of infection within the last 10 days [24].
Algorithms
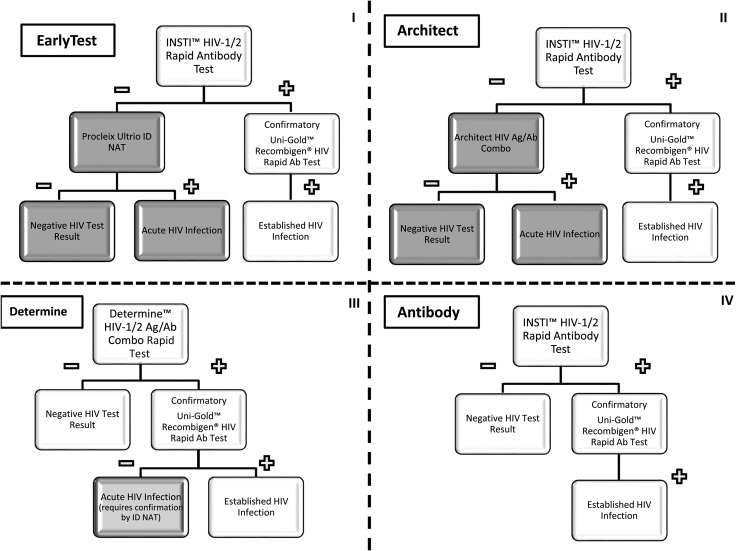
Annual costs were calculated for the year 2014 for the EarlyTest algorithm (ie, algorithm I) that includes POC HIV-1 Ab testing (INSTI HIV-1 Antibody Test, bioLytical Laboratories Inc., Richmond, Canada) [25] followed by ID-NAT (Procleix Ultrio, Hologic, Bedford, Massachusetts) in all individuals with negative or invalid POC test results. Study participants were notified of positive HIV NAT results approximately 4 days after their Ab test. We then compared costs to those estimated for an alternative testing algorithm (ie, algorithm II) that included POC HIV-1 Ab testing (INSTI) followed by Architect HIV Ag/Ab Combo (Abbott Diagnostics, Abbott Park, Illinois), that is, the Architect algorithm. We also compared costs of the EarlyTest and Architect algorithms to estimated costs of 2 rapid/POC algorithms: algorithm III, using the Determine HIV-1/2 Ag/Ab Combo test (Alere Inc, Waltham, Massachusetts; Determine algorithm), and algorithm IV, using POC HIV Ab testing (INSTI) alone (Antibody algorithm). All 4 algorithms are shown in Figure 1.
Figure 1.
The 4 algorithms evaluated in this cost analysis—EarlyTest (algorithm I), Architect (algorithm II), Determine (algorithm III), and Antibody only (algorithm IV). White shaded areas indicate point-of-care results. Gray shaded areas indicate results that require a second visit, light gray shaded area indicates that confirmation of the result requires a second visit. Abbreviations: Ab, antibody; Ag, antigen; HIV, human immunodeficiency virus; ID, individual donation; NAT, nucleic acid amplification test.
Performance calculations were carried out for all tests used in this cost analysis. Sensitivity of the Procleix Ultrio assay for HIV virus detection is 100% at 300 copies/mL and 99% at 100 copies/mL [26]. Summarizing previous studies, sensitivity of the Architect assay for seronegative AHI is 80% (n = 143/178 of samples positive; 95% confidence interval [CI], 74%–86%) [8–13, 27, 28], while sensitivity of the Determine combo is 50% (n = 51/102 samples positive; 95% CI, 40%–60%) [8, 29–32]. Specificities of the tests for HIV infection are listed in Supplementary Table 1. In algorithms that do not provide POC-positive results for AHI (ie, EarlyTest and Architect), a 5% (95% CI, 1%–9%) loss to follow-up (ie, clients not informed about AHI diagnosis) was estimated.
Costs per Test Results
The cost model was based on work-time estimates and compensation in the EarlyTest HIV screening program, San Diego. Results are expressed in 2014 US dollars. Details on calculations of cost per negative test result, established HIV diagnosis, and AHI diagnosis using the different algorithms are included in the Supplementary Materials.
Cost Thresholds
Calculated thresholds for cost-savings (ie, $22 909) and cost-effectiveness (ie, $63 053) per new HIV diagnosis derived by Farnham and colleagues were the basis for thresholds used in this study [33]. As those costs were calculated in 2009 US dollars, the thresholds were updated to 2014 US dollars by adding the cumulative rate of inflation (ie, 10.3%). This resulted in a cost-savings threshold of $25 269 and a cost-effectiveness threshold of $69 547. We therefore hypothesized that the incremental cost-effectiveness ratio (ICER) of EarlyTest in a population of MSM undergoing community-based HIV screening will be less than $25 269 per diagnosis of seronegative AHI when compared with the 3 alternative algorithms.
Incremental Cost-Effectiveness Ratios per AHI
ICERs were calculated by comparing 2 testing algorithms, with the numerator representing the differences in annual costs of the 2 algorithms and the denominator representing the difference in numbers of annual AHI diagnoses. Details are depicted in the supplement. ICERs were calculated for numbers of tests per year and prevalence of HIV and AHI in MSM who enrolled in the EarlyTest and SD PIRC between 2006 and 2014.
Sensitivity Analyses
We performed a 2-way sensitivity analysis by calculating ICERs per AHI for HIV prevalence rates that were less than those observed in the SD PIRC (range, 0.001–0.029) and 2 proportions of AHI (0.24 and 0.10 of all HIV diagnoses). While AHI cases represented 24% of all newly diagnosed HIV cases among MSM in the SD PIRC, a lower proportion of 10% may be more appropriate for settings in which clients undergo screening more infrequently [34, 35].
We also assessed the effect of a number of alternate plausible assumptions and used a probabilistic sensitivity analysis (PSA) to examine the impact of cost parameter uncertainty. For the PSA, we assigned uniformly distributed 95% CIs to applicable cost items (ie, all cost items except items that showed no variation, such as costs of tests, which were given by the manufacturer or represent actual costs in our setting, or salary; depicted in Table 1). To account for uncertainty in test sensitivities, we assigned normally distributed 95% CIs to AHI sensitivities of the Architect and Determine combo (ie, test performances were calculated using previous studies and tests; in contrast to ID-NAT, Architect and Determine were not routinely performed in the SD PIRC setting), as well as for loss to follow-up in algorithms that do not provide POC-positive results for AHI (ie, EarlyTest and Architect; all 95% CIs are listed in the algorithm paragraph of the Methods section). As specificities of all the evaluated tests are high (>99%) and 95% CIs are very small (due to the large number of HIV-negative samples tested previously), impact of specificity uncertainty on the cost model was shown to be minimal (details in Supplementary Material); therefore, we did not include 95% CIs for specificities into the PSA. To determine the frequency at which each algorithm was cost-saving/cost-effective at the given thresholds, we conducted Monte Carlo simulations to obtain 1000 samples from all distributions and used these samples to calculate means and 95% CIs for ICERs per AHI by using the 2.9% HIV prevalence rate and AHI proportions of 24% and 10%. We also used PSA to calculate ICERs for the lower 95% CIs of the sensitivity of the Determine combo in AHI samples (ie, 40% sensitivity).
Table 1.
Base Costs and Costs in Probabilistic Sensitivity Analysis per Negative Test, per Established Human Immunodeficiency Virus (HIV) Diagnosis and per Acute HIV Diagnosis in the Dual EarlyTest (Point-of-Care [POC] Antibody + Individual Donation Qualitative Nucleic Acid Amplification Test), the Architect combo, the Determine Combo, and the POC Antibody Alone Algorithms
| Cost Item/Diagnosis (in 2014 dollars) and Test Outcomes for Which Cost Items Are Applicable | Algorithm |
||||
|---|---|---|---|---|---|
| I Dual Early Test (EarlyTest) | II Architect Combo (Architect) | III Determine Combo (Determine) | IV POC Ab Alone (Antibody) | Details | |
| Personnel: Pretest counseling and testing related procedures. Negative, established HIV, AHI |
7.90 (95% CI, 5.79–10.53) | 7.90 (95% CI, 5.79–10.53) | 5.26 (95% CI, 3.68–6.32) | 5.26 (95% CI, 3.68–6.32) | Basis: SRA-1 level ($31.58 per hour); phlebotomy for EarlyTest and Architect, total 15 min (ie, 10 min pretest counseling, plus 5 min sample collection), 95% CI, 11–20 min; blood obtained from fingertip for Determine and Antibody, total 10 min (ie, 9 min pretest counseling, 1 min sample collection includes test processing), 95% CI, 7–12 min |
| POC/rapid Ab test (INSTI HIV-1 antibody test/Alere Determine HIV-1/2 combo test) Negative, established HIV, AHI |
8.33 (95% CI, 8.10–8.56) | 8.33 (95% CI, 8.10–8.56) | 18.22 (95% CI, 18.18–18.26) | 8.33 (95% CI, 8.10–8.56) | Determine p24 Ag/Ab used instead of INSTI in Determine Costs of INSTI: $6.99 (test) + $1.34 (costs per test for controls; assumption 1 vial set [ie, $5.00] per month) Costs of Determine: $18.00 (test) + $0.22 (costs per test for controls; assumption 1 set of controls [ie, $55.00] per month) Test costs are actual costs provided by manufacturer.95% CI calculated for costs of controls only. |
| Further investigations of false-positive INSTI/Determine combo test results (ie, supplemental Ab test [Uni-Gold Recombigen HIV Ab test] plus counseling for all 4 algorithms, plus costs for phlebotomy, ID-NAT, and second visit for Determine and Ab algorithms only) Negative |
0.06 (95% CI, .05–.06) | 0.07 (95% CI, .06–.08) | 0.69 (95% CI, .57–.86) | 0.43 (95% CI, .35–.53) | Calculation based on specificities of 99.5% (ie, 15 false positives per year) for INSTI and 99.2% (ie, 24 false positives per year) for Determine For all 4 algorithms costs of Uni-Gold: $8.25 (test) + $0.09 (costs per test for controls; assumption 1 set of controls [ie, $23.00] per month [250 tests per month]); 95% CI, $8.32–$8.37 plus costs of 5 min pretest counseling and test performance (95% CI, 3–7 min) by SRA-1 were added. Additional costs for performing ID-NAT instead of ARCHITECT have been included in Architect algorithm. For false-positives, Determine or INSTI in Determine or Antibody algorithm in addition to costs of phlebotomy, ID-NAT, second visit, and counseling have been included for false-positive Ab results. In contrast, costs for ID-NAT/Architect, second visit, and counseling are included for all cases in EarlyTest and Architect algorithm (see below). |
| Further investigations of true-positive INSTI/Determine combo test results, I; additional personnel for phlebotomy and supplemental Ab test in those with established HIV (Uni-Gold Recombigen HIV Ab test/INSTI HIV-1 Ab); pretest counseling and testing Established HIV |
2.63 (95% CI, 1.58–3.68) | 2.63 (95% CI, 1.58–3.68) | 5.26 (95% CI, 3.68–7.37) | 5.26 (95% CI, 3.68–7.37) | Basis: SRA-1 level ($31.58 per hour); 5 min for EarlyTest and Architect algorithms (pretest counseling and performance of Uni-Gold), 95%CI, 3–7 min; 10 min for Determine and Antibody algorithms (5 min for sample collection using phlebotomy, needed for further supplemental testing with, eg, Western blot, plus 5 min pretest counseling and performance of Uni-Gold), 95% CI, 7–14 min |
| Further investigations of true-positive INSTI/Determine combo test results, II; supplemental Ab test (Uni-Gold Recombigen HIV Ab test) Established HIV |
8.34 (95% CI, 8.32–8.37) | 8.34 (95% CI, 8.32–8.37) | 8.34 (95% CI, 8.32–8.37) | 8.34 (95% CI, 8.32–8.37) | Costs of Uni-Gold: $8.25 (test) + $0.09 (costs per test for controls; assumption 1 set of controls [ie, $23.00] per month [250 tests per month]). Test costs are actual costs provided by manufacturer.95% CI, calculated for costs of controls only. |
| Further investigations in true-positive Determine combo test results AHI |
… | … | 26.46 (95% CI, 24.45–29.01) | … | Contains costs for Uni-Gold Ab test to rule out established HIV ($8.34) plus costs for pretest counseling plus phlebotomy ($5.26 personnel costs for sample collection plus $12.86 for disposable items) |
| Miscellaneous disposable items Negative, AHI |
12.86 (95% CI, 12.45–13.27) | 12.86 (95% CI, 12.45–13.27) | 10.41 (95% CI, 10.05–10.77) | 10.41 (95% CI, 10.05–10.77) | Includes latex gloves, sterile wipes, gauze pads, adhesive bandages, phlebotomy equipment (needles, holders, blood tubes), absorbent workspace covers, biohazard waste-disposal bags, and laboratory supplies (pipettes, tubes). Items and costs differ between Determine and Antibody algorithms, where blood is obtained from fingertip, and EarlyTest and Architect algorithms, where phlebotomy is performed. |
| Miscellaneous disposable items Established HIV |
12.86 (95% CI, 12.45–13.27) | 12.86 (95% CI, 12.45–13.27) | 23.27 (95% CI, 22.50–24.04) | 23.27 (95% CI, 22.50–24.04) | Includes latex gloves, sterile wipes, gauze pads, adhesive bandages, phlebotomy equipment (needles, holders, blood tubes), absorbent workspace covers, biohazard waste-disposal bags, and laboratory supplies (pipettes, tubes). Items and costs differ between Determine and Antibody algorithms, where blood is obtained primarily from fingertip and then later again from phlebotomy, and EarlyTest and Architect algorithms, where phlebotomy is performed primarily. |
| Infrastructure Negative, Established HIV, AHI |
0 | 0 | 0 | 0 | Cost analyses conducted from an HIV testing program perspective, assuming that there is no extra cost for rooms/space for HIV counseling and testing. |
| Post-test counseling Negative |
5.26 (95% CI, 3.16–7.90) | 5.26 (95% CI, 3.16–7.90) | 5.26 (95% CI, 3.16–7.90) | 5.26 (95% CI, 3.16–7.90) | Basis: SRA-1 level ($31.58 per hour); 10 min for negative results (95% CI, 6 min–15 min). |
| Post-test counseling Established HIV |
15.79 (95% CI, 10.53–26.32) | 15.79 (95% CI, 10.53–26.32) | 15.79 (95% CI, 10.53–26.32) | 15.79 (95% CI, 10.53–26.32) | Basis: SRA-1 level ($31.58 per hour); 30 min for positive results (95% CI, 20 min–50 min). |
| Post-test counseling, POC results Acute HIV |
5.26 (95% CI, 3.16–7.90) | 5.26 (95% CI, 3.16–7.90) | 15.79 (95% CI, 10.53–21.05) | Basis: SRA-1 level ($31.58 per hour); 10 min for negative results in EarlyTest and Architect algorithms (95% CI, 6 min–15 min); 30 min (95% CI, 20 min–40 min) for positive result in Determine algorithm (which still needs to be confirmed by NAT). | |
| Sending samples via FedEx for HIV-1/HCV/HBV qualitative NAT testing (American Red Cross national testing lab in St. Louis, Missouri) Negative |
1.97 (95% CI, 1.90–2.04) | 0 | … | … | Costs calculated assuming 246 tests per month and shipments 3 times per week (ie, 13 shipments per month); ie, 19 samples per shipment; cost per shipment $27.43 + $10 per case (case costs $50.00 and assumed that every case is reused 5 times). For Architect algorithm, no costs for shipping of samples as ARCHITECT testing assumed available “in house.” |
| Sending samples via FedEx for HIV-1/HCV/HBV qualitative NAT testing (American Red Cross national testing lab in St. Louis, Missouri) (Acute HIV) |
1.97 (95% CI, 1.90–2.04) | 0 | 1.97 (95% CI, 1.90–2.04) | … | |
| HIV-1/HCV/HBV qualitative NAT testing (Procleix Ultrio assay) or ARCHITECT testing (Negative) |
21.00 | 20.00 | … | … | Actual test costs at UCSD/provided by manufacturer. No 95% CI calculated. |
| HIV-1/HCV/HBV qualitative NAT testing (Procleix Ultrio assay) or ARCHITECT testing (Acute HIV) |
21.00 | 20.00 | 21.00 | … | Actual test costs at UCSD/provided by manufacturer. No 95% CI calculated. |
| Data system maintenance (for delivering negative results) Negative |
13.60 (95% CI, 13.00–14.20) | 13.60 (95% CI, 13.00–14.20) | … | … | Assumption data system is available. Basis for costs salary of technician maintaining data system (ie, $3318.00 per month). To calculate costs per negative test result the monthly salary was divided by 244 (ie, number of negative tests per month). |
| Calling in those with positive NAT results (phone calls, messages) AHI |
7.90 (95% CI, 1.58–15.80) | 7.90 (95% CI, 1.58–15.80) | 7.90 (95% CI, 1.58–15.80) | … | Basis: SRA-1 level ($31.58 per hour); 15 min (95% CI, 3 min–30 min) Estimation: 5% of positives cannot be reached using EarlyTest and Architect algorithms; all clients reached in Determine algorithm (more extensive post-test counseling after positive Determine POC result). |
| Transportation costs for necessary second visit AHI |
10.00 | 10.00 | 10.00 | - | |
| Post-test counseling AHI |
15.79 (95% CI, 10.53–26.32) | 15.79 (95% CI, 10.53–26.32) | 15.79 (95% CI, 10.53–26.32) | Basis: SRA-1 level ($31.58 per hour); 30 min for positive results (95% CI, 20 min–50 min). | |
| Total base costs per negative test results | 70.98 | 68.02 | 39.84 | 29.69 | |
| Total base costs per established HIV diagnosis | 55.85 | 55.85 | 76.14 | 66.25 | |
| Total base costs per acute HIV diagnosis but lost to follow-up (ie, not informed about diagnosis) | 65.22 | 62.25 | … | … | Loss to follow-up only in algorithms that do not provide POC positive results for AHI (ie, EarlyTest and Architect algorithm) |
| Total base costs per acute HIV diagnosis | 91.01 | 88.04 | 132.80 | … | Acute HIV diagnosed in EarlyTest, Architect, and Determine algorithms only. |
| Total costs per negative test result in PSA (mean, SD) | 71.47 (2.02) | 68.51 (2.02) | 39.89 (1.61) | 29.73 (1.61) | |
| Total costs per established HIV diagnosis in PSA (mean, SD) | 58.78 (4.68) | 58.78 (4.68) | 78.93 (4.59) | 69.03 (4.58) | |
| Total costs per acute HIV diagnosis, acute HIV diagnosis but lost to follow-up in PSA (mean, SD) | 66.59 (4.57) | 63.63 (4.57) | … | … | |
| Total costs per acute HIV diagnosis in PSA (mean, SD) | 95.11 (6.33) | 92.14 (6.33) | 136.51(6.80) | … | |
Uniformly distributed 95% CIs were calculated for applicable cost items and used for PSA on costs per test result. The text in italics describes the total costs per diagnosis and the respective costs that were added.
Abbreviations: Ab, antibody; Ag, antigen; AHI, acute human immunodeficiency virus infection; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; ID-NAT, individual donation qualitative nucleic acid amplification test; POC, point-of-care; PSA, probabilistic sensitivity analysis; SD, standard deviation; SRA, Senior Research Associate; UCSD, University of California–San Diego.
RESULTS
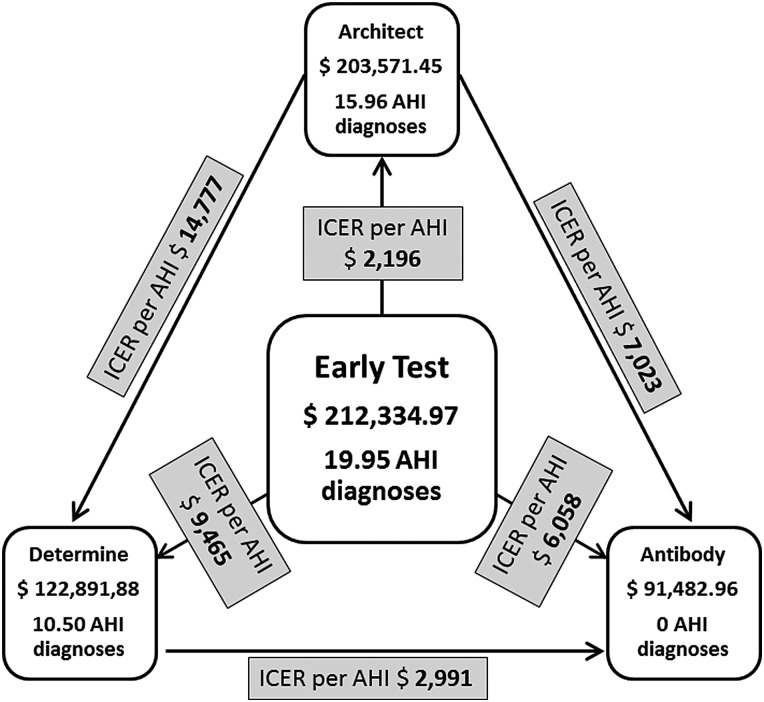
Cost items (including 95% CIs) as well as costs per test result in the 4 testing algorithms are depicted in Table 1. Estimated total annual costs associated with each algorithm using the SD PIRC MSM model (with 3000 annual HIV tests) are displayed in Figure 2 (for more detailed information, see Supplementary Table 2).
Figure 2.
Total annual costs and number of acute human immunodeficiency virus (HIV) infection diagnoses for each algorithm in the model of San Diego men who have sex with men (ie, base model). Costs per acute HIV diagnosis (ie, incremental cost-effectiveness ratio), by comparing each 2 of 4 testing algorithms, are indicated in the gray boxes. Abbreviations: AHI, acute human immunodeficiency virus infection; ICER, incremental cost-effectiveness ratio.
Incremental Cost-Effectiveness Ratio per AHI: Base Model
Costs per acute HIV diagnosis (ie, ICER), determined by comparing each 2 of the 4 testing algorithms, are depicted in Figure 2. AHI was detected in 21 individuals using the EarlyTest algorithm, and 5% of those were lost to follow-up. Based on a lower sensitivity of Architect (80%) compared with the Procleix Ultrio (markedly over 99%), 3.99 of these diagnoses would be missed by the Architect (“false-negative” result). These 3.99 cases of AHI are diagnosed only with EarlyTest, with an excess cost of $8764 when compared with Architect. The ICER of EarlyTest relative to Architect therefore is $2196 per AHI diagnosis (ie, $8764 divided by 3.99). ICERs per AHI diagnosis for EarlyTest compared with Determine (III) and for the 3 algorithms that detect AHI (ie, EarlyTest, Architect, and Determine) compared with Antibody (IV) alone are displayed in Table 2 and Figure 2. EarlyTest was cost-savings compared with the other 3 algorithms, Architect was cost-savings when compared with Determine (ICER $14,776.50) and Antibody, and Determine was cost-savings when compared with Antibody [36]. ICERs per AHI when assuming a lower proportion of AHI cases (10% of all new HIV diagnoses) are depicted in Table 2.
Table 2.
Costs per Acute Human Immunodeficiency Virus Diagnoses (ie, Incremental Cost Effectiveness Ratio) for Comparisons of the 4 Algorithms
| Costs per AHI Diagnoses (ICER) | Human Immunodeficiency Virus Prevalence/AHI Proportion (%) | Algorithm |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| EarlyTest (EarlyTest) (I) |
Architect (Architect) (II) |
Determine (Determine) (III) |
Point-of-Care Ab Alone (Ab) (IV) |
||||||
| Na | Cost, $b | Na | Cost, $b | Na | Cost, $b | Na | Cost, $b | ||
| ICER per AHI vs Point-of-Care Antibody Alone (Algorithm IV) | |||||||||
| ICER per AHI (base costs) | 0.029/24% | 19.95 | 6,057.74 | 15.96 | 7,023.09 | 10.50 | 2,991.33 | … | … |
| ICER per AHI, probabilistic sensitivity analysis (mean, SD) | 0.029/24% | 19.96 | 6,127.71 (281.80) | 15.98 | 7,117.69 (456.25) | 10.48 | 3,038.11 (326.62) | … | … |
| ICER per AHI, probabilistic sensitivity analysis (mean, SD) | 0.029/10% | 8.27 | 14,680.57 (679.16) | 6.62 | 17,056.27 (1,098.16) | 4.34 | 7,195.98 (788.62) | … | … |
| ICER per AHI, probabilistic sensitivity analysis (mean, SD) assuming 40% sensitivity of Determine (ie, lower 95% CI of sensitivity of the Determine combo) | 0.029/24% | … | … | 8.40 | 3,725.73 (62.85) | … | … | ||
| ICER per AHI vs Determine (Algorithm III) | |||||||||
| ICER per AHI (base costs) | 0.029/24% | 9.45 | 9,464.88 | 5.46 | 14,776.50 | … | … | … | … |
| ICER per AHI, probabilistic sensitivity analysis (mean, SD) | 0.029/24% | 9.48 | 9,736.52 (1,388.22) | 5.50 | 16,014.75 (5020.20) | … | … | … | … |
| ICER per AHI, probabilistic sensitivity analysis (mean, SD) | 0.029/10% | 3.93 | 23,422.08 (3,355.44) | 2.28 | 38,557.88 (12,121.14) | … | … | … | … |
| ICER per AHI, probabilistic sensitivity analysis (mean, SD) assuming 40% sensitivity of Determine (ie, lower 95% CI of sensitivity of the Determine combo) | 0.029/24% | 11.56 | 7879.02 (523.06) | 7.58 | 10,947.50 (1269.54) | … | … | … | … |
| ICER per AHI vs Architect (Algorithm II) | |||||||||
| ICER per AHI (base costs) | 0.029/24% | 3.99 | 2,196.37 | … | … | … | … | … | … |
| ICER per AHI, probabilistic sensitivity analysis (mean, SD) | 0.029/24% | 3.98 | 2,259.88 (386.30) | … | … | … | … | … | … |
| ICER per AHI, probabilistic sensitivity analysis (mean, SD) | 0.029/10% | 1.65 | 5,399.08 (928.38) | … | … | … | … | … | … |
Base costs and results of probabilistic sensitivity analyses are displayed.
Abbreviations: AHI, acute human immunodeficiency virus infection; CI, confidence interval; ICER, incremental cost-effectiveness ratio; SD, standard deviations.
a N corresponds to the total number of acute human immunodeficiency virus (HIV) diagnoses when compared with alternative algorithm.
b Cost corresponds to costs (in 2014) per single acute HIV diagnosis when compared with alternative algorithm.
Model for Community-based Screening Programs With Lower HIV Prevalence/2-way Sensitivity Analyses
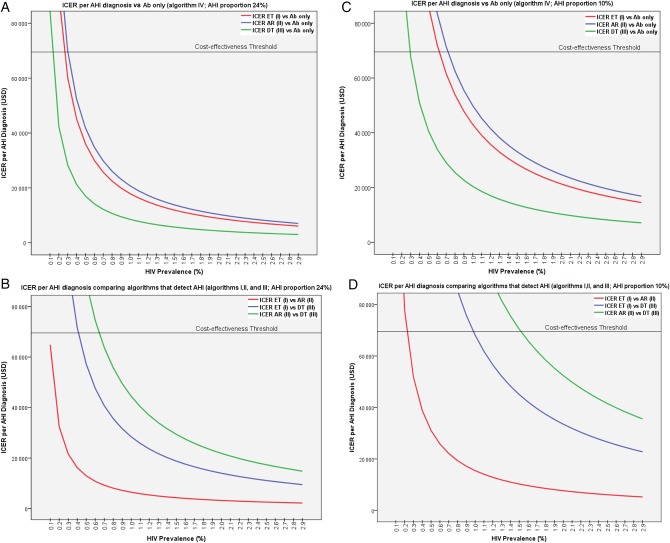
Comparing all 4 algorithms and assuming a 24% rate of AHI among all HIV diagnoses, EarlyTest was cost-effective (ie, ICER per AHI less than $69 547) for HIV prevalence rates >0.4%. Determine was cost-effective for prevalence rates between 0.1% and 0.4%. For ≤0.1% HIV prevalence, none of the 3 algorithms that detect AHI was cost-effective compared with Antibody alone (Figure 3). Architect was cost-effective when compared with Determine for HIV prevalence rates >0.6%.
Figure 3.
Model of incremental cost-effectiveness ratio per acute human immunodeficiency virus (HIV) infection (AHI) diagnosis for HIV prevalence rates between 0.1% and 2.9% assuming an AHI proportion of 24% (A and B, on the left) and 10% (C and D, on the right) of all new HIV diagnoses. A and C, Comparison of algorithms that detect AHI (I, EarlyTest; II, Architect; and III, Determine) compared with an algorithm (IV) that is based on point-of-care antibody testing alone. B and D, Comparison of the 3 algorithms that detect AHI. Cost-effectiveness threshold = $69 547; cost-savings threshold = $25 269 [33]. Abbreviations: Ab, antibody; AHI, acute human immunodeficiency virus infection; AR, Architect; DT, Determine; ET, EarlyTest; ICER, incremental cost-effectiveness ratio; USD, US dollars.
ICERs for AHI per HIV prevalence rates assuming a 10% rate of AHI among HIV diagnoses (ie, more applicable for other populations with less frequent screening) are depicted in Figure 3C and 3D. EarlyTest was cost-effective compared with the 3 other algorithms for HIV prevalence rates ≥1.0%. Determine was cost-effective for prevalence rates between ≥0.3% and 1.0%; below 0.3% HIV prevalence, none of the 3 algorithms that detect AHI was cost-effective compared with Antibody alone. When compared with Determine, Architect was cost-effective for HIV prevalence rates >1.5%.
Probabilistic Sensitivity Analysis
Results of the PSA for a 2.9% HIV prevalence with AHI proportions of 24% and 10%, including standard deviations, are listed in Table 2 (including ICERs calculated for the lower 95% CI of the Determine combo). EarlyTest compared favorably (ie, was below the ICER per AHI cost-effectiveness threshold) 100% of the time compared with the other 3 algorithms for both proportions of AHI. Architect and Determine were cost-effective 100% of the time compared with Antibody only. Finally, Architect was cost-effective compared with Determine 100% of the time at an AHI proportion of 24%, and 99% of the time at a proportion of 10%.
DISCUSSION
We conducted a cost analysis of the EarlyTest community-based HIV screening approach (ie, POC Ab plus routine ID-NAT in Ab-negative persons) and found that this approach was cost-savings (ie, ICERs less than $13.000 per AHI diagnosis) among a cohort of MSM when compared with 3 alternative testing strategies (using p24 Ag/HIV Ab detection and/or POC Ab alone). While cost-savings and cost-effectiveness cutoffs used in this study were calculations from a previous mathematical model [33], other cutoffs on the costs to be paid per AHI diagnosis have yet to be defined.
Diagnosis of HIV at acute stages followed by appropriate interventions to prevent further transmission may be a highly effective biomedical HIV prevention strategy [1, 37]. Cost models are critical for establishing the cost-effectiveness of community-based AHI screening, particularly given the additional costs associated with these screening methods. Here, we demonstrate that AHI screening is cost-effective among a metropolitan population of MSM.
The cost-effectiveness of using both that POC Ab test and quantitative NAT (with prompt initiation of antiretroviral treatment in those identified with HIV infection) when compared with HIV-Ab testing alone has been reported for persons who inject drugs while undergoing HIV screening every 3–6 months [38]. The routine addition of quantitative viral load testing for all annual HIV tests in MSM, however, has been estimated to increase the cost of screening by more than $100 000 per quality-adjusted life year gained [39]. Our results indicate that “cheaper” qualitative ID-NAT may provide an alternative cost-effective community-based screening technology for AHI in similar populations of MSM with an HIV prevalence >0.4%. The potential detection of hepatitis B virus (HBV) and hepatitis C virus (HCV) infections in persons at risk for HIV represents an additional benefit of the EarlyTest algorithm, as the Procleix Ultrio assay, which is used for HIV NAT screening, may also detect proportions of HBV and HCV infections [14, 15].
In our MSM model, EarlyTest compared favorably with the 2 other AHI algorithms based on p24 Ag detection. Similar to ID-NAT, Architect, even when performed “in house,” did not deliver POC results, and total costs of Architect therefore resembled those of the EarlyTest algorithm. The lower sensitivity of Architect for AHI compared with ID-NAT led to an unfavorable cost-effectiveness for Architect when compared with EarlyTest. Nevertheless, Architect compared favorably with Determine in settings with HIV prevalence >0.6%. Therefore, Architect may be a promising testing strategy in settings where it is readily available and ID-NAT is more expensive, especially because Architect may deliver results more rapidly than ID-NAT (ie, typically within 1 day).
In contrast, costs of the rapid Determine algorithm were markedly lower than those of EarlyTest. Cost items of Determine were similar to those for Antibody alone, with the major difference being that the Determine test may also detect some cases of AHI. While sensitivity of the Determine combo for AHI detection is disappointing, Determine may nevertheless represent a cost-effective alternative to community-based screening algorithms that use POC Antibody alone. This is true, in particular, for settings where limited resources may preclude use of ID-NAT or Architect screening and where there are populations with lower HIV prevalence rates. However, it has to be mentioned that sensitivities of the Determine test for AHI vary widely, with higher sensitivities found in frozen samples and even lower sensitivities in studies that evaluated real-life use [31, 32]. By assuming a 50% sensitivity of the test, which was calculated over all the studies (frozen samples and real life), our model suggests that Determine may be the only AHI screening algorithm that is cost-effective for very low HIV prevalence rates between 0.1% and 0.4% (ie, below the national average of 0.6% found in the United States).
Our study has several limitations. First, thresholds for defining cost-effectiveness and cost-savings relied on calculations from a previous cost model and may be subject to change. Also, calculations were based on 3000 tests per year, with proportions of 24% and 10% among all newly diagnosed HIV cases, being diagnosed at acute stage of infection (ie, AHI). The magnitude of effects will vary in other settings with differing numbers of annual tests and proportion of AHI diagnoses, although the qualitative result that EarlyTest is cost-effective for higher HIV prevalence rates is likely to hold over a wide range of parameter values. Further, cost items used to calculate costs of the algorithms may not reflect actual costs at other locations, especially in countries other than the United States. For these reasons, results are likely to differ in settings were NAT testing is much more expensive and the proportion of AHI diagnoses is ≤5%. As the model was limited to a 1-year time horizon, long-term cost-effectiveness was not assessed. Also, although we tried our best to estimate cost, the ultimate costs of fourth-generation HIV testing using Determine and Architect in a real-world setting are unknown. Finally, our model was based on an established HIV testing program (ie, infrastructure in place) and does not include the costs of program development.
In conclusion, community-based screening programs that use EarlyTest and Architect are associated with higher direct annual costs compared with programs that rely on POC testing only. Although more expensive, early identification of HIV infection using the dual EarlyTest screening algorithm may markedly reduce HIV transmission rates and, considering earlier models, is likely to be cost-effective not only among at-risk MSM in San Diego but also among other similar populations of MSM with HIV prevalence rates >0.4%. The Architect algorithm may be the second-best alternative for HIV prevalence rates ≥0.6%, while the Determine and Antibody algorithms may be promising only for HIV prevalence rates below the national average or for screening programs among populations that are tested less frequently and are therefore more likely to be diagnosed at the stage of established HIV infection.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by funds from the following: the Max Kade Foundation (Max Kade postdoctoral research grant), International Research Fellowship in NeuroAIDS (grant number R25-MH081482), and grants from the National Institutes of Health (grant numbers AI43638, AI074621, AI106039).
Potential conflicts of interest. M. H. served on the speakers' bureau of Merck. S. J. L. reported grant funding from Gilead Sciences, Inc. J. G.-Z. reports no conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Khanna AS, Goodreau SM, Gorbach PM, Daar E, Little SJ. Modeling the impact of post-diagnosis behavior change on HIV prevalence in southern California men who have sex with men (MSM). AIDS Behav 2014; 18:1523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009; 373:48–57. [DOI] [PubMed] [Google Scholar]
- 3.Paltiel AD, Walensky RP, Schackman BR et al. . Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med 2006; 145:797–806. [DOI] [PubMed] [Google Scholar]
- 4.Walensky RP, Freedberg KA, Weinstein MC, Paltiel AD. Cost-effectiveness of HIV testing and treatment in the United States. Clin Infect Dis 2007; 45(suppl 4):S248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxenius A, Price DA, Easterbrook PJ et al. . Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci U S A 2000; 97:3382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MS, Gay CL, Busch MP, Hecht FM. The detection of acute HIV infection. J Infect Dis 2010; 202(suppl 2):S270–7. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention (CDC). National HIV testing day and new testing recommendations. MMWR Morb Mortal Wkly Rep 2014; 63:537. [PMC free article] [PubMed] [Google Scholar]
- 8.Pilcher CD, Louie B, Facente S et al. . Performance of rapid point-of-care and laboratory tests for acute and established HIV infection in San Francisco. PLoS One 2013; 8:e80629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eshleman SH, Khaki L, Laeyendecker O et al. . Detection of individuals with acute HIV-1 infection using the ARCHITECT HIV Ag/Ab combo assay. J Acquir Immune Defic Syndr 2009; 52:121–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavez P, Wesolowski L, Patel P, Delaney K, Owen SM. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab combo assay. J Clin Virol 2011; 52(suppl 1):S51–5. [DOI] [PubMed] [Google Scholar]
- 11.Karris MY, Anderson CM, Morris SR, Smith DM, Little SJ. Cost savings associated with testing of antibodies, antigens, and nucleic acids for diagnosis of acute HIV infection. J Clin Microbiol 2012; 50:1874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos EM, Harb S, Dragavon J, Swenson P, Stekler JD, Coombs RW. Performance of an alternative HIV diagnostic algorithm using the ARCHITECT HIV Ag/Ab combo assay and potential utility of sample-to-cutoff ratio to discriminate primary from established infection. J Clin Virol 2013; 58(suppl 1):e38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krajden M, Cook D, Mak A et al. . Pooled nucleic acid testing increases the diagnostic yield of acute HIV infections in a high-risk population compared to 3rd and 4th generation HIV enzyme immunoassays. J Clin Virol 2014; 61:132–7. [DOI] [PubMed] [Google Scholar]
- 14.Stramer SL, Glynn SA, Kleinman SH et al. . Detection of HIV-1 and HCV infections among antibody-negative blood donors by nucleic acid-amplification testing. N Engl J Med 2004; 351:760–8. [DOI] [PubMed] [Google Scholar]
- 15.Dorsey KA, Moritz ED, Steele WR, Eder AF, Stramer SL. A comparison of human immunodeficiency virus, hepatitis C virus, hepatitis B virus, and human T-lymphotropic virus marker rates for directed versus volunteer blood donations to the American Red Cross during 2005 to 2010. Transfusion 2013; 53:1250–6. [DOI] [PubMed] [Google Scholar]
- 16.Seth P, Wang G, Collins NT, Belcher L. Identifying new positives and linkage to HIV medical care—23 testing site types, United States, 2013. MMWR Morb Mortal Wkly Rep 2015; 64:663–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Romley JA, Juday T, Solomon MD, Seekins D, Brookmeyer R, Goldman DP. Early HIV treatment led to life expectancy gains valued at $80 billion for people infected in 1996–2009. Health Aff (Millwood) 2014; 33:370–7. [DOI] [PubMed] [Google Scholar]
- 18.Facente SN, Pilcher CD, Hartogensis WE et al. . Performance of risk-based criteria for targeting acute HIV screening in San Francisco. PLoS One 2011; 6:e21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherlock M, Zetola NM, Klausner JD. Routine detection of acute HIV infection through RNA pooling: survey of current practice in the United States. Sex Transm Dis 2007; 34:314–6. [DOI] [PubMed] [Google Scholar]
- 20.Hoenigl M, Green N, Mehta SR, Little SJ. Risk factors for acute and early HIV infection among men who have sex with men (MSM) in San Diego, 2008–2014: a cohort study. Medicine 2015; 94:e1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoenigl M, Weibel N, Mehta SR et al. . Development and validation of the San Diego early test (SDET) score to predict acute and early HIV infection risk in men who have sex with men. Clin Infect Dis 2015; 61:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoenigl M, Anderson CM, Green N, Mehta SR, Smith DM, Little SJ. Repeat HIV-testing is associated with an increase in behavioral risk among men who have sex with men: a cohort study. BMC Med 2015; 13:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le T, Wright EJ, Smith DM et al. . Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013; 368:218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiebig EW, Wright DJ, Rawal BD et al. . Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003; 17:1871–9. [DOI] [PubMed] [Google Scholar]
- 25.Pavie J, Rachline A, Loze B et al. . Sensitivity of five rapid HIV tests on oral fluid or finger-stick whole blood: a real-time comparison in a healthcare setting. PLoS One 2010; 5:e11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. US Food and Drug Administration. Summary basis for regulatory action—Procleix Ultrio assay, 2015. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/BloodDonorScreening/InfectiousDisease/UCM244479.pdf. Accessed 2 July 2015.
- 27.Pandori MW, Hackett J Jr, Louie B et al. . Assessment of the ability of a fourth-generation immunoassay for human immunodeficiency virus (HIV) antibody and p24 antigen to detect both acute and recent HIV infections in a high-risk setting. J Clin Microbiol 2009; 47:2639–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghisetti V, Calcagno A, Burdino E, Orofino G, Bonora S. Acute HIV infection: improved algorithms for HIV testing. J Clin Virol 2015; 63:51–2. [DOI] [PubMed] [Google Scholar]
- 29.Faraoni S, Rocchetti A, Gotta F et al. . Evaluation of a rapid antigen and antibody combination test in acute HIV infection. J Clin Virol 2013; 57:84–7. [DOI] [PubMed] [Google Scholar]
- 30.Fox J, Dunn H, O'Shea S. Low rates of p24 antigen detection using a fourth-generation point of care HIV test. Sex Transm Infect 2011; 87:178–9. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg NE, Kamanga G, Phiri S et al. . Detection of acute HIV infection: a field evaluation of the determine(R) HIV-1/2 Ag/Ab combo test. J Infect Dis 2012; 205:528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duong YT, Mavengere Y, Patel H et al. . Poor performance of the determine HIV-1/2 Ag/Ab combo fourth-generation rapid test for detection of acute infections in a national household survey in Swaziland. J Clin Microbiol 2014; 52:3743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farnham PG, Sansom SL, Hutchinson AB. How much should we pay for a new HIV diagnosis? A mathematical model of HIV screening in US clinical settings. Med Decis Making 2012; 32:459–69. [DOI] [PubMed] [Google Scholar]
- 34.Patel P, Klausner JD, Bacon OM et al. . Detection of acute HIV infections in high-risk patients in California. J Acquir Immune Defic Syndr 2006; 42:75–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stekler J, Swenson PD, Wood RW, Handsfield HH, Golden MR. Targeted screening for primary HIV infection through pooled HIV-RNA testing in men who have sex with men. AIDS 2005; 19:1323–5. [DOI] [PubMed] [Google Scholar]
- 36.Farnham PG, Gopalappa C, Sansom SL et al. . Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: late versus early diagnosis and entry into care. J Acquir Immune Defic Syndr 2013; 64:183–9. [DOI] [PubMed] [Google Scholar]
- 37.Fox J, White PJ, Macdonald N et al. . Reductions in HIV transmission risk behaviour following diagnosis of primary HIV infection: a cohort of high-risk men who have sex with men. HIV Med 2009; 10:432–8. [DOI] [PubMed] [Google Scholar]
- 38.Cipriano LE, Zaric GS, Holodniy M, Bendavid E, Owens DK, Brandeau ML. Cost effectiveness of screening strategies for early identification of HIV and HCV infection in injection drug users. PLoS One 2012; 7:e45176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juusola JL, Brandeau ML, Long EF, Owens DK, Bendavid E. The cost-effectiveness of symptom-based testing and routine screening for acute HIV infection in men who have sex with men in the USA. AIDS 2011; 25:1779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.