While tenofovir-containing antiretroviral therapy is generally considered superior to zidovudine, our analysis demonstrates a higher rate of virologic failure when tenofovir, as compared with zidovudine, was combined with nevirapine-based first-line antiretroviral therapy in a Nigerian human immunodeficiency virus treatment program.
Keywords: zidovudine, tenofovir, nevirapine, virologic failure, antiretroviral therapy
Abstract
Background. Despite sparse efficacy data, tenofovir–emtricitabine or tenofovir–lamivudine plus nevirapine is used in many resource-constrained settings.
Methods. This retrospective cohort study included patients initiating nevirapine-based antiretroviral therapy (ART) with either tenofovir–emtricitabine or lamivudine (tenofovir group) or zidovudine–lamivudine (zidovudine group). Clinical, virologic, and immunologic evaluations were performed at baseline and every 6 months. Virologic failure was defined as 2 consecutive human immunodeficiency virus (HIV)-RNA values >1000 copies/mL. Patients were included from ART initiation until time of failure, regimen switch, discontinuation, or last HIV-RNA measurement. Cox proportional hazards regression was used to model factors influencing time to failure. Bias due to dependent censoring was investigated via inverse probability weighted pooled logistic regression.
Results. A total of 5547 patients were evaluated; 1484 (26.8%) were in the tenofovir group and 4063 (73.2%) were in the zidovudine group. In the adjusted model, tenofovir regimen (hazard ratio [HR], 1.47; 95% confidence interval [CI], 1.21–1.79) and higher baseline log10 HIV-RNA (HR, 1.15; 95% CI, 1.03–1.28) were associated with virologic failure. Higher baseline log10 CD4+ cell count (HR, 0.50; 95% CI, .40–.63) and increasing age (HR, 0.98; 95% CI, .97–.99) decreased the risk of virologic failure. Inverse probability weighting results were consistent with the primary analysis.
Conclusions. Compared with zidovudine–lamivudine, the use of tenofovir–lamivudine or emtricitabine in combination with nevirapine was a strong predictor of virologic failure in our cohort, which was not explained by other risk factors or criteria for regimen selection.
(See the Editorial Commentary by Manasa and Katzenstein on pages 519–20.)
Based on relative safety, efficacy, and convenience, the World Health Organization (WHO) recommends efavirenz, tenofovir, plus either emtricitabine or lamivudine as the sole, preferred first-line antiretroviral therapy (ART) regimen for human immunodeficiency virus (HIV)–infected adults living in resource-constrained settings [1]. When efavirenz cannot be used, nevirapine remains the alternate nonnucleoside reverse transcriptase inhibitor (NNRTI) option, given with the following 2 nucleoside reverse transcriptase inhibitors (NRTIs): zidovudine plus lamivudine or tenofovir plus either emtricitabine or lamivudine [1]. When combined with efavirenz, tenofovir is superior to zidovudine. Specifically, higher rates of virologic suppression were reported among patients on efavirenz-based ART who received tenofovir–emtricitabine vs zidovudine–lamivudine (81% vs 70%; P = .005) in addition to lower rates of ART discontinuation due to adverse events (4% vs 9%, respectively, P = .02) [2].
In clinical practice, the strength of tenofovir was extrapolated from these data to nevirapine-based regimens, despite the lack of associated outcome data. Simultaneously, there was a global focus on increasing access to tenofovir to avoid zidovudine-related toxicities. Consistent with WHO guidelines in 2008, approximately 20% of patients on first-line ART in our clinical program were receiving a tenofovir plus nevirapine-containing regimen; only nevirapine plus zidovudine and lamivudine was more commonly used. Since then, the efficacy of ART containing tenofovir and nevirapine has been questioned by results from small prospective [3–6] and larger retrospective studies [7–10], suggesting that higher rates of virologic failure or worse clinical outcomes occur with nevirapine plus tenofovir vs comparator regimens. Existing data are limited by the use of more potent comparator groups, small sample size, cross-sectional study design, or lack of virologic outcomes.
To address this gap, data from a large observational clinical cohort were used to conduct a robust comparison of virologic outcomes among HIV-infected patients on first-line, nevirapine-based ART. This study addresses the important question of which commonly used NRTI combination (zidovudine- vs tenofovir-containing) performs best with nevirapine; this is a question of great clinical significance for patients unable to take efavirenz. Accordingly, the primary objective was to evaluate the influence of the NRTI combination on time to virologic failure in our cohort.
METHODS
In this retrospective cohort study, prospectively collected data from a large clinical cohort in Nigeria were used. Between June 2004 and February 2012, the Harvard T. H. Chan School of Public Health (Harvard) collaborated with the AIDS Prevention Initiative in Nigeria Ltd./Gte. (APIN) to support HIV prevention, care, and treatment to over 160 000 patients, funded by the President's Emergency Plan for AIDS Relief (PEPFAR). Upon program entry, patients underwent a clinical examination and baseline laboratory testing including hematology and chemistry, CD4+ cell count (Cyflow, Partec), HIV-RNA (Cobas Amplicor Monitor Assay, Roche Diagnostics), hepatitis B virus (HBV) surface antigen (Monolisa HBsAg Ultra3, BioRad), and hepatitis C virus (HCV) antibody (DIA.PRO Diagnostic, Bioprobes srl) assessments. Patients eligible for ART, based on guidelines at the time of program entry, initiated standard first-line ART with 1 NNRTI and 2 NRTIs [11, 12]. Prescription refills were obtained monthly; clinical visits and laboratory tests were performed every 6 months, or earlier if necessary. All patient data were maintained in previously described electronic databases [13].
Ethical Considerations
All patients in the Harvard–APIN program provided written consent for care. Only those who consented to the use of their information for research were included in this analysis. Institutional review boards at Harvard and the individual clinics (Jos University Teaching Hospital, Nigerian Institute of Medical Research, 68 Military Hospital, and University of Maiduguri Teaching Hospital) approved the treatment protocol and data collection forms. Because we used de-identified data, the study was verified exempt from human subject review by institutional review boards.
Patient Selection
Antiretroviral-naive, HIV-1–infected adults (aged ≥18 years) who initiated nevirapine-based ART with zidovudine–lamivudine (zidovudine group) or nevirapine-based ART with tenofovir–emtricitabine or tenofovir–lamivudine (tenofovir group) from 2006 to 2008 were included. Patients with prior antiretroviral experience, missing baseline HIV-RNA or CD4+ cell count data, no follow-up HIV-RNA after at least 6 months on treatment, no ART refills, or inconsistent ART regimens over 6 months (ie, regimen changed with each refill) were excluded. Patient data were included from time of ART initiation (baseline) through 18 months post-ART initiation.
Patients in the zidovudine group received zidovudine 300 mg, lamivudine 150 mg, and nevirapine 200 mg twice daily as a fixed dose combination (FDC) tablet (Aspen Pharmacare or Aurobindo Pharma Limited). Patients in the tenofovir group received nevirapine (Aurobindo) 200 mg twice daily with a once-daily FDC tablet of tenofovir–emtricitabine 300 mg–200 mg (Truvada, Aspen Pharmacare) or tenofovir–lamivudine 300 mg–300 mg (Matrix Laboratories Limited); use of the emtricitabine- or lamivudine-containing product was based on availability during the study period.
Study Definitions
Sex, age, HBV or HCV coinfection, and WHO HIV clinical stage were evaluated at baseline. Tuberculosis coinfection and ART adherence were evaluated throughout the observation period. Adherence was estimated using ART refill data from baseline through the study-defined event or censoring [14, 15]. The average adherence rate was calculated as the proportion of time the patient had pills available (number of days supplied/number of days between refills) and was truncated at 100%. A prespecified adherence cutoff of 95% was used for categorical assessment of adherence.
Study events were confirmed virologic failure, ART switch, discontinuation, and unconfirmed virologic failure. Confirmed virologic failure was the primary outcome variable. In accordance with the clinical protocol, confirmed virologic failure was defined as 2 consecutive HIV-RNA >1000 copies/mL after 6 months on ART or switch to a second-line ART regimen, defined as protease inhibitor–based ART following an HIV-RNA >400 copies/mL. Time to virologic failure was the time from ART initiation to the first HIV-RNA >1000 copies/mL or the first protease inhibitor-based prescription. ART switch was defined as a change in ART for reasons other than virologic failure, with the exception of a switch between emtricitabine and lamivudine. The ART switch date was the date on which the new ART regimen was first dispensed. Discontinuation was defined as death, lost to follow-up (no ART refill for 6 months), or transferred or withdrew from the program. Time to discontinuation was calculated using the last recorded ART dispense date. Unconfirmed virologic failure was included to evaluate the potential influence of requiring 2 HIV-RNA results as our primary endpoint and was defined as HIV-RNA >1000 copies/mL without a consecutive confirmatory HIV-RNA measurement. Time to unconfirmed virologic failure was calculated using the date when the HIV-RNA measurement was >1000 copies/mL. Follow-up time was censored at the first event, and subsequent events were not included. For patients who did not experience a study event, follow-up was censored at last HIV-RNA within the study period.
Statistical Analyses
Subject demographics were compared between groups using the Wilcoxon rank sum test for continuous variables or the χ2 test of independence for categorical variables.
Time to Virologic Failure Regression Modeling
Bivariate analyses were used to evaluate variables for inclusion in the Cox proportional hazards model; categorical variables were evaluated using the log-rank test, and continuous variables were evaluated using Cox proportional hazards regression [16]. Variables with a bivariate P < .25 and clinically relevant variables (adherence, age, serum creatinine) were considered candidates for inclusion in the initial Cox model. Kaplan–Meier graphs were generated for categorical variables to reveal violations of the Cox model's assumption of proportionality.
A Cox proportional hazards model was fitted by means of manual backward elimination of single covariates based on the likelihood ratio test and the comparison of hazard ratios between the model with and without the covariate in question. All statistically significant covariates, clinically relevant covariates (significant or not), effect modifiers, and confounders were retained in the final model. The scale of continuous covariates was evaluated using the design variable method. All interactions were examined using the likelihood ratio test. The proportionality assumption was assessed for covariates using Schoenfeld residuals, and the overall fit of the model was evaluated using Cox–Snell residuals.
Evaluation of Dependent Censoring
The possibility of bias due to dependent censoring was evaluated by fitting a time-updated Cox regression model to assess the association between time-varying factors for each study-defined event [17]. Because clinical visits occur at 6-month intervals, laboratory values were carried forward up to 7 months to accommodate late visits. Percent adherence values were carried backward because adherence is calculated at the end of a prescription refill cycle. An inverse probability weighting (IPW) analysis was performed to assess potential bias due to dependent censoring by competing outcomes by fitting an IPW pooled logistic regression model using only uncensored person-time observations for the outcome of interest [18].
RESULTS
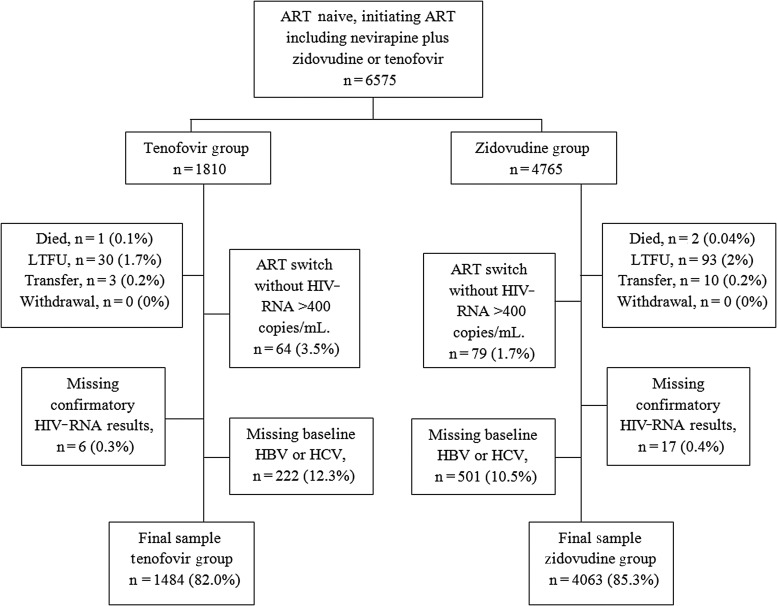
During the study period, 6575 patients were identified for inclusion and 5547 (84%) had data available for all variables in the final model—4063 (73.2%) in the zidovudine group and 1484 (26.8%) in the tenofovir group (Figure 1). The most common reason for exclusion was missing HBV or HCV status (11.0%). Baseline participant characteristics are summarized in Table 1. Consistent with clinical considerations for selecting an NRTI, HBV coinfection was more common among those in the tenofovir vs zidovudine group (27.2% vs 14.9%; P < .001), as was a lower median baseline hemoglobin (100 vs 110 g/L, P < .001). Proportionally, more women, patients with HCV coinfection, and those with more advanced HIV disease received tenofovir. Median (interquartile range [IQR]) duration of time to event or censor was slightly longer for the zidovudine group compared with the tenofovir group (392 [244] vs 376 [170] days, P < .001).
Figure 1.
Flow diagram of eligible patients included in the cohort analysis in the final Cox proportional hazards model. Abbreviations: ART, antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; LTFU, lost to follow-up.
Table 1.
Baseline Characteristics and Outcomes of Study Participants
| Tenofovir Group n = 1484 | Zidovudine Group n = 4063 | P Value | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age (y) | 33 (11) | 32 (11) | .41 |
| Female gender | 1255 (84.6) | 3085 (75.9) | <.001 |
| Hepatitis B virus coinfection | 404 (27.2) | 604 (14.9) | <.001 |
| Hepatitis C virus coinfection | 144 (9.7) | 294 (7.2) | .003 |
| CD4 count (cells/mm3) | 122 (122) | 143 (115) | <.001 |
| Human immunodeficiency virus-RNA (log10 copies/mL) | 4.86 (1.1) | 4.70 (1.2) | <.001 |
| World Health Organization Stage | n = 1335 | n = 3489 | <.001 |
| 1 | 236 (17.7) | 923 (26.5) | |
| 2 | 435 (32.6) | 1042 (29.9) | |
| 3 | 494 (37.0) | 1045 (30.0) | |
| 4 | 170 (12.7) | 479 (13.7) | |
| Tuberculosis coinfection | 294 (19.8) | 466 (11.5) | <.001 |
| Alanine transaminase (µkat/L) | 0.42 (0.37) | 0.40 (0.36) | .04 |
| Hemoglobin (g/L) | 100 (24), n = 1462 | 110 (20), n = 3948 | <.001 |
| Creatinine (μmol/L) | 77 (31), n = 1458 | 77 (31), n = 3880 | .943 |
| Study Outcomes | |||
| Virologic failure | 159 (10.7) | 298 (7.3) | <.001 |
| Antiretroviral therapy switch | 256 (17.3) | 622 (15.3) | .079 |
| Discontinue | 308 (20.8) | 649 (16.0) | <.001 |
| No event | 761 (51.3) | 2494 (61.4) | <.001 |
Values shown as either median (interquartile range) or n (%); percent is based on the total n (or category n) of the regimen group, as appropriate. The Wilcoxon rank sum test was used for continuous variables and the χ2 test of independence was used for categorical variables.
Among patients in the tenofovir group, 1043 (70.2%) received emtricitabine as their second NRTI, 190 (12.8%) received lamivudine, and 252 (19.8%) switched between emtricitabine and lamivudine during the study period (median [IQR] time to switch was 172 [97] days). The number of virologic failure events did not differ significantly between patients receiving emtricitabine or lamivudine (P = .47); therefore, data were combined in the final analysis irrespective of emtricitabine or lamivudine use.
Overall, the median ART adherence rate was 95.3%, which was similar between the zidovudine and tenofovir groups (95.2% vs 95.8%). Among those who experienced virologic failure, more patients had adherence <95% compared with ≥95% (56.4% vs 43.6%; P < .001), but there was no difference in median adherence between those patients with failure in the zidovudine or tenofovir groups (91.9% vs 93.6%; P = .36).
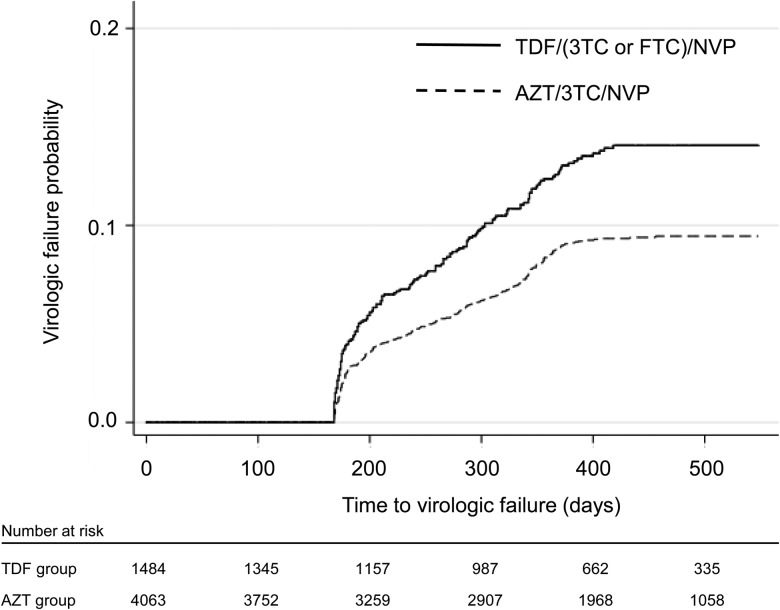
Patients in the tenofovir group had a higher rate of virologic failure and discontinuation (Table 1). Figure 2 represents time to virologic failure between the groups. Of the 957 patients with a discontinuation event, 14 (1.5%) died, 849 (88.7%) were lost to follow-up, 92 (9.6%) transferred to another site, and 2 (0.2%) withdrew from the program. These distributions were similar between NRTI groups (P = .50).
Figure 2.
Kaplan–Meier graph of the time to confirmed virologic failure, defined as the time of the first human immunodeficiency virus-RNA >1000 copies/mL, for the tenofovir (TDF) and zidovudine (AZT) groups. Time is represented as days after initiating nevirapine (NVP)-containing first-line antiretroviral therapy. Abbreviations: 3TC, lamivudine; FTC, emtricitabine.
Time to Virologic Failure
After adjusting for other baseline risk factors, patients in the tenofovir group had higher risk of virologic failure (adjusted hazard ratio [aHR], 1.47; 95% confidence interval [CI], 1.21–1.79; Table 2). Consistent with indicators of disease progression, the risk of virologic failure decreased with a higher baseline CD4+ cell count (aHR, 0.50; 95% CI, .40–.63), while a higher baseline HIV-RNA increased the risk of failure (aHR, 1.15; 95% CI, 1.03–1.28). Adherence was omitted from the final Cox model because it may have been on the causal pathway (ie, adherence is a potential mediator of the effect of regimen assignment) [19, 20]. Given its clinical importance, the Cox model that includes adherence is presented in Supplementary Table 1. While adherence <95% increased the risk of virologic failure (aHR, 1.28; 95% CI, 1.07–1.54), the inclusion of adherence in the Cox model had no notable effect on the HR or CI of the remaining covariates (tenofovir group aHR, 1.48; 95% CI, 1.21–1.80).
Table 2.
Risk Estimates and 95% Confidence Intervals for Virologic Failure During 18 Months of First Line Antiretroviral Therapy
| Factor | Cox Proportional Hazards Model (n = 5547 Patients) |
Inverse Probability Weighted Pooled Logistic Regression (5697 Patients, 81 642 Observations) |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | OR | 95% CI | P Value | |
| Tenofovir group | 1.47 | 1.21, 1.79 | .001 | 1.57 | 1.28, 1.94 | .0001 |
| Age | 0.98 | .97, .99 | .002 | 0.99 | .98, 1.00 | .122 |
| Female gender | 0.91 | .71, 1.17 | .474 | 0.88 | .69, 1.13 | .309 |
| Hepatitis B virus coinfection | 0.96 | .75, 1.23 | .717 | 0.98 | .78, 1.23 | .862 |
| Hepatitis C virus coinfection | 1.17 | .85, 1.61 | .326 | 0.91 | .66, 1.26 | .554 |
| CD4 count (log10) | 0.50 | .40, .63 | <.001 | 0.997 | .995, .998 | <.0001 |
| Human immunodeficiency virus-RNA (log10) | 1.15 | 1.03, 1.28 | .011 | 1.20 | 1.01, 1.32 | .018 |
| Tuberculosis coinfection | … | … | … | 0.72 | .50, 1.05 | .085 |
| Alanine transaminase (µkat/L) | … | … | … | 1.11 | 1.00, 1.22 | .052 |
| Hemoglobin (g/L) | … | … | … | 1.00 | .99, 1.00 | .415 |
| Creatinine (μmol/L) | … | … | … | 0.997 | .993, .999 | .013 |
All risk factors were measured at baseline.
Bold results indicate statistically significant results (P value <.05).
Abbreviations: CI, confidence interval; HR, hazard ratio; OR, odds ratio.
When the Cox analysis was restricted to virologic failures that occurred beyond 12 months, the regimen was no longer associated with virologic failure (aHR, 1.99; 95% CI, .94–4.21). However, the number of participants and the number of events that occurred during the 12–18 months were small (n = 3120 participants with 37 virologic failure events).
Evaluation of Other Outcome Differences or Dependent Censoring
Additional outcome-specific, time-updated Cox models were constructed for each of our defined outcomes to evaluate the effect of regimen on events outside of virologic failure (Supplementary Table 2). Discontinuation and unconfirmed virologic failure outcomes were not associated with treatment regimen. ART switch was associated with treatment regimen, indicating that patients in the tenofovir group were 34% more likely to switch regimens (aHR, 1.34; 95% CI, 1.15–1.57). The effect of regimen on time to virologic failure was consistent between the primary and time-updated models and reflected a 47% increase in the risk of virologic failure for patients in the tenofovir group (aHR, 1.47; 95% CI, 1.24–1.75). While these models did not rule out dependent censoring, we found an overall agreement between IPW analysis results (Table 2) and the primary Cox proportional hazards results related to the impact of the study group on virologic failure, demonstrating that dependent censoring did not compromise the original nontime-updated Cox model estimates.
DISCUSSION
The Harvard–APIN program initially identified a high rate of confirmed virologic failure among patients on nevirapine plus tenofovir and emtricitabine or lamivudine compared with other WHO-recommended or alternative first-line regimens [7]. To evaluate the specific impact of the NRTI combination with nevirapine, we focused on patients initiating nevirapine-based ART combined with either zidovudine or tenofovir using Cox proportional hazards, time varying analysis, and IPW modeling. These results indicate that among patients initiating nevirapine, the use of tenofovir carried a 47% greater risk of virologic failure compared with the use of zidovudine; this difference persisted after adjusting for other variables that may influence regimen selection and treatment outcomes (Table 2). Tenofovir benefits were not observed in other outcomes; specifically, the tenofovir group had higher risk for regimen switch and similar risk for discontinuation. Notably, we did not observe significantly higher virologic failure in the tenofovir group after 12 months of ART, suggesting that patients already virologically suppressed on nevirapine plus tenofovir may not be at increased risk for virologic failure. These data have significant clinical implications for adults living in low- and middle-income countries who require an alternative to efavirenz for first-line ART [1].
Our results are consistent with those from other evaluations of outcomes among patients who received ART containing nevirapine and tenofovir, thus supporting the belief that NNRTI effectiveness differs by NRTI selection. The DAUFIN study, which evaluated the effectiveness and tolerability of nevirapine given either with zidovudine–lamivudine or tenofovir–lamivudine in antiretroviral-naive patients, was terminated early due to high rates of early virologic failure in the tenofovir arm [6]; 9 of 36 patients receiving tenofovir experienced virologic failure, 8 during the first 12 weeks of the study, compared with only 1 of 35 patients receiving zidovudine. Lapadula et al also reported early virologic failure in patients receiving tenofovir–emtricitabine plus nevirapine compared with ritonavir-boosted atazanavir [5]. In another pilot study that described virologic outcomes among 23 patients receiving once-daily nevirapine plus tenofovir–lamivudine, only 10 patients achieved HIV-RNA <75 copies/mL by week 24 [3]. Turning to large clinical cohorts that evaluated this question, among 10 256 antiretroviral-naive patients initiating either zidovudine or tenofovir in combination with nevirapine in Zambia, 90-day mortality was higher among patients who received tenofovir (aHR, 1.45; 95% CI, 1.03–2.06); however, this difference did not remain after sensitivity analyses, including time-varying analysis [9]. In adjusted models from a large cohort study from southern Africa, patients on nevirapine plus tenofovir–emtricitabine or tenofovir–lamivudine experienced significantly higher rates of mortality but lower rates of loss to follow-up when compared with patients taking efavirenz plus similar NRTIs [21]. Consistent with these data, a metaanalysis of 33 studies comparing virologic efficacy among the WHO-recommended tenofovir-containing first-line ART regimens highlighted poorer outcomes with the nevirapine plus tenofovir-containing regimens [22].
The reason for the poorer performance of nevirapine–tenofovir ART is unclear. One proposed mechanism is an intracellular drug–drug interaction observed in vitro between tenofovir and nevirapine, resulting in lower intracellular concentrations of both antiretrovirals [23]. Differences in lamivudine vs emtricitabine pharmacokinetics have also been proposed. An observational Dutch cohort study identified a higher risk of virologic failure when lamivudine vs emtricitabine was combined with tenofovir and either nevirapine (aHR 2.01; 95% CI, 1.36–2.98) or efavirenz (aHR, 2.35; 95%CI, 1.61–3.42) [24]. Notably, our data did not identify a difference in outcomes among patients in the tenofovir group who received lamivudine or emtricitabine. Our results are consistent with those from a metaanalysis of 12 randomized controlled trials that compared 4913 patients randomized to lamivudine or emtricitabine in addition to various ART combinations and found that the 2 agents are clinically interchangeable [25].
Our findings are limited by the study's retrospective cohort design. At the time of data collection, WHO guidelines recommended the use of either nevirapine or efavirenz in combination with zidovudine and lamivudine or with tenofovir and emtricitabine or lamivudine for first-line ART [11]. Regimen selection may have been influenced by factors that we were unable to measure or control for in our analysis, indicated by differences in baseline characteristics (Table 1). To account for some potential biases from dependent censoring, we generated an IPW pooled logistic regression model, and the close correspondence between these models testifies to the robustness of the Cox estimates. Additionally, there may be limitations to measuring adherence based on prescription refill data, particularly as it relates to pill burden. Although all patients received nevirapine twice daily, there was a difference between groups in the total number of pills per day. The zidovudine group received nevirapine plus a twice-daily FDC tablet of zidovudine–lamivudine (4 pills daily) or, beginning early 2007, a twice-daily full regimen FDC tablet (2 pills daily). The tenofovir group received nevirapine plus a once-daily FDC tablet of tenofovir–emtricitabine or tenofovir–lamivudine (3 pills daily). For twice-daily regimens, higher pill burden has previously been associated with lower rates of both adherence and virologic suppression (Spearman correlation, −0.67 and −0.75, respectively; both P < .01) [26]. However, a difference in adherence was not identified between our study groups and did not influence the associated risk of virologic failure related to regimen selection.
In conclusion, ART that includes tenofovir and nevirapine should be initiated with caution. The WHO recommends nevirapine as the alternative for patients who cannot take efavirenz, and nevirapine remains widely used for first-line ART in low- and middle-income countries [1, 27]. These results offer important information to guide selection of the NRTI combination when designing an HIV treatment regimen, indicating a preference for zidovudine over tenofovir when combined with nevirapine. These findings support the need for systematic evaluations of ART combinations before their inclusion in ART guidelines and subsequent widespread implementation.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. The authors gratefully acknowledge the patients and the clinical, data, and laboratory staff at all of the Harvard–APIN PEPFAR ART sites for their incredible work and dedication.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The contents are solely the responsibility of the authors and do not represent the official views of the funding institutions.
Financial support. This work was supported, in part, through grants from the US Department of Health and Human Services, Health Resources and Services Administration (grant number U51HA02522), the Centers for Disease Control and Prevention through a cooperative agreement with APIN (grant number PS 001058), and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant number AI104459).
Potential conflicts of interest. R. L. M. is a member of the Data Safety Monitoring Board for Gilead Sciences. P. J. K., P. O., and O. O. A. received grant support from Diagnostics for the Real World, University of Cambridge, United Kingdom. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: World Health Organization, 2013. [PubMed] [Google Scholar]
- 2.Gallant JE, DeJesus E, Arribas JR et al. . Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med 2006; 354:251–60. [DOI] [PubMed] [Google Scholar]
- 3.Towner W, Kerrigan HL, LaRiviere M et al. . Efficacy of a once daily (QD) regimen of nevirapine (NVP), lamivudine (3TC), and tenofovir (TDF) in treatment-naïve HIV infected patients: a pilot study [abstract P49]. In: 7th International Congress on Drug Therapy in HIV Infection, Glasgow, 14–18 November 2004. [Google Scholar]
- 4.Clumeck N, Mwamba C, Kabeya K et al. . First-line antiretroviral therapy with nevirapine versus lopinavir-ritonavir based regimens in a resource-limited setting. AIDS 2014; 28:1143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapadula G, Costarelli S, Quiros-Roldan E et al. . Risk of early virological failure of once-daily tenofovir-emtricitabine plus twice-daily nevirapine in antiretroviral therapy-naive HIV-infected patients. Clin Infect Dis 2008; 46:1127–9. [DOI] [PubMed] [Google Scholar]
- 6.Rey D, Hoen B, Chavanet P et al. . High rate of early virological failure with the once-daily tenofovir/lamivudine/nevirapine combination in naive HIV-1-infected patients. J Antimicrob Chemother 2009; 63:380–8. [DOI] [PubMed] [Google Scholar]
- 7.Darin K, Scarsi K, Meloni S et al. . Clinical and virologic outcomes of six first-line regimens in a large ART program in Nigeria [abstract THPE0117]. In: XVIII International AIDS Conference, Vienna, 18–23 July 2010. [Google Scholar]
- 8.Amoroso A, Etienne-Mesubi M, Edozien A et al. . Treatment outcomes of recommended first-line antiretroviral regimens in resource-limited clinics. J Acquir Immune Defic Syndr 2012; 60:314–20. [DOI] [PubMed] [Google Scholar]
- 9.Chi BH, Mwango A, Giganti MJ et al. . Comparative outcomes of tenofovir-based and zidovudine-based antiretroviral therapy regimens in Lusaka, Zambia. J Acquir Immune Defic Syndr 2011; 58:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shearer K, Fox MP, Maskew M, Berhanu R, Long L, Sanne I. The impact of choice of NNRTI on short-term treatment outcomes among HIV-infected patients prescribed tenofovir and lamivudine in Johannesburg, South Africa. PloS One 2013; 8:e71719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Geneva, Switzerland: World Health Organization, 2006. rev. [PubMed] [Google Scholar]
- 12. Federal Ministry of Health. National guidelines for HIV and AIDS treatment and care in adolescents and adults. Abuja, Nigeria: Federal Ministry of Health, 2007. [Google Scholar]
- 13.Chaplin B, Meloni S, Eisen G et al. . Scale-up of networked HIV treatment in Nigeria: creation of an integrated electronic medical records system. Int J Med Inform 2015; 84:58–68. [DOI] [PubMed] [Google Scholar]
- 14.Bisson GP, Gross R, Bellamy S et al. . Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Med 2008; 5:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sangeda RZ, Mosha F, Prosperi M et al. . Pharmacy refill adherence outperforms self-reported methods in predicting HIV therapy outcome in resource-limited settings. BMC Public Health 2014; 14:1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosmer D, Lemeshow S, May S. Applied survival analysis. 2nd ed Hoboken, NJ: John Wiley & Sons, 2008. [Google Scholar]
- 17.Andersen P, Gill R. Cox's regression model for counting processes: a large sample study. Ann Stat 1982; 10:1100–20. [Google Scholar]
- 18.Fewell Z, Hernán M, Wolfe F, Tilling K, Choi H, Sterne J. Controlling for time-dependent confounding using marginal structural models. Stata J 2004; 4:402–20. [Google Scholar]
- 19.Cole SR, Hernan MA. Fallibility in estimating direct effects. Int J Epidemiol 2002; 31:163–5. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum PR. The consequences of adjustment for a concomitant variable that has been affected by the treatment. J Roy Statist Soc A 1984; 147;656–66. [Google Scholar]
- 21.Wandeler G, Moyo C, Ehmer J et al. . Nevirapine vs efavirenz in first-line ART containing tenofovir disoproxil fumarate: Southern Africa. In: 19th Conference on Retroviruses and Opportunistic Infections, Seattle, 5–8 March 2012 Paper #635. [Google Scholar]
- 22.Tang MW, Kanki PJ, Shafer RW. A review of the virological efficacy of the 4 World Health Organization-recommended tenofovir-containing regimens for initial HIV therapy. Clin Infect Dis 2012; 54:862–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liptrott NJ, Curley P, Moss D, Back DJ, Khoo SH, Owen A. Interactions between tenofovir and nevirapine in CD4+ T cells and monocyte-derived macrophages restrict their intracellular accumulation. J Antimicrob Chemother 2013; 68:2545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rokx C, Fibriani A, van de Vijver DA et al. . Increased virological failure in naive HIV-1-infected patients taking lamivudine compared with emtricitabine in combination with tenofovir and efavirenz or nevirapine in the Dutch nationwide ATHENA cohort. Clin Infect Dis 2015; 60:143–53. [DOI] [PubMed] [Google Scholar]
- 25.Ford N, Shubber Z, Hill A et al. . Comparative efficacy of lamivudine and emtricitabine: a systematic review and meta-analysis of randomized trials. PloS One 2013; 8:e79981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nachega JB, Parienti JJ, Uthman OA et al. . Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis 2014; 58:1297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joint WHO/UNAIDS Annual Consultation with Pharmaceutical Companies and Stakeholders on Forecasting Global Demand of Antiretroviral Drugs for 2013–2016. 25–26 November 2013, Geneva, Switzerland, March 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.